Abstract
Approximately 280 Escherichia coli isolates were isolated from a bovine feedlot at the University of Connecticut campus via enrichment in lauryl tryptose broth and random selection from MacConkey plates. The E. coli subspecies diversity was estimated by employing whole-cell BOX-PCR genomic fingerprints. A total of 89 distinct operational taxonomic units (OTUs) were identified by employing a criterion of 85% fingerprint similarity as a surrogate for an OTU, while the Chao1 index estimated the E. coli population richness at 128 OTUs. One genotype (at a similarity level of 60%) dominated the population at 66% regardless of sampling depth or location, while no significant vertical distribution pattern was observed in terms of genotype, mobility, antibiotic resistance profile, or biofilm-forming ability. Motility, measured by a soft agar assay, had a very broad range among the E. coli population and was positively correlated with biofilm-forming ability in minimal medium (Spearman's rank correlation coefficient r = 0.619, P < 10−4) but not in Luria broth. Only an estimated 48% of the population possessed gene agn43, which encodes Ag43, a phase-variable outer membrane protein that has been implicated in biofilm formation in minimal medium. We observed significantly more biofilm formation in both minimal medium and Luria broth for agn43+ strains, with a larger effect in minimal medium. This study represents an exhaustive inventory of extant E. coli population diversity at a bovine feedlot and reveals significant subspecies heterogeneity in interfacial behavior.
Escherichia coli is a consistent and predominant facultative inhabitant of the human gastrointestinal tract (10). The regular presence of E. coli in the intestine and feces of warm-blooded animals makes this bacterium an indicator of fecal pollution. The grazing of cattle and land application of animal wastes may lead to the occurrence of enteric pathogens in nearby surface and groundwaters. This potential contamination due to animal husbandry operations can be a serious threat to public health (52). Therefore, the fate and transport of pathogenic microorganisms that are shed from cattle operations must be understood to evaluate and possibly mitigate the contamination of water supplies.
Soil exhibits a filtering capacity for microorganisms by the combined actions of straining, adsorption, and adhesion onto soil surfaces. Adhesion is commonly thought to be the main factor retarding bacterial transport in soil. The main factors that affect bacterial adhesion are ionic strength, the pH of the aqueous phase, and the surface properties of the geological matrix and bacterial cell (35, 39, 47, 58). The degree of adhesion to a solid surface can, however, change dramatically with the physiological state of the bacterium, due to changes in cell surface properties (4, 7, 27, 47). Whether this described soil filtering capacity explains the retardation or distribution of E. coli in soils affected by cattle activity remains untested.
The fate and distribution of a species in a natural environment may, in part, be governed by diversity within the species; hence, estimating this diversity is requisite. Several high-resolution molecular fingerprinting techniques have been used to reveal species and subspecies diversity (41, 46, 54). Ribotyping (1, 38) and repetitive extragenic palindromic PCR (2, 9) techniques have been successfully applied to cluster E. coli strains according to host type.
If the fate and transport of E. coli in soil-dominated environments are governed by interactions with solid matrices, then subspecies variability in genotype or phenotype related to surface adhesion might be expected to establish its population ecology. One mechanism for ensuring survival in the environment might be a differential biofilm-forming ability within a natural E. coli population. Although biofilm formation is the net result of multiple interacting molecular events (14, 22) and is most conveniently measured at the phenotypic level, a few discrete genetic systems may be essential to adhesion properties, and any population level variability in their occurrence appears worthy of study. Motility, for example, is a variable property within E. coli that may influence surface attachment and detachment (30, 55) and is required for biofilm formation in both rich and minimal environments (8, 40). Therefore, differences in motility may affect transport in the environment, as they might facilitate transport through porous media (42) or towards a surface (30, 40).
At the genotypic level, there are two phase-variable surface proteins, type 1 fimbriae and antigen 43 (Ag43), encoded by the fim gene cluster (25, 36) and agn43 (20), respectively, which have been suggested as critical in determining the adhesion properties of E. coli. Type 1 fimbriae are the most common adhesins produced by E. coli associated with colonization of extraintestinal locations such as the urinary tract (6). Located at the tip of each fimbria and also interspersed along the length (23), the FimH protein has been implicated in biofilm formation on abiotic surfaces under static growth conditions (40). On the other hand, Ag43, which extends beyond the lipopolysaccharide structure, is the most abundant phase-variable outer membrane protein in E. coli (37), and is regulated by competition between deoxyadenosine methyltransferase and the global regulator OxyR (56). Expression of Ag43 has not yet been tested as relevant for intestinal colonization but is implicated in biofilm formation in glucose-minimal but not in rich media (8). The natural habitat of E. coli is the gastrointestinal tract, where conditions are very different from the soil or laboratory environment in terms of nutrient composition, pH, and oxygen availability. Adhering to animal tissue or soil particles might be of fundamental importance in a bacterial life cycle. It is expected that conditions in the gastrointestinal tract would favor expression of FimH (34), while repression of Ag43 expression may provide a selective advantage by lowering susceptibility to phage infection (11). Furthermore, it has been suggested that fimbrial expression per se negatively affects the expression of agn43 by affecting the thiol-disulfide status of OxyR (44, 45). Phase variation, regulating the expression of fimbriae and Ag43 in a population, may result in subpopulations of cells with very different adhesion properties and may be an important factor in the selective colonization of surfaces.
The purpose of the present study was to isolate and describe the strain diversity of an E. coli population retrieved from a long-term operating bovine feedlot by employing a whole-genome fingerprinting technique. Additional parameters (the presence of fimH and agn43, motility, biofilm formation ability, and resistance to certain antibiotics) were also investigated to examine possible correlations with the vertical distribution of population diversity in the soil profile.
MATERIALS AND METHODS
Sample collection, bacterial enumeration and coliform isolation.
