Abstract
Natural product drug discovery programs often rely on the use of silica (Si) gel, reversed- phase media, or size-exclusion resins (e.g., RP-C18, Sephadex LH-20) for compound purification. The synthetic polymer-based sorbent Diaion™ HP20SS (cross-linked polystyrene matrix) is used as an alternative to prepare purified natural product libraries. To evaluate the impact of chromatographic media on the isolation of biologically active, yet chromatographically unstable natural products, Diaion HP20SS was evaluated side-by-side with normal-phase sorbents for irreversible binding of extract constituents and their effects on bioactivity. An array of chemically diverse natural product-rich extracts was selected as a test panel and a cell-based reporter assay for hypoxia-inducible factor-1 (HIF-1) was employed to monitor potential change(s) in bioactivity. Silica gel caused significant irreversible binding of three out of ten extracts. Curcuma longa, Saururus cernuus and Citrus reticulata extracts showed decreased HIF-1 inhibitory activity after elution through Si gel. An additional non-polar column wash of HP20SS with EtOAc retained considerable bioactivities of active extracts. In general, Si gel produced the greatest loss of bioactivity. However, HP20SS elution reduced significantly HIF-1 inhibitory activity of certain extracts (e.g., Asimina triloba).
Natural products and natural product-rich extracts have been used worldwide for centuries in traditional medicinal systems. Over the last three decades, 34% of small molecules approved by the U.S. FDA for therapeutic uses were either natural products or derived from natural product drug leads.1 Traditionally, a chemistry-based phytochemical approach is used to purify bioactive compounds from natural sources. Accompanying the shift to an emphasis on molecular-targeted drug discovery, bioassay-guided fractionation has gained considerable popularity. The bioassay-guided approach has the advantage of deselecting inactive fractions in favor of active constituents. With the development of high-throughput screening (HTS) technologies, the pharmaceutical industry has shifted from the use of natural products to strategies that rely on synthetic compound libraries as a primary source of drug leads.2 However, natural products remain an unparalleled source of chemically and mechanistically novel drug leads.1 To improve screening efficiency, semi-purified and purified natural product libraries are often prepared and these compound libraries are gradually replacing the traditional extract-based sample libraries.3 Both pure compound- and extract-based libraries are generated at the U.S. National Cancer Institute (NCI) for drug discovery.4
Regardless of chemistry- or bioassay-guided isolation effort, the selection of chromatographic sorbents is a critical step for the success of natural product-based drug discovery. Exposure of certain compounds to inappropriate chromatographic media can cause catalytic degradation or irreversible binding to the solid phase.5–8 Incorrect sorbent use in a single fractionation step can result in overall bioactivity loss and/or generate chemical artifacts that appear in subsequent fractions.6,8,9 The commonly used chromatographic sorbent can be broadly classified into normal-phase or bonded normal-phase (Si gel and diol), styrene- divinylbenzene polymers, and bonded reversed-phase (C8, C18, and phenyl-hexyl) media. Si gel is inexpensive and often used for initial normal-phase fractionation. However, Si gel can irreversibly bind certain natural products and promote acid-catalyzed rearrangement reactions that may cause the degradation of active compounds.7,8 Diol bonded-phase sorbent is used as a less chemically reactive alternative for normal-phase separations where Si gel-like elution properties are desired.10,11 Sephadex LH-20 (hydroxypropylated dextran) is a gel permeation sorbent that is often used in natural product isolation.12 However, the large-scale preparative use of bonded-phase sorbent and LH-20 is often limited by their relatively high cost. Alternatives, such as C8, C18, and phenyl-hexyl media are used for reversed-phase chromatography and can provide versatility in the separation process.13 Diaion HP20SS is a synthetic styrene-divinylbenzene polymer used for reversed-phase like separation. Diaion HP20SS provides an aromatic surface that adsorbs aromatic molecules by van der Waal’s forces.14 Diaion HP20SS is less reactive, reusable, and has emerged as a possible substitute for Si gel for use in large-scale separations.15
The focus of the present study was to compare these chromatographic media side-by-side for their advantages/disadvantages in natural product drug discovery efforts. To monitor the impact of chromatographic sorbent selection on bioactive natural products, a cell-based reporter assay16 for hypoxia-inducible factor-1 (HIF-1) was employed as an exemplary bioassay. The transcription factor HIF-1 regulates cellular adaptation and survival under hypoxic conditions and has emerged as an important molecular target for anticancer drug discovery.17 This study has compared the potential of commonly used chromatographic media to irreversibly bind natural products from plant extracts, characterized the elution profile of the alternative chromatographic sorbent HP20SS, and examined the potential of these alternative media to reduce the loss of bioactivity as assessed by a cell-based molecular-targeted bioassay.
