Abstract
Differentiation of naïve CD4+ T cells has been considered to be an irreversible event and, in particular, the plasticity is believed to be completely lost in Th1 subset in vitro after multiple stimulations. However, here we demonstrate that highly polarized myelin oligodendrocyte glycoprotein (MOG)- and herpes simples virus-specific Th1 clones were still capable of producing IL-17 upon superantigen stimulation. Anti-MHC class-II and anti-TCR αβ chains partially blocked superantigen-induced IL-17 production. These findings suggest that fully differentiated Th1 cells still have capability to produce cytokines of other Th subsets and production of IL-17 by MOG-specific Th1 cells may have implication in initiation and/or exacerbation of neurological autoimmune diseases.
Keywords: Superantigen, Th1, Th17, MOG35-55
1. Introduction
Microbial superantigens are polypeptide molecules produced by pathogens and have been implicated in autoimmune disease initiation and/or exacerbation [1, 2]. Indeed, superantigens can cause exacerbation of several animal models of autoimmune diseases including experimental autoimmune encephalomyelitis (EAE), experimental autoimmune uveoretinitis and collagen-induced arthritis [1]. However, the mechanism of superantigen mediated autoimmune disease exacerbation is not well understood. In humans, CD4+ T cell clones from normal individuals and patients with multiple sclerosis (MS) that are specific to myelin proteins can be expanded by superantigens such as staphylococcal enterotoxins (SEA and SEB) and toxic shock syndrome toxin-1 (TSST-1) [3]. CD4+ T cell clones from MS patients and normal individuals proliferate and secrete interferon (IFN)- in response to myelin oligodendrocyte glycoprotein (MOG) peptides [4]. This suggests that these clones are Th1 cells. However, several lines of evidence suggests a pathogenic role of IL-17 and Th17 cells in autoimmune diseases including MS [5]. The question arises as to whether the cytokine profile secreted from those cloned T cells is changed after stimulation with superantigens. In particular, there is scarce of data with regard to production of IL-17 from these cloned T cells since many of the studies involving superantigen stimulation of myelin protein or MOG specific T cells were performed prior to the recognition of the pathogenic role of IL-17 and Th17 cells.
It has been reported that fully differentiated Th17cells can be redirected to produce IFN-when they are stimulated by IL-12. In contrast, in vitro fully differentiated Th1 cells exhibit little plasticity, that is, it is very difficult to redirect Th1 cells to become Th17 cells [6]. In the present study we challenged this dogma by taking advantage of the property of superantigens and well established antigen specific, highly polarized Th1 clones. We demonstrated that highly polarized Th1 clones are still capable of producing IL-17 upon superantigen stimulation while their antigen specificity remains unaltered. These data provide support to the potential role of superantigens in exacerbation of autoimmune diseases.
2. Material and Methods
2.1. Antigen specific CD4+ T cell clones
Human MOG35-55- and herpes simplex virus (HSV)-specific T cell clones were established by limiting dilution and repeated antigen stimulation as previously described [7]. To avoid possible T and NK cell contamination in antigen presenting cells (APC), T and NK cells were removed from peripheral blood mononuclear cells (PBMC) by a negative selection using anti-CD3, anti-CD56 and subsequently Dynabeads-conjugated anti-mouse Ig (Invitrogen ) plus irradiation (2500 rad).
2.2. Superantigen stimulation
Cloned CD4+ T cells were incubated with 100 ng/ml staphylococcal endotoxin B (SEB) or 100 ng/ml toxic shock syndrome toxin 1 (TSST-1) (Sigma) with or without APC for 72 hours. IL-17 and IFN- level in the supernatant was quantified by ELISA (eBiosciences). In blocking assay, anti-HLA DR, DP and DQ (BD Biosciences) mixture at a total concentration of 20 μg/ml or 10 μg/ml anti-TCR αβ chains (BD Biosciences) were added into cloned cells at 4°C 4 hours before superantigen stimulation.
2.3. Flow cytometry
Cloned CD4+ T cells were stained intracellularly with anti-IL-17 and anti-IFN- antibodies according to manufacturer’s instruction (BD Pharmingen). Clones were pre-stimulated with 500 ng/ml phorbol 12-myristate 13-acetate (PMA) and 50 ng/ml ionomycin for 5 hours at 37°C in the presence of GolgiPlug.