Three soil cores (SC-01, SC-02, and SC-03), 1.5 in. in diameter and 37 in. long, separated by 3 to 5 ft were collected at a long-term operating bovine feedlot at the University of Connecticut in mid-December 2000. The cores were collected by Geoprobe sampling in ethanol-sterilized sleeves, capped, and stored on ice before being transported to the lab. Sleeves were cut into 1-in. sections for the top 20 in. and 2-in. sections for the 20- to 37-in. interval. One gram of soil from each section was added to 3 ml of eluent buffer (0.1% Na4P2O7, 0.05% polyvinyl-pyrrolidone [pH 7.2]) and vortexed thoroughly for 5 min. Serial dilutions were made from the resulting supernatant in phosphate-buffered saline (0.1 M NaCl, 0.02 M sodium phosphate [pH 7]). Coliform and heterotroph cell enumerations were performed by direct plating on lactose MacConkey (Difco Laboratories, Detroit, Mich.) and nutrient agar (Difco) plates, respectively. Another 1 g of soil from each section was added to 5 ml of lauryl tryptose broth (LTB) (Difco) with inverted fermentation vials and incubated at 37°C for 24 h. Aliquots from gas-producing tubes were streaked on MacConkey agar plates and incubated at 37°C for 24 h. Five to seven lactose-fermenting coliform colonies were randomly selected from each plate. Enterobacteriaceae type strains used in this study for comparison with feedlot isolates are listed in Table 1.
TABLE 1.
Enterobacteriaceae and other type strains used in this study
| Strain | Serotype | Source |
|---|---|---|
| Serratia marcescens | ATCC 8195 | |
| Serratia marcescens | ATCC 8100 | |
| Salmonella choleraesuis | ATCC 700720 | |
| Shigella flexneri | ATCC 29903 | |
| Klebsiella pneumoniae | ATCC 700831 | |
| Enterobacter aerogenes | ATCC 13048 | |
| Pseudomonas aeruginosa | ATCC 27853 | |
| Escherichia coli | ATCC 25922 | |
| Escherichia coli | O157:H7 | ATCC 700728 |
| Escherichia coli | O157:NM | ATCC 700375 |
| Escherichia coli | O19a,19b:K:H7 | ATCC 23514 |
| Escherichia coli | O55:K59(B5):H− | ATCC 12014 |
| Escherichia coli | O16:K92:H− | ATCC 35860 |
| Escherichia coli | O91:H21 | ATCC 51435 |
| Escherichia coli | O157:H8 | *a |
| Escherichia coli | O157:H16 | * |
| Escherichia coli | O157:H43 | * |
| Escherichia coli | O18:H7 | * |
| Escherichia coli | O18:H7 | * |
| Escherichia coli | O2:H7 | * |
| Escherichia coli | H7 | * |
| Escherichia coli | O5:H11 | * |
| Escherichia coli | O26:H11 | * |
| Escherichia coli | O18a,18c:K77:H7 | * |
| Escherichia coli | O18:H14 | * |
| Escherichia coli | O6:K2:H1 | * |
| Escherichia coli | O75:NM | * |
| Escherichia coli | O45:H19 | * |
*, E. coli strain provided by L. A. McLandsborough (28).
PCR conditions.
Bacterial cultures were pregrown in nutrient broth (Difco) for 8 h at 30°C and transferred by a microbial replicator tool to yield cell templates. A BLAST search was performed by using the Escherichia coli antigen 43 precursor gene as query sequence. Genes encoding Ag43-like proteins, including the Cah (calcium binding antigen 43 homologue) family (51), were used to identify consensus sequences, and a primer set was designed for a 499-bp fragment (GenBank accession number U24429; nucleotides [nt] 4799 to 5297) (Table 2). PCR was carried out in 96-well plates in a 20-μl volume containing 1× buffer, 2.5 mM MgCl2, 200 μM deoxynucleoside triphosphate, 0.6 U of AmpiTaq Gold (Perkin-Elmer Corp.), 0.5 μM (each) primer for agn43 and fimH, and 0.9 μM BOX A1R in an automated thermal cycler (GeneAmp PCR system 9700; Perkin-Elmer Corp.). PCR conditions were as listed in Table 2.
TABLE 2.
Primer sequences and PCR conditions
| Primer | Nucleotide sequence (GenBank accession number) | PCR conditions | Reference or source |
|---|---|---|---|
| agn43 | 5′-GACTATGACCGGATTSTGGCAGGCT-3′ (U24429; nt 4799 to 4823) | Initial denaturation at 95°C for 10 min, followed by 10 cycles consisting of 94°C for 30 s, 72 to 62°C for 1 min, and 72°C | This study |
| 5′-GTGGCTCCAGCATCAGRTTGTCAG-3′ (U24429; nt 5274 to 5297) | for 30 s, and then 35 cycles consisting of 94°C for 30 s, 62°C for 1 min, 72°C for 30 s, and a final extension cycle at 72°C for 5 min | ||
| fimH | 5′-CGTGCTTATTTTGCGACAGA-3′ | Initial denaturation at 95°C for 10 min, followed by 35 cycles | This study |
| (AF317710; nt 496 to 515) | consisting of 94°C for 30 s, 60°C for 1 min, 72°C for 30 s, | ||
| 5′-CTCCGGTACGTGCGTAATTT-3′ (AF317710; nt 877 to 896) | and a final extension cycle at 72°C for 5 min | ||
| BoxA1R | 5′-CTACGGCAAGGCGACGCTGACG-3′ | Initial denaturation at 95°C for 10 min, followed by 35 cycles consisting of 94°C for 30 s, 50°C for 1 min, 65°C for 8 min, and a final extension cycle at 65°C for 8 min | 9 |
Computer-assisted analysis of BOX-PCR DNA fingerprints.
PCR mixtures were electrophoresed on a 0.6% agarose gel supplemented with 0.4% SynerGel (Diversified Biotech) for 5.5 h at 74 V. Gels were stained with ethidium bromide (0.5 μg/ml) and destained in water for 20 min with shaking at room temperature. Images were captured and saved directly as TIFF files and processed by BioNumerics 3.0 (Applied Maths, Belgium). Fragments smaller than 500 bp were excluded from cluster analysis. Spectral analysis was applied to individual images to determine the optimal parameters for the least-square filtering and rolling-disk background subtraction. Similarity matrices of densitometric curves were calculated by Pearson's product-moment correlation coefficient with a position tolerance of 1.42%, calculated from optimizing the position tolerance of six groups of E. coli type strains so that maximum group contrast was revealed. Cluster analyses of similarity matrices were performed by an unweighted pair group method with arithmetic mean (UPGMA) algorithm. The correlation was expressed as percent similarity.
