Abstract
Cajal bodies (CBs) are subnuclear domains that participate in the biogenesis of small nuclear ribonucleoproteins (snRNPs) and telomerase. CBs are found in cells with high splicing demands, such as neuronal and cancer cells. The purpose of this review is to highlight what is known about the signals that impact the formation and activity of CBs. Particular attention is paid to phosphorylation as a major regulator of CB formation and composition, but a non-biochemical mediated pathway (mechanotransduction) that impacts CBs is also discussed. Amongst the CB components, recently published work on coilin (the CB marker protein) strongly suggests that this protein, and the CB by extension, is a global sensor that responds to environmental signals. Disruption of these signals, which would result in a decreased capacity to generate snRNPs and telomerase, is predicted to be beneficial in the treatment of cancer.
Keywords: coilin, snRNP, phosphorylation, telomerase
1. Introduction
The nucleus is highly organized and contains various compartments such as the Cajal body (CB). The CB is involved in ribonucleoprotein (RNP) biogenesis (Machyna et al., 2013). Specifically, the CB is a maturation point for RNPs involved in splicing, histone mRNA processing and telomere formation. In transcriptionally active cells (such as neuronal and cancer cells) that require high levels of RNPs, the CB serves as an efficiency platform for modification reactions. In regards to spliceosomal small nuclear RNPs (snRNPs), the small nuclear RNA (snRNA) component of the snRNP is modified in the CB with the guidance of small Cajal body-specific RNAs (scaRNAs) (Machyna, Heyn, 2013). It is also possible that CBs participate in the initial processing of the nascent snRNA since CBs associate with certain U snRNA gene loci (Frey et al., 1999, Frey and Matera, 1995, Jacobs et al., 1999, Shevtsov and Dundr, 2011, Smith et al., 1995, Suzuki et al., 2010). In addition to snRNPs, CBs contain the RNA component of telomerase (TERC, also known as hTR) (Zhu et al., 2004). It is possible that the CB helps to traffic hTR to telomeres during S phase for holoenzyme assembly with the telomerase reverse transcriptase component (TERT). Both scaRNAs and hTR are targeted to CBs via interactions with the protein WRAP53 (also known as TCAB1 and WDR79), which binds a conserved sequence element present in these RNAs (the CAB box) (Venteicher et al., 2009) (Tycowski et al., 2009). Some mutations in WRAP53 result in the mislocalization of telomerase to the nucleolus (Batista et al., 2011). WRAP53 has been shown to associate with two major components of the CB, SMN and coilin (Mahmoudi et al., 2010). SMN (survivor of motor neuron) is mutated in most cases of spinal muscular atrophy (SMA), and plays a crucial role in the cytoplasmic phase of snRNP biogenesis. The role of SMN in the CB is less clear, but may involve snRNA regeneration (Pellizzoni et al., 1998) and/or tri-snRNP assembly (Boulisfane et al., 2011, Forthmann et al., 2013). Coilin is known as the CB marker protein and is essential for canonical CB formation (Figure 1).
Figure 1. The CB is a dynamic nuclear domain responsive to many stimuli.