2.4. ELISPOT assay
ELISPOT was performed by following the standard protocol of Cellular Technology Limited (CTL). Briefly, plates with PVDF 0.45 μM filter membrane (Millipore) were coated with purified anti-IL-17, anti-IFN- or both antibodies. 1 × 105 cloned T cells/well with T- and NK-deleted APC at ratio 1:1 in formulated serum-free medium were incubated with MOG35-55 or SEB for 24 hours and followed by incubation of biotin-anti-IL-17 and FITC-conjugated anti-IFN- Southern BioTech) overnight at 4°C. Diluted streptavidin-alkaline phosphotase or anti-FITC-horseradish peroxidase were added and incubated for 1 hour at room temperature. Vector Blue solution (blue) and AEC (red) were added consequently and incubated for 30 minutes each for color development. Two-color image analysis for spot forming cells (SFC) was independently performed by CTL.
The study was approved by Oregon Health & Science University Institute of Review Board.
3. Results and Discussion
3.1. Proinflammatory cytokines did not induce IL-17 from Th1 clones
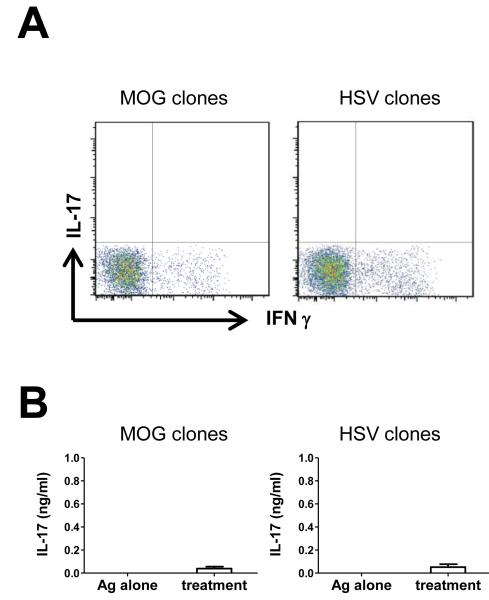
It has been well documented that IFN- and IL-17 co-producing cells exist in vivo and these cells can be induced in vitro during Th17 polarization. However, it is generally believed that fully differentiated Th1 cells have little or no plasticity to be redirected to become Th17 cells. Here we investigate whether conditions used to differentiate Th17 cells will be able to redirect fully differentiated antigen specific cloned Th1 cells to produce IL-17. First, cytokine profile of CD4+ T cell clones was examined by flow cytometry. As shown in Fig 1A, the exclusive IFN- production confirmed that these CD4+ T cell clones were Th1 type without production of IL-17. Cytokine production by CD4+ T cells is highly influenced by other cytokines in the microenvironment. Since IL-1, IL-6, IL-23 and TGF- have been reported as Th17 polarizing cytokines in vitro, we next examined whether combination of these cytokines can induce IL-17 from Th1 clones. Recombinant IL-1, IL-6, IL-23, TGF- and anti-IFN- and anti-IL-12 antibodies were added in order to mimic a proinflammatory microenvironment. Under this condition, CD4+ T cell clones in the presence of freshly prepared APC were stimulated with each specific antigen respectively for 72 hours. There is negligible level of IL-17 in the supernatant (Fig 1B). Therefore, conditions used to differentiate Th17 cells failed to redirect cloned Th1 cells to produce IL-17.
Figure 1. Th17 polarizing cytokines failed to induce IL-17 from antigen-specific Th1 clones.
(A) MOG35-55- and HSV-specific T cell clones were stimulated with PMA and ionomycin and intracellular IFN- and IL-17 were evaluated by flow cytometry (representative of three experiments). (B) MOG35-55- and HSV-specific T cell clones were respectively stimulated by each antigen with or without addition of IL-1, IL-6, IL-23 and anti-IFN- . IL-17 level in the supernatant was quantified by ELISA (pooled data of 4 experiments).
