Abstract
We have observed substantial differences in angiogenic responsiveness in mice and have mapped the genetic loci responsible for these differences. We have found that the albino mutation is one of the loci responsible for such differences. Using B6.A consomic strains, we determined that chromosome 7 bears a locus that inhibits VEGF-induced corneal neovascularization. F2 crosses between B6.A<Chromosome 7> consomic mice and C57BL/6J parents along with AXB and BXA recombinant inbred strains demonstrated highest linkage near the tyrosinase gene. This region was named AngVq4. Congenic animals confirmed this locus, but could not demonstrate that the classical tyrosinase albino (c) mutation was causative because of the existence of additional linked loci in the congenic region. However, in 1970, a second tyrosinase albino mutation (c-2J) arose in the C57BL/6J background at Jackson Labs. Testing this strain (C57BL/6J<c-2J>) demonstrated that the albino mutation is sufficient to completely explain the alteration in angiogenic response that we observed in congenic animals. Thus, we conclude that the classical tyrosinase mutation is responsible for AngVq4. In contrast to the cornea, where pigmented animals exhibit increased angiogenic responsiveness, iris neovascularization was inhibited in pigmented animals. These results may partially explain increased aggressiveness in amelanotic melanoma, as well as ethnic differences in diabetic retinopathy and macular degeneration.
Introduction
Angiogenesis is the process by which new vessels are formed and is vital for growth and development. In normal adults, however, angiogenesis only occurs during wound healing and during the reproductive cycle. On the other hand it is a hallmark of several pathologies including cancer, arthritis, cardiovascular disease, macular degeneration, and diabetic retinopathy. Angiogenesis is regulated by the balance of stimulators and inhibitors, however we have observed that the interpretation of this balance by the organism differs in a genetically-controlled manner, with some inbred mouse strains much more responsive to angiogenic stimuli than others [1–7].
Tyrosinase is a copper-dependant enzyme that catalyzes the oxidation of tyrosine to dopaquinone using molecular oxygen and releasing water [8]. Dopaquinone then spontaneously cyclizes to give rise to dopachrome—an indolic eumelanin precursor, or, in combination with cysteine, 5-S-cysteinyldopa—a pheomelanin precursor. Spontaneous elimination of CO2 from dopachrome will yield 5,6-dihydroxyindole (DHI), or dopachrome tautomerase can catalyze the formation of 5,6-dihydroxycarboxylic acid (DHICA). Further oxidation and polymerization of these products results in melanin formation. As is true of most enzymes that use molecular oxygen, tyrosinase can also generate reactive oxygen species (ROS), including superoxide and peroxide, under catalytic conditions. In addition, these reactive oxygen can be both produced and scavenged by melanin and melanin precursors. In addition to reactive oxygen, tyrosinase produces other small molecules. Dopaquinone can spontaneously disproportionate with phenols in aqueous solution. In the case of tyrosine this results in the production of dihydroxyphenylalanine (DOPA), a dopamine precursor. In addition to tyrosine, tyrosinase will catalyze the oxidation of several other phenols, as well as the oxidation of catechols. Indeed, DOPA or dopamine oxidation is required to activate partially reduced met-tyrosinase. Thus, tyrosinase can give rise to as well as catabolize several molecules that can regulate angiogenesis including dopamine and other catecholamines [9,10], as well as reactive oxygen species [11].
A tyrosinase null, or albino, mutation in mice has been known since ancient times [12]. Indeed, this mutation factors importantly in the history of genetics. The first published demonstration of linkage was made using this locus and the pink-eyed dilution locus [13]. In addition to pigment deficiencies, other abnormalities have been noted in albino animals. For example, they exhibit abnormal optic chiasma and other neural anomolies attributable to abnormal neuronal guidance. In addition to developmental neural anomolies, Page-McCaw et al (2004) have found that ongoing tyrosinase activity, and not merely initial pigment production, is required for normal retinal light sensitization in zebrafish. This deficit is partially rescued by the addition of L-DOPA [14] demonstrating that tyrosinase DOPA production can be physiologically important.
Materials and Methods
Mouse strains and corneal micropocket assay
The corneal micropocket assay was performed as described [15] using pellets containing 10 ng of FGF2 or 200 ng carrier free human recombinant VEGF 165 (R&D Systems, Minneapolis, Minnesota). The area of vascular response was assessed on the fifth (FGF2) or sixth (VEGF) postoperative day using a slit lamp. Vessel area was calculated using the formula 0.2π × VL × CH where VL is vessel length from the limbus in mm and CH is clock hours around the cornea. Attempts were made to make comparable measurements by holding mice until several strains could be assayed at once thus reducing lot-to-lot variability in the pellets and reducing pellet age differences. This resulted in mice of several different ages being assayed, however in cases where the same strain was assayed at different ages there was no significant difference in results. At least five mice (10 eyes) per strain were analyzed and similar numbers of control C57BL/6J were included in each assay to confirm consistency. All mouse strains were obtained from Jackson Laboratories (Bar Harbor, Maine) and were housed in Children’s Hospital’s animal facility on standard diet and bedding until the assay was performed. All animal studies were conducted according to protocols approved by the Institutional Animal Care and Use Committee of Children’s Hospital.