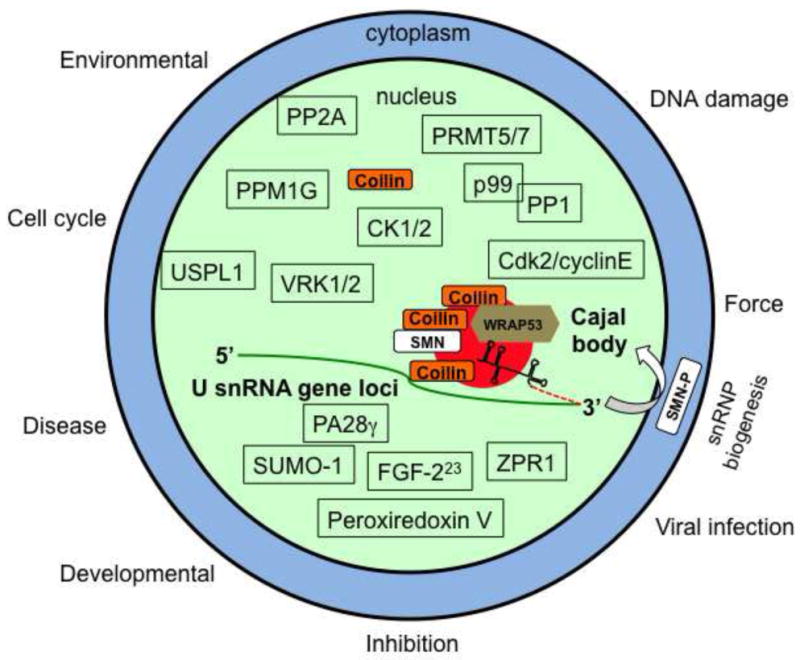
Major structural components of the CB include coilin, WRAP53, SMN and snRNPs. Since CBs are known to associate with many gene loci, including those giving rise to U snRNA, such as U2 snRNA, it is possible that the CB participates in the initial 3′ end processing event of the nascent pre-U snRNA (stem loop structure with dashed red line). Mature snRNPs, scaRNAs and TERC are not shown. Besides accumulations in the CB, every known protein component of the CB also resides elsewhere in the cell. The majority of coilin, for example, is nucleoplasmic. Shown above the CB are enzymes known or suspected to modify coilin or other components of the CB: PPM1G (protein phosphatase, Mg2+/Mn2+ dependent, 1G) (Hearst et al., 2009, Petri, Grimmler, 2007), CK1/2 (casein kinase 1 (our unpublished results) and 2) (Hebert and Matera, 2000), VRK1/2 (vaccinia-related kinase 1 and 2) (Sanz-Garcia et al., 2011), PRMT5/7 (protein arginine methyltrasferase 5 and 7) (Boisvert, Cote, 2002, Gonsalvez, Tian, 2007), protein phosphatases PP2A and PP1(γ), with adaptor subunit p99 (PNUTS) (Kitao et al., 2008, Lyon et al., 1997, Moorhead et al., 2007, Renvoise et al., 2012) and Cdk2/cyclinE (Liu et al., 2000). The ubiquitin-specific protease-like 1 (USPL1) SUMO isopeptidase protein has been shown to impact coilin localization (Schulz, Chachami, 2012). Shown below the CB are signaling molecules that impact or are found in CBs: PA28γ (proteasome activator) (Cioce et al., 2006), FGF-223 (fibroblast growth factor) (Bruns et al., 2009), ZPR1 (zinc finger protein 259) (Gangwani et al., 2001), SUMO-1 (Navascues, Bengoechea, 2008) and Peroxiredoxin V (Kropotov et al., 2004). The biogenesis of U1, U2, U4 and U5 snRNPs require cytoplasmic processing steps under the control of SMN. Cytoplasmic SMN is hyperphosphorylated (SMN-P) relative to that found in the nucleus, due in part to the action of the nuclear phosphatase PPM1G (Grimmler, Bauer, 2005, Petri, Grimmler, 2007). Arrayed along the outside of the cell are stimuli that affect CB assembly.
2. Functions
Many lines of investigation reinforce the concept that the CB increases the RNP biogenesis efficiency of the cell (Klingauf et al., 2006). For example, coilin reduction in zebrafish (which abolishes CBs) is associated with an embryonic lethal phenotype that can be partially rescued by the addition of mature snRNPs (Strzelecka et al., 2010b). Interestingly, the CB may have other functions centering upon response to stress. Viral infection, UV-C exposure, ionizing radiation, and treatment with the DNA damaging agents cisplatin and etoposide all disrupt CBs in different ways (Hebert, 2010). UV-C and adenovirus infection, for example, trigger the formation of coilin containing microfoci. Interestingly, the UV-C pathway for CB disruption requires the proteasome activator subunit PA28γ, which does not localize to CBs, but may impact their formation via interactions with the nucleoplasmic pool of coilin. As opposed to the microfoci formed with UV-C treatment, herpes virus infection relocalizes coilin to damaged centromeres in a process termed the interphase centromere damage response (iCDR) (Morency et al., 2007). Ionizing radiation and exposure to cisplatin or etoposide, in contrast, disrupt CBs and cause a relocalization of coilin to the nucleolus (Gilder et al., 2011). Although the reasons for changes in CB formation in response to these cellular insults are not clear, the fact that they occur implicate the CB in some aspect of the stress response pathway.
