Abstract
Earlier studies have shown that cardiac myosin binding protein-C (cMyBP-C) is easily releasable into the circulation following myocardial infarction (MI) in animal models and patients. However, since its release kinetics has not been clearly demonstrated, no parameters are available to judge its efficacy as a bona fide biomarker of MI in patients with MI. To make this assessment, plasma levels of cMyBP-C and six known biomarkers of MI were determined by sandwich enzyme-linked immunosorbent assay in patients with MI who had before and after Percutaneous Transcoronary Angioplasty (PTCA), as well as healthy controls. Compared to healthy controls (22.3 ± 2.4 ng/mL (n=54)), plasma levels of cMyBP-C were significantly increased in patients with MI (105.1 ± 8.8 ng/mL (n=65), P<0.001). Out of 65 patients, 24 had very high levels of plasma cMyBP-C (116.5 ± 13.3 ng/mL), indicating high probability of MI. Importantly, cMyBP-C levels were significantly decreased in patients (n=40) at 12 hours post-PTCA (41.2 ± 9.3 ng/mL, P<0.001), compared to the patients with MI. Receiver operating characteristic analysis revealed that a plasma cMyBP-C reading of 68.1 ng/mL provided a sensitivity of 66.2% and a specificity of 100%. Also, myoglobin, carbonic anhydrase and creatine kinase-MB levels were significantly increased in MI patients who also had higher cMyBP-C levels. In contrast, levels of cardiac troponin I, glycogen phosphorylase and heart-type fatty acid binding protein were not significantly changed in the samples, indicating the importance of evaluating the differences in release kinetics of these biomarkers in the context of accurate diagnosis. Our findings suggest that circulating cMyBP-C is a sensitive and cardiac-specific biomarker with potential utility for the accurate diagnosis of MI.
Keywords: Acute coronary syndrome, cardiac biomarker, cardiac myosin binding protein-C, contractile protein, cMyBP-C, myocardial infarction
Introduction
Acute coronary syndrome (ACS) is a major cause of morbidity and mortality worldwide, and it incurs huge healthcare expenditures [1-4]. In the United States alone, 683,000 discharge occurrences resulting from ACS were reported in 2009 [5,6]. Remarkably, 1,190,000 secondary discharges were associated with ACS, of which 829,000 were attributed to myocardial infarction (MI) alone [5,6]. ACS is caused by the sudden obstruction of a coronary artery, and the primary symptom is acute chest pain with additional symptoms, such as sweating, nausea, vomiting, and shortness of breath. Early diagnosis and prompt treatment of ACS continues to be a diagnostic challenge in medicine, especially since ACS constitutes a large spectrum of clinical conditions, such as unstable angina (UA, absence of MI), non-ST elevation MI (NSTEMI), and acute ST-elevation MI (STEMI). These three categories are normally classified based on the presence or absence of ST segment elevation on the electrocardiogram and increased plasma levels of myocardial biomarkers. However, blood levels of the current gold standard cardiac biomarkers are very low during the initial stages of MI, making early diagnosis difficult. This results in patients waiting for 6 to 12 hours for accurate clinical differentiation between UA and NSTEMI before the appropriate treatment method can be chosen. Therefore, a robust biomarker able to determine the presence and the severity of MI at the earliest onset (0-6 hours) is urgently needed.
The timing and concentration of currently preferred diagnostic biomarkers, such as circulating cardiac troponin I and T (cTnI, cTnT), depend on local blood flow, infarct-related artery patency, infarct size, individual biomarker localization, molecular weight, and half-life (clearance rate) in the blood. Nonspecific cTnI false-positive results are often reported among hospitalized patients and can easily lead to incorrect prognoses [7]. Recent American College of Cardiology/American Heart Association guidelines report high mortality among patients with MI in the absence of cTnI, indicating that measurement of plasma cTnI levels is not necessarily an accurate evaluation of atherosclerotic coronary artery disease [8-10]. Studies have shown that assessment of heart-type-fatty acid-binding protein (H-FABP) within the first 4 hrs of MI is superior to cTnT [11] and that testing for both H-FABP and cTnI is a reliable tool for the early diagnosis of MI/ACS, as well as a valuable rule-out test for patients presenting at 3 to 6 hours after chest pain onset [12]. Cardiac myosin binding protein-C (cMyBP-C) is a cardiomyocyte specific sarcomeric assembly protein, which regulates both sarcomeric structure and function.
We previously reported that cMyBP-C is a potential biomarker of MI by its dephosphorylation, proteolysis and subsequent release into the circulation post-MI in a rat model and patients with MI [13]. However, since the number of subjects in this human study was limited to fifteen, the results were insufficient to confirm whether cMyBP-C is a bona fide biomarker of MI equal to, or exceeding, the diagnostic performance currently preferred biomarkers, including the gold standard cTnl and cTnT, as noted above.
Therefore, the aim of the present study was to determine the level of cMyBP-C compared to the currently preferred diagnostic biomarkers and define the level of cMyBP-C in patients with MI after Percutaneous Transcoronary Angioplasty (PTCA), compared to the baseline levels in healthy controls. Our findings suggest that circulating cMyBP-C is a sensitive and cardiac-specific biomarker with potential utility for the accurate diagnosis of MI.