Genotyping and Mapping
Windows QTL Cartographer version 2.5 [16] was used throughout for linkage analysis. Default settings were used throughout unless otherwise noted.
Genotypes for AXB and BXA strains and linkage map data were obtained from the most recent (3rd August 2005) version of the Wellcome-CTC Mouse Strain SNP Genotype Set, (http://www.well.ox.ac.uk/mouse/INBREDS/index.shtml). Genotyping of F2 animals was performed by PCR using primers (Sigma-Proligo, St. Louis, Missouri) annotated in the MGD and DNA purified directly from the animals assayed using Qiagen DNeasy Tissue Kits (Valencia, California). Cycling profiles were determined empirically and performed in a PTC-200 thermal cycler (MJ Research, Reno, Nevada). Taq polymerase and other PCR reagents were from Invitrogen (Carlsbad, California). Physical map positions were obtained from Build 37.1 of the C57BL/6J mouse genome (ftp://ftp.ncbi.nih.gov/genomes/MapView/Mus_musculus/sequence/BUILD.37.1/). For linkage analysis, positions were converted to the centimorgan scale using the mouse map converter (http://cgd.jax.org/mousemapconverter/).
Generation of congenics
B6.AChr7 subcongenic animals by generating by intercrossing the progeny of a C57BL/6J x B6.AChr7 mating to generate F2 animals. These animals were genotyped and animals exhibiting appropriate crossover events were then selected for backcrossing with C57BL/6J animals. Progeny bearing the recombinant chromosome were intercrossed and animals homozygous for the recombinant chromosome were selected as founders for the subcongenic lines.
Migration Assays
Human microvascular endothelial cells (Cambrex, Walkersville, MD) were maintained in EGM-2 (Cambrex, Walkersville, MD) according to the vendors instructions and used before passage 7. Bovine capillary endothelial cells (a kind gift of Dr. Judah Folkman) were maintained in DMEM containing 10% FBS (Gibco, Carlsbad, CA), 1x penicillin, streptomycin, glutamine (Gibco), and 2ng/ml bFGF. Polycarbonate transwell inserts, 6.5 mm diameter with 8.0 um pores, were coated with fibronectin (BD BioScience, San Jose, CA), Type I rat tail collagen (Upstate, Lake Placid, NY), or left uncoated. Cells were harvested and resuspended in EBM (Cambrex, Rockland, ME) containing 0.1% BSA (Fisher Chemical). 10,000–20,000 cells/well were plated onto wells containing media alone. These wells were suspended above wells containing medium conditioned by either pigmented Melan-c cells, or their unpigmented counterparts. Alternatively, cells were suspended above EGM-2 with or without purified tyrosinase (Enzo Life Sciences, Farmingdale, NY); 50 μM 3,4-DOPA (Sigma-Aldrich, St. Louis, MO), or 100 μM 5,6-DHI (Chem-Impex International, Wood Dale, IL). Cells were allowed to migrate for 4 hours. Membranes were rinsed once in PBS, then fixed and processed using Diff-Quick (Dade diagnostics, Aguada, PR). Cells on the top of the membrane were removed using cotton-tipped applicators and the membrane was removed from the insert using a scalpel. Membranes were then mounted on slides, and the number of cells in a microscopic field was counted either manually, or with computer assistance using Scion Image.