3.2. Superantigen stimulation promoted IL-17 production from Th1 clones
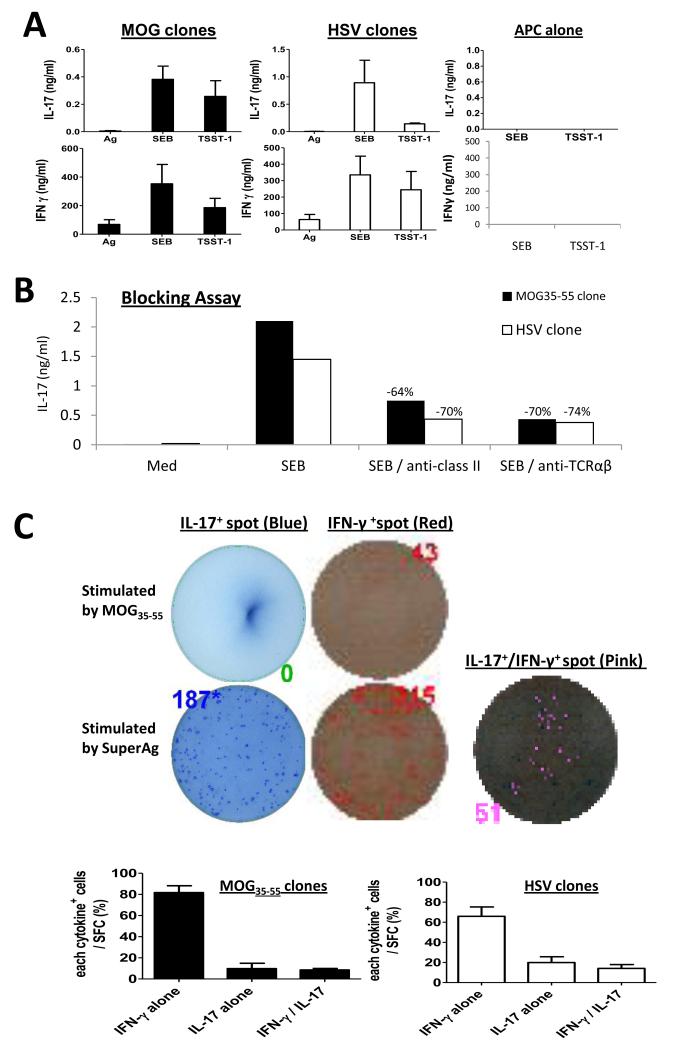
Microbial superantigens are powerful activators of T cells that stimulate the release of a large quantity of cytokines. We asked whether superantigens can stimulate IL-17 production from cloned Th1 cells. Th1 clones with freshly prepared APC were stimulated with superantigens for 72 hours. Both MOG35-55- and HSV-specific clones produced IL-17 under superantigen stimulation (Fig 2A). SEB was stronger than TSST-1 in promoting IL-17. Meanwhile IFN- production was also boosted by superantigens compared to specific antigen stimulation (Fig 2A). Since other cell types other than T cells are known to produce IL-17, we first eliminated the possibility that IL-17 producing cells such as NK cells in the APC preparation contribute to IL-17 production from Th1 clones. We used both anti-CD3 and anti-CD56 antibodies to remove T and NK cells and followed by irradiation. When APC alone were co-cultured with superantigens there was no measurable IL-17, nor IFN- (Fig 2A) detected in the supernatant. In contrast, IL-17 production was detected when cloned Th1 cells alone (with no APC added) were stimulated with superantigens (see ELISOPT data). These results confirmed that IL-17 is produced from Th1 cells. When both SEB and TSST-1 were added each at a concentration of 100 ng/ml, an additive effect on promoting IL-17 was observed with greater than 2 ng/ml/105 cells was produced in both clones.
Figure 2. IL-17 and IFN- production by superantigen stimulation and reduction by anti-MHC class-II and anti-TCR αβ chains.
(A) MOG35-55- and HSV-specific T cell clones were respectively stimulated by specific antigen (10 μg/ml MOG35-55 or HSV protein) or SEB and TSST-1 in the presence of T- and NK-deleted APC for 72 hours (pooled data of three experiments). (B) Anti-HLA DR, DP and DQ mixture at a total concentration of 20 μg/ml and 10 μg/ml anti-human TCR αβ chains were respectively added to Th1 clones under superantigen-stimulation for 72 hours. IFN- and IL-17 production in the supernatant was measured by ELISA (representative of two experiments). (C) ELISPOT assay showing IFN- and IL-17 producing cells stimulated by superantigen. SFC for IL-17+ in blue, IFN- + in red and IL-17+/IFN- + labeled in pink were numerated by CTL suing a quantitative SFC (representative of two experiments).