Microbial richness estimation.
Ten independent BOX-PCRs were performed on six randomly selected E. coli strains from Table 1. The minimum similarity within a strain was used as the criterion to define an operational taxonomic unit (OTU), while statistical analyses of microbial richness were computed by using EstimateS (version 5; R. K. Colwell, University of Connecticut, Storrs [http://viceroy.eeb.uconn.edu/estimates]). OTU richness was estimated by employing the Chao1 estimator, a nonparametric estimator suitable for microbial diversity analysis (21):
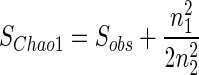 |
where Sobs is the number of observed OTU, n1 is the number of singletons (OTUs observed once), and n2 is the number of doubletons (OTUs observed twice) (3, 21).
Biochemical confirmation.
A total of 54 randomly selected feedlot isolates were inoculated into API-20E strips (BioMerieux Co.) according to the manufacturer's manual.
Motility assays.
Individual bacterial cultures were inoculated in the center of 0.35% nutrient swimming agar plates by using a sterile toothpick. After incubation at 25°C for 48 h, the diameter of migration and growth was measured in centimeters.
Antibiotic susceptibility test.
Antibiotic disks (BBL Sensi-Disk antimicrobial disks; BD Diagnostic Systems) were deposited on a nutrient agar plate inoculated with a high density of an individual culture resulting in a bacterial lawn. The diameter of the clear zone around the disk was measured after incubation for 24 h at 25°C. Antibiotic disks used in this study were tetracycline (30 μg), streptomycin (10 μg), polymyxin (300 U), chloramphenicol (30 μg), carbenicillin (100 μg), and erythromycin (15 μg).
Biofilm formation.
Biofilm formation was assayed in polystyrene microwell plates (Costar; Fisher Scientific) in M63 medium supplemented with 0.8% glucose as minimal medium and in Luria broth (LB) as rich medium as described by Danese et al. (8). Polystyrene-attached cells were stained with 1% crystal violet, rinsed, and thoroughly dried. Biofilms were then dissolved in dimethyl sulfoxide, and the solubilized crystal violet was transferred to a fresh 96-well polystyrene dish. Absorbance at 570 nm was then determined with a microplate reader (SpectraMax 190; Molecular Devices), and the readings were normalized to the medium used in individual tests. Each strain was tested in triplicate, and averages were reported.
Statistical analyses.
Correlations between quantitative properties were evaluated by employing the nonparametric Spearman’s rank correlation coefficient; differences between means were tested by using a one-tailed Student's t test (48). All statistical tests were performed at a significance level (α) of 0.05 by commercial software (XLSTAT, version 6.0; Addinsoft, Brooklyn, N.Y.).
RESULTS
Coliform distribution in the test bovine feedlot.
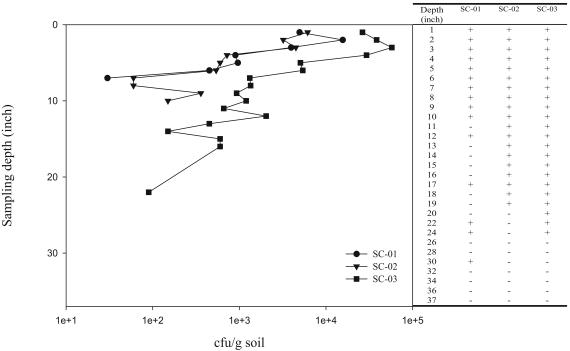
Three soil cores (SC-01, SC-02, and SC-03) were collected from a bovine feedlot and analyzed for coliform content as a function of soil depth and E. coli subspecies diversity. The coliform density decreased dramatically from 5.8 × 104 CFU per g of soil at the surface to undetectable levels at a depth of 8 in. (SC-01), 11 in. (SC-02), and 23 in. (SC-03). The profile of coliform density with depth was very similar for all three cores, although SC-03 had a significantly higher coliform content than the other two (Fig. 1). The total coliform fraction never exceeded 0.5% of the total heterotrophic count as recovered on nutrient agar plates.
FIG. 1.
Coliform density as a function of sampling depth (left panel). Coliform occurrence at each depth was estimated after enrichment in LTB (right panel). +, coliform presence; −, coliform absence.
To evaluate the maximum depth of coliform occurrence, enrichments in LTB were performed with soil sections of increasing depth. The maximum depth of E. coli occurrence was 30, 19, and 24 in. for SC-01, SC-02, and SC-03, respectively (Fig. 1). Five to seven lactose-fermenting strains, randomly selected from each MacConkey plate inoculated with LTB enrichments from all soil depths, were collected to yield 326 coliform isolates for further characterization.
BOX-PCR and identification of Escherichia coli strains.
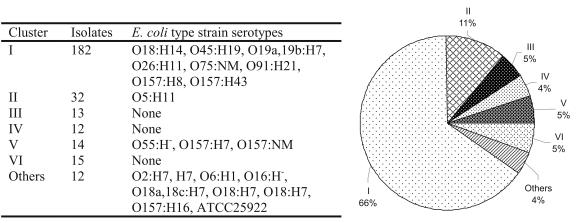
BOX-PCR was performed on the coliform isolates and other Enterobacteriaceae type strains (Table 1). Isolates that did not yield a recognizable BOX-PCR pattern were excluded from further analysis (14 of 326). Curve-based product-moment correlation coefficients were used for pairwise fingerprint comparison, and UPGMA was used to perform cluster analysis on all isolates and type strains. Feedlot isolates that clustered with Enterobacteriaceae other than E. coli were also excluded from further analysis (26 of 326). Of the remaining 286 feedlot coliform isolates that clustered with E. coli type strains, 55 were randomly selected for confirmation of an E. coli biochemical profile by API-20E testing. Only 1 of 55 did not display the expected E. coli biochemical profile, indicating that the BOX-PCR-based identification method effectively groups bacteria at the species level. Other isolates (6 of 286) that clustered with this non-E. coli strain in the BOX-PCR analysis were also excluded from further analysis. Based on BOX-PCR pattern similarity, 280 E. coli feedlot isolates were identified, and six main clusters were defined at a similarity level of 60%. One genotype, comprising 66% of the population, was dominant regardless of sampling site or depth (Fig. 2).
