Abstract
Context
Alternatives to sedative medications are needed to reduce anxiety in mechanically ventilated patients. Music is an integrative therapy without adverse effects that may alleviate the anxiety associated with ventilatory support.
Objective
To test whether patient-directed, self-initiated music listening can reduce anxiety and sedative exposure during ventilatory support in critically ill patients as compared with 2 control conditions.
Design, Setting, and Patients
Randomized, controlled trial that enrolled 373 ICU patients from the Minneapolis-St. Paul area receiving acute mechanical-ventilatory support for respiratory failure between September 2006 and March 2011. Patients were Caucasian (86%), female (52%), with mean age 59 (SD 14), APACHE III 63 (SD 21.6), on protocol 5.7 (SD 6.4) days.
Intervention
Patients (1) self-initiated music listening (patient-directed music; PDM) with preferred selections tailored by a music therapist whenever desired while receiving ventilatory support, (2) self-initiated use of noise-abating headphones (HP), or (3) received usual ICU care (UC).
Main Outcome Measures
Daily assessments of anxiety (100-mm visual analog scale) and two aggregate measure of sedative exposure (sedation intensity and sedation frequency).
Results
Mixed-models analysis showed that PDM patients had decreased levels of anxiety compared with the UC group of −19.5 (p=.003). By the fifth study day anxiety was reduced by 36.5% in PDM patients. The interaction between treatment and time showed PDM significantly reduced both measures of sedative exposure. PDM reduced sedation intensity by −.18 (−.36, −.004) points per day and frequency by −.21 (−.37, −.05) points per day compared to UC (p = .05, .01 respectively). PDM reduced sedation frequency by −.18 (−.36, −.004) points per day compared to HP (p = .04). By the fifth study day, PDM patients received two fewer sedative doses (reduction of 38%) and had a reduction of 36% in sedation intensity. There was no significant decline in sedation intensity compared to HP (p = .32).
Conclusions
Among ICU patients receiving acute ventilatory support for respiratory failure, self-initiated music resulted in greater reduction in anxiety compared with usual care. Concurrently, PDM resulted in greater reduction in sedation frequency compared with usual care or self-initiated noise-abating headphones, and greater reduction in sedation intensity compared with usual care. Combining a modest reduction in sedative exposure while maintaining a lower level of anxiety, PDM is an ideal non-pharmacological intervention to improve patients' tolerance of mechanical ventilation.
Critically ill mechanically ventilated patients (MVPs) receive intravenous sedative and analgesic medications to reduce anxiety and promote comfort and ventilator synchrony. These potent medications are often administered at high doses for prolonged periods and are associated with adverse effects such as bradycardia, hypotension, gut dysmotility, immobility, weakness, and delirium.1–3 Despite protocols and sedation-assessment tools that guide clinicians, patients still experience significant levels of anxiety.4,5 Unrelieved anxiety and fear are not only unpleasant symptoms that clinicians want to palliate, but increased sympathetic nervous system (SNS) activity can cause dyspnea and increased myocardial oxygen demand.6 Sustained anxiety and SNS activation can decrease the ability to concentrate, rest, or relax.6,7 Mechanically ventilated patients have little control over pharmacological interventions to relieve anxiety; dosing and frequency of sedative and analgesic medications are controlled by ICU clinicians. Interventions are needed that reduce anxiety, actively involve patients, and minimize the use of sedative medications.
Non-pharmacological interventions such as relaxing music are effective in reducing anxiety while reducing medication administration.8,9 Music is a powerful distractor that can alter perceived levels of anxiety10 by occupying attention channels in the brain with meaningful, auditory stimuli11 rather than stressful environmental stimuli. Listening to preferred, relaxing music reduces anxiety in MVPs in limited trials.12–15 It is not known if music can reduce anxiety throughout the course of ventilatory support, or reduce exposure to sedative medications. We evaluated if a patient-directed music intervention could reduce anxiety and sedative exposure in ICU patients receiving mechanical ventilation.
METHODS
Design
A 3-group, randomized controlled design was used. A computer-generated random numbers list allocated patients to 1 of 3 groups by each of the 5 participating hospitals: 1) patient-directed music intervention (PDM); 2) active control with noise-abating headphones only (HP); or 3) usual ICU care (UC). Group assignment was concealed in an opaque envelope.