RESULTS AND DISCUSSION
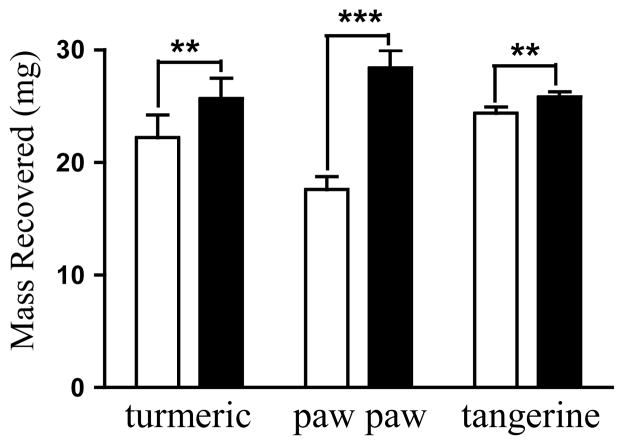
Ten chemically diverse plant extracts were selected to evaluate the effects of chromatographic media on natural product metabolite recovery and HIF-1 inhibitory activity (Table 1). The samples (30 mg each) were first subjected to column chromatography with Si gel or HP20SS as specified, eluted with typically used solvent systems [hexanes, EtOAc, MeOH, and water for Si gel; and MeOH-water (1:1), MeOH, EtOAc and hexanes for HP20SS], dried, weighed, and the mass compared to that of the original sample. Among the extracts examined, spray-dried Vaccinium macrocarpon Ait. (Ericaceae) juice and extracts of Asimina triloba (L.) Dunal. (Annonaceae), and Punica granatum L. (Lythraceae) showed a significant level of solid-phase binding-associated sample loss on Si gel (15% to 39% sample loss, Figure 1). Drastic losses in the masses of Curcuma longa L. (Zingiberaceae), Citrus reticulata Blanco. (Rutaceae), and Saururus cernuus L. (Saururaceae) extracts were observed (~50%, data not shown) on both HP20SS and Si gel. Rather than irreversible binding, this observation suggested that the HP20SS columns were insufficiently eluted by the initial solvent systems. Therefore, a relatively non-polar solvent such as EtOAc was used for final elution. Three representative samples (Cu. longa, Asi. triloba, and Ci. reticulata extracts) were subjected to HP20SS column chromatography, eluted with the commonly used MeOH solvent system, with or without a final EtOAc wash/elution step, and the fractions collected and dried. The weights of the combined fractions were used to calculate total sample recovery. In the case of Cu. longa, the total sample recovery was significantly increased (p = 0.0015) when a final EtOAc wash/elution step was added (Figure 2). Asi. triloba and Ci. reticulata extract recovery also showed significant improvement when the columns were subjected to a final EtOAc wash (Figure 2). The application of previously reported water-acetone gradients18 produced a similarly complete elution of the Cu. longa extract from the HP20SS columns (data not shown).
Table 1.
Plant materials evaluated and known bioactive constituents from these plants.
| Sample ID | Potential antitumor constituents |
|---|---|
| Pomegranate - Punica granatum L. (Lythraceae) | flavonoids, proanthocyanidins19 |
| Pomegranate juice - P. granatum juice | flavonoids, anthocyanins19 |
| Cranberry – Vaccinium macrocarpon Aiton (Ericaceae) freeze-dried juice | polyphenols20 |
| Rooibos - Aspalathus linearis (Burm.f.) R.Dahlgren (Fabaceae) | flavonoids21 |
| Honeybush - Cyclopia intermedia E.Mey. (Fabaceae) | flavonoid glycosides22 |
| Tangerine - Citrus reticulata Blanco (Rutaceae) | flavonoid glycosides23 |
| Turmeric - Curcuma longa L. (Zingiberaceae) | curcuminoids24 |
| Lizard’s tail - Saururus cernuus L. (Saururaceae) sec-butanol partitionate | sesquineolignans, dineolignans16 |
| Paw Paw - Asimina triloba (L.) Dunal. (Annonaceae) | annonaceous acetogenins25 |
| Mayapple – Podophyllum peltatum L. (Berberidaceae) | lignans26 |
Figure 1.
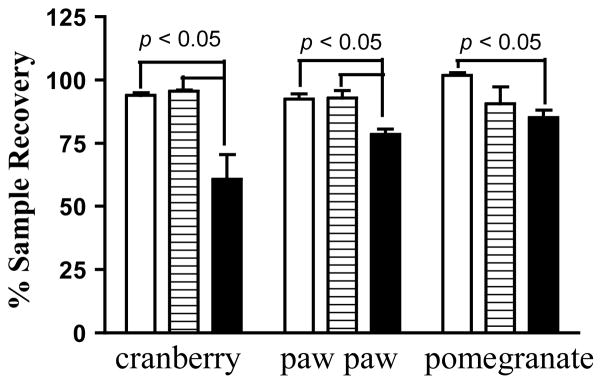
Chromatographic medium-dependent sample recovery of plant extracts. Samples of cranberry (Vaccinium macrocarpon), paw paw (Asimina triloba), and pomegranate (Punica granatum) extracts (30 mg; 150 μL stock solution) were subjected to HP20SS (striped bar) and Si gel (solid bar) column chromatography. The amount of recovered sample was presented as “% Sample Recovery.” As a control for drying-associated sample loss, extract samples were placed in vials, dried, and the dried materials served as sample recovery control (open bar). Data shown are average + standard deviation from three separate columns or vials, as appropriate. One-way ANOVA with the Bonferroni post hoc test was applied to analyze the data using GraphPad Prism 5.0 and differences between data sets were considered statistically significant when p < 0.05.