FIG. 2.
Clustering of feedlot E. coli isolates based on BOX-PCR fingerprint pattern. The number of isolates and the E. coli type strain serotypes falling into each main cluster are indicated.
Subspecies diversity of E. coli.
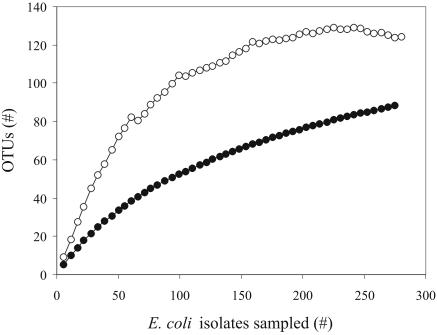
To define OTUs, it was critical to examine the reproducibility of BOX-PCR patterns. The patterns from six randomly chosen E. coli type strains, obtained from 10 independent gels and at least 10 different PCR runs, were compared. From the results, two isolates were grouped into the same OTUs if their patterns were ≥85% similar and into different OTUs if their patterns were <85% similar. By this definition, the 280 isolates fell into 89 distinct OTUs. Although the rarefaction curve did not reach an asymptote after the 280 sampling events, it was far beyond the linear range (Fig. 3), indicating that our sampling was representative. The Chao1 estimator of the feedlot E. coli subspecies diversity was 125 OTUs (95% confidence interval, 109 to 141) and was adequately sampled at about 200 isolates.
FIG. 3.
Observed and estimated OTU richness of the E. coli feedlot population versus sampling size. The accumulation curve (rarefaction curve) averaged over 50 simulations (•), and the Chao1 estimated OTU richness (○) are shown.
Motility and biofilm formation on polystyrene plates.
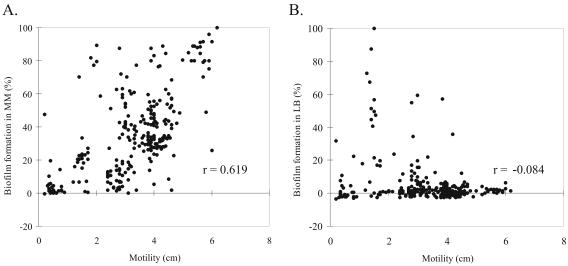
Motility was measured by a nutrient soft agar assay. The average distance of bacterial migration was 3.3 ± 1.4 cm. The E. coli isolates exhibited a very broad range of motility; 90% of the isolates displayed a migration front between 1.1 and 4.6 cm, and the highest motility was 6.2 cm. Biofilm formation was quantified by measuring the amount of crystal violet retained in the biofilm of each isolate grown in individual polystyrene wells. Measured values for biofilm formation in minimal medium followed a normal distribution, while those measured in LB were strongly skewed towards lower absorbance values. An isolate's biofilm formation was expressed as a percentage of the highest biofilm formed in the respective growth medium. A positive linear relationship (Spearman's rank correlation coefficient r = 0.619, P < 10−4) was observed between motility and biofilm formation in minimal medium, while no significant correlation was observed between motility and biofilm formation in LB (Fig. 4).
FIG. 4.
Correlation (r) between motility and biofilm formation in minimal medium (MM) supplemented with glucose (A) and LB (B) for E. coli isolates. Biofilm intensity was expressed as a percentage of the highest biofilm formed in each medium. Each dot is the average of three independent experiments for each isolate.
Distribution of fimH and agn43 genes in the E. coli population.
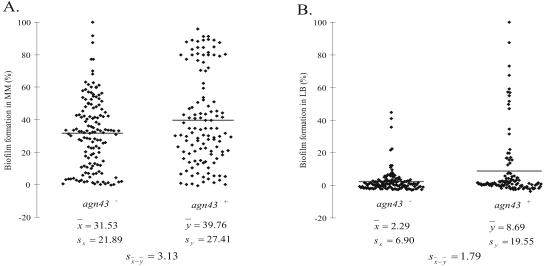
The presence of two phase-variable genes, fimH and agn43, was assayed in all of the 280 E. coli isolates by PCR using gene-specific primers (Table 2). All isolates possessed fimH, but only 48% of the population possessed agn43. Biofilm formation in minimal and rich media was affected by agn43, with agn43+ isolates forming significantly thicker biofilms (P < 10−3) (Fig. 5).
FIG. 5.
Biofilm formation in minimal medium (MM) supplemented with glucose (A) and LB (B). Biofilm intensity was expressed as a percentage of the highest biofilm formed in each medium. Each dot is the average from three independent experiments for each isolate. The horizontal bar is the mean value of the biofilm formation in that subpopulation. X̄, mean biofilm formation in agn43-deficient subpopulation; Ȳ, mean biofilm formation in agn43+ subpopulations; SX and Sy, estimated standard deviations of x and y, respectively; Sx̄−ȳ, estimated standard deviation of x̄ − ȳ.
Correlation of genotypic clustering with phenotypic characters.