Insight into the rationale for the CB in the stress response comes from studies on coilin. We have found that coilin levels impact the cell response to cisplatin and modulate the association of RNA pol I with rDNA (Gilder, Do, 2011). We have also found that coilin binds both DNA and RNA, and has RNase activity (Broome and Hebert, 2012b). The RNase activity of coilin appears to be most specific for the 3′ end of U2 snRNA and hTR, suggesting that coilin participates in the biogenesis of these non-coding RNAs (Broome et al., 2013, Broome and Hebert, 2012a). Alteration of coilin levels correlates with changes in the steady state levels of several non-coding RNAs, including U2 snRNA, rRNA (pol I derived) and hTR, and their pre-processed pools (Broome, Carrero, 2013, Broome and Hebert, 2012b). These findings indicate that coilin may influence the transcription and/or processing of select non-coding RNAs. In support of this hypothesis, we have shown by chromatin immunoprecipitation (ChIP) that coilin interacts with U1 and U2 snRNA gene loci (Broome, Carrero, 2013). Additional we have observed that coilin associates with various non-coding RNAs, such as the 47/45S rRNA precursor, U2 snRNA and hTR, and the association of coilin with these RNAs changes upon exposure to cisplatin or etoposide (Broome, Carrero, 2013). Hence, experimental evidence strongly suggests that coilin and the CB take part in stress response pathways that regulate RNP biogenesis and rRNA transcription/processing.
3. Cascades
Despite the fact that certain treatments, such as cisplatin exposure can disrupt CBs, little is known about how stress response pathways interconnect with the CB. In addition to the treatments listed above that disrupt CBs, other situations and conditions are known to impact CBs. Environmental factors (such as temperature), developmental changes (such as nuclear organization in fetal vs. adult cells), and disease state (such as normal vs. transformed) all impact the nucleus and the CB (Carmo-Fonseca et al., 1993, Spector et al., 1992, Young et al., 2001) (Strzelecka et al. 2010a). Very interestingly, local dynamic force on the surface of the cell via integrins has been shown to cause disruptions in the association of certain proteins within the CB (Poh et al., 2012). For example, following surface force, the interaction between coilin and SMN, as measured by fluorescence resonance energy transfer (FRET), is altered. It is not thought that a biochemical cascade triggered by the surface force is responsible for these changes in CB protein:protein interactions. Rather, the force on the surface of the cell activates a mechanotransduction pathway dependent on (among other things) intact F-actin and cytoskeletal tension and Lamin A/C. Hence, any discussion of signals controlling CB formation must also take into account mechanical as well as biochemical transduction pathways.
What indicates that the CB is subject to traditional signal transduction pathways? One line of evidence is that CBs are impacted when several different types of inhibitors are used. Inhibitors of transcription, translation, nuclear export and kinase and phosphatase activity all cause CB disassembly and/or coilin mis-localization (Hebert, 2010). Additionally, as a dynamic nuclear body, the CB, like the nucleus and nucleolus, disassembles during mitosis and reforms during the G1 phase of the cell cycle (Carmo-Fonseca, Ferreira, 1993). Since phosphorylation plays a crucial role in the cell cycle regulated disassembly of the nucleus and nucleous, it is likely that this modification also controls CB disassembly and reformation that occurs during the cell cycle.