Figure 2.
Ethyl acetate wash over the HP20SS column increases extract recovery. Samples of turmeric (Curcuma longa), paw paw (Asimina triloba), and tangerine (Citrus reticulata) extracts (30 mg) were subjected to HP20SS column chromatography with a water-MeOH system. The mass recovered without (open bar) or with (solid bar) an EtOAc wash was recorded. Data shown are average + standard deviation from three separate columns. Student’s paired t-test (two-tailed) was performed to compare the data using GraphPad Prism 5.0. Differences between data sets were considered statistically significant when p < 0.05 (** p < 0.01, *** p < 0.001).
To monitor potential chromatography-associated chemical alteration or compound loss, TLC profile analysis was performed on both the original and recovered extract samples (Figure S1A–C, Supporting Information). The chemical composition of the Podophyllum peltatum L. (Berberidaceae) extract after passing through Si gel was noticeably altered. The TLC profiles of the Ci. reticulata and Cu. longa extracts indicated that considerable compound loss occurred on HP20SS unless a final wash with a non-polar solvent such as EtOAc was performed.
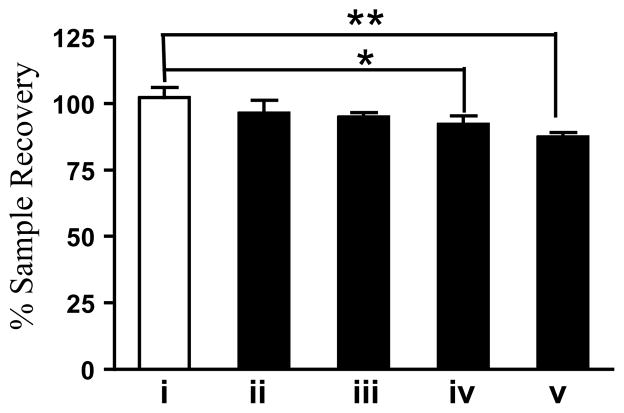
Diol bonded-phase media have been used by Dr. Kirk Gustafson’s group to generate natural product libraries at the U.S. National Cancer Institute, Frederick, Maryland.27 Diol bonded-phase media has been used to isolate natural products that bind phorbol ester receptors,28 and in the chromatography of antioxidants29 and pesticides30 from olive oil. To evaluate the elution properties of diol columns, Ci. reticulata, Cu. longa, and S. cernuus extracts were subjected to diol-bonded phase chromatography. Of the three extracts, the Ci. reticulata extract showed a significant loss of mass (Figure 3). The Ci. reticulata and Cu. longa extracts were similarly partitioned by MeOH elution on Sephadex LH-20. The Ci. reticulata extract also exhibited a significant loss of recovery from Sephadex LH-20 (Figure 3).
Figure 3.
Recovery of C. reticulata extract from various chromatographic columns. The conditions are: i – drying control, ii – HP20SS, iii – Si Gel, iv – diol, and v – Sephadex LH-20. Results shown are average + standard deviation (n = 3). Data were analyzed by one-way ANOVA with the Bonferroni post hoc test using GraphPad Prism 5.0. Differences between datasets were considered statistically significant when p < 0.05 (* p < 0.05, ** p < 0.01).
These results indicate the potential of Si gel, relative to other chromatographic sorbents, to cause irreversible binding or chemical alteration of the plant extract chemical constituents. Use of Si gel as a chromatographic sorbent for fractionation of extracts obtained from terrestrial or marine organisms with an unknown chemical profile can cause a loss of potential chemical diversity, sample quantity, or produce corresponding experimental artifacts. While alternative media such as diol bonded-phase and Sephadex LH-20 also caused observable losses in sample recovery that were elution protocol dependent, Si gel caused the greatest sample recovery losses. Water-isopropanol solvent systems commonly used for the elution of HP20SS columns appear to be insufficient for complete extract elution, especially for extracts with lipophilic constituents. Elution of HP20SS with relatively non-polar solvents such as EtOAc can prevent the loss of the intermediate to highly non-polar compounds. Some natural product drug discovery programs may choose to exclude lipophilic constituents that may not serve as promising drug candidates. However, these compounds may serve ultimately as valuable molecular probes or as potential “template” molecules from which to construct more suitable drug candidates. Such factors should be considered in the overall strategy and goals of the research program when selecting a protocol to produce natural product-rich extracts/fraction libraries for drug discovery. Another noteworthy observation is that the use of non-polar solvents such as EtOAc can cause elution of fine HP20SS particles during the separation process. However, washing the HP20SS columns first with EtOAc and then washing the column with MeOH prevented the subsequent loss of fine particles during the separation procedures.