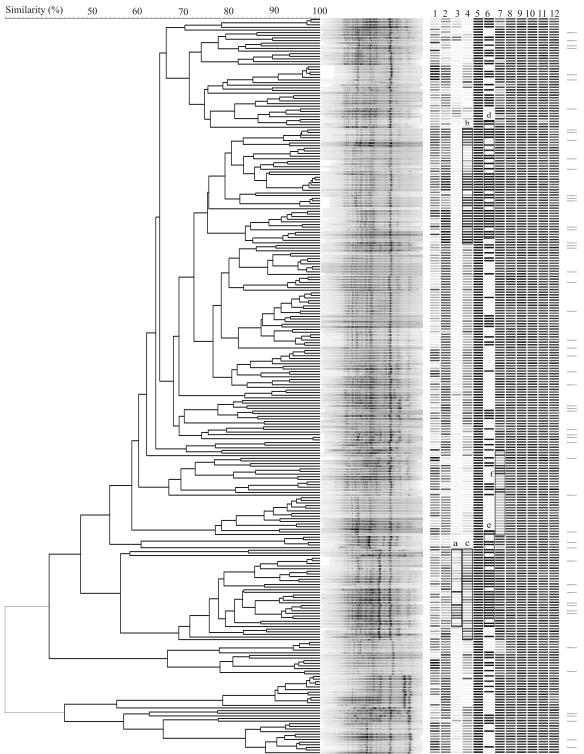
The distribution of phenotypic characters was compared with the genotypic clustering of the isolates to examine possible congruence (Fig. 6). Isolates with confirmed E. coli biochemical profiles are indicated and are evenly distributed among the genotypic clusters. No population-wide accordance between genotype and phenotype was observed. However, some clusters were characterized by a consistent phenotype such as biofilm-forming intensity in LB (Fig. 6, box a), biofilm-forming intensity in minimal medium (boxes b and c), the presence of agn43 (boxes d and e), and tetracycline sensitivity (box f). No significant correlations were observed in terms of sampling depth, motility, and other antibiotic resistance profiles. The correlation between different phenotypic characteristics and their relationship to the isolates' depths of origin are summarized in Table 3. No significant correlation was observed between genotypic or phenotypic characteristics and sampling depths of the isolates. Significant correlations were observed between motility and biofilm-forming ability in minimum medium and between the presence of agn43 and biofilm-forming ability in both rich and minimum media.
FIG. 6.
Clustering of E. coli feedlot isolates based on BOX-PCR fingerprint pattern. Corresponding phenotypes are shown on the right panel as gray bars: depth of origin (lane 1), motility (lane 2), biofilm formation in LB (lane 3), biofilm formation in minimal medium (lane 4), presence of fimH (lane 5), presence of agn43 (lane 6), and resistance to tetracycline (lane 7), streptomycin (lane 8), polymyxin (lane 9), chloramphenicol (lane 10), carbenicillin (lane 11), and erythromycin (lane 12). The gray scale of the bars was obtained as follows: depth is shown as the proportion to total sampling depth, the degree of motility is shown relative to maximum motility, biofilm formation is shown relative to the highest biofilm formed in each medium, fimH and agn43 are shown as present (black) or absent (white), and antibiotic resistance is shown as the relative size of the clear zone compared to the maximum clear zone for the individual antibiotic. Horizontal bars at the far right indicate isolates that were confirmed to have E. coli biochemical fingerprints by API-20E testing.
TABLE 3.
Correlation of genotype, phenotype, and sampling depth
| Parameter | Characteristic | Clustering or correlation (r) | P value |
|---|---|---|---|
| Genotypea | Depth | None | |
| Motility | None | ||
| Biofilm in MMd | Some | ||
| Biofilm in LB | Some | ||
| agn43 | Some | ||
| Tetracycline | Some | ||
| Other antibiotics | None | ||
| agn43b | Biofilm in MM | Yes | 0.005 |
| Biofilm in LB | Yes | <10−4 | |
| Depth | No | 0.211 | |
| Motility | No | 0.196 | |
| Motilityc | Biofilm in MM | Yes (0.619) | <10−4 |
| Biofilm in LB | No | 0.080 | |
| Depth | No | 0.138 | |
| Biofilm in MMc | Depth | No | 0.431 |
| Biofilm in LBc | Depth | No | 0.104 |
| Biofilm in MMc | Biofilm in LB | No | 0.167 |
| Antibiotic resistancec | Depth | No | >0.044 |
Visual observation was used to compare the relationship between genotype and other examined characteristics.
Student's t test was used to compare the means in a one-tailed test at a significance level of α = 0.05.
Correlation was determined by a nonparametric Spearman's test at a significance level of α = 0.05.
MM, minimal medium.
DISCUSSION
The E. coli population comprised 86% of the enterobacterial strains isolated from feedlot soil cores recovered on MacConkey agar after LTB enrichment. This finding is consistent with E. coli being the most common enterobacterial species recovered from mammalian hosts, where it can account for up to 46% of the facultative flora (12). One genomic cluster comprised the majority (66%) of all feedlot isolates; whether this dominance reflected a metabolic advantage in growth or survival in soil associated with this cluster or was a simple reflection of the dominance in the intestinal host environment was not ascertained.
BOX-PCR was selected as the molecular typing technique for the E. coli isolates because it is reproducible, rapid, easy to perform, and highly discriminatory at the subspecies level (33), yielding results that correlate well with pairwise DNA-DNA analyses (41). The BOX-PCR genomic fingerprint patterns were analyzed by a curve-based protocol, which retains more information than merely the number and position of fingerprint fragments (15). Curve-based Pearson product-moment correlation coefficients provided estimators of pairwise similarities, and clustering was performed by using UPGMA. We assessed the reproducibility of our fingerprinting techniques by examining 10 independently obtained fingerprints (different PCRs, different electropherograms) of six E. coli type strains. Similarity coefficients of the replicate genomic fingerprints exceeded 85% for each test strain, with 80% used as a cutoff value for identical genotypes based on BOX-PCR fingerprints (5, 32).
When microbial species diversity is inferred from molecular fingerprints or sequence information, individual OTUs must be defined as species surrogates, although no consistent definitions of OTUs are currently employed, leading to noncomparable diversity estimates (26, 29, 50). When subspecies diversity is examined according to the method used in the present study, similar descriptors of diversity within the species can be employed with an OTU based on molecular data with higher genomic resolution (such as BOX-PCR fingerprints) than that required for species diversity estimation. With a criterion of 85% fingerprint similarity as a cutoff for an OTU, 89 distinct OTUs were identified among the 280 E. coli typed isolates.