4. Key molecules
If, like the nucleus and nucleolus, phosphorylation impacts CB formation and activity, what proteins in the CB are modified by phosphorylation and what kinases and phosphatases take part in this process? There are at least 20 different proteins in the CBs that are phosphorylated (Hebert, 2010). Because every protein found in the CB is also found in other cellular locales, it is not initially obvious if phosphorylation regulates the activity of only the CB fraction of these proteins. Studies have found that the phosphorylation status of both coilin and SMN influences the interaction of these proteins with each other and snRNPs (Grimmler et al., 2005, Petri et al., 2007, Toyota et al., 2009). Since WRAP53 is also phosphorylated, it is likely that phosphorylation regulates the interaction between coilin, SMN and WRAP53, and these interactions are critical for canonical CB formation. What kinases and phosphatases modify CB proteins? Figure 1 shows enzymes that can or are suspected to modify coilin or other CB proteins.
Besides altering protein:protein interactions that underlie CB formation, phosphorylation may also impact CB activity. We have found that the RNase activity of coilin is decreased in a phosphomutant background (S489D) (Broome, Carrero, 2013). We have also found that the association of coilin with various non-coding RNAs changes upon hyperphosphorylation (Broome, Carrero, 2013), which correlates with decreased coilin self-association and CB disassembly that normally take place during mitosis. The phosphorylation/dephosphorylation of various CB components, therefore, is the end result of signaling pathways that respond to the transcriptional and splicing needs of the cell. These pathways likely regulate the nuclear and cytoplasmic steps of snRNP biogenesis. In addition to phosphorylation, PRMT5 and 7, which generate symmetrical dimethylarginine, modify coilin and other components of the CB (Boisvert et al., 2002, Clelland et al., 2009, Gonsalvez et al., 2007, Hebert et al., 2002). As with phosphorylation, this modification plays a significant regulatory role in controlling protein:protein interactions and localization, and thus also impacts CB formation and activity (Tapia et al., 2010). Lastly, sumoylation of CB components may also contribute to the regulation of this subnuclear domain (Navascues et al., 2008, Schulz et al., 2012). In addition to phosphorylation and other forms of posttranslational modification, several signaling proteins impact CB formation or alter its composition (Figure 1).
5. Associated pathologies and therapeutic implications
A clear understanding of the signals that control CB formation and activity have the potential to clarify detrimental abnormalities in spinal muscular atrophy, dyskeratosis congenita and cancer. Although much needs to be learned, the limited knowledge we have about coilin phosphorylation and CB formation in normal and transformed cells allows for the formulation of a model with potential new targets for anti-cancer therapeutics (Figure 2). For such a model to translate to the clinic however, more effort is needed to identify the factors, such as kinase and phosphatases, that modify CB proteins. Studies also need to be conducted to functionally characterize how modifications impact the activity of proteins in the CB. With this knowledge, CBs can be connected to various known signaling pathways. This will result in a better understanding of how these pathways control CB activity and thus the RNP biogenesis capacity of the cell.
Figure 2. Potential mechanism for inhibiting CB function in transformed cells.