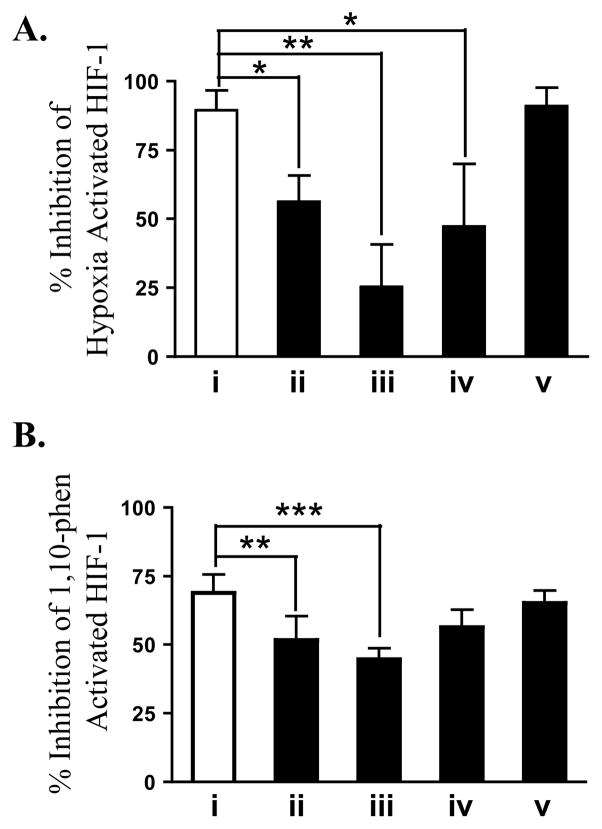
To evaluate the impact of chromatographic medium exposure on bioactive natural products, a T47D cell-based reporter assay16 was employed to monitor the potential HIF-mediated antitumor activity of the extracts. Of the ten extracts, Asi. triloba (0.5 μg/mL), Ci. reticulata (50 μg/mL), Po. peltatum (50 μg/mL), S. cernuus (50 μg/mL), and Cu. longa (50 μg/mL) extracts inhibited HIF-1 activation (Figure S2, Supporting Information), while the other extracts were inactive. The Po. peltatum extract was excluded from further evaluation because it was unstable and decomposed during storage.31 A comparative study was first performed to determine the consequence of HP20SS, Si gel, diol, and Sephadex LH-20 media exposure on bioactivity, using the Curc. longa extract as an example. All fractions obtained from each extract/column combination were recombined to assess the impact of exposure to each sorbent on HIF-1 inhibitory activity. The reconstituted Cu. longa extracts that had been chromatographed on the HP20SS, Si gel, and diol columns were significantly less active at suppressing hypoxia-induced HIF-1 activation, relative to the original extract (Figure 4A). Similarly, exposure to either Si gel or HP20SS decreased the activity of Cu. longa extract to inhibit 1,10-phenanthroline-induced HIF-1 activation (Figure 4B). No loss in activity was observed with the reconstituted Cu. longa fractions that had been passed over Sephadex LH-20 columns in both assays (Figures 4A and 4B). A similar study with the Ci. reticulata extract revealed that Si gel exposure markedly reduced the HIF-1 inhibitory activity of this extract (Figure S3, Supporting Information).
Figure 4.
Impact of chromatographic sorbent exposure on the HIF-1 inhibitory activity of Curcuma longa extract. Recombined fractions obtained by passing Cu. longa extract over various media were evaluated at 25 μg mL−1 for their effects on hypoxia- (A) and 1,10-phenanthroline (1,10-phen)-induced (B) HIF-1 activation in a T47D cell-based reporter assay. The chromatography conditions are: i – drying control, ii – HP20SS, iii – Si Gel, iv – diol, and v – Sephadex LH-20. Bars represent average + standard deviation from two independent experiments (n = 4 for i, and n = 6 for ii through v). Data were analyzed by one-way ANOVA with the Bonferroni post hoc test using GraphPad Prism 5.0. Differences between data sets were considered statistically significant when p < 0.05. (* p < 0.05, ** p < 0.01, *** p < 0.001).