Methods
Human samples
Categorization of ACS followed the guidelines of the American Heart Association/American College of Cardiology [8-10]. A total of 65 patients (aged 62 ± 15 years; 52 males and 13 females) were admitted to the ER (Base, MI) with complaint of acute coronary syndrome. Of these patients, a total of 40 underwent Percutaneous Transcoronary Angioplasty. Samples from all 65 patients were measured to determine the level of plasma cMyBP-C, and those subjects undergoing PTCA were measured 12 hours post-procedure. A diagnosis of MI was determined by the presence of STEMI. These samples were previously procured in a subclinical study to determine the inflammatory response to Brachytherapy following PTCA. For negative controls, 54 plasma samples were obtained from volunteers (aged 25 ± 20 years; 32 males and 22 females). The Institutional Review Board at Loyola University Chicago approved the protocol for the use of de-identified human samples previously used for research studies. No other sample information was available to the investigators or technicians in the laboratory.
Sandwich ELISA assay for cMyBP-C
Plasma level of cMyBP-C was determined by sandwich ELISA as described previously [13]. Reproducibility, variability, and detection limits were tested by coating Sigma-Nunc-ImmunoTM MicroWellTM96 MaxiSorpTM polystyrene solid plates (Catalog # 449824, Thermo Scientific Nunc, USA) overnight with capture mouse monoclonal antibody raised against cMyBP-C C0 region (Catalog # sc-137180 Clone E7, Santa Cruz Biotechnology, Santa Cruz, CA). The unbound capture antibody was removed by washing with phosphate buffered saline (PBS)-Tween 20 (0.01%)using an ELISA plate washer (Immuno wash® 1575, Bio-Rad, Hercules, CA). The plate was then blocked using 1x-blocking buffer (Catalog # 11921673001, Roche Diagnostics Corp., Indianapolis, IN 46250) and incubated on an ELISA plate shaker at 200 rpm/min (IKA-Werke, Germany) for 1 hour at room temperature. Subsequently, the plates were washed, as detailed earlier, and a dilution series (recombinant mouse cMyBP-C N’-terminal peptide 40 kDa), ranging from 0.096 ng/ml to 1500 ng/ml, was used to obtain standard curves. The plate was washed as detailed earlier. As detection antibody, rabbit polyclonal cMyBP-C (residues 2-14) at 1:1000 dilution in PBS was used [13]. The plate was then incubated for 1 hour at room temperature, while shaking at 200 rpm/min. The plate was washed again, and HRP-conjugated donkey anti-rabbit IgG antibody (Catalog # sc-2313, Santa Cruz Biotechnology, Santa Cruz, CA) was added at a dilution of 1:2000 in PBS, followed by 30 min incubation, while shaking at 200 rpm/min at room temperature. The plate was washed again, and for signal detection, 1-step 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS, Catalog # 37615, Thermo Scientific, USA) was added. The plate was incubated at 37°C for 5 minutes. The absorbance was measured at 405 nm using the BioTek Epoch microplate reader. The data were analyzed using the Gen5TM 5.1.11 Data Analysis Software (BioTek). Standard curve was fitted with a 4-parameter curve at 1/y2 weighting.
Multiplex assay for existing cardiac biomarkers
A six-plex determination of existing cardiac biomarkers, cTnI, MYO, CK-MB, GPBB, CAIII, and H-FABP, was performed using the Randox biochip array technology [14] on the Evidence Investigator analyser (Catalog # EV3602, Randox Laboratories, Crumlin, United Kingdom). The Cardiac Plus Array kit and Cardiac Plus control were used according to the manufacturer’s instructions (Catalog # EV3511, EV3558, Randox Laboratories Limited, Crumlin, United Kingdom).
Statistical analysis
The data are represented as percent of mean ± standard error of the mean (SEM). Comparisons between groups (Base MI, 12 hrs post-PTCA and control) were made using pairwise multiple comparison procedures (Holm-Sidak method, Sigma Plot, Systat Software, San Jose, CA). A value of P<0.001 was considered statistically significant. One-way ANOVA analysis was used to compare the level of cMyBP-C with other biomarkers. Receiver operating characteristic (ROC) curve was generated by plotting sensitivity versus specificity to assess the possible predictive value of each cMyBP-C value in classifying patients into positive or negative status (GraphPad Prism 5, La Jolla, CA).
Results
Plasma cMyBP-C level is elevated in patients with MI
cMyBP-C is a predominant cardiomyocyte-specific structural protein that regulates cardiac structure and function [15-18]. Importantly, cMyBP-C differs from its skeletal isoforms by having an N’-terminal C0 domain (99 residues) and being exclusively expressed in the heart (Figure 1) [19]. Using the first 14 residues of cMyBP-C-specific C0 domain, we previously generated a rabbit polyclonal (cMyBP-C2-14) antibody for detection in the sandwich ELISA assay [13]. The capture mouse monoclonal antibody was generated against the entire C0 domain (Clone E7, Santa Cruz, CA). Sandwich ELISA was previously developed using both of these antibodies to determine the level of cMyBP-C in either serum or plasma samples [13]. The sandwich ELISA was characterized by comparing actual (spiked) and expected (calculated) values to determine coefficient of variation, recovery percentages, dynamic range and reproducibility of the assay (Table 1).
Figure 1.

Schematic structure of sarcomere showing the localization of cardiac myosin binding protein-C (cMyBP-C). The basic functional unit of cardiac muscle is the sarcomere, which consists of both thin and thick filament proteins. Thick filament proteins are titin, myosin and cMyBP-C, whereas thin filament proteins are actin, α-tropomyosin and troponin complex that includes troponin C, I and T. The increased level of cTnI and cTnT in the blood has been extensively characterized, and both indicators are the current gold standard biomarkers of MI. However, the release of other sarcomeric proteins has not been completely characterized. Particularly, cMyBP-C forms 7-9 transverse stripes at regular intervals of 43 nm in the C-zone of the sarcomeric A-band, which contribute to 2% of total myofibril mass. Unlike cTnI and cTnT, cMyBP-C protein belongs to the intracellular immunoglobulin (Ig) superfamily.
