Abstract
For the past thirty years, since IL-1β and TNFα were first cloned, there have been efforts to measure plasma cytokine concentrations in patients with severe sepsis and trauma, and to use these measurements to predict clinical outcome and response to therapies. The numbers of cytokines and chemokines that have been measured in the plasma have literally exploded with the development of multiplex immune approaches. Dozens of relatively small cohort studies have shown plasma cytokine concentrations correlating with outcome in sepsis and trauma. Despite what appears to be a consensus that plasma cytokine concentrations should be useful in the clinical setting, only two cytokines, IL-6 and procalcitonin, have approached routine clinical use. IL-6 has been used as a research tool for entry into sepsis-intervention trials, while procalcitonin is being used clinically at a large number of institutions to distinguish sepsis from other inflammatory processes. For most cytokines, the relative lack of sensitivity and specificity of individual or multiplex cytokine measurements has hindered their utility to predict clinical trajectory in individual patients. The problem rests with a general misunderstanding of cytokine biology, failing to appreciate the general paracrine nature of these mediators, the presence of binding proteins, chaperones and inhibitors in the plasma, and the rapid clearance of these proteins by binding to cell receptors and clearance predominantly by the kidney. The future of using plasma cytokine measurements as an indicator of sepsis/trauma severity or predicting outcome is generally behind us, although there is optimism that procalcitonin measurements may ultimately prove to have utility in the diagnosis of severe sepsis.
Keywords: TNFα, IL-1β, IL-6, procalcitonin, chemokines, clinical trials
Despite years of investigation and advances in intensive care management, morbidity and mortality from sepsis and severe traumatic injury remain unacceptably high [1, 2]. Mortality from sepsis and septic shock was traditionally presumed to be a consequence of an overabundant early innate immune response, caused by an over-production of early pro-inflammatory mediators, notably tumor necrosis factor-alpha (TNFα), interleukin (IL)-6, IL-1, and IL-8, that contributed to tissue damage, multiple organ failure (MOF), and ultimately leading to death [3, 4]. Many of these are proximal, proinflammatory cytokines that have the ability not only to induce expression of chemokines and other inflammatory mediators, and at the same time, activate endothelial cell dysfunction and prothrombotic events [5-11]. More recent studies have clearly demonstrated a simultaneous immune compromised state evidenced by increased anti-inflammatory and immunosuppressive cytokines in the blood, most notably IL-10, that can lead to secondary nosocomial or opportunistic infections [12, 13].
Inflammation and survival from sepsis and trauma
The optimism for a biological response modifier-based therapy to improve survival from severe sepsis and trauma in humans by modifying the endogenous host immune response began nearly 25 years ago when mice and then primates showed radical improvements in survival to a lethal injection of endotoxin or live bacteria by blocking a single inflammatory cytokine [14]. Since then, there have been over 100 clinical trials utilizing various drug therapies; most consisting of agents to suppress or block this proinflammatory response, which have been unsuccessful in reducing the mortality from sepsis [15-17]. The inevitable question has always been why the clinical trials have failed when studies in rodents and primates have almost always been successful. The answers are clearly multifactoral, but much of the responsibility of early failures falls both on an over interpretation of the value of rodent and primate models of sepsis, as well as the early models that employed a bolus injection of either endotoxin or living bacterial [18, 19]. In retrospect, these early models emphasize the proinflammatory component of sepsis, and poorly reflect the complexity of sepsis that originates from a nidus of infection, rather than a bolus administration. A clear signal that we were on the wrong track was that plasma TNFα and IL-1β concentrations were often several logs higher in these models than they were in human sepsis or trauma (Table 1). More recent models that have been employed over the last decade include the commonly used models of polymicrobial abdominal sepsis, including the cecal ligation and puncture (CLP) model [20] and the colon ascendence stent peritonitis model (CASP) [21]. Early on, these models were thought to incite not only the early proinflammatory SIRS component of sepsis, but also, the later anti-inflammatory response, representing the early SIRS-CARS model proposed by Bone and colleagues [22]. However, as time has progressed, so has our understanding of sepsis, and the traditional SIRS-CARS model continued to evolve, especially as the results of studies utilizing these models began to call in to question the “compensatory nature” of the anti-inflammatory response [23, 24]. Traditionally, the most commonly used model of murine trauma, has been the trauma-hemorrhage models, which includes hemorrhagic shock followed by a simple, laparotomy [25-27]. Recently, the validity of this and all murine models have been called into question as recent reports have found significant differences between human trauma, burns, and endotoxicosis, and traditional murine models of these same disease entities [28]. Although it has been increasingly recognized that murine models do not fully mimic the human condition [19], which is likely a significant contributor to failure of translation of successful preclinical models to the clinical setting; further research into the utility of murine models for trauma and sepsis research is needed, as other reports show that by refining currently used models, to ones that better recapitulate the human condition, cytokine, phenotypic, and genomic responses can be improved [29].
Table 1.
Plasma Cytokine Concentrations in Murine and Primate Models of Lethal Endotoxicosis and Gram negative Bacteremia, and in Human Endotoxin Administration and Severe Sepsis.
| Lethal Murine Endotoxicosis | Sublethal Primate Endotoxicosis | Lethal Primate E. coli Shock | Mild Human Endotoxinemia | Severe Human Sepsis | Severe Human Trauma | References | |
|---|---|---|---|---|---|---|---|
| TNFα pg/ml | 1440 - 1600 | 1,200 | 11,000-13,500 | 250 - 525 | 0 – 459 | 0 -210 | [4, 100-102] [51, 103-105] |
| IL-1β pg/ml | 0 – 500 | 0 | 1368 -2,500 | 0 | 0 - 143 | 6 - 25 | [4, 100-104, 106] [51] |
| IL-6 pg/ml | 13,500 - 720,000 | 3400 | 325,000 – 584,000 | 800 - 1250 | 0-49,827 | 310 -7,610 | [4, 51, 100-106] |
| IL-8 pg/ml | No mouse homologue | 2700 | 3,000 -5,200 | 1250 - 2000 | 0 – 3,338 | 75 - 2,300 | [4, 51, 102, 105, 107, 108] |
| KC/GRO pg/ml | 21,000 – 1,000,000 | No Primate homologue | No Primate homologue | No human homologue | No human homologue | No human homologue | [103, 106, 109] |
We do not believe that the failure of biological response modifiers in severe sepsis and trauma can be explained entirely by imprecise rodent and nonhuman primate models. The lack the complexity of the human condition, including pre-existing comorbidities, age, guided antibiotic therapy, and intensive care unit (ICU) care and interventions are important explanations. One principle failure has been the inability to identify prospectively those patients who would benefit from biological response modifiers. It is generally accepted that many of these treatment failures have been due to the inability to select patients who will benefit from such therapy. Most clinical trials in sepsis are inundated with patients who would either survive or die, regardless of the intervention, and these populations clearly dilute any drug-based effect [30]. Additionally, multiple studies have revealed that treatment with biological response modifiers can actually be harmful when used indiscriminately in less severely ill patients [31-33]. Unfortunately, drug trials to treat patients with severe sepsis have been unable to identify patients prospectively who will or will not benefit from interventional therapies.