3.3. Role of MHC class-II and TCR Vβ in superantigen stimulation
Superantigens cross link MHC II and TCR but signal T cells through an alternative pathway to the canonical TCR signaling cascade [8]. We examined the role of MHC class-II and TCR Vβ in superantigen stimulation of IL-17 production from Th1 clones. When anti-HLA DR, DP, and DQ mixture at 20 μg/ml was added to Th1 clones 4 hours before superantigen stimulation, a greater than 60% reduction of IL-17 was observed in both Th1 clones. Similarly, 10 g/ml anti-human TCR αβ chains blocked 70% of IL-17 production by these Th1 clones (Fig. 2B). Thus, we demonstrated that superantigen-stimulated IL-17 production by cloned Th1 cells is TCR V and MHC class-II partially dependent which is consistent with the known pathway of superantigen activation of T cells [2]. Recently, Arad et al uncovered that superantigens bind to MCH II and TCR, then engage CD28 to form a quaternary molecule complex [9] to induce IL-2, IFN- and TNF production. It would be interesting to determine whether CD28 is involved in superantigen-induced IL-17 production by cloned Th1 cells.
3.4. Co-expression IL-17 and IFN- by same Th1 cells
The ELISPOT assay was chosen to determine and quantify the cellular source for IL-17 and IFN- upon superantigen stimulation. An MOG35-55 specific T cell clone at 100,000 cells per well alone were stimulated with 10 μg/ml MOG35-55 or 100 ng/ml SEB for 24 hours. As shown in Fig 2C, under MOG35-55-stimulation there was no IL-17+ but 43 IFN- + SFC detected, while under SEB stimulation, 187 IL-17+ SFC were counted in single-colored (blue), 315 IFN- + SFC in single-colored (red) and 51 IL-17+/IFN- + SFC in double-colored plates (labeled in pink) were detected. The IL-17+/IFN- + SFC represented approximately 30% of total IL-17+ SFC induced by SEB stimulation. These data suggest that superantigens can induce IL-17 single producing and IL-17/IFN- coproducing cells from previously polarized IFN- producing Th1 cells.
It was thought that cytokine profiles of helper T cells are imprinted by specific signal transductions, transcription factors and epigenetic modifications [10]. In Th1 cells, repressive epigenetic modification, histon-H3 lysine 27 trimethylation, are broadly distributed on loci of il-17 and rorc [11] and the master transcription factor of Th1, T-bet is known to repress expression of the master transcription factor of Th17, retinoid-related orphan receptor (ROR)- t, through suppressing RUNX 1 [12]. Therefore, it is considered to be difficult to promote IL-17 production from Th1 cells. Indeed, as demonstrated in the current study, cytokines that are known to promote polarization for Th17 cells from naïve CD4+ T cells failed to stimulate IL-17 production from cloned Th1 cells. However, superantigens were able to induce IL-17 from polarized antigen specific Th1 clones, which suggests that the repression of IL-17 gene expression in Th1 cells can be reversed by stronger and comprehensive T cell activation. Superantigens signal T cells through an alternative pathway which is different from the canonical pathway that antigen-TCR interaction signals T cells. Superantigen stimulation of T cells does not require CD4 as co-receptor and can bypass Lck dependent pathway [13]. Further detailed analyses of signaling pathways by superantigens will be required to understand how these superantigens induced IL-17 production in those cloned Th1 cells. It is noteworthy that our data are derived from highly polarized T cell clones, therefore it is may not be extended to primary Th1 cells.
Finally, findings of the current study have potentially important clinical relevance. Microbial infection has been thought to play a major role in the development of autoimmune diseases. Staphylococcus aureus primarily colonize the skin in the nasal area in adults. Interestingly, superantigenic Staphylococcus aureus stimulate IL-17 production from CD4+ T cells of adults with memory phenotypes but not from those CD4+ T cells from cord blood or infants with naïve phenotypes [14]. It can be speculated that when autoreactive Th1 cells such as MOG specific Th1 cells are encountered with superantigens they may be stimulated to produce IL-17 to cause inflammation and demyelination in the central nervous system. Further in vivo experiments in autoimmune disease models will be required to formally prove this hypothesis. For example, in a transfer model of EAE, MOG35-55-specific Th1 clones can be stimulated by sugerantigens either in vitro or in vivo to assess the capacity of these Th1 clones in production of IL-17 and induction and/or exacerbation of EAE.