Table 1.
Analytical parameters of sandwich ELISA in quantitating plasma cMyBP-C levels
| Theoretical value of calibrators (ng/ml) | Calculated mean values (ng/ml) | Standard Deviation | Coefficient of Variation (%) | Recovery % |
|---|---|---|---|---|
| 0.096 | 0 | 0 | 0 | 0 |
| 0.48 | 0.58 | 0.167 | 28.6 | 121 |
| 2.4 | 2.15 | 0.36 | 16.8 | 90 |
| 12 | 12.76 | 0.75 | 5.9 | 106 |
| 60 | 57.94 | 2.93 | 5.1 | 97 |
| 300 | 308.7 | 44.4 | 14.4 | 103 |
| 1500 | 1564.2 | 131.7 | 8.4 | 105 |
The detection values are as follows: LLOD (1.41 ± 0.39 ng/ml), LLOQ (2.4 ng/ml), ULOD (1500 ng/ml) and ULOQ (1500 ng/ml).The intra- and interplate variability are 6.2 ± 2.1% and 13.3 ± 4.4%, respectively (triplicates).
Sandwich ELISA data showed that plasma level of cMyBP-C is significantly elevated in MI samples (105.08 ± 8.80 ng/mL, P<0.0001, n=65), compared to control samples (22.27 ± 2.36 ng/mL, n=54, Figure 2A-C). A 4.78-fold increase in plasma cMyBP-C levels was observed in patients with MI, compared to the controls. Samples of these patients were collected at the time of their admission to the ER. Furthermore, at 12 hours post-PTCA, the level of cMyBP-C was significantly decreased in patients who underwent the minimally invasive procedure (41.21 ± 9.30 ng/mL). Although the level was still significantly higher than the controls, the fold change was decreased from 4.78 to 1.88, relative to the base MI. Interestingly, 24 MI patients out of 65 MI patients presented with higher plasma cMyBP-C levels (116.53 ±13.32 ng/mL) with a minimal value of 45.45 ng/mL. In total, sixty-six percent of MI samples had higher cMyBP-C levels than the control group, a datum that is also reflected in the ROC curve (Figure 2D). Importantly, the ROC curve showed that a cMyBP-C plasma level of 68.1 ng/mL is predictive of MI with specificity of 100% and sensitivity of 66.2% (area under curve (AUC) of 0.89).
Figure 2.
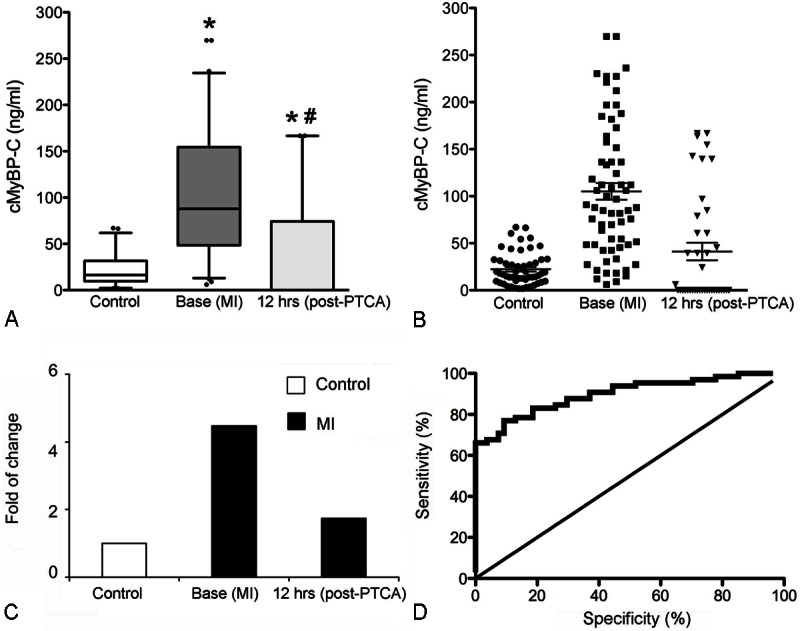
Elevated level of plasma cMyBP-C in patients with MI. Data are shown as box-andwhisker plots of cMyBP-C measured in MI patients prior to PTCA (Base, n=65) and 12 hrs post-PTCA (n=40), in comparison with healthy control samples (n=54, A). *P<0.001 versus control; #P<0.001 versus base. Distribution of plasma cMyBP-C levels in control samples and MI samples (B). cMyBP-C level was measured in control, Base (MI) and 12 hrs post-PTCA (MI groups as shown in panel A, and data are displayed for individual patients. Line and whiskers: 22.27 ± 2.36 ng/ml and 105.08 ± 8.80 ng/ml. Fold change of cMyBP-C level was determined in Base (MI) and 12 hrs post-PTCA (MI) groups, compared to controls (C). ROC curve for plasma cMyBP-C levels comparing MI patients (n=45) and controls (n=45, D). Area under the curve (AUC)=0.89, 95% CI: 0.83 to 0.95. cMyBP-C plasma level of 68.1 ng/ml has 100% specificity and 66.2% sensitivity.