With the implementation of the Surviving Sepsis Campaign, early diagnosis of those at risk for severe sepsis and at an increased risk for death is imperative [34, 35]. Currently, sepsis is diagnosed and monitored using physiologic parameters combined with bacterial cultures, although positive cultures are not found in close to one third of patients exhibiting a clinical diagnosis of sepsis [36, 37]. Such scoring systems as the Acute Physiology and Chronic Health Evaluation (APACHE) II score, the sequential organ failure assessment (SOFA) score, and the multiple organ dysfunction (MOD) scoring system (Denver or Marshall) are often utilized to classify the physiological derangements produced by sepsis and trauma, and organ function. These scoring systems however, do not measure the magnitude of either the inflammatory response, or the adaptive immune dyscrasia that accompanies trauma and sepsis. This is an important weakness since most of the current biological response modifiers that have been or are in clinical trials are directed against these responses. These scoring systems can only crudely predict which patients will have a complicated course ending in death or who will benefit from immunomodulatory therapy. This has led many researchers to investigate the utility of plasma cytokine measurements as biomarkers to identify an immunological profile that can identify which patients with sepsis or trauma have increased risk from mortality, and to direct therapy with immune modulating drugs [37]. For the past two decades, researchers have sought that magical or mystical cytokine(s) whose persistent or continued elevation is associated with poor outcomes, while decreasing levels can signal response to therapy and eventual recovery in severe sepsis or trauma.
Unfortunately, with few exceptions discussed below, none have proven efficacious enough for mainstream use to help predict outcome and guide therapy [38, 39] and currently the role for the use of biomarkers remains undefined, and according to the recently updated Surviving Sepsis Guidelines, “No recommendation can be given for the use of these markers to distinguish between severe infection and other acute inflammatory states” [35].
Capturing the early inflammatory response via plasma cytokine concentrations
Conceptually, the idea that plasma cytokine concentrations could predict the severity of the inflammatory response in sepsis and trauma is sound. In practice, it has proven more challenging. Since it has been determined that early activation of the innate immune system with release of proinflammatory cytokines is in part responsible for the early systemic inflammatory response syndrome (SIRS), the value of utilizing measurements of these cytokines would be to allow the clinician a glimpse of the patients immunological status beyond the routine physiologic or anatomical markers of injury or sepsis severity. Almost thirty years ago, we speculated that because of the rapid onset of the inflammatory response and innate immune activation, treatments initiated hours after the onset of symptoms would inevitably miss the early cytokine and inflammatory mediator release [38]. By the time that SIRS is recognized and sepsis is diagnosed, and the therapeutic agent prepared and administered, early inflammatory mediator release had already peaked and the inflammatory cascade well initiated. Biomarker therapy using early inflammatory cytokines would in turn allow for early intervention, thus improving a patient's prognosis. Early inflammatory cytokines such as IL-1β and TNFα are produced early in response to pathogen invasion, and are believed to be responsible for the early SIRS response [6, 40].
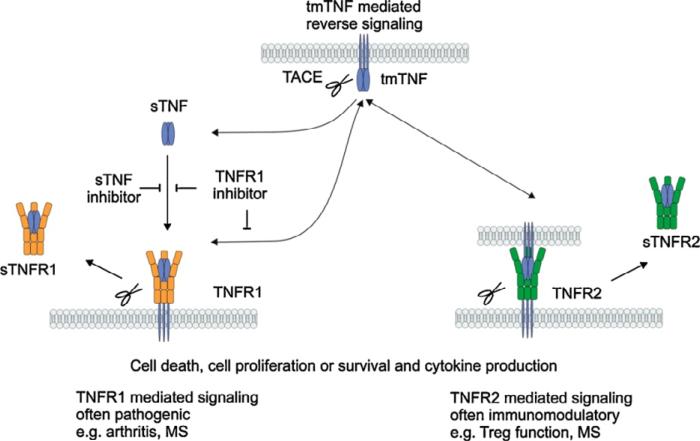
Unfortunately, we couldn't have chosen a worse pair of cytokines to measure in plasma. At the time, we readily assumed that these two proximal mediators were readily released into the circulation, and their plasma concentrations reflected tissue production. This was simplistically based on observations from mice and humans where massive doses of endotoxin or live bacteria were used [7, 41-43]. We couldn't have been more wrong. After twenty years of study, we recognize that TNFα and its other family members are not primarily secreted proteins, but exist predominantly as cell-associated homotrimers [44, 45] (Figure 1). Their primary functions are paracrine, and their appearance in the circulation requires both up-regulation of their expression, but also successful cleavage of the cell-associated form by the cell membrane metalloproteinase, ADAM17 (TACE) [46]. In addition, the shed receptors of TNFα are also released and bind circulating TNFα, often making it undetectable if not biologically inactive [47].
Figure 1.
Diagrammatic representation of TNFa processing initially as a cell-associated homodimer, processed by the matrix metalloproteinase TACE (ADAM17) to release the soluble form. sTNFa can bind the shed sTNFRI or sTNFRII, blocking its bioactivity. Reprinted with the permission of Cytokine and Growth Factor Reviews, vol 22, 319, 2011.
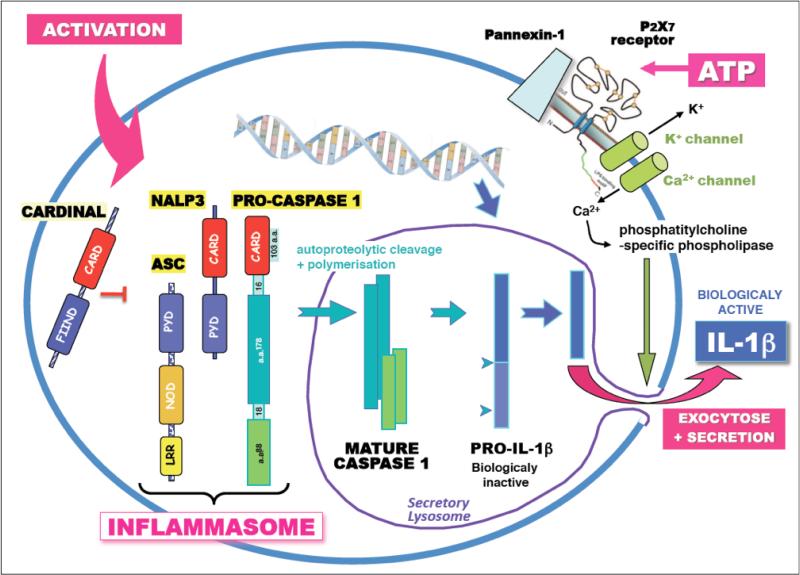
The story for IL-1β is even more problematic from a plasma measurement point of view. IL-1β is produced as an inactive intracellular protein without a classic signal sequence, and must first be processed by caspase-1 and the inflammasome before it can be released [48, 49] (Figure 2). How it is released is still controversial, and it has generally been assumed that a primary mechanism is through cell death. Like TNFα, IL-1β has a dummy receptor, the type II receptor that is shed and can bind the protein in the circulation [50]. Although these cytokines are perhaps the most proximal mediators of inflammation, they would not be a good first choice for their use as plasma biomarkers.