Analogous to the use of species richness and species evenness concepts to describe microbial community diversity, microbial population diversity can be measured as subspecies richness, graphically presented as a subspecies accumulation curve or rarefaction curve (19), or captured in a single value estimator, such as the Chao1 index, which has been found adequate for describing microbial community diversity (3, 21, 31). Although the rarefaction curve for feedlot isolates did not reach an asymptote after the 280 sampling events, it was far beyond the linear range (Fig. 3), indicating that our sampling was representative. Similarly, the Chao1 estimator indicated adequate sampling at about 200 isolates. The retrieved E. coli population diversity was, therefore, representative of the true extant diversity with 89 measured OTUs divided into six main clusters.
Although multiple-antibiotic resistance profiles have been successfully used to differentiate E. coli from different sources (13, 16), the resistance profiles of the tested antibiotics (tetracycline, streptomycin, polymyxin, chloramphenicol, carbenicillin, and erythromycin) were not sufficiently discriminating to cluster the E. coli strains retrieved in this study (Fig. 6), possibly because of the similar host environment to which all isolates were originally exposed. Congruence with genomic clustering could not be tested; only tetracycline resistance provided some resolving power, with shared low tetracycline resistance in one genomic cluster (cluster f, Fig. 6).
It has been suggested that flagellum-mediated motility influences the attachment and detachment rates of E. coli to a glass surface (30) and is required for initial cell attachment during biofilm formation in both rich and minimal media (8, 40), possibly because flagellum-mediated motility may assist bacteria in overcoming repulsive interfacial forces. As a first examination of phenotypic variability at the population level, we tested the range of displayed motilities within the E. coli population; a very broad range in motility was observed. Furthermore, motility, as measured by the nutrient soft agar assay, showed a positive correlation with biofilm-forming ability on a polystyrene surface in minimal medium but not in rich medium (Fig. 4). Because flagellum biosynthesis and, hence, motility are highly responsive to environmental conditions (49, 57), it will be critical to evaluate whether the observed population-level differences might result in differential survival (due to biofilm formation) in the anticipated low-nutrient environment.
In addition to cellular motility, the phase-variable adhesins Ag43 and fimbriae, whose expression is tightly controlled by environmental conditions, have been implicated in the interfacial and biofilm behavior of E. coli (18, 43). Ag43 is a self-recognizing protein located in the outer membrane and promotes biofilm development by inducing microcolony formation (8, 24). The presence of fimbriae, however, can physically block Ag43-mediated interactions (17), while fimbrial biosynthesis per se might down-regulate agn43 expression (44), making fimbriation dominant to Ag43 expression.
The presence of the fimH and agn43 genes in the retrieved E. coli isolates was evaluated. The consensus PCR primers were designed to permit retrieval of the highly conservative fimH gene (53) and known agn43 loci as well as the locus encoding the homologous Cah protein (51). While all feedlot E. coli isolates possessed fimH, only 48% possessed agn43. The agn43+ isolates form significantly more biofilm in both minimal and rich media (Fig. 5), with the larger effect in the former. Hence, the population-level effect of agn43 presence on biofilm formation is consistent with the effect of agn43 expression on biofilm formation observed in a laboratory E. coli strain as inferred from mutant analysis (8).
This report presents an exhaustive inventory of an extant E. coli population at a bovine feedlot. Overall, the vertical distribution of the retrieved E. coli isolates did not correlate with any phenotypic or genotypic characteristic examined. Recognition of the heterogeneity of the E. coli population in terms of motility, biofilm formation potential, and presence of the phase-variable protein encoding the agn43 gene calls into question the values of these phenotypic traits in predicting or determining the fate of E. coli after it is shed from the bovine host. Rather, it appears that the various subspecies taxonomic units exhibit a broad degree of variability in these presumably fate-related traits, indicating that survival or a competitive advantage may result from diversification of behavior within individual populations. Our ongoing studies aim to further delineate variability across the population in terms of surface behavior and fate during transport in porous media.
Acknowledgments
This work was supported by a grant from the U.S. Department of Agriculture National Research Initiative Competitive Grants Program (USDA/NRICGP; 99-35102-8593).
REFERENCES
- 1.Carson, C. A., B. L. Shear, M. R. Ellersieck, and A. Asfaw. 2001. Identification of fecal Escherichia coli from humans and animals by ribotyping. Appl. Environ. Microbiol. 67:1503-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carson, C. A., B. L. Shear, M. R. Ellersieck, and J. D. Schnell. 2003. Comparison of ribotyping and repetitive extragenic palindromic-PCR for identification of fecal Escherichia coli from humans and animals. Appl. Environ. Microbiol. 69:1836-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chao, A. 1984. Non-parametric estimation of the number of classes in a population. Scand. J. Stat. 11:265-270. [Google Scholar]
- 4.Chavant, P., B. Martinie, T. Meylheuc, M.-N. Bellon-Fontaine, and M. Hebraud. 2002. Listeria monocytogenes LO28: surface physicochemical properties and ability to form biofilms at different temperatures and growth phases. Appl. Environ. Microbiol. 68:728-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho, J.-C., and J. M. Tiedje. 2000. Biogeography and degree of endemicity of fluorescent Pseudomonas strains in soil. Appl. Environ. Microbiol. 66:5448-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connell, H., W. Agace, P. Klemm, M. Schembri, S. Marild, and C. Svanborg. 1996. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc. Natl. Acad. Sci. USA 93:9827-9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowell, B. A., M. D. Willcox, B. Herbert, and R. P. Schneider. 1999. Effect of nutrient limitation on adhesion characteristics of Pseudomonas aeruginosa. J. Appl. Microbiol. 86:944-954. [DOI] [PubMed] [Google Scholar]
- 8.Danese, P. N., L. A. Pratt, S. L. Dove, and R. Kolter. 2000. The outer membrane protein, antigen 43, mediates cell-to-cell interactions within Escherichia coli biofilms. Mol. Microbiol. 37:424-432. [DOI] [PubMed] [Google Scholar]
- 9.Dombek, P. E., L. K. Johnson, S. T. Zimmerley, and M. J. Sadowsky. 2000. Use of repetitive DNA sequences and the PCR to differentiate Escherichia coli isolates from human and animal sources. Appl. Environ. Microbiol. 66:2572-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drasar, B. S., and P. S. Barrow. 1985. Aspects of microbiology 10. Intestinal microbiology. American Society for Microbiology, Washington, D.C.