Results
The albino mutation affects VEGF-induced angiogenesis
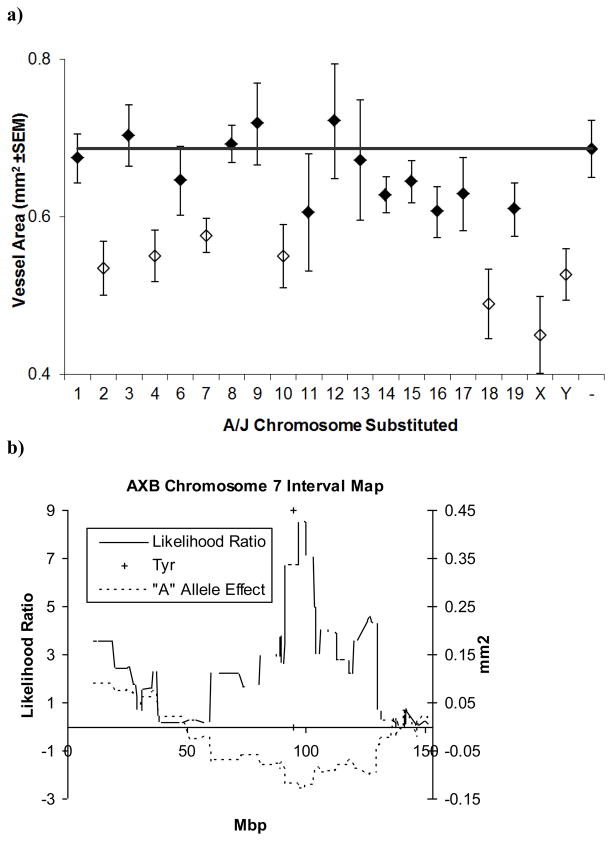
In order to ascertain which chromosomes might harbor angiogenesis-regulating loci, we screened the B6.A consomic strain set. This consists of a set of strains in which a chromosome from C57BL/6J has been replaced with the corresponding A/J chromosome [17,18]. This screen identified differences in angiogenic responsiveness resulting from loci on chromosomes 2, 4, 7, 10, 18, X, and Y (Figure 1a). Because we had observed linkage on chromosome 7 in separate studies on bFGF responsiveness [19], we chose this chromosome for further mapping of the loci responsible for differences in angiogenic responsiveness. To do this, we used two strategies, F2 intercross, and recombinant inbred strain cross.
Figure 1. Identification of AngVq4.
a) Results of corneal neovascularization assay in C57BL/6J-A/J consomic mouse strains using 200 ng VEGF pellets. Open symbols indicate strains with an angiogenic response that is significantly different (p<0.05 by ANOVA) than control (C57BL/6J) b) Interval mapping of chromosome 7 using BXA, AXB strains. Error bars show standard error of the mean.
Using the consomic C57BL/6J Chr7A strain (which bears the classical albino mutation and is thus white in color) and C57BL/6J as parents, we generated 56 F2 progeny. These progeny are B6 at all but chromosome 7, where they are a mixture of homozygotes and heterozygotes for each of the parental alleles. Genotyping and phenotyping revealed that markers near the albino locus showed the strongest association with angiogenic responsiveness (Table I) and albino-bearing subcongenic lines generated from this cross also showed significant decreases in angiogeenic responsiveness (Figure 2a). We observed a similar association in recombinant inbred mice. All available members of the BXA and AXB recombinant inbred strain sets were typed. These animals were generated by repeated sib-mating of the progeny of A/J x C57BL/6J and C57BL/6J x A/J crosses. During this process, recombinations are generated and then fixed as a result of inbreeding. Thus they represent a homozygous recombination of the C57BL/6J and A/J genomes with a higher recombination frequency than typically observed in F2 animals. Using only marker data from chromosome 7, we observed that markers near the albino locus were also most strongly associated with the decrease in vessel area observed in the C57BL/6J Chr7A consomic animals (Figure 1b), with this locus explaining up to 25% of the variance observed in this strain set.
Table I.
Association between marker genotype and corneal neovascularization in a C57BL/6J x C57BL/6J-Chr7A/J/NaJ F2 cross.
| Marker | b0 | b1 | F | %Var | P |
|---|---|---|---|---|---|
| D7Mit229 | 0.787 | −0.220 | 5.265 | 8.88% | 0.025 |
| D7Mit318 | 0.781 | −0.248 | 8.825 | 14.05% | 0.004 |
| D7Mit62 | 0.780 | −0.239 | 8.094 | 13.04% | 0.006 |
| Tyr | 0.767 | −0.234 | 8.863 | 14.10% | 0.004 |
| D7Mit301 | 0.773 | −0.210 | 8.600 | 13.74% | 0.005 |
| D7Mit238 | 0.770 | −0.201 | 8.048 | 12.97% | 0.006 |
| D7Mit186 | 0.761 | −0.156 | 4.514 | 7.71% | 0.037 |
| D7Mit332 | 0.763 | −0.177 | 6.884 | 11.31% | 0.011 |
Marker = marker tested; %Var = fraction of the experimental variance attributable to genotype at the marker indicated under an additive model; P = likelihood that there is no relationship between the marker genotype and choroidal neovascularization area (by F test), when the data are fit to the simple linear regression model y = b0 + b1 × + e. The results give the estimates for b0, b1 for each marker. b0 is approximately the average area of C57BL/6J-allele-containing strains. b1 is an indication of the effect of substitution of an A/J allele at that marker.