Figure 2.
Daigrammatic representation of IL-1β processing. IL-1β is expressed as an inactive precursor protein that must be cleaved to an active form by caspase 1 in secretory lysosomes. Since it has no signal sequence, it is not actively secreted, and its exit from the cell is not completely known, although exocytosis and cell death is known to release the mature active protein. Reprinted with permission from Institute Pasteur. http://www.pasteur.fr/ip/easysite/pasteur/en/research/scientific-departments/infection-and-epidemiology/units-and-groups/cytokines-e-inflammation/figures
Cytokines as plasma biomarkers
The search for prognostic biomarkers in sepsis or trauma based on immunological measures has been substantial; however, the results of clinical studies examining the role of cytokines in patients with varying degrees of sepsis have often been conflicting, and only one cytokine, procalcitonin (PCT)) has shown any value for routine clinical use. The numbers of cytokines detected in the circulation of patients with severe sepsis and trauma are overwhelming; a partial list includes over 15 cytokines, 4 members of the TNF superfamily, at least 10 chemokines, and at least 5 cytokine receptors, binding proteins and antagonists (Table 2). Multiplex approaches to the measurement of plasma cytokines have revolutionized the field, and if you have the money, you can measure almost as many cytokines as you desire [51]. Routine commercially available multiplex kits measure as many as 31 cytokines simultaneously from 25 μL of plasma, and the limits are more financial than theoretical. We have routinely measured anywhere from 10 to 31 plasma cytokines simultaneously in pharmaceutical-driven studies in sepsis and trauma, and tend to find that the concentrations appear to be strongly correlated, when they do appear in the circulation. One of the most common traits that we have seen with cytokine measurements in blood is that their appearance is rarely normally distributed. Most commonly, there are significant numbers of patients in whom concentrations are undetectable or at the lower limits at detection, with a smaller number of patients with marked variation in their concentrations. Not unexpectedly, some of the highest plasma cytokine concentrations and most labile, involve chemokines. When one considers their role in regulating the efflux of leukocyte populations from one compartment to another and the need to create a gradient across different tissues, it is not surprising that the plasma concentrations of these proteins would be so variable. We have found that the common chemokines, IL-8 (CXCL8), MCP1 (CCL7), IP-10 (CXCL10), SDF1 (CXCL12), MIP1a (CCL3) are often as reliable as IL-6 in measuring the magnitude of the injury or sepsis response [51, 52]. Additionally, there are a number of studies that have claimed that combinations of individual cytokines, or even ratios of proinflammatory to anti-inflammatory cytokines are more predictive than individual cytokines alone [53-55], however, this has yet to be consistently proven in the literature.
Table 2.
Cytokines and Chemokines Readily Detectable in the Circulation During Lethal Inflammation. The list is not inclusive of all cytokines measured, but those that have been measured frequently.
| Cytokines | Chemokines | TNF Super Family | Cytokine Antagonists | Interferons | Misc. |
|---|---|---|---|---|---|
| IL-1β | IL-8 (CXCL8) | TNFα | IL-1ra | IFNγ | MIF |
| IL-2 | RANTES (CCL5) | TNFβ | sIL1RI | IFNβ1 | |
| IL-3 | MIP1α (CCL3) | sFASL | sIL1RII | IFNα2 | |
| G-CSF | MIP1β (CCL4) | TRAIL | sIL2R | ||
| GM-CSF | IP-10 (CXCL10) | LIGHT | sIL6R | ||
| IL-4 | SDF1(CXCL12) | sIL18R | |||
| IL-5 | MCP1 (CCL2) | sTNFRI | |||
| IL-6 | Eotaxin (CCL11) | sTNFRII | |||
| LIF | |||||
| IL-10 | |||||
| IL-12p40 | |||||
| IL-12p70 | |||||
| IL-15 | |||||
| IL-17 | |||||
| IL-18 | |||||
| IL-22 | |||||
| IL-23 | |||||
| IL-33 |
Other cytokines that have been found to be predictive of sepsis-related mortality include IL-8, monocyte chemotactic protein-1 (MCP-1), sRAGE, and the immunosuppressive cytokine IL-10 [56, 57]. Out of a panel of cytokines obtained from patients diagnosed with severe sepsis, Bozza, et al. showed that IL-8 and MCP-1 had the best accuracy for predicting 28 day mortality, while IL-6 and IL-8 were good predictors of worsening organ dysfunction [58]. Additionally, Bopp et al. showed that sRAGE measured by enzyme linked immunoassay (ELISA) was elevated in non-survivors compared to survivors in a 29 patient observational study [59]. Other studies have focused on anti-inflammatory cytokines. In fact de Pablo et al found that it was the anti-inflammatory mediators sTNFRI, sTNFRII, and IL-1ra that were the best predictors of mortality in septic shock [60].
Despite an overwhelming body of literature too large to review, none of these most studied cytokines above exhibit either the sensitivity or the specificity to be reliably used alone as a marker or predictor of prognosis in sepsis. Additionally, the literature is variable, as many studies have found no correlation between cytokine measurements and outcome or prognosis [61-63]. Recently, attention has turned to the utilization of several biomarkers to create a “bioscore” that could be used for early sepsis diagnosis and outcome prediction that were found to be superior to individual biomarkers alone [55, 64]. In a prospective pilot study of 29 patients, Anuluz-Ojeda et al. recently showed that IL-6, IL-8, IL-10, and MCP-1 levels were elevated in patients who died compared to those who survived [65]. Additionally, they found that IL-6, IL-8, and IL-10 levels were associated with mortality early, on day three, and later on day 28. Therefore they developed a bioscore based on these cytokines, which was able to better predict mortality than the individual cytokines alone [65]. Likewise, Gibot et al. showed that when utilizing a bioscore consisting of levels of procalcitonin, sTREM-1, and PMN CD64 index, proved to be useful in the rapid diagnosis of sepsis vs. SIRS, with a diagnostic accuracy of greater than 80% [55]. The use of a composite score of multiple cytokines is likely to be better than individual cytokines alone as is shown in the previous studies, and may eventually be of potential clinical use for the early detection of sepsis. Additionally, these efforts are more in line with the complex immune response the body generates toward sepsis; however, their clinical application to date has been limited by their complexity and their cost.