Comparison between cMyBP-C levels and those of other cardiac biomarkers
Next, twenty-four samples that represented base MI with high levels of cMyBP-C (116.53 ± 13.32 ng/mL) were further used for simultaneous determination of plasma levels of cTnI, MYO, CK-MB, GPBB, CAIII, and H-FABP with a biochip-multiplexing assay (Table 2). Quantification of plasma levels of all the cardiac biomarkers in this study is summarized in Table 3. Levels of cMyBP-C, CK-MB, MYO, and GP-BB were all significantly higher in MI patients compared to control samples (P<;0.001). Elevated levels of plasma cTnI, H-FABP, CKMB and GP-BB were observed, but they were not significantly higher than control. Strikingly higher than the controls in these 24 samples, the mean value of cMyBP-C was predominant and robust at 116.53 ± 13.32 ng/mL, as shown in Figure 3 and Table 3. None of these six biomarkers had higher protein concentration in the circulation than cMyBP-C, indicating that increased plasma level of cMyBP-C is a strong determinant for the presence of MI.
Table 2.
Functional sensitivity and limit of detection of other biomarkers with the cardiac biochip array (multiplex) as provided by the manufacturer.The respective cut off values and release kinetics are also indicated
| Calibrators | LLOD (ng/ml) | ULOD (ng/ml) | Cut off value Normal (ng/l) | Cut off valueMI (ng/ml) | Onset (hrs) | Peak (hrs) | Duration (hrs) | Reference |
|---|---|---|---|---|---|---|---|---|
| GP-BB | 1.97 | 200 | 2-4 | 8-12 | 24-36 | [40,41] | ||
| CAIII | 0.2 | 200 | Skeletal Muscle Only | |||||
| MYO | 1.8 | 700 | 6-85 | 450 | 1-4 | 6-7 | 18-24 | [42,43] |
| CK-MB | 0.4 | 100 | 3-4 | 12 | 3-12 | 14-16 | 24-36 | [43-45] |
| H-FABP | 0.15 | 100 | 0 | 5 | 1-3 | 6 | 18-30 | [43,45,46] |
| cTnI | 0.18 | 50 | 0 | 5 | 3-12 | 18-24 | 216 | [43,47] |
Carbonic anhydrase III (CAIII) is expressed exclusively in skeletal muscle and restricted to skeletal muscle injury in association with MI [48].
Table 3.
Comparison of plasma cMyBP-C levels with other biomarkers of MI. The values are expressed as mean values ± SEM values (ng/ml). Data show that plasma levels of cMyBP-C in patients with MI at base are significantly higher, compared to controls, for the selected 24 samples presenting high levels of cMyBP-C (>45.45 ng/mL)
| cMyBP-C (ng/ml) | cTnI (ng/ml) | CK-MB (ng/ml) | MYO (ng/ml) | GP-BB (ng/ml) | H-FABP (ng/ml) | CAIII (ng/ml) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Control | Base (MI) | Control | Base (MI) | Control | Base (MI) | Control | Base (MI) | Control | Base (MI) | Control | Base (MI) | Control | Base (MI) | |
| Mean (ng/ml) | 18.51 ± 2.10 | 116.53 ± 13.32 | 0.19 ± 0.02 | 0.22 ± 0.01 | 0.66 ± 0.08 | 2.10 ± 0.4 | 6.09 ± 1.64 | 40.78 ± 5.13 | 6.11 ± 1.38 | 10.17 ± 1.46 | 1.97 ± 0.9 | 3.13 ± 0.46 | 4.40 ± 1.43 | 26.28 ± 4.61 |
| Fold change | 6.3 | 1.15 | 3.18 | 6.69 | 1.66 | 1.58 | 5.97 | |||||||
| P-value | <0.001 | 0.14 | 0.00 | <0.001 | 0.05 | 0.25 | <0.001 | |||||||
| Min (ng/ml) | 1.81 | 45.45 | 0.11 | 0.18 | 0.4 | 0.4 | 0.4 | 14.81 | 2.00 | 2.00 | 0.15 | 1.12 | 0.20 | 9.35 |
| Max (ng/ml) | 38.04 | 236.36 | 0.36 | 0.31 | 1.61 | 9.90 | 39.8 | 60.96 | 20.91 | 20.54 | 17.62 | 11.91 | 34.89 | 86.07 |
| n | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 |
Figure 3.

Comparison of plasma cMyBP-C levels with existing cardiac biomarkers. Randox Cardiac Plus Biochip Multiplex Assay was used to determine the level of cardiac biomarkers, including cTnI (A), H-FABP (B), CAIII (C) GPBB (D), MYO (E), CK-MB (F), in patients with MI (Base, MI) at the time of admission to the ER, compared to controls. On the same set of samples, cMyBP-C level was determined by sandwich ELISA (G). Box-and-whisker plots were used to demonstrate the deviation and distribution. *P><;0.001, Control (24) vs. MI base samples (24). The data values are summarized in Table 3.