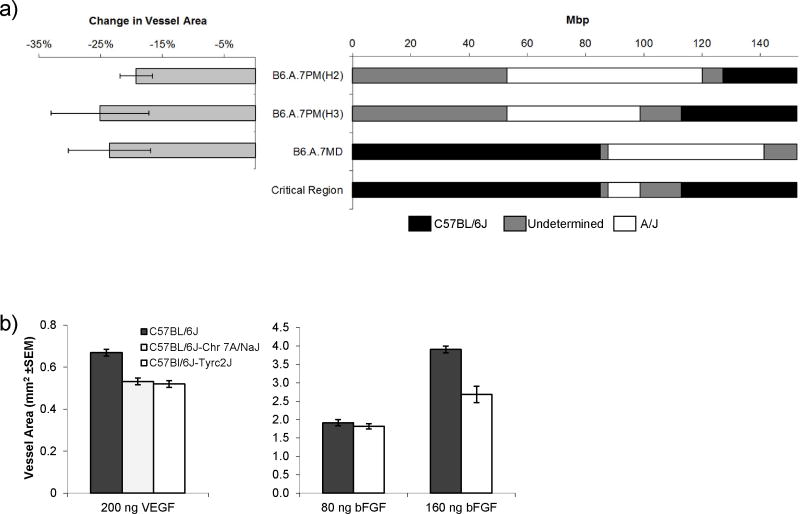
Figure 2. The tyrosinase albino mutation can explain AngVq4.
a) Angiogenic responsiveness to VEGF (left) and associated congenic region (right) for the three B6.A.Chr7 subcongenics that define the AngVq4 critical region (shown below). b) Corneal neovascularization in response to 160 ng VEGF, for C57BL/6J, C57BL/6JA/J-Chr7, and C57BL/6JTyr-c2J (left) or bFGF for C57BL/6J and C57BL/6JTyr-c2J (right). Note that the A/J-Chr7 and Tyr-c2J strains bear the classical (c) and a newer (c-2J) albino mutation of the tyrosinase gene. All differences with C57BL/6J are statistically significant (P<0.05 by ANOVA) except where 80 ng bFGF is the angiogenic stimulus. Error bars show standard error of the mean.
To test the hypothesis that the albino mutation itself is responsible for the differences that we observed in consomic, F2, and RI strains, we turned to the c-2J mutation. While the classical albino mutation (c), has been propagated in several classical mouse strains, the c-2J mutation arose independently in 1970 at the Jackson Laboratory in the C57BL/6J background. The C57BL/6Jc-2J strain thus contains a single difference with the parental strain (i.e. it is not a congenic with multiple differences carried along with the mutation to be tested). VEGF-induced corneal neovascularization was reduced in C57BL/6Jc-2J mice to the same extent as in C57BL/6J Chr7A animals (Figure 2b) indicating that the albino mutation is sufficient to account for the decrease in VEGF-induced corneal neovascularization seen in the consomic animals. Histological analysis of corneas from the two strains showed no structural differences between them.
Interestingly, we had previously observed no significant difference in bFGF-induced corneal neovascularization between C57BL/6Jc-2J and C57BL/6J mice [1]. This might have been due to the fact that the C57BL/6Jc-2J strain had undergone two additional backcrosses in the intervening time, leading to the possibility that genetic drift in the animals used in the original results may have masked a difference. To confirm this we repeated those bFGF experiments (Figure 2b). Again, we found no difference in bFGF-induced corneal neovascularization with 80 ng pellets, however with 160 ng pellets we saw a decrease in angiogenic response in the C57BL/6Jc-2J mice consistent with that observed with 200 ng pellets of VEGF.
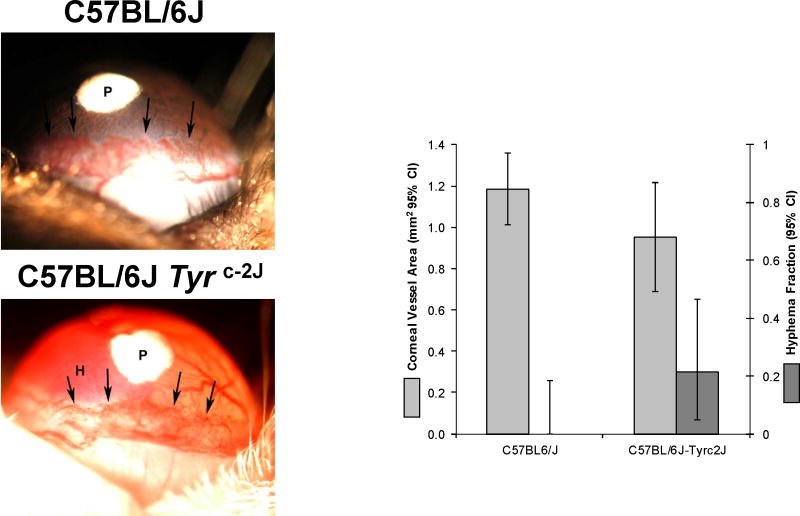
While we had previously observed no difference in bFGF-induced corneal neovascularization with 80 ng pellets, we had observed a significant increase in iris neovascularization (with hyphema formation) in C57BL/6Jc-2J animals at high bFGF doses. To test whether this difference is also observed at high VEGF doses, we performed the corneal neovasculariztion assay with pellets containing 640 ng VEGF (Figure 3). This resulted in significant hyphema formation in C57BL/6Jc-2J, but not in the pigmented controls. These same animals exhibited significantly lower corneal neovascularization than pigmented wild-type animals demonstrating that differences VEGF dose cannot account for these differences. Histological analysis demonstrated that C57BL/6Jc-2J irises were slightly thinner than wild-type, consistent with the absence of pigment granules in these eyes.