It has been our experience that measuring the plasma concentrations of cytokines has been helpful in cohort studies to determine the severity of the inflammatory response, and the response to therapy, but has lacked the specificity and sensitivity to be useful for predicting outcome or trajectory in individual patients. The explanations vary for each individual cytokine. However, blood leukocytes are not the primary source of most cytokines in the plasma [41].
Since most cytokines are meant to signal in a local paracrine environment, appearance in the plasma is often a byproduct of local production. Most cytokines are cleared from the circulation by either binding to their receptors or clearance via the kidney, as well as by binding to specific binding proteins, and the plasma concentration varies over minutes to hours. Thus, for the most part, we have argued that in general, plasma cytokine concentrations are a relatively crude and inefficient measure of the immunological state of the patient [66, 67]. In many cases, more is not better than less, and the availability of large multiplex measurements doesn't necessarily mean that they are more informative, at least for evaluating the overall inflammatory response.
Rather, we have argued that a judicious use of inflammatory cytokines combined with physiological or anatomical scoring systems may be more beneficial in predicting response to therapy in severe trauma patients [68], but importantly, these findings have not been validated prospectively.
Plasma IL-6 measurements
Since the 1980's many preclinical and clinical studies studies have shown IL-6 measurements in the blood to be a reliable marker to predict the severity of sepsis [64, 69-72]. We were the first to show that IL-6 circulates in the plasma of humans; those studies were conducted in human volunteers administered endotoxin [73]. Interestingly, IL-6 is a highly and variably glycosylated cytokine and it is presumed that this glycosylation prolongs its biological half-life. Many individuals have natural antibodies to IL-6, and some have proposed that these antibodies are not inhibitory, but serve as chaperone proteins, again extending their half-lives [74].
IL-6 is a novel cytokine that plays many roles. Thought to be both pro-inflammatory and anti-inflammatory at the same time, it is now recognized as an essential player in cell development. In addition, IL-6 is a key cytokine in initiation of innate immunity and functions in adaptive immunity as well [75]. It's attractiveness as a biomarker has nothing to do with its function, but rather lies in the fact that it is known to be elevated early, and can reach peak concentration within two hours under experimental conditions [75, 76], thus potentially being informative prior to the onset of clinical symptoms. Initially, scientists found that elevated levels of IL-6 were associated with abnormal physiologic measurements and routine laboratory measurements such as heart rate, mean arterial pressure, lactate levels, and platelet levels [77]. Additionally, many studies found that early levels of IL-6 were of prognostic significance [37, 69, 72, 78-81]. For instance, we showed that in a prospective randomized double-blind placebo controlled trial that baseline IL-6 concentrations were higher in patients with septic shock and those who went on to die by 28 days [37].
Although the success of plasma IL-6 measurements seemed to be promising, and there have been efforts to develop an IL-6 assay approved by the FDA, plasma IL-6 measurements have still not been integrated into the clinical armamentarium. The reason clearly is not technical. The validity of these measurements has been well demonstrated and even “fast” IL-6 measurements that can be done at the bedside have been used in research protocols. The failure to use IL-6 routinely in the clinical setting has more to do with the interpretation of the results, and their value when compared to existing biomarkers. At present, IL-6 is primarily used to assess the severity of the inflammatory response, and simply put; most clinicians see no compelling need to add an additional biomarker. The general consensus is that existing diagnostics, such as total and differential white blood cell count, C-reactive protein and the clinical condition of the patient are adequate to judge the severity of the inflammatory insult. Mouse studies by Remick et al have shown that early IL-6 concentrations could both predict mortality in mice, but also response to therapy. Until a similar human prospective study demonstrates that addition of IL-6 measurements can better identify individual patient trajectories or responses to therapy, plasma IL-6 will likely remain a research tool.
Procalcitonin
Procalcitonin is a calcitonin precursor involved in calcium homeostasis that is released during the inflammatory response that is currently one of the most well-described inflammatory mediators thought to be predictive of outcome in sepsis, as it has been shown to correlate with the severity of sepsis and organ failure [82]. Unfortunately, one of the greatest downsides of procalcitonin is that it has been found to be elevated in a number of inflammatory states other than in in sepsis [38]. High procalcitonin levels are also found in other states of generalized inflammation, including trauma and burns [83], post-operatively, in cardiogenic shock [55], pancreatitis, or heatstroke [84]; therefore it's elevation may be nonspecific, and cannot be attributed to a single disorder alone, like an ongoing infection. For example, in a study by de Werra et al. examining procalcitonin levels among patients with septic shock, cardiogenic shock, and bacterial pneumonia, they found that concentrations in patients with septic shock were similar in magnitude to the patients with cardiogenic shock and bacterial pneumonia and were not predictive of outcome [85].
Despite the growing body of literature promoting procalcitonin as a diagnostic and predictive biomarker [86], we remain concerned that the marker may lack the requisite sensitivity and specificity to be of value. Although numerous studies have revealed that procalcitonin may be useful in the diagnosis of and as a prognosticator of severe sepsis [82, 87, 88], other studies have shown conflicting results and revealed that procalcitonin is not useful in these realms [56, 89-92]. In a meta-analysis of 14 studies meeting criteria performed by Tang et al., they showed that the diagnostic performance of procalcitonin for differentiating sepsis from SIRS was low in critically ill patients [93]. More interestingly, a recent meta-analysis published in Lancet-Infectious Diseases evaluated 30 clinical studies of 3844 septic patients. A simple bivariate analysis of sepsis presence or absence gave a mean sensitivity of 0.77 and specificity of 0.79 with the area under the receiver operating characteristic curve was 0.85 [94]. The studies had significant heterogeneity and none of the common variables including population demographics, admission criteria, assay used, and severity of disease could account for the heterogeneity.
One realm where the use of procalcitonin may be beneficial is by using the fact that a patient has low levels to help guide clinicians in the discontinuation of empiric antibiotic therapy that was started in a patient with suspected sepsis, as is recommended by the most recent Surviving Sepsis Guidelines, however, there is limited prospective data to support the use of this strategy [35, 95]. Additionally, procalcitonin assays have not yet been found to be neither sensitive nor specific enough for individual patients in order to be reliable enough to direct therapy.