Discussion
cMyBP-C is a new and promising cardiac-specific marker for the early detection of MI [20-23]. It is a thick filament assembly protein in the sarcomere, which interacts with titin, myosin and actin to regulate the structure and function of the heart. Phosphorylation of cMyBP-C at Ser-273, Ser-282, and Ser-302 regulates myocardial function and confers resistance to proteolysis, preserving cardiac function post-MI [24-28]. In contrast, dephosphorylation at Ser-273 and Ser282 facilitates cMyBP-C degradation and the release of a 40 kDa N’-terminal fragment, which is toxic to cardiomyocytes and significantly impairs contractility and Ca2+ handling via inhibition of actomyosin function [19]. In fact, our previous studies have demonstrated that cMyBP-C is easily soluble and releasable from the sarcomere [21]. Moreover, the large size of cMyBP-C and its early release into the circulation in response to ischemic injury make it an ideal biomarker candidate for early detection of MI [29]. The present study, moreover, confirmed that cMyBP-C could act as a robust biomarker for MI in patients with ACS. In addition, the presence of N’terminal fragments of cMyBP-C in myocardial injuries can contribute to altered protein-protein interaction, impaired Ca2+ handling, and contractile dysfunction [19]. Regulation at these phosphorylation sites may play an important role in the degradation and release kinetics of cMyBP-C, but further research is necessary to determine whether this fully explains the mechanism underlying cMyBP-C release into the circulation post-MI. We recently determined that after ligation of the coronary artery in adult swine, the levels of cMyBP-C were elevated in the circulation within 30 minutes post MI [30]. Importantly, we also determined that plasma cMyBP-C levels were significantly raised within 30 minutes in humans with hypertrophic cardiomyopathy undergoing transcoronary ablation of septal hypertrophy [30], suggesting that cMyBP-C is an ultraearly biomarker of MI.
When the plasma levels of cMyBP-C from patients were compared with the levels of other existing cardiac biomarkers of MI, such as cTnI, MYO, CK-MB, GP-BB, H-FABP, and CAIII, the increase of cMyBP-C was comparable or higher. Among the 65 MI patients studied, plasma levels of cMyBP-C declined 12 hours post-PTCA, indicating that cMyBP-C may be more useful than the gold standard biomarker cTnI, which is normally detected 6-12 hours after onset of MI and persists in the blood for more than 2 weeks. Diagnostic testing for MI under ER conditions (within 3-6 hours of onset) may benefit from combining several biomarkers to realize a synergistically more sensitive and specific “multimarker” regimen for the early and accurate detection of acute MI [31-34]. CK-MB and the troponins do not show significantly increased sensitivity, but combinations of MYO with CK-MB [32,33,35] or troponins [32,33] were promising. In a study of 6,352 chest pain patients, with 814 patients suffering from acute MI, MYO, CK-MB, or combination of these two were sensitive for acute MI only 64%, 52%, and 72%, respectively, with specificities of 90%, 96%, and 88% [36]. CK-MB, MYO, cTnI, and cTnT plasma markers all appear within 3-6 hours post-MI [37]. Studies have shown that assessment of heart-type-fatty acid-binding protein (H-FABP) within the first 4 hrs of MI is superior to cTnT [11], and testing with both H-FABP and cTnI is a reliable tool for the early diagnosis of MI/ACS and a valuable rule-out test for patients presenting at 3 to 6 hours after chest pain onset [12].
However, to increase the specificity of such “multimarker” regimens, it is advantageous to add a biomarker shown to be a comparatively robust, specific and sensitive determinant of MI. Furthermore, to reduce potential false-positive analytic results, the lowest cutoff of sensitivity and specificity of troponin assays should be set above the 99th percentile, with 10% coefficient of variation. This requirement can be attributed to the lack of precision in low concentration ranges of the biomarker [38]. Thus, very low cTnI levels in multiplexing may help exclude MI diagnoses, but cross validation with a more sensitive biomarker, such as cMyBP-C, will support a definitive diagnosis of MI based on the higher presence of detectable cMyBP-C in plasma. Both MI and control sample sizes were small in this study and a systematic timepoint study is required to more precisely characterize the release kinetics of cMyBP-C post-MI. Nonetheless, the data derived from this study have sufficiently demonstrated that cMyBP-C is a sensitive and early cardiac-specific biomarker of MI, compared to the currently preferred diagnostic biomarkers, and may prove to be an extremely useful tool in providing rapid and accurate point-of-care diagnoses and treatments for ACS patients [29].
In summary, we have demonstrated that the robust release of cMyBP-C is useful as a sensitive, cardiac-specific biomarker of MI. In a study of MI patients with ACS, data confirmed that plasma cMyBP-C levels were significantly higher than those of cTnl, suggesting that cMyBP-C could be a more effective biomarker by its consistently increased titers over those of the gold standard marker cTnl. It was also suggested that the inclusion of cMyBP-C as a robust component of a “multimaker” test regimen could lead to more accurate and timely, i.e., within 3 to 6 hours, diagnosis of MI patients with ACS [30]. Protein release time-course experiments and exact cutoff values in healthy normal versus patients with MI will be required to ascertain whether cMyBP-C can provide greater sensitivity during earlier time points after MI, which is an ongoing project in our group [29,30].
Acknowledgment
These studies were supported by National Institutes of Health grants R01HL105826 and K02HL114749 to Dr. Sadayappan and American Heart Association Midwest Postdoctoral Fellowship 13POST14720024 (Dr. Govindan). We are also thankful to Eric D. Grassman, Rohit Sundrani, and Matt Hutchins for their skills in procuring the plasma samples from the patients included in this study. We also thank María Luz Rodríguez and Mary Jo Kurth, Randox Laboratories Limited, Crumlin, United Kingdom, for support in the preparation of this manuscript.
Authors' note
A poster presentation of this study was given before the American College of Cardiology annual meeting, March 25, 2012, Chicago, IL, USA [39].