Figure 3. Corneal and iris neovascularization assays using high-dose VEGF pellets in C57BL/6J and C57BL/6JTyrc-2J mice.
i Left. Representative photomicrographs showing hyphema formation in mice with high dose (800 ng) VEGF pellets implanted in the cornea. Pellet location is indicated with the “P”. The location of the advancing vessel front in the cornea is indicated with the arrows to help distinguish corneal vessels from those on the iris immediately below. Right. Vessel area in the cornea and fraction of animals exhibiting hyphema. Difference between mutant and wild type mice is statistically significant in both cases (P<0.05). Error bars indicate the 95% confidence interval for the mean vessel area and fraction of animals exhibiting hyphema.
Pigment production can alter endothelial cell activity in vitro
In order to ascertain whether the inhibitory effects that we observed in the iris were a direct result of pigment production, or the animal’s adaptation to differential pigment production in the two strains, we assayed the ability of melanocytes to affect angiogenesis and in vitro endothelial cell function. To do this, we used the Melan-c cell line and a derivative of that cell line in which the albino mutation was corrected using RNA-DNA oligonucleotides [20]. We observed that the ability of bovine capillary endothelial cells and human dermal microvascular endothelial cells to migrate to serum-containing media were reduced in the presence of tyrosinase, pigmented melanocytes, or conditioned media from those cells compared to albino controls (Figure 4a). Similarly, the tyrosinase products L-DOPA and DHI inhibited human dermal microvascular endothelial cell migration (Figure 4b). These results indicate that intermediate products of pigment formation can inhibit angiogenic processes.
Figure 4. The effect of tyrosinase and tyrosinase products on endothelial cell migration.
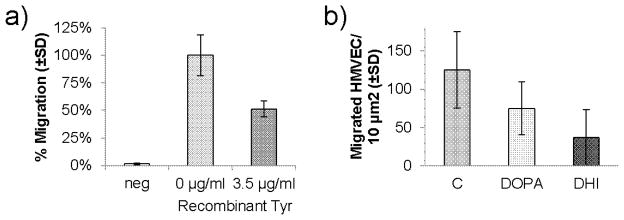
a) Migration of HMVEC-d to serum-free wells (neg) and wells containing serum plus the indicated amount of recombinant tyrosinase. All three values are statistically significantly different (p<0.05 by ANOVA). Error bars show standard deviation of the mean. b) Migration of HMVEC-d to wells containing serum plus carrier “C”, L-DOPA (50 μM), or DHI (100 μM). Both tyrosinase products significantly inhibit migration (p<0.05 by ANOVA). Error bars show standard deviation of the mean.
Discussion
These results demonstrate a causal connection between melanin production and angiogenic differences in animals. There are at least two major classes of tyrosinase products that may be responsible for the differences in angiogenic responsiveness. First, melanin intermediate products including DHI and catecholamines such as DOPA which are produced as side products of tyrosine oxidation, but can also serve as substrates for, and thus be consumed by, tyrosinase [21–24]. Second, reactive oxygen species that are produced as a result of tyrosinase activity and eliminated by the antioxidant characteristics of melanin have been shown to alter angiogenic processes [25].
One of the most intriguing outcomes of this study is the observation that the same mutation can have opposite effects on the same process in different tissues. The albino mutation increased VEGF responsiveness in the cornea, but decreased it in the iris. One explanation for this difference may be differing sensitivities of different endothelial cell types to melanin intermediate products. Alternatively, differences in the tissue levels of various intermediate melanin products may explain these results. For example, different catecholamines produce pro-and antiangiogenic effects. While dopamine is antiangiogenic at pharmacologic doses [9], the other products of dopamine beta-hydroxylase (including epinephrine and norepinephrine) are proangiogenic and are required for normal angiogenesis in a hindlimb ischemia model [26]. Interestingly the inhibitory effect which we observe of the melanin intermediate 5,6-dihydroxyindole (DHI) on endothelial cell migration has not previously been observed and may contribute to the lower level of iris neovascularization in C57BL/6J.