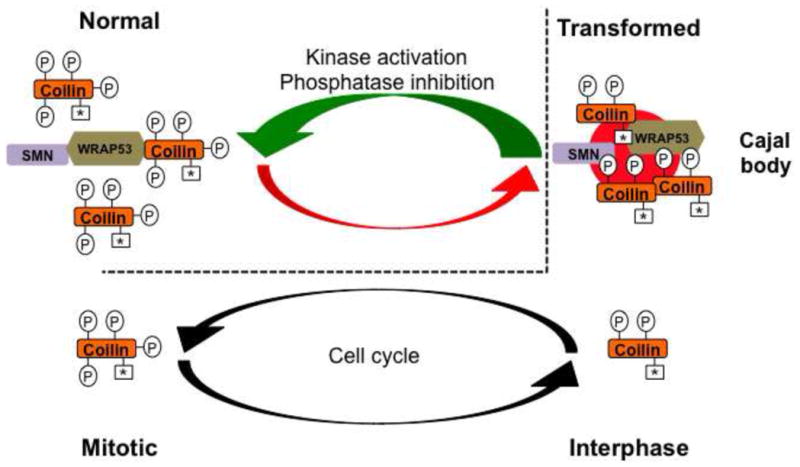
Coilin is relatively hyperphosphorylated in most normal cells (denoted by four Ps) compared to that in transformed cells (two Ps). Because hyperphosphorylated coilin has reduced self-association, CBs do not form in most normal cells. However, the extent of coilin phosphorylation is reduced in transformed cells, which correlates with increased coilin self-association and CB formation. A parallel can be drawn between the amount of phosphorylation of coilin in normal cells to that of mitotic coilin in transformed cells (dashed line). In transformed cells, interphase coilin is phosphorylated but can still self-associate and form CBs (two Ps). When transformed cells enter mitosis, however, CBs disassemble and this disassembly correlates with increased coilin phosphorylation (four Ps). We speculate that this same level or extent of coilin phosphorylation in mitotic transformed cells is also present during interphase of normal cells (note the four Ps in normal cells), and this accounts for the lack of CBs in most normal cell types. Strategies that could activate kinases or inhibit phosphatases that act on coilin would be detrimental to transformed cells (green arrow) because the lack of CBs would decrease the available snRNP and telomerase resources, and thus make these cells more “normal” in regards to nuclear organization. Symmetrical dimethylarginine modifications on coilin are indicated by an asterisk (*).
Key facts.
Cajal bodies (CBs) play a crucial role in ribonucleoprotein (RNP) biogenesis, and are found in cells with high transcription and splicing demands.
Several conditions, such as DNA damage, disrupt CBs or alter their composition, implying that the CB is responsive to many different signaling pathways including those dealing with stress.
One likely regulatory mechanism for controlling CB formation and activity is phosphorylation.
The modification of known CB proteins by phosphorylation, including the CB marker protein coilin, may underlie CB induction observed in the transformation process.
Acknowledgments
This work was supported by The National Institute of General Medical Sciences of the National Institutes of Health under award number R01GM081448. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Batista LF, Pech MF, Zhong FL, Nguyen HN, Xie KT, Zaug AJ, et al. Telomere shortening and loss of self-renewal in dyskeratosis congenita induced pluripotent stem cells. Nature. 2011;474:399–402. doi: 10.1038/nature10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisvert FM, Cote J, Boulanger MC, Cleroux P, Bachand F, Autexier C, et al. Symmetrical dimethylarginine methylation is required for the localization of SMN in Cajal bodies and pre-mRNA splicing. J Cell Biol. 2002;159:957–69. doi: 10.1083/jcb.200207028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulisfane N, Choleza M, Rage F, Neel H, Soret J, Bordonne R. Impaired minor tri-snRNP assembly generates differential splicing defects of U12-type introns in lymphoblasts derived from a type I SMA patient. Hum Mol Genet. 2011;20:641–8. doi: 10.1093/hmg/ddq508. [DOI] [PubMed] [Google Scholar]