Abbreviations
- ABTS
1-step 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)
- ACS
acute coronary syndrome
- AUC
area under the curve
- α-TM
α-tropomyosin
- cTnI
cardiac troponin I
- cTnT
cardiac troponin T
- CAIII
carbonic anhydrase III
- CK-MB
creatine kinase-MB
- cMyBP-C
cardiac myosin binding protein-C
- ELISA
enzyme-linked immunosorbent assay
- GP-BB
glycogen phosphorylase BB
- H-FABP
heart-type fatty acid-binding protein
- IR
ischemia-reperfusion
- LOD
limit of detection
- LLOD
lower limit of detection
- LLOQ
lower limit of quantitation
- NSTEMI
non-ST elevation MI
- ng/ml
nanogram/milliliter
- MYO
myoglobin
- MI
myocardial infarction
- PBS
phosphate buffered saline
- PTCA
Percutaneous Transcoronary Angioplasty
- ROC
Receiver Operating Characteristic
- SEM
standard error of mean
- STEMI
ST-elevation MI
- TASH
transcoronary ablation of septal hypertrophy
- UA
unstable angina
- ULOD
upper limit of detection
- ULOQ
upper limit of quantitation
Disclosure of conflict of interest
A full patent application is pending (Application Serial No. 13/464,466, Pub. No. US 2012/0282618 A1 and Date: 05/04/12) to determine the risk factors associated with cMyBP-C degradation and release into human body fluid.
References
- 1.Turpie AG. Burden of disease: medical and economic impact of acute coronary syndromes. Am J Manag Care. 2006;12:S430–434. [PubMed] [Google Scholar]
- 2.Torpy JM, Burke AE, Glass RM. JAMA patient page. Acute coronary syndromes. JAMA. 2010;303:90. doi: 10.1001/jama.303.1.90. [DOI] [PubMed] [Google Scholar]
- 3.Arbab-Zadeh A, Nakano M, Virmani R, Fuster V. Acute coronary events. Circulation. 2012;125:1147–1156. doi: 10.1161/CIRCULATIONAHA.111.047431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kehl DW, Iqbal N, Fard A, Kipper BA, De La Parra Landa A, Maisel AS. Biomarkers in acute myocardial injury. Transl Res. 2012;159:252–264. doi: 10.1016/j.trsl.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alcalai R, Planer D, Culhaoglu A, Osman A, Pollak A, Lotan C. Acute coronary syndrome vs nonspecific troponin elevation: clinical predictors and survival analysis. Arch Intern Med. 2007;167:276–281. doi: 10.1001/archinte.167.3.276. [DOI] [PubMed] [Google Scholar]
- 8.Wright RS, Anderson JL, Adams CD, Bridges CR, Casey DE Jr, Ettinger SM, Fesmire FM, Ganiats TG, Jneid H, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP, Antman EM, Califf RM, Chavey WE 2nd, Hochman JS, Levin TN. 2011 ACCF/AHA focused update of the Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction (updating the 2007 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American College of Emergency Physicians, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;57:1920–1959. doi: 10.1016/j.jacc.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Wright RS, Anderson JL, Adams CD, Bridges CR, Casey DE Jr, Ettinger SM, Fesmire FM, Ganiats TG, Jneid H, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP, Jacobs AK. 2011 ACCF/AHA Focused Update of the Guidelines for the Management of Patients With Unstable Angina/ Non-ST-Elevation Myocardial Infarction (Updating the 2007 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:2022–2060. doi: 10.1161/CIR.0b013e31820f2f3e. [DOI] [PubMed] [Google Scholar]
- 10.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, Chavey WE 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC Jr. 2011 ACCF/AHA Focused Update Incorporated Into the ACC/ AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:e426–579. doi: 10.1161/CIR.0b013e318212bb8b. [DOI] [PubMed] [Google Scholar]
- 11.McCann CJ, Glover BM, Menown IB, Moore MJ, McEneny J, Owens CG, Smith B, Sharpe PC, Young IS, Adgey JA. Novel biomarkers in early diagnosis of acute myocardial infarction compared with cardiac troponin T. Eur Heart J. 2008;29:2843–2850. doi: 10.1093/eurheartj/ehn363. [DOI] [PubMed] [Google Scholar]
- 12.McMahon CG, Lamont JV, Curtin E, McConnell RI, Crockard M, Kurth MJ, Crean P, Fitzgerald SP. Diagnostic accuracy of heart-type fatty acid-binding protein for the early diagnosis of acute myocardial infarction. Am J Emerg Med. 2012;30:267–274. doi: 10.1016/j.ajem.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 13.Govindan S, McElligott A, Muthusamy S, Nair N, Barefield D, Martin JL, Gongora E, Greis KD, Luther PK, Winegrad S, Henderson KK, Sadayappan S. Cardiac myosin binding protein-C is a potential diagnostic biomarker for myocardial infarction. J Mol Cell Cardiol. 2012;52:154–164. doi: 10.1016/j.yjmcc.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitzgerald SP, Lamont JV, McConnell RI, Benchikh el O. Development of a high-throughput automated analyzer using biochip array technology. Clin Chem. 2005;51:1165–1176. doi: 10.1373/clinchem.2005.049429. [DOI] [PubMed] [Google Scholar]