An additional mechanism by which tyrosinase might regulate angiogenesis is reactive oxygen species. High concentrations of reactive oxygen is believed to be responsible for a general cytotoxicity observed in and adjacent to cells producing melanin. However lower levels of exogenous ROS can stimulate VEGF production in endothelial cells [27] as well as induce in vitro behavior in endothelial cells that is predictive of angiogenesis such as migration and proliferation [25]. The signaling mechanism involved in these changes is currently being defined. Reactive oxygen species can modify a number of angiogenesis-signaling molecules within cells including tyrosine and serine phosphatases, transcription factors, and lipids leading to altered signaling in endothelial cells [28] as well as associated cells [29]. In vivo, ROS appear to be produced as second messengers by a class of non-phagocytic NAD(P)H oxidases (Nox) [30]. For example, in scratch migration assays, ROS are induced in migrating cells and Nox2 is translocated to the leading edge of the cells. Inhibition of ROS accumulation by Nox2 siRNA, PEG-catalase, or N-acetylecysteine inhibited cell migration suggesting a requirement for ROS for full migratory activity in this assay [31]. Whether reactive oxygen products contribute to either corneal or iris neovascularization in this model remains to be determined.
These findings in this paper correlate with an observation in melanoma research. Amelanotic melanoma is believed to be biologically more aggressive than melanin-producing melanoma [32]. This difference may be attributable to differences in angiogenesis. Radial growth is much more common in melanotic melanomas than in amelanotic melanomas [33]. Since such growth may be a result of a failure to initiate angiogenesis efficiently [34], this may be a result of inhibition of angiogenesis similar to that observed in the iris of pigmented animals. This also indicates that pigment production per se, through its effects on angiogenesis, may be responsible for differences in growth characteristics in melanoma.
Choroidal pigmentation varies dramatically among various ethnic groups [35]. Marked racial differences have been reported for choroidal neovascularization in Age-Related Macular Degeneration (ARMD). While Caucasians and African-Americans have a similar prevalence of nonexudative form of ARMD, there is a disparity in the prevalence of neovascular form. Gregor and Joffe demonstrated that Caucasians had a 3.5% incidence of exudative ARMD compared to 0.1% for African-Americans [36]. The Baltimore Eye Survey reported that ARMD accounted for 30% of bilateral blindness among Caucasians (usually the resulting from the exudative form of ARMD) as compared to 0% for African-Americans [37]. Thus angiogenesis is lower in the human pigmented choroid in much the same way that it is lower in the iris of pigmented mice.
In contrast, in the unpigmented retina, severe diabetic retinopathy has been found to be more frequent in African Americans than in whites. While these differences have traditionally been explained by differences in blood sugar control, recent studies demonstrate that hemoglobin A1c levels (a surrogate for blood sugar control) cannot control for these differences. This indicates that differences in diabetic retinopathy may arise from genetic differences among individuals of differing ethnicity [38]. These effects in the unpigmented retina, which lies above the pigmented choroid, mimics our observations in the unpigmented cornea which lies above the pigmented iris. In both cases the tissue containing pigmented melanocytes has reduced angiogenesis whereas the nonpigmented adjacent tissue has higher angiogenic responsiveness. Thus diffusible versus nondiffusible angiogenesis regulating factors produced by melanocytes may contribute to the differences in the angiogenic balance immediately adjacent to melanocytes versus the neighboring tissues..
Acknowledgments
Supported by National Institutes of Health Grants R01 EY12726
References
- 1.Rohan RM, Fernandez A, Udagawa T, Yuan J, D’Amato RJ. Genetic heterogeneity of angiogenesis in mice. Faseb J. 2000;14 (7):871–876. doi: 10.1096/fasebj.14.7.871. [DOI] [PubMed] [Google Scholar]
- 2.Rogers MS, Rohan RM, Birsner AE, D’Amato RJ. Genetic loci that control vascular endothelial growth factor-induced angiogenesis. Faseb J. 2003 doi: 10.1096/fj.03-0246fje. [DOI] [PubMed] [Google Scholar]