Highlights.
Superantigens stimulate IL-17 production by well differentiated cloned Th1 cells
Superantigen-induced IL-17 production is partially MHC II and TCR dependent
Superantigen-induced IL-17 production may be involved in exacerbation of autoimmune diseases associated with infections
Acknowledgement
This work was supported by a grant from NIH to CQC (AR055254) and Portland VA Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Delogu LG, Deidda S, Delitala G, Manetti R. Infectious diseases and autoimmunity. Journal of infection in developing countries. 2011;5:679–87. doi: 10.3855/jidc.2061. [DOI] [PubMed] [Google Scholar]
- [2].Herman A, Kappler JW, Marrack P, Pullen AM. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annual review of immunology. 1991;9:745–72. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- [3].Zhang J, Vandevyver C, Stinissen P, Mertens N, van den Berg-Loonen E, Raus J. Activation and clonal expansion of human myelin basic protein-reactive T cells by bacterial superantigens. Journal of autoimmunity. 1995;8:615–32. doi: 10.1016/0896-8411(95)90012-8. [DOI] [PubMed] [Google Scholar]
- [4].Raddassi K, Kent SC, Yang J, Bourcier K, Bradshaw EM, Seyfert-Margolis V, et al. Increased frequencies of myelin oligodendrocyte glycoprotein/MHC class II-binding CD4 cells in patients with multiple sclerosis. J Immunol. 2011;187:1039–46. doi: 10.4049/jimmunol.1001543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. The American journal of pathology. 2012;181:8–18. doi: 10.1016/j.ajpath.2012.03.044. [DOI] [PubMed] [Google Scholar]
- [6].Zhu J, Paul WE. CD4+ T cell plasticity-Th2 cells join the crowd. Immunity. 2010;32:11–3. doi: 10.1016/j.immuni.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chou YK, Henderikx P, Vainiene M, Whitham R, Bourdette D, Chou CH, et al. Specificity of human T cell clones reactive to immunodominant epitopes of myelin basic protein. Journal of neuroscience research. 1991;28:280–90. doi: 10.1002/jnr.490280215. [DOI] [PubMed] [Google Scholar]
- [8].Zamoyska R. Superantigens: supersignalers? Science’s STKE : signal transduction knowledge environment. 2006;2006:pe45. doi: 10.1126/stke.3582006pe45. [DOI] [PubMed] [Google Scholar]
- [9].Arad G, Levy R, Nasie I, Hillman D, Rotfogel Z, Barash U, et al. Binding of superantigen toxins into the CD28 homodimer interface is essential for induction of cytokine genes that mediate lethal shock. PLoS biology. 2011;9:e1001149. doi: 10.1371/journal.pbio.1001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].O’Shea JJ, Lahesmaa R, Vahedi G, Laurence A, Kanno Y. Genomic views of STAT function in CD4+ T helper cell differentiation. Nature reviews Immunology. 2011;11:239–50. doi: 10.1038/nri2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–67. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lazarevic V, Chen X, Shim JH, Hwang ES, Jang E, Bolm AN, et al. T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORgammat. Nat Immunol. 2011;12:96–104. doi: 10.1038/ni.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bueno C, Lemke CD, Criado G, Baroja ML, Ferguson SS, Rahman AK, et al. Bacterial superantigens bypass Lck-dependent T cell receptor signaling by activating a Galpha11-dependent, PLC-beta-mediated pathway. Immunity. 2006;25:67–78. doi: 10.1016/j.immuni.2006.04.012. [DOI] [PubMed] [Google Scholar]
- [14].Islander U, Andersson A, Lindberg E, Adlerberth I, Wold AE, Rudin A. Superantigenic Staphylococcus aureus stimulates production of interleukin-17 from memory but not naive T cells. Infection and immunity. 2010;78:381–6. doi: 10.1128/IAI.00724-09. [DOI] [PMC free article] [PubMed] [Google Scholar]