- 15.Barefield D, Sadayappan S. Phosphorylation and function of cardiac myosin binding protein-C in health and disease. J Mol Cell Cardiol. 2010;48:866–875. doi: 10.1016/j.yjmcc.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhandapany PS, Sadayappan S, Xue Y, Powell GT, Rani DS, Nallari P, Rai TS, Khullar M, Soares P, Bahl A, Tharkan JM, Vaideeswar P, Rathinavel A, Narasimhan C, Ayapati DR, Ayub Q, Mehdi SQ, Oppenheimer S, Richards MB, Price AL, Patterson N, Reich D, Singh L, Tyler-Smith C, Thangaraj K. A common MYBPC3 (cardiac myosin binding protein C) variant associated with cardiomyopathies in South Asia. Nat Genet. 2009;41:187–191. doi: 10.1038/ng.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris SP, Lyons RG, Bezold KL. In the thick of it: HCM-causing mutations in myosin binding proteins of the thick filament. Circ Res. 2011;108:751–764. doi: 10.1161/CIRCRESAHA.110.231670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Previs MJ, Beck Previs S, Gulick J, Robbins J, Warshaw DM. Molecular mechanics of cardiac myosin-binding protein C in native thick filaments. Science. 2012;337:1215–1218. doi: 10.1126/science.1223602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Govindan S, Sarkey J, Ji X, Sundaresan NR, Gupta MP, de Tombe PP, Sadayappan S. Pathogenic properties of the N-terminal region of cardiac myosin binding protein-C in vitro. J Muscle Res Cell Motil. 2012;33:17–30. doi: 10.1007/s10974-012-9292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker JO, Devaraj R, Reinhold J, Kanaganayagam GS, Sadayappan S, Gautel M, Redwood C, Marber M. Cardiac myosin binding protein-C (cMyBP-C) as a potential new serum biomarker of myocardial infarction. Circulation. 2010;122:A15438. [Google Scholar]
- 21.Govindan S, McElligott A, Muthusamy S, Nair N, Barefield D, Martin JL, Gongora E, Greis KD, Luther PK, Winegrad S, Henderson KK, Sadayappan S. Cardiac myosin binding protein-C is a potential diagnostic biomarker for myocardial infarction. J Mol Cell Cardiol. 2012;52:154–164. doi: 10.1016/j.yjmcc.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Govindan S, Kahn DJ, Grassman ED, Sundrani R, Jeske WP, Hutchins M, Walenga JM, Leya F, Hoppensteadt D, Fareed J, Sadayappan S. Cardiac myosin binding protein-C: a new biomarker in patients with acute coronary syndrome. J Am Coll Cardiol. 2012;59:E404. [Google Scholar]
- 23.Jacquet S, Yin X, Sicard P, Clark J, Kanaganayagam GS, Mayr M, Marber MS. Identification of cardiac myosin-binding protein C as a candidate biomarker of myocardial infarction by proteomics analysis. Mol Cell Proteomics. 2009;8:2687–2699. doi: 10.1074/mcp.M900176-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bardswell SC, Cuello F, Rowland AJ, Sadayappan S, Robbins J, Gautel M, Walker JW, Kentish JC, Avkiran M. Distinct sarcomeric substrates are responsible for protein kinase D-mediated regulation of cardiac myofilament Ca2+ sensitivity and cross-bridge cycling. J Biol Chem. 2010;285:5674–5682. doi: 10.1074/jbc.M109.066456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Decker RS, Decker ML, Kulikovskaya I, Nakamura S, Lee DC, Harris K, Klocke FJ, Winegrad S. Myosin-binding protein C phosphorylation, myofibril structure, and contractile function during low-flow ischemia. Circulation. 2005;111:906–912. doi: 10.1161/01.CIR.0000155609.95618.75. [DOI] [PubMed] [Google Scholar]
- 26.Sadayappan S, Gulick J, Klevitsky R, Lorenz JN, Sargent M, Molkentin JD, Robbins J. Cardiac myosin binding protein-C phosphorylation in a {beta}-myosin heavy chain background. Circulation. 2009;119:1253–1262. doi: 10.1161/CIRCULATIONAHA.108.798983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadayappan S, Gulick J, Osinska H, Martin LA, Hahn HS, Dorn GW 2nd, Klevitsky R, Seidman CE, Seidman JG, Robbins J. Cardiac myosin- binding protein-C phosphorylation and cardiac function. Circ Res. 2005;97:1156–1163. doi: 10.1161/01.RES.0000190605.79013.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadayappan S, Osinska H, Klevitsky R, Lorenz JN, Sargent M, Molkentin JD, Seidman CE, Seidman JG, Robbins J. Cardiac myosin binding protein C phosphorylation is cardioprotective. Proc Natl Acad Sci U S A. 2006;103:16918–16923. doi: 10.1073/pnas.0607069103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadayappan S. Cardiac myosin binding protein-C: a potential early-stage, cardiac-specific biomarker of ischemia-reperfusion injury. Biomark Med. 2012;6:69–72. doi: 10.2217/bmm.11.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuster DW, A CO, Miller L, Troidl C, Nef HM, Liebetrau D, Helge M, Pieper KS, Mahaffey KW, Kleiman NS, Stuyvers BD, Marian AJ, Sadayappan S. Cardiac Myosin Binding Protein C is an Ultra-early and Cardiac-specific Biomarker of Myocardial Necrosis. Circulation Research. 2012;111:e380. [Google Scholar]
- 31.Jurlander B, Clemmensen P, Wagner GS, Grande P. Very early diagnosis and risk stratification of patients admitted with suspected acute myocardial infarction by the combined evaluation of a single serum value of cardiac troponin-T, myoglobin, and creatine kinase MB (mass) Eur Heart J. 2000;21:382–389. doi: 10.1053/euhj.1999.1760. [DOI] [PubMed] [Google Scholar]