The Asi. triloba and S. cernuus extracts were also subjected to HP20SS and Si gel column chromatography. Both the original extracts and reconstituted Asi. triloba and S. cernuus fractions inhibited hypoxia-induced HIF-1 activation (Figure 5A). The S. cernuus extract lost activity upon Si gel elution, but a similar loss was not observed upon passage over HP20SS. The reconstituted Asi. triloba extract lost considerable HIF-1 inhibitory activity when passed over HP20SS but retained its activity when eluted through Si gel. The Ci. reticulata and Cu. longa extracts were selected as an example to determine the consequence of HP20SS column chromatography on bioactive natural products. When Ci. reticulata and Cu. longa extracts were passed over HP20SS, active material remained on the columns until ultimately washed with EtOAc. The material recovered from the EtOAc wash retained the HIF-1 inhibitory activity (Figure 5B).
Figure 5.
Impact of HP20SS and Si gel column chromatography on bioactive natural products. (A) Asimina triloba and Saururus cernuus extracts were passed over various media (ii – HP20SS and iii – Si gel) and the fractions combined. An identical volume of the stock solution used to load the columns was placed in vials and dried (i – Control). The samples were evaluated at specified concentrations (0.05 μg mL−1 Asi. triloba extract; and 3.13 μg mL−1 S. cernuus extract) in a T47D cell-based reporter assay for their effects on hypoxia-induced HIF-1 activation. The bars represent average + standard deviation (n = 6). Data were analyzed by one-way ANOVA with the Bonferroni post hoc test. Differences between datasets were considered statistically significant when p < 0.05 (* p <0.05, *** p < 0.001). (B) Citrus reticulata and Curcuma longa extracts were passed over HP20SS columns eluted with EtOAc. Samples recovered were assayed for HIF-1 inhibitory activity in the T47D cell-based reporter assay, and compared to that of the original extract (“Control”). All samples were tested at 50 μg mL−1. The HIF-1 inducing conditions were hypoxia (1% O2, 16 h) and 1,10-phen. (10 μM 1,10-phenanthroline, 16 h), respectively. Data shown are average + standard deviation (n = 4 for control, and n = 6 for HP20SS).
Bioassay-guided isolation protocols are commonly used to identify molecular-targeted potential antitumor natural products. Since bioassay-guided fractionation relies on the activity of extracts and subsequent fractions, loss of activity due to inappropriate chromatographic sorbent selection, can impact the overall success of the drug discovery process significantly. Three of the four extracts that inhibited HIF-1 activation lost activity when eluted through Si gel. The Cu. longa extract lost no detectable sample mass upon passage over HP20SS or diol, but lost a significant level of bioactivity. Only the extracts of Asi. triloba and Cu. longa lost HIF-1 inhibitory activity upon passage over HP20SS. Moreover, neither the Asi. triloba extract nor the Cu. longa extract lost significant mass upon passage over HP20SS, but the Asi. triloba extract lost mass upon Si gel elution. As the Asi. triloba extract contains active lipophilic acetogenin constituents, it is possible that these active constituents were strongly bound to the HP20SS by π-bonding. However, because these extremely potent acetogenins are relatively minor extract components, the overall loss of sample mass was not statistically significant. Eluting HP20SS with a non-polar solvent such as CH2Cl2 or hexanes can prevent such sample losses. While the bioactivity loss upon Si gel elution for some extracts was apparent, passage over HP20SS can also cause bioactivity loss. When possible, chromatographic medium selection should place a priority away from the use of Si gel in favor of alternative chromatographic sorbent, but elution solvent choices should be carefully considered when HP20SS is used. Unless the aim of the separation is to include a “defatting” step to remove lipophilic compounds, HP20SS elution protocols should include a final relatively non-polar wash with EtOAc, CH2Cl2 or other lipophilic solvent to ensure more complete sample elution than may be obtained with commonly used aqueous-MeOH or aqueous-isopropanol gradients. To avoid the elution of small HP20SS particles when non-polar solvents are used to wash the column, the HP20SS should be prewashed with EtOAc and subsequently washed with MeOH.
EXPERIMENTAL SECTION
General Experimental Procedures
TLC was performed using Merck Si60F254 or Si60RP18F254 plates, visualized under UV light at 254 nm, and heated after spraying with 10% H2SO4 in EtOH. The Si gel (32–63 μm) was from Selecto Scientific (Suwanee, GA, USA). HP20SS (Diaion 75–100 μm, Supelco), diol (50 μm, Discovery® Supelco), and Sephadex LH-20 were from Sigma (St. Louis, MO, USA). Solvents (HPLC grade for HPLC and reagent grade for extractions and column chromatography) were from Fisher Scientific unless otherwise specified.