- 3.Rogers MS, Rohan RM, Birsner AE, D’Amato RJ. Genetic loci that control the angiogenic response to basic fibroblast growth factor. Faseb J. 2004;18 (10):1050–1059. doi: 10.1096/fj.03-1241com. [DOI] [PubMed] [Google Scholar]
- 4.Nakai K, Rogers MS, Baba T, Funakoshi T, Birsner AE, Luyindula DS, D’Amato RJ. Genetic loci that control the size of laser-induced choroidal neovascularization. Faseb J. 2009 doi: 10.1096/fj.08-124321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers MS, Birsner AE, D’Amato RJ. The mouse cornea micropocket angiogenesis assay. Nat Protoc. 2007;2 (10):2545–2550. doi: 10.1038/nprot.2007.368. [DOI] [PubMed] [Google Scholar]
- 6.Rogers MS, D’Amato RJ. The effect of genetic diversity on angiogenesis. Exp Cell Res. 2006;312 (5):561–574. doi: 10.1016/j.yexcr.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Shaked Y, Bertolini F, Man S, Rogers MS, Cervi D, Foutz T, Rawn K, Voskas D, Dumont DJ, Ben-David Y, Lawler J, Henkin J, Huber J, Hicklin DJ, D’Amato RJ, Kerbel RS. Genetic heterogeneity of the vasculogenic phenotype parallels angiogenesis; Implications for cellular surrogate marker analysis of antiangiogenesis. Cancer Cell. 2005;7 (1):101–111. doi: 10.1016/j.ccr.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 8.Land EJ, Ramsden CA, Riley PA. Tyrosinase autoactivation and the chemistry of ortho-quinone amines. Acc Chem Res. 2003;36 (5):300–308. doi: 10.1021/ar020062p. [DOI] [PubMed] [Google Scholar]
- 9.Basu S, Nagy JA, Pal S, Vasile E, Eckelhoefer IA, Bliss VS, Manseau EJ, Dasgupta PS, Dvorak HF, Mukhopadhyay D. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat Med. 2001;7 (5):569–574. doi: 10.1038/87895. [DOI] [PubMed] [Google Scholar]
- 10.Chakroborty D, Sarkar C, Basu B, Dasgupta PS, Basu S. Catecholamines regulate tumor angiogenesis. Cancer Res. 2009;69 (9):3727–3730. doi: 10.1158/0008-5472.CAN-08-4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ushio-Fukai M, Nakamura Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett. 2008;266 (1):37–52. doi: 10.1016/j.canlet.2008.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beermann F, Orlow SJ, Lamoreux ML. The Tyr (albino) locus of the laboratory mouse. Mamm Genome. 2004;15 (10):749–758. doi: 10.1007/s00335-004-4002-8. [DOI] [PubMed] [Google Scholar]
- 13.Haldane JBS, Sprunt AD, Haldane NM. Reduplication in Mice. J Genet. 1915;5 (2):133–135. [Google Scholar]
- 14.Page-McCaw PS, Chung SC, Muto A, Roeser T, Staub W, Finger-Baier KC, Korenbrot JI, Baier H. Retinal network adaptation to bright light requires tyrosinase. Nat Neurosci. 2004;7 (12):1329–1336. doi: 10.1038/nn1344. [DOI] [PubMed] [Google Scholar]
- 15.Kenyon BM, Voest EE, Chen CC, Flynn E, Folkman J, D’Amato RJ. A model of angiogenesis in the mouse cornea. Invest Ophthalmol Vis Sci. 1996;37 (8):1625–1632. [PubMed] [Google Scholar]
- 16.Wang S, Basten CJ, Zeng Z-B. Windows QTL Cartographer 2.5. 2.5 2007. [Google Scholar]
- 17.Nadeau JH, Singer JB, Matin A, Lander ES. Analysing complex genetic traits with chromosome substitution strains. Nat Genet. 2000;24 (3):221–225. doi: 10.1038/73427. [DOI] [PubMed] [Google Scholar]
- 18.Singer JB, Hill AE, Burrage LC, Olszens KR, Song J, Justice M, O’Brien WE, Conti DV, Witte JS, Lander ES, Nadeau JH. Genetic dissection of complex traits with chromosome substitution strains of mice. Science. 2004;304 (5669):445–448. doi: 10.1126/science.1093139. [DOI] [PubMed] [Google Scholar]
- 19.Rogers MS, Boyartchuk V, Rohan RM, Birsner AE, Dietrich WF, D’Amato RJ. The classical pink-eyed dilution mutation affects angiogenic responsiveness. PLoS One. 2012;7 (5):e35237. doi: 10.1371/journal.pone.0035237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexeev V, Yoon K. Stable and inheritable changes in genotype and phenotype of albino melanocytes induced by an RNA-DNA oligonucleotide. Nat Biotechnol. 1998;16 (13):1343–1346. doi: 10.1038/4322. [DOI] [PubMed] [Google Scholar]
- 21.Pham CL, Leong SL, Ali FE, Kenche VB, Hill AF, Gras SL, Barnham KJ, Cappai R. Dopamine and the dopamine oxidation product 5,6-dihydroxylindole promote distinct on-pathway and off-pathway aggregation of alpha-synuclein in a pH-dependent manner. J Mol Biol. 2009;387 (3):771–785. doi: 10.1016/j.jmb.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Chesney J, Metz C, Bacher M, Peng T, Meinhardt A, Bucala R. An essential role for macrophage migration inhibitory factor (MIF) in angiogenesis and the growth of a murine lymphoma. Mol Med. 1999;5 (3):181–191. [PMC free article] [PubMed] [Google Scholar]