- 11.Gabig, M., A. Herman-Antosiewicz, M. Kwiatkowska, M. Los, M. S. Thomas, and G. Wegrzyn. 2002. The cell surface protein Ag43 facilitates phage infection of Escherichia coli in the presence of bile salts and carbohydrates. Microbiology 148:1533-1542. [DOI] [PubMed] [Google Scholar]
- 12.Gordon, D. M., and F. FitzGibbon. 1999. The distribution of enteric bacteria from Australian mammals: host and geographical effects. Microbiology 145:2663-2671. [DOI] [PubMed] [Google Scholar]
- 13.Graves, A. K., C. Hagedorn, A. Teetor, M. Mahal, A. M. Booth, and R. B. Reneau, Jr. 2002. Antibiotic resistance profiles to determine sources of fecal contamination in a rural Virginia watershed. J. Environ. Qual. 31:1300-1308. [DOI] [PubMed] [Google Scholar]
- 14.Hall-Stoodley, L., and P. Stoodley. 2002. Developmental regulation of microbial biofilms. Curr. Opin. Biotechnol. 13:228-233. [DOI] [PubMed] [Google Scholar]
- 15.Hane, B. G., K. Jager, and H. G. Drexler. 1993. The Pearson product-moment correlation coefficient is better suited for identification of DNA fingerprint profiles than band matching algorithms. Electrophoresis 14:967-972. [DOI] [PubMed] [Google Scholar]
- 16.Harwood, V. J., J. Whitlock, and V. Withington. 2000. Classification of antibiotic resistance patterns of indicator bacteria by discriminant analysis: use in predicting the source of fecal contamination in subtropical waters. Appl. Environ. Microbiol. 66:3698-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasman, H., T. Chakraborty, and P. Klemm. 1999. Antigen-43-mediated autoaggregation of Escherichia coli is blocked by fimbriation. J. Bacteriol. 181:4834-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasman, H., M. A. Schembri, and P. Klemm. 2000. Antigen 43 and type 1 fimbriae determine colony morphology of Escherichia coli K-12. J. Bacteriol. 182:1089-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heck, K. J., G. van Belle, and D. Simberloff. 1975. Explicit calculation of the rarefaction diversity measurement and the determination of sufficient sample size. Ecology 56:1459-1461. [Google Scholar]
- 20.Henderson, I. R., M. Meehan, and P. Owen. 1997. Antigen 43, a phase-variable bipartite outer membrane protein, determines colony morphology and autoaggregation in Escherichia coli K-12. FEMS Microbiol. Lett. 149:115-120. [DOI] [PubMed] [Google Scholar]
- 21.Hughes, J. B., J. J. Hellmann, T. H. Ricketts, and B. J. M. Bohannan. 2001. Counting the uncountable: statistical approaches to estimating microbial diversity. Appl. Environ. Microbiol. 67:4399-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson, D. W., K. Suzuki, L. Oakford, J. W. Simecka, M. E. Hart, and T. Romeo. 2002. Biofilm formation and dispersal under the influence of the global regulator CsrA of Escherichia coli. J. Bacteriol. 184:290-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones, C., J. Pinkner, R. Roth, J. Heuser, A. Nicholes, S. Abraham, and S. Hultgren. 1995. FimH adhesin of type 1 pili is assembled into a fibrillar tip structure in the Enterobacteriaceae. Proc. Natl. Acad. Sci. USA 92:2081-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kjaergaard, K., M. A. Schembri, C. Ramos, S. Molin, and P. Klemm. 2000. Antigen 43 facilitates formation of multispecies biofilms. Environ Microbiol. 2:695-702. [DOI] [PubMed] [Google Scholar]
- 25.Klemm, P., and G. Christiansen. 1987. Three fim genes required for the regulation of length and mediation of adhesion of Escherichia coli type 1 fimbriae. Mol. Gen. Genet. 208:439-445. [DOI] [PubMed] [Google Scholar]
- 26.Kroes, I., P. W. Lepp, and D. A. Relman. 1999. Bacterial diversity within the human subgingival crevice. Proc. Natl. Acad. Sci. USA 96:14547-14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landini, P., and A. J. B. Zehnder. 2002. The global regulatory hns gene negatively affects adhesion to solid surfaces by anaerobically grown Escherichia coli by modulating expression of flagellar genes and lipopolysaccharide production. J. Bacteriol. 184:1522-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, J., and L. A. McLandsborough. 1999. The effects of the surface charge and hydrophobicity of Escherichia coli on its adhesion to beef muscle. Int. J. Food Microbiol. 53:185-193. [DOI] [PubMed] [Google Scholar]
- 29.McCaig, A. E., L. A. Glover, and J. I. Prosser. 1999. Molecular analysis of bacterial community structure and diversity in unimproved and improved upland grass pastures. Appl. Environ. Microbiol. 65:1721-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McClaine, J. W., and R. M. Ford. 2002. Characterizing the adhesion of motile and nonmotile Escherichia coli to a glass surface using a parallel-plate flow chamber. Biotechnol. Bioeng. 78:179-189. [DOI] [PubMed] [Google Scholar]
- 31.Nicholas, J. G., and C. K. Robert. 2001. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 4:379-391. [Google Scholar]
- 32.Oda, Y., W. Wanders, L. A. Huisman, W. G. Meijer, J. C. Gottschal, and L. J. Forney. 2002. Genotypic and phenotypic diversity within species of purple nonsulfur bacteria isolated from aquatic sediments. Appl. Environ. Microbiol. 68:3467-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olive, D. M., and P. Bean. 1999. Principles and applications of methods for DNA-based typing of microbial organisms. J. Clin. Microbiol. 37:1661-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olsen, P. B., M. A. Schembri, D. L. Gally, and P. Klemm. 1998. Differential temperature modulation by H-NS of the fimB and fimE recombinase genes which control the orientation of the type 1 fimbrial phase switch. FEMS Microbiol. Lett. 162:17-23. [DOI] [PubMed] [Google Scholar]