Setting
Patients were enrolled from 12 ICUs in 5 hospitals in the Minneapolis-St. Paul area from September 2006–March 2011.
Sample
Patients were invited to participate in the study if they were: receiving ventilatory support for acute respiratory failure; alert; participating in their daily care routines; appropriately following commands; cognitively intact to participate in the consent process; and had adequate/corrected vision and hearing. Patients were not approached if they were: receiving aggressive ventilatory support; requiring vasopressors; unresponsive or delirious; receiving chronic ventilator support prior to hospitalization, or had a documented mental incompetence (e.g., Alzheimer's disease).
The target sample size of 286 was based on power-analysis calculations that required 48 hours or more of protocol data and allowed for 20% attrition. Other parameters were alpha = .05 and power = 80%, based on a repeated measures ANCOVA, which provides a good approximation for mixed models. In prior studies, the visual analog scale-anxiety (VAS-A; 0–100 mm scale) had a mean of 50.5 with SD of 29.2.16 A difference of 15.2 or greater would be detected as a statistically significant difference between groups. For the sedative-exposure aim, previous data gave a mean estimate of 6.5 with SD of 4.3.17 Using the sample size determined for the VAS-A, any difference of 1.8 or greater in the sedation intensity would be detected as a statistically significant difference between groups.
Study approval was obtained from the University of Minnesota's Institutional Review Board (IRB) and from the participating hospitals' IRBs. Given the patient-directed nature of the protocol, the IRB required patients to provide their own written informed consent. To validate patient understanding of the study's risks, benefits, and procedures, the patient had to answer 7 “yes or no” questions correctly to the research nurses. If any one of the questions was answered incorrectly, that patient was not enrolled that day but remained eligible to be re-approached if mental status improved and inclusion criteria were still met. Trained research nurses obtained all written consents.
Measures and Instrumentation
Demographic and Study Entry Data
Data were obtained on gender, age, days mechanically ventilated and ICU days prior to enrollment, diagnoses, ventilator settings, and all medications received 24 hours prior to enrollment. Data from ICU admission day were abstracted from the medical record to calculate the Acute Physiology, Age and Chronic Health Evaluation (APACHE) III score, which was used as a covariate to control for illness severity.
Anxiety
Anxiety was defined as a state marked by apprehension, agitation, increased motor activity, arousal, and fearful withdrawal.18 Anxiety was assessed via self-report at study entry and daily while ventilated using the100-mm VAS-A which was presented to patients with a vertical orientation like a thermometer. The scale's bottom was anchored by the statement “not anxious at all” and the top was anchored by “most anxious ever”. Patients indicated their current level of anxiety in response to “how are you feeling today?” The VAS-A score was the number of millimeters from the bottom edge of the line anchor to the subject's mark. The VAS-A and the Spielberger state anxiety inventory are correlated r = .4916 to .82,19 demonstrating concurrent validity.
Sedative Exposure
Sedative exposure was determined for all patients who received any of 8 commonly administered sedative and analgesic medications in the ICU (midazolam, lorazepam, propofol, dexmedetomidine, morphine, fentanyl, hydromorphone, haloperidol) 24 hours prior to enrollment and each day on protocol. Sedative exposure was operationalized as a daily sedative drug intensity score and sedative-dose frequency.17 The usual practice on the participating ICUs consisted of physicians writing orders for sedation therapy per their individual preferences with the nurses managing administration of these medications within the parameters of the orders. Sedative administration was not directed by a specific unit protocol orby a study protocol.