- 23.Rosengren E, Bucala R, Aman P, Jacobsson L, Odh G, Metz CN, Rorsman H. The immunoregulatory mediator macrophage migration inhibitory factor (MIF) catalyzes a tautomerization reaction. Mol Med. 1996;2 (1):143–149. [PMC free article] [PubMed] [Google Scholar]
- 24.Khoufache K, Bazin S, Girard K, Guillemette J, Roy MC, Verreault JP, Al-Abed Y, Foster W, Akoum A. Macrophage migration inhibitory factor antagonist blocks the development of endometriosis in vivo. PLoS One. 2012;7 (5):e37264. doi: 10.1371/journal.pone.0037264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yasuda M, Ohzeki Y, Shimizu S, Naito S, Ohtsuru A, Yamamoto T, Kuroiwa Y. Stimulation of in vitro angiogenesis by hydrogen peroxide and the relation with ETS-1 in endothelial cells. Life Sci. 1999;64 (4):249–258. doi: 10.1016/s0024-3205(98)00560-8. [DOI] [PubMed] [Google Scholar]
- 26.Chalothorn D, Zhang H, Clayton JA, Thomas SA, Faber JE. Catecholamines augment collateral vessel growth and angiogenesis in hindlimb ischemia. Am J Physiol Heart Circ Physiol. 2005;289 (2):H947–959. doi: 10.1152/ajpheart.00952.2004. [DOI] [PubMed] [Google Scholar]
- 27.Chua CC, Hamdy RC, Chua BH. Upregulation of vascular endothelial growth factor by H2O2 in rat heart endothelial cells. Free Radic Biol Med. 1998;25 (8):891–897. doi: 10.1016/s0891-5849(98)00115-4. [DOI] [PubMed] [Google Scholar]
- 28.Ushio-Fukai M, Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase. Mol Cell Biochem. 2004;264 (1–2):85–97. doi: 10.1023/b:mcbi.0000044378.09409.b5. [DOI] [PubMed] [Google Scholar]
- 29.Sato H, Sato M, Kanai H, Uchiyama T, Iso T, Ohyama Y, Sakamoto H, Tamura J, Nagai R, Kurabayashi M. Mitochondrial reactive oxygen species and c-Src play a critical role in hypoxic response in vascular smooth muscle cells. Cardiovasc Res. 2005;67 (4):714–722. doi: 10.1016/j.cardiores.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 30.Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, Fujimoto M, Quinn MT, Pagano PJ, Johnson C, Alexander RW. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2002;91 (12):1160–1167. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- 31.Ikeda S, Yamaoka-Tojo M, Hilenski L, Patrushev NA, Anwar GM, Quinn MT, Ushio-Fukai M. IQGAP1 regulates reactive oxygen species-dependent endothelial cell migration through interacting with Nox2. Arterioscler Thromb Vasc Biol. 2005;25 (11):2295–2300. doi: 10.1161/01.ATV.0000187472.55437.af. [DOI] [PubMed] [Google Scholar]
- 32.Notani K, Shindoh M, Yamazaki Y, Nakamura H, Watanabe M, Kogoh T, Ferguson MM, Fukuda H. Amelanotic malignant melanomas of the oral mucosa. Br J Oral Maxillofac Surg. 2002;40 (3):195–200. doi: 10.1054/bjom.2001.0713. [DOI] [PubMed] [Google Scholar]
- 33.Ohashi K, Kasuga T, Tanaka N, Enomoto S, Horiuchi J, Okada N. Malignant melanomas of the oral cavity: heterogeneity of pathological and clinical features. Virchows Arch A Pathol Anat Histopathol. 1992;420 (1):43–50. doi: 10.1007/BF01605983. [DOI] [PubMed] [Google Scholar]
- 34.Marcoval J, Moreno A, Graells J, Vidal A, Escriba JM, Garcia-Ramirez M, Fabra A. Angiogenesis and malignant melanoma. Angiogenesis is related to the development of vertical (tumorigenic) growth phase. J Cutan Pathol. 1997;24 (4):212–218. doi: 10.1111/j.1600-0560.1997.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 35.Weiter JJ, Delori FC, Wing GL, Fitch KA. Retinal pigment epithelial lipofuscin and melanin and choroidal melanin in human eyes. Invest Ophthalmol Vis Sci. 1986;27 (2):145–152. [PubMed] [Google Scholar]
- 36.Gregor Z, Joffe L. Senile macular changes in the black African. Br J Ophthalmol. 1978;62 (8):547–550. doi: 10.1136/bjo.62.8.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sommer A, Tielsch JM, Katz J, Quigley HA, Gottsch JD, Javitt JC, Martone JF, Royall RM, Witt KA, Ezrine S. Racial differences in the cause-specific prevalence of blindness in east Baltimore. N Engl J Med. 1991;325 (20):1412–1417. doi: 10.1056/NEJM199111143252004. [DOI] [PubMed] [Google Scholar]
- 38.Emanuele N, Sacks J, Klein R, Reda D, Anderson R, Duckworth W, Abraira C. Ethnicity, race, and baseline retinopathy correlates in the veterans affairs diabetes trial. Diabetes Care. 2005;28 (8):1954–1958. doi: 10.2337/diacare.28.8.1954. [DOI] [PubMed] [Google Scholar]