Plant Material
Podophyllum peltatum root material was collected May 1, 2005 from along Old Taylor Road (N 34° 24.175′; W 089° 30.056′) on the University of Mississippi campus in Oxford, Mississippi and stored at −80 °C. The samples were from fresh collections (collection voucher DN-MS-223) of routinely accessed Old Taylor Road UM populations identified by R. Moraes and C. M. Burandt, National Center for Natural products Research, University of Mississippi. The Citrus reticulata fruit (collection voucher BM-T-1) were purchased (February 15, 2007) from a local store and the peels were air-dried. Voucher samples of powders and plant samples used to prepare extracts were previously processed and retained in the Research Institute of Pharmaceutical Sciences and Department of Biology Herbaria at the University of Mississippi, University, Mississippi.
Column Preparation
Silica gel, HP20SS, diol, and Sephadex LH-20 columns were prepared by packing pasture pipettes with each respective chromatographic sorbent. Each column was 7.5 cm long with 0.55 cm internal diameter. The Si gel and diol were activated by heating at 110 °C for 30 min and then cooling to room temperature immediately before packing the columns. The HP20SS columns were prewashed with EtOAc followed by MeOH wash prior to use.
Extract Preparation and Elution
The freeze-dried Punica granatum juice,32 spray-dried Vaccinium macrocarpon juice,33 and Pu. granatum,32 Aspalathus linearis (Burm.f.) R.Dahlgren (Fabaceae),34 Cyclopia intermedia E. Mey. (Fabaceae),35 and Asimina triloba25 extracts were prepared and used in previously published studies. Curcuma longa powder (McCormick) was purchased and extracted with four volumes of EtOH (500 mL) at 24 h intervals at room temperature until the supernatant was nearly colorless. Air-dried (40 °C) Po. peltatum roots and rhizomes were crushed in a mortar and pestle and extracted with EtOH (500 mL) at room temperature four times at 24 h intervals until the extract was nearly colorless. Saururus cernuus sec-butanol/water partition was previously obtained by Dr. Chowdhury Faiz Hossain.36 Briefly, S. cernuus roots were extracted with CH2Cl2 and MeOH (50% v/v). The extract was first dissolved in CHCl3 and then partitioned with water. The water residue was partitioned with sec- butanol. The air-dried Ci. reticulata fruit peels were extracted four times with EtOH until the supernatant was nearly colorless.
The extracts were weighed at accuracy up to one-tenth of a milligram in small vials and dissolved in EtOH to obtain 200 mg/mL stock solutions. Extracts that were not completely soluble in EtOH were first dissolved with a H2O–EtOH (50:50) mixture (approximately 10–20 μL), then diluted with EtOH to achieve the desired concentration. The columns were loaded with samples in a volume of 150 μL (30 mg). For the Sephadex LH-20 columns, Cu. longa and Ci. reticulata extracts were dissolved in MeOH and filtered, with no substantial residue on the filter paper. The columns were loaded with the MeOH soluble portion of the extracts (150 μL). The extracts were allowed to sit on the surface of the columns for 30–45 min. For the diol columns, the extracts were allowed to sit overnight. The Si gel columns were eluted successively with hexanes (2 mL), EtOAc (5 mL), MeOH (5 mL) and water (2 mL) and the HP20SS columns were eluted successively with 50% MeOH–H2O (2 mL), MeOH (5 mL), EtOAc (5 mL) and hexanes (2 mL). Step gradients of water in isopropanol (100:0, 75:25, 50:50, 25:75, 0:100; 6 mL each) were applied for the elution of Cu. longa, Ci. reticulata and S. cernuus extracts. The combined dry weights of these fractions were used to calculate the total extract recoveries. Because regular solvent systems were unable to completely elute Ci. reticulata and Cu. longa from the columns (yellow color remained on the columns, but the sample recovery loss was not statistically significant), the experiments were repeated using a water-acetone step gradient in the same manner as the water-isopropanol system. Step gradients of hexanes–CH2Cl2 (9:1; 6 mL), CH2Cl2–EtOAc (20:1; 6 mL), EtOAc (6 mL), EtOAc-MeOH (5:1; 6 mL) and MeOH (6 mL) were passed over diol-bonded phase columns. Sephadex LH-20 columns were eluted with 100% MeOH. The stock solutions of extracts (30 mg, 150 μL) were placed directly in pre-weighed vials, dried, and weighed. These served as controls for the losses in recovery associated with sample handling.
Cell-based HIF-1 Reporter Assay
Cell maintenance, experimental procedures, and data presentation for the human breast tumor T47D cell-based reporter assay were the same as previously described.16 All extract, fraction, and compound samples were prepared as stock solutions in DMSO (final solvent concentration was less than 0.5% v/v). The following formula was used to calculate the % inhibition data:
Statistical Analysis
Data analyses were performed using Student’s t-test, one-way ANOVA and Bonferroni post hoc analyses (GraphPad Prism 5.0). Differences between data sets were considered statistically significant when p < 0.05.