The sedative drug intensity score aggregated dose amounts of medications from disparate drug classes by using a weight-adjusted dose (adjusting for differing patient weights) of each sedative administered during 4-hour time blocks during mechanical ventilation. Every drug amount, for instance, 2 mg of lorazepam administered between noon and 4 pm, was then placed into quartiles created by using all patients' lorazepam data during their entire time on the study protocol; 2 mg of lorazepam might fall into quartile 2. If fentanyl was also given at a dose that fell into quartile 3 for all fentanyl doses within the entire sample, then the noon-4 pm value was 5 (2+3). If none of the 8 medications were given, the value was 0. The values were summed over the 6 4-hour blocks to produce a daily sedative drug intensity score. For dose frequency, a 24-hour day was divided into 6 4-hour time blocks and, for each of the 8 drugs, the occurrences in which a sedative was administered at least once during that interval were summed. This approach to sedative exposure accounts for medications administered to patients from non-equivalent, disparate drug classes.17
Environmental Scan. The Environmental Scan form was developed for this study to collect data on the overall activity level in the patient's room each shift and on ICU nursing experience. Nurses were invited to provide any comments about the study protocol. This paper and pencil form was adhered to a brightly colored clip-board kept at each participant's bed side.
Protocol
Experimental PDM Group
A starter set of 6 compact discs (CDs) were reviewed with the patient by the research nurse to provide for immediate listening upon randomization to PDM. The starter-set included relaxing music played on piano, harp, guitar, and Native American flute. The research nurse oriented the patient to CD player and headphone operation. A standard CD/MP3 player with comfortable, noise-abating headphones was kept within easy reach to allow the patient to self-initiate music listening.
Within 24 hours of randomization, the music therapist completed a music preference assessment on each PDM patient using a tool designed to assess music preferences of MVPs with a simple yes/no format.20 Patients were prompted verbally and with posted signs to use music at least twice a day when feeling anxious and/or to provide relaxation, but were encouraged to self-initiate music listening as frequently as desired. Nursing staff were encouraged to offer music at least twice during their shift, but were reminded by the research staff that the decision to listen was determined by the patient. A data-logger system on the headphones captured each PDM session and total daily music-listening time; system details are described elsewhere.21
Control Groups
Patients randomized to the active control of noise-abating headphones (HP) group were encouraged to wear headphones whenever they wanted to block out ICU noise or have some quiet time. As with the PDM group, HP patients self-initiated headphone use. Patients randomized to the usual-care (UC) control group received standard ICU care for that respective unit.
Patients had daily assessment visits by a research nurse who administered the VAS-A. Patients remained on protocol up to 30 days as long as they were receiving ventilatory support.
Analysis
Descriptive statistics and graphing were performed on all study data to assess the variables' distributions. We used bivariate associations to identify covariates to be considered in subsequent analyses. Covariates were not included to assess their effect per se or adjust for imbalance among groups, but were included if significantly associated with the outcome to subtract the variability piece they represent and thus gain efficiency.
Patients with at least 2 days of VAS-A and sedative-exposure data were used in the change over time analyses. Change over time was assessed as the slope of the outcome variables determined from one day to the next by the best fitting line. We used mixed-effects models to analyze anxiety and sedative exposure (sedation intensity scores and sedation frequency) as they accommodate measures that are correlated from one time point to another and have variances that are not constant from one time point to another, which would be expected in a repeated measures analysis of covariance (ANCOVA). This is the recommended modeling for intent-to-treat analyses.22 Using the data as is within a mixed model analysis has a lower type I error and higher power than any type of imputation method used for missing data, which would be needed for a repeated measures ANCOVA. Also, imputation may result in biased estimates of effects and standard errors. A series of models were estimated and compared with Aikake's information criterion (AIC) and the Bayesian information criterion (BIC) to determine the best model of change for the anxiety and sedative-exposure data.
An unconditional means model was used to assess 2 null hypotheses: (a) no change across occasions; and (b) no variation between subjects. Rejecting these null hypotheses warrants further analysis.
An unconditional growth model with DAY added as a predictor incorporated estimation of change coefficients. Models with several within-person error covariance structures that were compatible with the correlation pattern between anxiety measures and sedative-exposure measures at different time points were explored. The best fit was the autoregressive + random effects covariance structure that assumes correlations decrease as the lag time increases and that covariance also comes from measures within subject. An unconditional growth model with a quadratic term was also explored to assess if there were non-linear changes in sedative-exposure measures over time.
A conditional growth model introduced the effect of the intervention and included any covariates found to be associated with the outcome. These were included in the analysis to eliminate the variability attached to them and improve the precision of the beta estimates. Post hoc multiple comparisons were completed within the mixed modeling controlling the overall alpha level at .05.