- 35.Otto, K., H. Elwing, and M. Hermansson. 1999. Effect of ionic strength on initial interactions of Escherichia coli with surfaces, studied on-line by a novel quartz crystal microbalance technique. J. Bacteriol. 181:5210-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otto, K., H. Elwing, and M. Hermansson. 1999. The role of type 1 fimbriae in adhesion of Escherichia coli to hydrophilic and hydrophobic surfaces. Colloids Surf. B 15:99-111. [Google Scholar]
- 37.Owen, P., P. Caffrey, and L. Josefsson. 1987. Identification and partial characterization of a novel bipartite protein antigen associated with the outer membrane of Escherichia coli. J. Bacteriol. 169:3770-3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parveen, S., K. M. Portier, K. Robinson, L. Edmiston, and M. L. Tamplin. 1999. Discriminant analysis of ribotype profiles of Escherichia coli for differentiating human and nonhuman sources of fecal pollution. Appl. Environ. Microbiol. 65:3142-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Powelson, D. K., and A. L. Mills. 2001. Transport of Escherichia coli in sand columns with constant and changing water contents. J. Environ. Qual. 30:238-245. [DOI] [PubMed] [Google Scholar]
- 40.Pratt, L. A., and R. Kolter. 1998. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 30:285-293. [DOI] [PubMed] [Google Scholar]
- 41.Rademaker, J., B. Hoste, F. Louws, K. Kersters, J. Swings, L. Vauterin, P. Vauterin, and F. de Bruijn. 2000. Comparison of AFLP and rep-PCR genomic fingerprinting with DNA-DNA homology studies: Xanthomonas as a model system. Int. J. Syst. E vol. Microbiol. 50:665-677. [DOI] [PubMed] [Google Scholar]
- 42.Reynolds, P. J., P. Sharma, G. E. Jenneman, and M. J. McInerney. 1989. Mechanisms of microbial movement in subsurface materials. Appl. Environ. Microbiol. 55:2280-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schembri, M. A., G. Christiansen, and P. Klemm. 2001. FimH-mediated autoaggregation of Escherichia coli. Mol. Microbiol. 41:1419-1430. [DOI] [PubMed] [Google Scholar]
- 44.Schembri, M. A., and P. Klemm. 2001. Coordinate gene regulation by fimbriae-induced signal transduction. EMBO J. 20:3074-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schembri, M. A., D. W. Ussery, C. Workman, H. Hasman, and P. Klemm. 2002. DNA microarray analysis of fim mutations in Escherichia coli. Mol. Genet. Genomics 267:721-729. [DOI] [PubMed] [Google Scholar]
- 46.Schloter, M., M. Lebuhn, T. Heulin, and A. Hartmann. 2000. Ecology and evolution of bacterial microdiversity. FEMS Microbiol. Rev. 24:647-660. [DOI] [PubMed] [Google Scholar]
- 47.Smets, B. F., D. Grasso, M. A. Engwall, and B. J. Machinist. 1999. Surface physicochemical properties of Pseudomonas fluorescens and impact on adhesion and transport through porous media. Colloids Surf. B 14:121-139. [Google Scholar]
- 48.Snedecor, G. W., and W. G. Cochran. 1989. Statistical methods, 8th ed. Iowa State University Press, Ames, Iowa.
- 49.Soutourina, O., A. Kolb, E. Krin, C. Laurent-Winter, S. Rimsky, A. Danchin, and P. Bertin. 1999. Multiple control of flagellum biosynthesis in Escherichia coli: role of H-NS protein and the cyclic AMP-catabolite activator protein complex in transcription of the flhDC master operon. J. Bacteriol. 181:7500-7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suau, A., R. Bonnet, M. Sutren, J.-J. Godon, G. R. Gibson, M. D. Collins, and J. Dore. 1999. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl. Environ. Microbiol. 65:4799-4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torres, A. G., N. T. Perna, V. Burland, A. Ruknudin, F. R. Blattner, and J. B. Kaper. 2002. Characterization of Cah, a calcium-binding and heat-extractable autotransporter protein of enterohaemorrhagic Escherichia coli. Mol. Microbiol. 45:951-966. [DOI] [PubMed] [Google Scholar]
- 52.U.S. Environmental Protection Agency. 2003. Concentrated animal feeding operations (CAFO). Fed. Regist. 68:7176. [PubMed] [Google Scholar]
- 53.Vandemaele, F., D. Vandekerchove, M. Vereecken, J. Derijcke, M. Dho-Moulin, and B. M. Goddeeris. 2003. Sequence analysis demonstrates the conservation of fimH and variability of fimA throughout avian pathogenic Escherichia coli (APEC). Vet. Res. 34:153-163. [DOI] [PubMed] [Google Scholar]
- 54.Vaneechoutte, M. 1996. DNA fingerprinting techniques for microorganisms. A proposal for classification and nomenclature. Mol. Biotechnol. 6:115-142. [DOI] [PubMed] [Google Scholar]
- 55.Vigeant, M. A.-S., R. M. Ford, M. Wagner, and L. K. Tamm. 2002. Reversible and irreversible adhesion of motile Escherichia coli cells analyzed by total internal reflection aqueous fluorescence microscopy. Appl. Environ. Microbiol. 68:2794-2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waldron, D. E., P. Owen, and C. J. Dorman. 2002. Competitive interaction of the OxyR DNA-binding protein and the Dam methylase at the antigen 43 gene regulatory region in Escherichia coli. Mol. Microbiol. 44:509-520. [DOI] [PubMed] [Google Scholar]
- 57.Wei, B. L., A. M. Brun-Zinkernagel, J. W. Simecka, B. M. Pruss, P. Babitzke, and T. Romeo. 2001. Positive regulation of motility and flhDC expression by the RNA-binding protein CsrA of Escherichia coli. Mol. Microbiol. 40:245-256. [DOI] [PubMed] [Google Scholar]
- 58.Williams, V., and M. Fletcher. 1996. Pseudomonas fluorescens adhesion and transport through porous media are affected by lipopolysaccharide composition. Appl. Environ. Microbiol. 62:100-104. [DOI] [PMC free article] [PubMed] [Google Scholar]