Supplementary Material
Acknowledgments
The authors thank Dr. D. Ferreira (University of Mississippi) for providing some of the extract samples evaluated, Drs. I. A. Khan and Y.-H. Wang (University of Mississippi) for the HPLC analysis of the Cu. longa extract, and Dr. S. L. McKnight (University of Texas Southwestern Medical Center at Dallas) for providing the pTK-HRE3-luc construct. This work was supported in part by the National Institutes of Health National Cancer Institute (grant CA98787) and NOAA NURP/NIUST grant NA16RU1496. This investigation was conducted in a facility constructed with Research Facilities Improvement Grant C06 RR-14503 from the National Institutes of Health.
Footnotes
The authors declare no competing financial interests.
Supporting Information: TLC analysis of the extracts and fractions exposed to various chromatographic sorbents, inhibition of HIF-1 activation by extract samples, impact of chromatographic media on the HIF-1 inhibitory activity of Ci. reticulata extract, HPLC analysis of the Cu. longa extract. The material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Newman DJ, Cragg GM. J Nat Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McChesney JD, Venkataraman SK, Henri JT. Phytochemistry. 2007;68:2015–2022. doi: 10.1016/j.phytochem.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 3.Bugni TS, Harper MK, McCulloch MW, Reppart J, Ireland CM. Molecules. 2008;13:1372–1383. doi: 10.3390/molecules13061372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. [accessed December 1, 2012];National Institutes of Health Molecular Libraries Program: Pathways to Discovery. http://mli.nih.gov/mli/secondary-menu/nih-resources/
- 5.Hostettmann K, Marston A, Hostettmann M. Preparative Chromatography Techniques: Applications in Natural Product Isolation. 2. Springer; New York: 1998. p. 57. [Google Scholar]
- 6.Cimino G, Sodano G, Spinella A. J Nat Prod. 1988;51:1010–1011. doi: 10.1021/np50059a039. [DOI] [PubMed] [Google Scholar]
- 7.Posner GH. Angew Chem Int Edit. 1978;17:487–496. [Google Scholar]
- 8.Shamma M, Rahimizadeh M. J Nat Prod. 1986;49:398–405. [Google Scholar]
- 9.Middleditch BS. Journal of Chromatography Library. Vol. 44. Elsevier; Amsterdam: 1989. Analytical Artifacts: gc, ms, hplc, tlc, and pc. [Google Scholar]
- 10.Kazoka H. J Chromatogr A. 2008;1189:52–58. doi: 10.1016/j.chroma.2007.10.083. [DOI] [PubMed] [Google Scholar]
- 11.Kim IW, Lee HS, Lee YK, Jang MD, Par JH. J Chromatogr A. 2001;915:35–42. doi: 10.1016/s0021-9673(01)00628-8. [DOI] [PubMed] [Google Scholar]
- 12.a) Seidel V. In: In Natural Products Isolation. 2. Sarker SD, Latif Z, Gray AI, editors. Humana Press; Totowa, NJ: 2005. pp. 27–46. [Google Scholar]; b) Mansson M, Phipps RK, Gram L, Munro MHG, Larsen TO, Nielsen KF. J Nat Prod. 2010;73:1126–1132. doi: 10.1021/np100151y. [DOI] [PubMed] [Google Scholar]; c) Gafner S, Bergeron C, Villinski JR, Godejohann M, Kessler P, Cardellina JH, II, Ferreira D, Feghali K, Grenier D. J Nat Prod. 2011;74:2514–2519. doi: 10.1021/np2004775. [DOI] [PubMed] [Google Scholar]; d) Chen JT, Su HJ, Huang JW. J Agric Food Chem. 2012;60:7341–7344. doi: 10.1021/jf301570y. [DOI] [PubMed] [Google Scholar]
- 13.a) Reid RG. In: Methods in Biotechnology, Vol. 20, Natural Products Isolation. 2. Sarker SD, Latif Z, Gray AI, editors. Chapter 5. Humana Press Inc; Totowa, NJ: 2005. pp. 117–157. [Google Scholar]; b) Latif Z. In: Methods in Biotechnology, Vol. 20, Natural Products Isolation. 2. Sarker SD, Latif Z, Gray AI, editors. Chapter 8. Humana Press Inc; Totowa, NJ: 2005. pp. 213–232. [Google Scholar]; c) Houssen WE, Jaspers M. In: Methods in Biotechnology, Vol. 20, Natural Products Isolation. 2. Sarker SD, Latif Z, Gray AI, editors. Chapter 14. Humana Press Inc; Totowa, NJ: 2005. pp. 353–390. [Google Scholar]; d) Ghisalberti EL. In: Bioactive Natural Products: Detection, Isolation, And Structural Determination. 2. Colegate SM, Molyneux RJ, editors. Chapter 2. CRC Press; Boca Raton, FL: 2008. p. 39. [Google Scholar]; e) Beutler JA. Curr Protoc Pharmacol. 2009;46:9.11.1–9.11.21. doi: 10.1002/0471141755.ph0911s46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimizu Y, Li B. Purification of Water-Soluble Natural Products. In: Sarker SD, Latif Z, Gray AI, editors. Natural products isolation. 2. Humana Press; Totowa, N.J: 2005. pp. 415–438. [Google Scholar]