- 32.Stork TV, Wu AH, Muller-Bardorff M, Gareis R, Muller R, Hombach V, Katus H, Mockel M North-Wurttemberg Infarction Study (NOWIS) Group. Diagnostic and prognostic role of myoglobin in patients with suspected acute coronary syndrome. North-Wurttemberg Infarction Study (NOWIS) Group. Am J Cardiol. 2000;86:1371–1374. A5. doi: 10.1016/s0002-9149(00)01246-7. [DOI] [PubMed] [Google Scholar]
- 33.McCord J, Nowak RM, McCullough PA, Foreback C, Borzak S, Tokarski G, Tomlanovich MC, Jacobsen G, Weaver WD. Ninety-minute exclusion of acute myocardial infarction by use of quantitative point-of-care testing of myoglobin and troponin I. Circulation. 2001;104:1483–1488. doi: 10.1161/hc3801.096336. [DOI] [PubMed] [Google Scholar]
- 34.Apple FS, Christenson RH, Valdes R Jr, Andriak AJ, Berg A, Duh SH, Feng YJ, Jortani SA, Johnson NA, Koplen B, Mascotti K, Wu AH. Simultaneous rapid measurement of whole blood myoglobin, creatine kinase MB, and cardiac troponin I by the triage cardiac panel for detection of myocardial infarction. Clin Chem. 1999;45:199–205. [PubMed] [Google Scholar]
- 35.Lindahl B, Venge P, Wallentin L. Early diagnosis and exclusion of acute myocardial infarction using biochemical monitoring. The BIOMACS Study Group. Biochemicals Markers of Acute Coronary Syndromes. Coron Artery Dis. 1995;6:321–328. doi: 10.1097/00019501-199504000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Gibler WB, Hoekstra JW, Weaver WD, Krucoff MW, Hallstrom AP, Jackson RE, Sayre MR, Christenson J, Higgins GL, Innes G, Harper RJ, Young GP, Every NR. A randomized trial of the effects of early cardiac serum marker availability on reperfusion therapy in patients with acute myocardial infarction: the serial markers, acute myocardial infarction and rapid treatment trial (SMARTT) J Am Coll Cardiol. 2000;36:1500–1506. doi: 10.1016/s0735-1097(00)00897-4. [DOI] [PubMed] [Google Scholar]
- 37.Fesmire FM, Decker WW, Diercks DB, Ghaemmaghami CA, Nazarian D, Brady WJ, Hahn S, Jagoda AS. Clinical policy: critical issues in the evaluation and management of adult patients with non-ST-segment elevation acute coronary syndromes. Ann Emerg Med. 2006;48:270–301. doi: 10.1016/j.annemergmed.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Apple FS, Wu AH, Jaffe AS. European Society of Cardiology and American College of Cardiology guidelines for redefinition of myocardial infarction: how to use existing assays clinically and for clinical trials. Am Heart J. 2002;144:981–986. doi: 10.1067/mhj.2002.124048. [DOI] [PubMed] [Google Scholar]
- 39.Govindan S, Kahn D, Sundrani R, Jeske WP, Walenga J, Leya F, Hoppensteadt D, Fareed J, Sadayappan S. Cardiac myosin binding protein-C: a new biomarker in patients with acute coronary syndrome. J Am Coll Cardiol. 2012;59:E404. [Google Scholar]
- 40.Jordanova N, Gyongyosi M, Khorsand A, Falkensammer C, Zorn G, Wojta J, Anvari A, Huber K. New cut-off values of cardiac markers for risk stratification of angina pectoris. Int J Cardiol. 2005;99:429–435. doi: 10.1016/j.ijcard.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Kemp M, Donovan J, Higham H, Hooper J. Biochemical markers of myocardial injury. Br J Anaesth. 2004;93:63–73. doi: 10.1093/bja/aeh148. [DOI] [PubMed] [Google Scholar]
- 42.Stone MJ, Waterman MR, Harimoto D, Murray G, Willson N, Platt MR, Blomqvist G, Willer-son JT. Serum myoglobin level as diagnostic test in patients with acute myocardial infarction. Br Heart J. 1977;39:375–380. doi: 10.1136/hrt.39.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Azzazy HM, Pelsers MM, Christenson RH. Unbound free fatty acids and heart-type fatty acid-binding protein: diagnostic assays and clinical applications. Clin Chem. 2006;52:19–29. doi: 10.1373/clinchem.2005.056143. [DOI] [PubMed] [Google Scholar]
- 44.Kratz A, Ferraro M, Sluss PM, Lewandrowski KB. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Laboratory reference values. N Engl J Med. 2004;351:1548–1563. doi: 10.1056/NEJMcpc049016. [DOI] [PubMed] [Google Scholar]
- 45.Alhadi HA, Fox KA. Heart-Type Fatty Acid-Binding Protein in the Early Diagnosis of Acute Myocardial Infarction: The potential for influencing patient management. Sultan Qaboos Univ Med J. 2010;10:41–49. [PMC free article] [PubMed] [Google Scholar]
- 46.Kumagai K, Ohnaka H, Okamoto F, Yasuda M, Kamegai H, Ohmichi M. Early diagnosis of postpartum acute myocardial infarction with combined use of troponin T and heart-type fatty acid-binding protein rapid assay. J Obstet Gynaecol Res. 2011;37:1484–1488. doi: 10.1111/j.1447-0756.2011.01535.x. [DOI] [PubMed] [Google Scholar]
- 47.Lurati Buse GA, Koller MT, Grapow M, Bolliger D, Seeberger M, Filipovic M. The prognostic value of troponin release after adult cardiac surgery - a meta-analysis. Eur J Cardiothorac Surg. 2010;37:399–406. doi: 10.1016/j.ejcts.2009.05.054. [DOI] [PubMed] [Google Scholar]
- 48.Kato K, Mokuno K. Distribution of immunoreactive carbonic anhydrase III in various human tissues determined by a sensitive enzyme immunoassay method. Clin Chim Acta. 1984;141:169–177. doi: 10.1016/0009-8981(84)90008-1. [DOI] [PubMed] [Google Scholar]