Conclusions and Recommendations
Severe sepsis, trauma and burn injury remain a significant cause of morbidity and mortality. Identifying severely inflamed patients early who will have a complicated clinical outcome, and thus are more likely to benefit from innunomodulatory therapy has been difficult. Interventions in critically ill patients need to be early to be most effective. Existing criteria for biological response modifiers are primarily physiologic and are limited to nonspecific inflammatory responses and overall organ injury, and despite the complexity of the host immune response, we know that not all patients respond to sepsis in the same manner [96]. Consequently, the well-established clinical criteria used to enter patients into sepsis trials includes individuals who will either not benefit from the therapy or may actually be harmed [30]. This has led researchers to explore cytokines as biomarkers to predict clinical trajectory and outcomes, and to initiate treatment based on the magnitude of the early inflammatory response. These efforts have generally failed [97, 98].
Although researchers have been able to elucidate distinct cytokine profiles associated with sepsis severity, organ failure, and mortality [58], there has yet to be a way to make these measurements useful prospectively in clinical practice. Current literature offers no consensus opinion, as it is plagued with a multitude of studies both in support of and against the usefulness of cytokines as prognostic biomarkers, mostly hampered by variable patient populations, small sample sizes, and hetergenous biomarker assays [77]. Multiplex cytokine approaches have also been employed [99], but have not been readily accepted into clinical practice. Perhaps, only a single cytokine has any probability of entering the routine clinical practice in the immediate future, procalcitonin [86]. Procalcitonin has been promulgated for the identification of sepsis and to guide the discontinuation of antibiotic therapy when levels are not elevated [35]. Both are controversial, and the defining study demonstrating their utility has not yet been performed. Thus, the quest continues for early biomarkers as a means to identify patients with adverse clinical outcomes who might benefit from interventional therapies.
Highlights.
Some believe plasma cytokine levels can assess the inflammatory state of patients.
TNFα and IL-1β were initially considered good biomarkers in trauma and sepsis.
Concentrations of anti-inflammatory cytokines (IL-6, etc) have proven more reliable.
Only procalcitonin can be considered useful as a clinical adjunct.
It's unclear if plasma cytokines will ever be able to reliably predict clinical outcome.
Acknowledgments
Supported in part by grants GM-040586-24 and GM-081923-06, awarded by the National Institute of General Medical Sciences (NIGMS), P.H.S. LFG, AGC and ELV were supported by a T32 training grant in burns and trauma awarded by the NIGMS (T32 GM-008721-14). AGC was also supported by an individual training grant from NIGMS (F32 GM093665-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9(5):517–24. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 2.Probst C, et al. 30 years of polytrauma care: An analysis of the change in strategies and results of 4849 cases treated at a single institution. Injury. 2009;40(1):77–83. doi: 10.1016/j.injury.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Bone RC, Grodzin CJ, Balk RA. Sepsis: a new hypothesis for pathogenesis of the disease process. Chest. 1997;112(1):235–43. doi: 10.1378/chest.112.1.235. [DOI] [PubMed] [Google Scholar]
- 4.Van Zee KJ, et al. IL-8 in septic shock, endotoxemia, and after IL-1 administration. J Immunol. 1991;146(10):3478–82. [PubMed] [Google Scholar]
- 5.Okusawa S, et al. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest. 1988;81(4):1162–72. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinarello CA. The biology of interleukin 1 and comparison to tumor necrosis factor. Immunol Lett. 1987;16(3-4):227–31. doi: 10.1016/0165-2478(87)90151-9. [DOI] [PubMed] [Google Scholar]
- 7.Fong Y, et al. Antibodies to cachectin/tumor necrosis factor reduce interleukin 1 beta and interleukin 6 appearance during lethal bacteremia. J Exp Med. 1989;170(5):1627–33. doi: 10.1084/jem.170.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gershenwald JE, et al. Interleukin 1 receptor blockade attenuates the host inflammatory response. Proc Natl Acad Sci U S A. 1990;87(13):4966–70. doi: 10.1073/pnas.87.13.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Poll T, et al. Differential effects of anti-tumor necrosis factor monoclonal antibodies on systemic inflammatory responses in experimental endotoxemia in chimpanzees. Blood. 1994;83(2):446–51. [PubMed] [Google Scholar]
- 10.Levi M, et al. Inhibition of endotoxin-induced activation of coagulation and fibrinolysis by pentoxifylline or by a monoclonal anti-tissue factor antibody in chimpanzees. J Clin Invest. 1994;93(1):114–20. doi: 10.1172/JCI116934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Poll T, et al. Elimination of interleukin 6 attenuates coagulation activation in experimental endotoxemia in chimpanzees. J Exp Med. 1994;179(4):1253–9. doi: 10.1084/jem.179.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacLean LD, et al. Host resistance in sepsis and trauma. Ann Surg. 1975;182(3):207–17. doi: 10.1097/00000658-197509000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parsons PE, et al. Circulating IL-1ra and IL-10 levels are increased but do not predict the development of acute respiratory distress syndrome in at-risk patients. Am J Respir Crit Care Med. 1997;155(4):1469–73. doi: 10.1164/ajrccm.155.4.9105096. [DOI] [PubMed] [Google Scholar]
- 14.Delano MJ, Moldawer LL. Magic bullets and surrogate biomarkers circa 2009. Crit Care Med. 2009;37(5):1796–8. doi: 10.1097/CCM.0b013e3181a09440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boomer JS, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. Jama. 2011;306(23):2594–605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hotchkiss RS, Opal S. Immunotherapy for sepsis--a new approach against an ancient foe. N Engl J Med. 2010;363(1):87–9. doi: 10.1056/NEJMcibr1004371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riedemann NC, Ward PA. Anti-inflammatory strategies for the treatment of sepsis. Expert Opin Biol Ther. 2003;3(2):339–50. doi: 10.1517/14712598.3.2.339. [DOI] [PubMed] [Google Scholar]
- 18.Marshall JC, et al. Preclinical models of shock and sepsis: what can they tell us? Shock. 2005;24(Suppl 1):1–6. doi: 10.1097/01.shk.0000191383.34066.4b. [DOI] [PubMed] [Google Scholar]