- 15.Bugni TS, Richards B, Bhoite L, Cimbora D, Harper MK, Ireland CM. J Nat Prod. 2008;71:1095–1098. doi: 10.1021/np800184g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodges TW, Hossain CF, Kim YP, Zhou YD, Nagle DG. J Nat Prod. 2004;67:767–771. doi: 10.1021/np030514m. [DOI] [PubMed] [Google Scholar]
- 17.Semenza GL. Trends Pharmacol Sci. 2012;33:207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boonlarppradab C, Faulkner DJ. J Nat Prod. 2007;70:846–848. doi: 10.1021/np060472c. [DOI] [PubMed] [Google Scholar]
- 19.Seeram NP, Schulman RN, Heber D. Pomegranates: Ancient Roots to Modern medicine. CRC/Taylor & Francis; Boca Raton, FL: 2006. p. 244. [Google Scholar]
- 20.Neto CC. J Nutr. 2007;137:186S–193S. doi: 10.1093/jn/137.1.186S. [DOI] [PubMed] [Google Scholar]
- 21.Bramati L, Minoggio M, Gardana C, Simonetti P, Mauri P, Pietta P. J Agric Food Chem. 2002;50:5513–5519. doi: 10.1021/jf025697h. [DOI] [PubMed] [Google Scholar]
- 22.Kamara BI, Brandt EV, Ferreira D, Joubert E. J Agric Food Chem. 2003;51:3874–3879. doi: 10.1021/jf0210730. [DOI] [PubMed] [Google Scholar]
- 23.Khan MA, Ali M, Alam P. Nat Prod Res. 2010;24:610–620. doi: 10.1080/14786410802425787. [DOI] [PubMed] [Google Scholar]
- 24.Roth GN, Chandra A, Nair MG. J Nat Prod. 1998;61:542–545. doi: 10.1021/np970459f. [DOI] [PubMed] [Google Scholar]
- 25.Coothankandaswamy V, Liu Y, Mao SC, Morgan JB, Mahdi F, Jekabsons MB, Nagle DG, Zhou YD. J Nat Prod. 2010;73:956–961. doi: 10.1021/np100228d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moraes RM, Bedir E, Barrett H, Burandt C, Jr, Canel C, Khan IA. Planta Med. 2002;68:341–344. doi: 10.1055/s-2002-26740. [DOI] [PubMed] [Google Scholar]
- 27.Gustafson K. Personal communication. National Cancer Institute; Frederick, MD: 2008. [Google Scholar]
- 28.Beutler JA, Alvarado AB, Schaufelberger DE, Andrews P, McCloud TG. J Nat Prod. 1990;53:867–874. doi: 10.1021/np50070a014. [DOI] [PubMed] [Google Scholar]
- 29.Fu S, Arraez-Roman D, Menendez JA, Segura-Garretero A, Fernandez-Gutierrez A. Rap Commun Mass Spectrom. 2009;23:51–59. doi: 10.1002/rcm.3855. [DOI] [PubMed] [Google Scholar]
- 30.Amvrazi EG, Albanis TA. J Agric Food Chem. 2006;54:9642–9651. doi: 10.1021/jf061375s. [DOI] [PubMed] [Google Scholar]
- 31.van Maanen JM, Verkerk UH, Broersen J, Lafleur MV, De Vries J, Retel J, Pinedo HM. Free Radic Res Commun. 1988;4:371–384. doi: 10.3109/10715768809066905. [DOI] [PubMed] [Google Scholar]
- 32.Kasimsetty SG, Bialonska D, Muntha KR, Thorton C, Willet KL, Ferreira D. J Agric Food Chem. 2009;57:10636–10644. doi: 10.1021/jf902716r. [DOI] [PubMed] [Google Scholar]
- 33.Foo LY, Lu Y, Howell AB, Vorsa N. Phytochemistry. 2000;54:173–181. doi: 10.1016/s0031-9422(99)00573-7. [DOI] [PubMed] [Google Scholar]
- 34.Rabe C, Steenkamp JA, Joubert E, Burger JFW, Ferreira D. Phytochemistry. 1994;35:1559–1565. [Google Scholar]
- 35.Ferreira D, Kamara BI, Brandt EV, Joubert E. J Agric Food Chem. 1998;46:3406–3410. doi: 10.1021/jf980258x. [DOI] [PubMed] [Google Scholar]
- 36.Hossain CF, Kim YP, Baerson SR, Zhang L, Bruick RK, Mohammed KA, Agarwal AK, Nagle DG, Zhou YD. Biochem Biophys Res Commun. 2005;333:1026–1033. doi: 10.1016/j.bbrc.2005.05.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.