- Broome HJ, Carrero ZI, Douglas HE, Hebert M. Phosphorylation regulates coilin activity and RNA association. Biology Open. 2013 doi: 10.1242/bio.20133863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome HJ, Hebert MD. Coilin Displays Differential Affinity for Specific RNAs In Vivo and Is Linked to Telomerase RNA Biogenesis. J Mol Biol. 2012a doi: 10.1016/j.jmb.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome HJ, Hebert MD. In Vitro RNase and Nucleic Acid Binding Activities Implicate Coilin in U snRNA Processing. PLoS One. 2012b;7:e36300. doi: 10.1371/journal.pone.0036300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns AF, van Bergeijk J, Lorbeer C, Nolle A, Jungnickel J, Grothe C, et al. Fibroblast growth factor-2 regulates the stability of nuclear bodies. Proc Natl Acad Sci U S A. 2009;106:12747–52. doi: 10.1073/pnas.0900122106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Fonseca M, Ferreira J, Lamond AI. Assembly of snRNP-containing Coiled Bodies Is Regulated in Interphase and Mitosis - Evidence that the Coiled Body Is a Kinetic Nuclear Structure. J Cell Biol. 1993;120:841–52. doi: 10.1083/jcb.120.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioce M, Boulon S, Matera AG, Lamond AI. UV-induced fragmentation of Cajal bodies. J Cell Biol. 2006;175:401–13. doi: 10.1083/jcb.200604099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland AK, Kinnear NP, Oram L, Burza J, Sleeman JE. The SMN protein is a key regulator of nuclear architecture in differentiating neuroblastoma cells. Traffic. 2009;10:1585–98. doi: 10.1111/j.1600-0854.2009.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forthmann B, Brinkmann H, Ratzka A, Stachowiak MK, Grothe C, Claus P. Immobile survival of motoneuron (SMN) protein stored in Cajal bodies can be mobilized by protein interactions. Cell Mol Life Sci. 2013 doi: 10.1007/s00018-012-1242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey MR, Bailey AD, Weiner AM, Matera AG. Association of snRNA genes with coiled bodies is mediated by nascent snRNA transcripts. Curr Biol. 1999;9:126–31. doi: 10.1016/s0960-9822(99)80066-9. [DOI] [PubMed] [Google Scholar]
- Frey MR, Matera AG. Coiled Bodies Contain U7 Small Nuclear RNA and Associate with Specific DNA Sequences in Interphase Cells. Proc Natl Acad Sci USA. 1995;92:5915–9. doi: 10.1073/pnas.92.13.5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangwani L, Mikrut M, Theroux S, Sharma M, Davis RJ. Spinal muscular atrophy disrupts the interaction of ZPR1 with the SMN protein. Nat Cell Biol. 2001;3:376–83. doi: 10.1038/35070059. [DOI] [PubMed] [Google Scholar]
- Gilder AS, Do PM, Carrero ZI, Cosman AM, Broome HJ, Velma V, et al. Coilin participates in the suppression of RNA polymerase I in response to cisplatin-induced DNA damage. Mol Biol Cell. 2011;22:1070–9. doi: 10.1091/mbc.E10-08-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalvez GB, Tian L, Ospina JK, Boisvert FM, Lamond AI, Matera AG. Two distinct arginine methyltransferases are required for biogenesis of Sm-class ribonucleoproteins. J Cell Biol. 2007;178:733–40. doi: 10.1083/jcb.200702147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimmler M, Bauer L, Nousiainen M, Korner R, Meister G, Fischer U. Phosphorylation regulates the activity of the SMN complex during assembly of spliceosomal U snRNPs. EMBO Rep. 2005;6:70–6. doi: 10.1038/sj.embor.7400301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearst SM, Gilder AS, Negi SS, Davis MD, George EM, Whittom AA, et al. Cajal-body formation correlates with differential coilin phosphorylation in primary and transformed cell lines. J Cell Sci. 2009;122:1872–81. doi: 10.1242/jcs.044040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert MD. Phosphorylation and the Cajal body: modification in search of function. Arch Biochem Biophys. 2010;496:69–76. doi: 10.1016/j.abb.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert MD, Matera AG. Self-association of coilin reveals a common theme in nuclear body localization. Mol Biol Cell. 2000;11:4159–71. doi: 10.1091/mbc.11.12.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert MD, Shpargel KB, Ospina JK, Tucker KE, Matera AG. Coilin methylation regulates nuclear body formation. Dev Cell. 2002;3:329–37. doi: 10.1016/s1534-5807(02)00222-8. [DOI] [PubMed] [Google Scholar]