- 19.Dyson A, Singer M. Animal models of sepsis: why does preclinical efficacy fail to translate to the clinical setting? Crit Care Med. 2009;37(1 Suppl):S30–7. doi: 10.1097/CCM.0b013e3181922bd3. [DOI] [PubMed] [Google Scholar]
- 20.Cuenca AG, et al. Cecal ligation and puncture. Curr Protoc Immunol. 2010 doi: 10.1002/0471142735.im1913s91. Chapter 19: p. Unit 19 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Traeger T, et al. Colon ascendens stent peritonitis (CASP)--a standardized model for polymicrobial abdominal sepsis. J Vis Exp. 2010;(46) doi: 10.3791/2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bone RC. Toward a theory regarding the pathogenesis of the systemic inflammatory response syndrome: what we do and do not know about cytokine regulation. Crit Care Med. 1996;24(1):163–72. doi: 10.1097/00003246-199601000-00026. [DOI] [PubMed] [Google Scholar]
- 23.Eskandari MK, et al. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J Immunol. 1992;148(9):2724–30. [PubMed] [Google Scholar]
- 24.Remick D, et al. Blockade of tumor necrosis factor reduces lipopolysaccharide lethality, but not the lethality of cecal ligation and puncture. Shock. 1995;4(2):89–95. doi: 10.1097/00024382-199508000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Wang P, et al. Trauma-hemorrhage and resuscitation in the mouse: effects on cardiac output and organ blood flow. Am J Physiol. 1993;264(4 Pt 2):H1166–73. doi: 10.1152/ajpheart.1993.264.4.H1166. [DOI] [PubMed] [Google Scholar]
- 26.Venet F, et al. Lymphocytes in the development of lung inflammation: a role for regulatory CD4+ T cells in indirect pulmonary lung injury. J Immunol. 2009;183(5):3472–80. doi: 10.4049/jimmunol.0804119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gill R, et al. Systemic inflammation and liver injury following hemorrhagic shock and peripheral tissue trauma involve functional TLR9 signaling on bone marrow-derived cells and parenchymal cells. Shock. 2011;35(2):164–70. doi: 10.1097/SHK.0b013e3181eddcab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seok J, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gentile LF, et al. Identification and Description of a Novel Murine Model for Polytrauma and Shock. Crit Care Med. 2013 doi: 10.1097/CCM.0b013e318275d1f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marshall JC. Such stuff as dreams are made on: mediator-directed therapy in sepsis. Nat Rev Drug Discov. 2003;2(5):391–405. doi: 10.1038/nrd1084. [DOI] [PubMed] [Google Scholar]
- 31.Eichacker PQ, et al. Risk and the efficacy of antiinflammatory agents: retrospective and confirmatory studies of sepsis. Am J Respir Crit Care Med. 2002;166(9):1197–205. doi: 10.1164/rccm.200204-302OC. [DOI] [PubMed] [Google Scholar]
- 32.Abraham E, et al. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. N Engl J Med. 2005;353(13):1332–41. doi: 10.1056/NEJMoa050935. [DOI] [PubMed] [Google Scholar]
- 33.Gentile L, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, Moldawer LL, Moore FA. Peristent inflammation and immunosupression: A common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72(6):1–11. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zambon M, et al. Implementation of the Surviving Sepsis Campaign guidelines for severe sepsis and septic shock: we could go faster. J Crit Care. 2008;23(4):455–60. doi: 10.1016/j.jcrc.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Dellinger RP, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 36.Marshall JC, Reinhart K, F. International Sepsis Biomarkers of sepsis. Crit Care Med. 2009;37(7):2290–8. doi: 10.1097/CCM.0b013e3181a02afc. [DOI] [PubMed] [Google Scholar]
- 37.Oberholzer A, et al. Plasma cytokine measurements augment prognostic scores as indicators of outcome in patients with severe sepsis. Shock. 2005;23(6):488–93. [PubMed] [Google Scholar]
- 38.Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock. 2001;16(2):83–96. doi: 10.1097/00024382-200116020-00001. [DOI] [PubMed] [Google Scholar]
- 39.Schuetz P, Christ-Crain M, Muller B. Biomarkers to improve diagnostic and prognostic accuracy in systemic infections. Curr Opin Crit Care. 2007;13(5):578–85. doi: 10.1097/MCC.0b013e3282c9ac2a. [DOI] [PubMed] [Google Scholar]
- 40.Beutler B, Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med. 1987;316(7):379–85. doi: 10.1056/NEJM198702123160705. [DOI] [PubMed] [Google Scholar]
- 41.Fong YM, et al. The acute splanchnic and peripheral tissue metabolic response to endotoxin in humans. J Clin Invest. 1990;85(6):1896–904. doi: 10.1172/JCI114651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marano MA, et al. Cachectin/TNF production in experimental burns and Pseudomonas infection. Arch Surg. 1988;123(11):1383–8. doi: 10.1001/archsurg.1988.01400350097015. [DOI] [PubMed] [Google Scholar]
- 43.Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229(4716):869–71. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 44.Keogh C, et al. Identification of a novel tumor necrosis factor alpha/cachectin from the livers of burned and infected rats. Arch Surg. 1990;125(1):79–84. doi: 10.1001/archsurg.1990.01410130085011. discussion 85. [DOI] [PubMed] [Google Scholar]
- 45.Bauer S, et al. Structure-activity profiles of Ab-derived TNF fusion proteins. J Immunol. 2006;177(4):2423–30. doi: 10.4049/jimmunol.177.4.2423. [DOI] [PubMed] [Google Scholar]
- 46.Black RA, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385(6618):729–33. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 47.Van Zee KJ, et al. Tumor necrosis factor soluble receptors circulate during experimental and clinical inflammation and can protect against excessive tumor necrosis factor alpha in vitro and in vivo. Proc Natl Acad Sci U S A. 1992;89(11):4845–9. doi: 10.1073/pnas.89.11.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boraschi D, et al. Structure-function relationship in the IL-1 family. Front Biosci. 1996;1:d270–308. doi: 10.2741/a132. [DOI] [PubMed] [Google Scholar]
- 49.Kostura MJ, et al. Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc Natl Acad Sci U S A. 1989;86(14):5227–31. doi: 10.1073/pnas.86.14.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colotta F, et al. The type II ‘decoy’ receptor: a novel regulatory pathway for interleukin 1. Immunol Today. 1994;15(12):562–6. doi: 10.1016/0167-5699(94)90217-8. [DOI] [PubMed] [Google Scholar]