- Jacobs EY, Frey MR, Wu W, Ingledue TC, Gebuhr TC, Gao L, et al. Coiled bodies preferentially associate with U4, U11, and U12 small nuclear RNA genes in interphase HeLa cells but not with U6 and U7 genes. Mol Biol Cell. 1999;10:1653–63. doi: 10.1091/mbc.10.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitao S, Segref A, Kast J, Wilm M, Mattaj IW, Ohno M. A compartmentalized phosphorylation/dephosphorylation system that regulates U snRNA export from the nucleus. Mol Cell Biol. 2008;28:487–97. doi: 10.1128/MCB.01189-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingauf M, Stanek D, Neugebauer KM. Enhancement of U4/U6 small nuclear ribonucleoprotein particle association in Cajal bodies predicted by mathematical modeling. Mol Biol Cell. 2006;17:4972–81. doi: 10.1091/mbc.E06-06-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropotov AV, Grudinkin PS, Pleskach NM, Gavrilov BA, Tomilin NV, Zhivotovsky B. Downregulation of peroxiredoxin V stimulates formation of etoposide-induced double-strand DNA breaks. FEBS Lett. 2004;572:75–9. doi: 10.1016/j.febslet.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Liu J, Hebert MD, Ye Y, Templeton DJ, Kung H, Matera AG. Cell cycle-dependent localization of the CDK2-cyclin E complex in Cajal (coiled) bodies. J Cell Sci. 2000;113 (Pt 9):1543–52. doi: 10.1242/jcs.113.9.1543. [DOI] [PubMed] [Google Scholar]
- Lyon CE, Bohmann K, Sleeman J, Lamond AI. Inhibition of protein dephosphorylation results in the accumulation of splicing snRNPs and coiled bodies within the nucleolus. Exp Cell Res. 1997;230:84–93. doi: 10.1006/excr.1996.3380. [DOI] [PubMed] [Google Scholar]
- Machyna M, Heyn P, Neugebauer KM. Cajal bodies: where form meets function. Wiley interdisciplinary reviews. RNA. 2013;4:17–34. doi: 10.1002/wrna.1139. [DOI] [PubMed] [Google Scholar]
- Mahmoudi S, Henriksson S, Weibrecht I, Smith S, Soderberg O, Stromblad S, et al. WRAP53 is essential for Cajal body formation and for targeting the survival of motor neuron complex to Cajal bodies. PLoS Biol. 2010;8:e1000521. doi: 10.1371/journal.pbio.1000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorhead GB, Trinkle-Mulcahy L, Ulke-Lemee A. Emerging roles of nuclear protein phosphatases. Nat Rev Mol Cell Biol. 2007;8:234–44. doi: 10.1038/nrm2126. [DOI] [PubMed] [Google Scholar]
- Morency E, Sabra M, Catez F, Texier P, Lomonte P. A novel cell response triggered by interphase centromere structural instability. J Cell Biol. 2007;177:757–68. doi: 10.1083/jcb.200612107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navascues J, Bengoechea R, Tapia O, Casafont I, Berciano MT, Lafarga M. SUMO-1 transiently localizes to Cajal bodies in mammalian neurons. J Struct Biol. 2008;163:137–46. doi: 10.1016/j.jsb.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Pellizzoni L, Kataoka N, Charroux B, Dreyfuss G. A novel function for SMN, the spinal muscular atrophy disease gene product, in pre-mRNA splicing. Cell. 1998;95:615–24. doi: 10.1016/s0092-8674(00)81632-3. [DOI] [PubMed] [Google Scholar]
- Petri S, Grimmler M, Over S, Fischer U, Gruss OJ. Dephosphorylation of survival motor neurons (SMN) by PPM1G/PP2Cgamma governs Cajal body localization and stability of the SMN complex. J Cell Biol. 2007;179:451–65. doi: 10.1083/jcb.200704163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poh YC, Shevtsov SP, Chowdhury F, Wu DC, Na S, Dundr M, et al. Dynamic force-induced direct dissociation of protein complexes in a nuclear body in living cells. Nature communications. 2012;3:866. doi: 10.1038/ncomms1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renvoise B, Querol G, Verrier ER, Burlet P, Lefebvre S. A role for protein phosphatase PP1gamma in SMN complex formation and subnuclear localization to Cajal bodies. J Cell Sci. 2012;125:2862–74. doi: 10.1242/jcs.096255. [DOI] [PubMed] [Google Scholar]