- 51.Jastrow KM, 3rd, et al. Early cytokine production risk stratifies trauma patients for multiple organ failure. J Am Coll Surg. 2009;209(3):320–31. doi: 10.1016/j.jamcollsurg.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 52.Xiao W, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208(13):2581–90. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gogos CA, et al. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis. 2000;181(1):176–80. doi: 10.1086/315214. [DOI] [PubMed] [Google Scholar]
- 54.van Dissel JT, et al. Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet. 1998;351(9107):950–3. doi: 10.1016/S0140-6736(05)60606-X. [DOI] [PubMed] [Google Scholar]
- 55.Gibot S, et al. Combination Biomarkers to Diagnose Sepsis in the Critically Ill Patient. Am J Respir Crit Care Med. 2012 doi: 10.1164/rccm.201201-0037OC. [DOI] [PubMed] [Google Scholar]
- 56.Lichtenstern C, et al. Predictors of survival in sepsis: what is the best inflammatory marker to measure? Curr Opin Infect Dis. 2012;25(3):328–36. doi: 10.1097/QCO.0b013e3283522038. [DOI] [PubMed] [Google Scholar]
- 57.Partrick DA, et al. Jack A. Barney Resident Research Award winner. The inflammatory profile of interleukin-6, interleukin-8, and soluble intercellular adhesion molecule-1 in postinjury multiple organ failure. Am J Surg. 1996;172(5):425–9. doi: 10.1016/s0002-9610(96)00252-8. discussed 429-31. [DOI] [PubMed] [Google Scholar]
- 58.Bozza FA, et al. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care. 2007;11(2):R49. doi: 10.1186/cc5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bopp C, et al. sRAGE is elevated in septic patients and associated with patients outcome. J Surg Res. 2008;147(1):79–83. doi: 10.1016/j.jss.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 60.de Pablo R, et al. Mortality in patients with septic shock correlates with anti-inflammatory but not proinflammatory immunomodulatory molecules. J Intensive Care Med. 2011;26(2):125–32. doi: 10.1177/0885066610384465. [DOI] [PubMed] [Google Scholar]
- 61.Lvovschi V, et al. Cytokine profiles in sepsis have limited relevance for stratifying patients in the emergency department: a prospective observational study. PLoS One. 2011;6(12):e28870. doi: 10.1371/journal.pone.0028870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Selberg O, et al. Discrimination of sepsis and systemic inflammatory response syndrome by determination of circulating plasma concentrations of procalcitonin, protein complement 3a, and interleukin-6. Crit Care Med. 2000;28(8):2793–8. doi: 10.1097/00003246-200008000-00019. [DOI] [PubMed] [Google Scholar]
- 63.Heper Y, et al. Evaluation of serum C-reactive protein, procalcitonin, tumor necrosis factor alpha, and interleukin-10 levels as diagnostic and prognostic parameters in patients with community-acquired sepsis, severe sepsis, and septic shock. Eur J Clin Microbiol Infect Dis. 2006;25(8):481–91. doi: 10.1007/s10096-006-0168-1. [DOI] [PubMed] [Google Scholar]
- 64.Gaini S, et al. Procalcitonin, lipopolysaccharide-binding protein, interleukin-6 and C-reactive protein in community-acquired infections and sepsis: a prospective study. Crit Care. 2006;10(2):R53. doi: 10.1186/cc4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Andaluz-Ojeda D, et al. A combined score of pro- and anti-inflammatory interleukins improves mortality prediction in severe sepsis. Cytokine. 2012;57(3):332–6. doi: 10.1016/j.cyto.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 66.Rogy MA, et al. Correlation between Acute Physiology and Chronic Health Evaluation (APACHE) III score and immunological parameters in critically ill patients with sepsis. Br J Surg. 1996;83(3):396–400. doi: 10.1002/bjs.1800830333. [DOI] [PubMed] [Google Scholar]
- 67.Espat NJ, et al. CMNSG Guest Lecture. Interleukin-1, interleukin-1 receptor, and interleukin-1 receptor antagonist. Proc Nutr Soc. 1994;53(2):393–400. doi: 10.1079/pns19940044. [DOI] [PubMed] [Google Scholar]
- 68.Oberholzer A, et al. Functional modification of dendritic cells with recombinant adenovirus encoding interleukin 10 for the treatment of sepsis. Shock. 2005;23(6):507–15. [PubMed] [Google Scholar]
- 69.Hack CE, et al. Increased plasma levels of interleukin-6 in sepsis. Blood. 1989;74(5):1704–10. [PubMed] [Google Scholar]
- 70.Damas P, et al. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg. 1992;215(4):356–62. doi: 10.1097/00000658-199204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Panacek EA, et al. Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab')2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit Care Med. 2004;32(11):2173–82. doi: 10.1097/01.ccm.0000145229.59014.6c. [DOI] [PubMed] [Google Scholar]
- 72.Patel RT, et al. Interleukin 6 is a prognostic indicator of outcome in severe intra-abdominal sepsis. Br J Surg. 1994;81(9):1306–8. doi: 10.1002/bjs.1800810914. [DOI] [PubMed] [Google Scholar]
- 73.Fong Y, et al. Endotoxemia elicits increased circulating beta 2-IFN/IL-6 in man. J Immunol. 1989;142(7):2321–4. [PubMed] [Google Scholar]
- 74.May LT, et al. Antibodies chaperone circulating IL-6. Paradoxical effects of anti-IL-6 “neutralizing” antibodies in vivo. J Immunol. 1993;151(6):3225–36. [PubMed] [Google Scholar]
- 75.Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–78. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 76.Biffl WL, et al. Interleukin-6 in the injured patient. Marker of injury or mediator of inflammation? Ann Surg. 1996;224(5):647–64. doi: 10.1097/00000658-199611000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ventetuolo CE, Levy MM. Biomarkers: diagnosis and risk assessment in sepsis. Clin Chest Med. 2008;29(4):591–603. vii. doi: 10.1016/j.ccm.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 78.Vyas D, et al. Early antibiotic administration but not antibody therapy directed against IL-6 improves survival in septic mice predicted to die on basis of high IL-6 levels. Am J Physiol Regul Integr Comp Physiol. 2005;289(4):R1048–53. doi: 10.1152/ajpregu.00312.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harbarth S, et al. Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. Am J Respir Crit Care Med. 2001;164(3):396–402. doi: 10.1164/ajrccm.164.3.2009052. [DOI] [PubMed] [Google Scholar]
- 80.Kuster H, et al. Interleukin-1 receptor antagonist and interleukin-6 for early diagnosis of neonatal sepsis 2 days before clinical manifestation. Lancet. 1998;352(9136):1271–7. doi: 10.1016/S0140-6736(98)08148-3. [DOI] [PubMed] [Google Scholar]
- 81.Iskander KN, et al. Cecal ligation and puncture-induced murine sepsis does not cause lung injury. Crit Care Med. 2013;41(1):159–70. doi: 10.1097/CCM.0b013e3182676322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Giamarellos-Bourboulis EJ, et al. Procalcitonin: a marker to clearly differentiate systemic inflammatory response syndrome and sepsis in the critically ill patient? Intensive Care Med. 2002;28(9):1351–6. doi: 10.1007/s00134-002-1398-z. [DOI] [PubMed] [Google Scholar]