- Sanz-Garcia M, Vazquez-Cedeira M, Kellerman E, Renbaum P, Levy-Lahad E, Lazo PA. Substrate profiling of human vaccinia-related kinases identifies coilin, a Cajal body nuclear protein, as a phosphorylation target with neurological implications. Journal of proteomics. 2011;75:548–60. doi: 10.1016/j.jprot.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Schulz S, Chachami G, Kozaczkiewicz L, Winter U, Stankovic-Valentin N, Haas P, et al. Ubiquitin-specific protease-like 1 (USPL1) is a SUMO isopeptidase with essential, non-catalytic functions. EMBO Rep. 2012;13:930–8. doi: 10.1038/embor.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevtsov SP, Dundr M. Nucleation of nuclear bodies by RNA. Nat Cell Biol. 2011;13:167–73. doi: 10.1038/ncb2157. [DOI] [PubMed] [Google Scholar]
- Smith K, Carter K, Johnson C, Lawrence J. U2 and U1 snRNA gene loci associate with coiled bodies. J Cell Biochem. 1995;59:473–85. doi: 10.1002/jcb.240590408. [DOI] [PubMed] [Google Scholar]
- Spector DL, Lark G, Huang S. Differences in snRNP localization between transformed and nontransformed cells. Mol Biol Cell. 1992;3:555–69. doi: 10.1091/mbc.3.5.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strzelecka M, Oates AC, Neugebauer KM. Dynamic control of Cajal body number during zebrafish embryogenesis. Nucleus. 2010a;1:96–108. doi: 10.4161/nucl.1.1.10680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strzelecka M, Trowitzsch S, Weber G, Luhrmann R, Oates AC, Neugebauer KM. Coilin-dependent snRNP assembly is essential for zebrafish embryogenesis. Nat Struct Mol Biol. 2010b;17:403–9. doi: 10.1038/nsmb.1783. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Izumi H, Ohno M. Cajal body surveillance of U snRNA export complex assembly. J Cell Biol. 2010;190:603–12. doi: 10.1083/jcb.201004109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia O, Bengoechea R, Berciano MT, Lafarga M. Nucleolar targeting of coilin is regulated by its hypomethylation state. Chromosoma. 2010;119:527–40. doi: 10.1007/s00412-010-0276-7. [DOI] [PubMed] [Google Scholar]
- Toyota CG, Davis MD, Cosman AM, Hebert MD. Coilin phosphorylation mediates interaction with SMN and SmB′. Chromosoma. 2009 doi: 10.1007/s00412-009-0249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tycowski KT, Shu MD, Kukoyi A, Steitz JA. A conserved WD40 protein binds the Cajal body localization signal of scaRNP particles. Mol Cell. 2009;34:47–57. doi: 10.1016/j.molcel.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venteicher AS, Abreu EB, Meng Z, McCann KE, Terns RM, Veenstra TD, et al. A human telomerase holoenzyme protein required for Cajal body localization and telomere synthesis. Science. 2009;323:644–8. doi: 10.1126/science.1165357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young PJ, Le TT, Dunckley M, Nguyen TM, Burghes AH, Morris GE. Nuclear gems and Cajal (coiled) bodies in fetal tissues: nucleolar distribution of the spinal muscular atrophy protein, SMN. Exp Cell Res. 2001;265:252–61. doi: 10.1006/excr.2001.5186. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Tomlinson RL, Lukowiak AA, Terns RM, Terns MP. Telomerase RNA accumulates in Cajal bodies in human cancer cells. Mol Biol Cell. 2004;15:81–90. doi: 10.1091/mbc.E03-07-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]