- 83.Haasper C, et al. Prognostic value of procalcitonin (PCT) and/or interleukin-6 (IL-6) plasma levels after multiple trauma for the development of multi organ dysfunction syndrome (MODS) or sepsis. Technol Health Care. 2010;18(2):89–100. doi: 10.3233/THC-2010-0571. [DOI] [PubMed] [Google Scholar]
- 84.Carlet J. Rapid diagnostic methods in the detection of sepsis. Infect Dis Clin North Am. 1999;13(2):483–94. xi. doi: 10.1016/s0891-5520(05)70087-8. [DOI] [PubMed] [Google Scholar]
- 85.de Werra I, et al. Cytokines, nitrite/nitrate, soluble tumor necrosis factor receptors, and procalcitonin concentrations: comparisons in patients with septic shock, cardiogenic shock, and bacterial pneumonia. Crit Care Med. 1997;25(4):607–13. doi: 10.1097/00003246-199704000-00009. [DOI] [PubMed] [Google Scholar]
- 86.Uusitalo-Seppala R, et al. Early detection of severe sepsis in the emergency room: diagnostic value of plasma C-reactive protein, procalcitonin, and interleukin-6. Scand J Infect Dis. 2011;43(11-12):883–90. doi: 10.3109/00365548.2011.600325. [DOI] [PubMed] [Google Scholar]
- 87.Clec'h C, et al. Diagnostic and prognostic value of procalcitonin in patients with septic shock. Crit Care Med. 2004;32(5):1166–9. doi: 10.1097/01.ccm.0000126263.00551.06. [DOI] [PubMed] [Google Scholar]
- 88.Brunkhorst FM, et al. Procalcitonin for early diagnosis and differentiation of SIRS, sepsis, severe sepsis, and septic shock. Intensive Care Med. 2000;26(Suppl 2):S148–52. doi: 10.1007/BF02900728. [DOI] [PubMed] [Google Scholar]
- 89.Ugarte H, et al. Procalcitonin used as a marker of infection in the intensive care unit. Crit Care Med. 1999;27(3):498–504. doi: 10.1097/00003246-199903000-00024. [DOI] [PubMed] [Google Scholar]
- 90.Tang BM, Eslick GD. Procalcitonin for sepsis: methodological issues in meta-analysis lead to further uncertainty. Crit Care Med. 2007;35(2):679. doi: 10.1097/01.CCM.0000254967.79846.6A. author reply 679-80. [DOI] [PubMed] [Google Scholar]
- 91.Tschaikowsky K, et al. Predictive value of procalcitonin, interleukin-6, and C-reactive protein for survival in postoperative patients with severe sepsis. J Crit Care. 2011;26(1):54–64. doi: 10.1016/j.jcrc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 92.Ruiz-Alvarez MJ, et al. Diagnostic efficacy and prognostic value of serum procalcitonin concentration in patients with suspected sepsis. J Intensive Care Med. 2009;24(1):63–71. doi: 10.1177/0885066608327095. [DOI] [PubMed] [Google Scholar]
- 93.Tang BM, et al. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet Infect Dis. 2007;7(3):210–7. doi: 10.1016/S1473-3099(07)70052-X. [DOI] [PubMed] [Google Scholar]
- 94.Wacker C, et al. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis. 2013 doi: 10.1016/S1473-3099(12)70323-7. [DOI] [PubMed] [Google Scholar]
- 95.Heyland DK, et al. Procalcitonin for reduced antibiotic exposure in the critical care setting: a systematic review and an economic evaluation. Crit Care Med. 2011;39(7):1792–9. doi: 10.1097/CCM.0b013e31821201a5. [DOI] [PubMed] [Google Scholar]
- 96.Cohen J, et al. New strategies for clinical trials in patients with sepsis and septic shock. Crit Care Med. 2001;29(4):880–6. doi: 10.1097/00003246-200104000-00039. [DOI] [PubMed] [Google Scholar]
- 97.Abraham E, et al. p55 Tumor necrosis factor receptor fusion protein in the treatment of patients with severe sepsis and septic shock. A randomized controlled multicenter trial. Ro 45-2081 Study Group. JAMA. 1997;277(19):1531–8. [PubMed] [Google Scholar]
- 98.Abraham E, et al. Lenercept (p55 tumor necrosis factor receptor fusion protein) in severe sepsis and early septic shock: a randomized, double-blind, placebo-controlled, multicenter phase III trial with 1,342 patients. Crit Care Med. 2001;29(3):503–10. doi: 10.1097/00003246-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 99.Slotman GJ. Prospectively validated prediction of physiologic variables and organ failure in septic patients: The Systemic Mediator Associated Response Test (SMART). Crit Care Med. 2002;30(5):1035–45. doi: 10.1097/00003246-200205000-00014. [DOI] [PubMed] [Google Scholar]
- 100.Rosenberg JJ, et al. Development of a novel, nonimmunogenic, soluble human TNF receptor type I (sTNFR-I) construct in the baboon. J Appl Physiol. 2001;91(5):2213–23. doi: 10.1152/jappl.2001.91.5.2213. [DOI] [PubMed] [Google Scholar]
- 101.Alvarez SM, et al. Low-dose steroid alters in vivo endotoxin-induced systemic inflammation but does not influence autonomic dysfunction. J Endotoxin Res. 2007;13(6):358–68. doi: 10.1177/0968051907086465. [DOI] [PubMed] [Google Scholar]
- 102.Friedland JS, et al. Plasma proinflammatory cytokine concentrations, Acute Physiology and Chronic Health Evaluation (APACHE) III scores and survival in patients in an intensive care unit. Crit Care Med. 1996;24(11):1775–81. doi: 10.1097/00003246-199611000-00003. [DOI] [PubMed] [Google Scholar]
- 103.Remick DG, et al. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock. 2000;13(2):110–6. doi: 10.1097/00024382-200013020-00004. [DOI] [PubMed] [Google Scholar]
- 104.Fischer E, et al. Interleukin-1 receptor blockade improves survival and hemodynamic performance in Escherichia coli septic shock, but fails to alter host responses to sublethal endotoxemia. J Clin Invest. 1992;89(5):1551–7. doi: 10.1172/JCI115748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wittebole X, et al. Nicotine exposure alters in vivo human responses to endotoxin. Clin Exp Immunol. 2007;147(1):28–34. doi: 10.1111/j.1365-2249.2006.03248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lamkanfi M, et al. Caspase-7 deficiency protects from endotoxin-induced lymphocyte apoptosis and improves survival. Blood. 2009;113(12):2742–5. doi: 10.1182/blood-2008-09-178038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Van Zee KJ, et al. Protection against lethal Escherichia coli bacteremia in baboons (Papio anubis) by pretreatment with a 55-kDa TNF receptor (CD120a)-Ig fusion protein, Ro 45-2081. J Immunol. 1996;156(6):2221–30. [PubMed] [Google Scholar]
- 108.Coyle SM, Calvano SE, Lowry SF. Gender influences in vivo human responses to endotoxin. Shock. 2006;26(6):538–43. doi: 10.1097/01.shk.0000232589.39001.4d. [DOI] [PubMed] [Google Scholar]
- 109.Call DR, et al. Differential local and systemic regulation of the murine chemokines KC and MIP2. Shock. 2001;15(4):278–84. doi: 10.1097/00024382-200115040-00005. [DOI] [PubMed] [Google Scholar]