We used SPSS v.17 and SAS v.9.2. Final parameter estimates were considered significant at p ≤ .05 with a 2-sided alpha.
RESULTS
Demographic Characteristics and Intervention Use Times
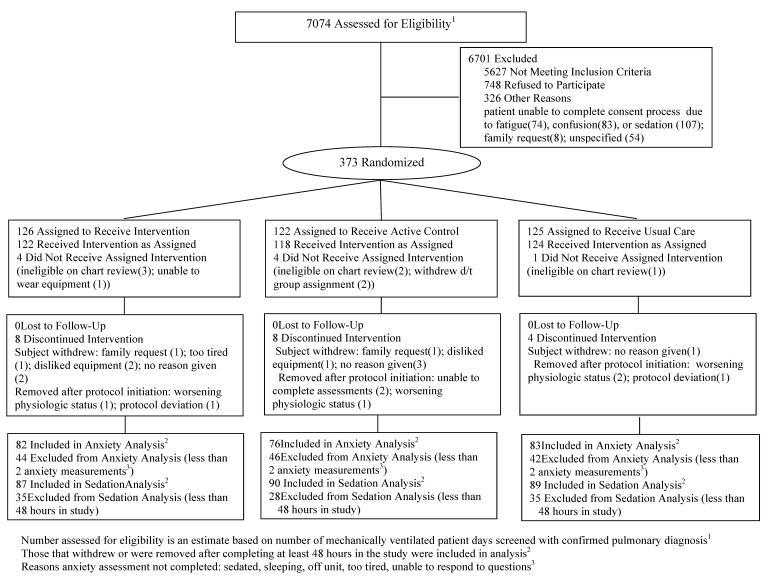
Table 1 summarizes the characteristics of patients. Mean age was 59 years with a wide range of APACHE III scores. The primary indication for mechanical ventilation was respiratory failure or distress. Only median ICU days prior to enrollment were significantly different at study entry; HP patients were in the ICU 1–2 days longer prior to enrollment than PDM or UC patients. Patients remained on protocol for 5.7 (SD 6.4) days (median 3.2; range 1–30). Figure 1 details subject flow through the study.
Table 1.
Demographic and Clinical Characteristics of Subjects (n = 373)
| Variable | Patient-directed Music N = 126 | Headphones N = 122 | Usual Care N = 125 | p-value |
|---|---|---|---|---|
|
| ||||
| Mean age (SD) | 60.4 (15.4) | 59.4 (14.3) | 57.8 (13.5) | .37 |
|
| ||||
| Female gender | 68 (54%) | 56 (46%) | 69 (55%) | .28 |
|
| ||||
| Mean APACHE III (SD) | 63.1 (18.7) | 62.2 (22.3) | 65.6 (23.5) | .43 |
|
| ||||
| Median ICU days prior to study entry (range) | 6(0–40) | 8(1–85) | 7(0–33) | .02 * |
|
| ||||
| Median ventilator days prior to study entry (range) | 4.5(0–35) | 6(1–79) | 6(0–38) | .11 |
|
| ||||
| Median ventilator days total (prior + study days) | 7.5 (1–53) | 7.3 (1–47) | 7.7 (1–46) | .74 |
|
| ||||
| Median days enrolled on protocol (range) | 3 (1–27) | 3.6 (1–30) | 3.8 (1–30) | .66 |
|
| ||||
| Mean study entry VAS-A mm(SD) | 51.9mm(32.4) | 49.0mm(30.1) | 52.3mm(29.7) | .66 |
|
| ||||
| Median sedation intensity score 24 hours prior to enrollment (range) | 4.0(0–12) | 3.5(0–10) | 4.0(0–12) | .07 |
|
| ||||
| Median sedation frequency 24 hours prior to enrollment (range) | 7.0(0–18) | 6.0(0–19) | 6.0(0–14) | .14 |
|
| ||||
| Extubated at end of study | 89 (72%) | 67 (56%) | 83 (67%) | .02 * |
|
| ||||
| Alive at end of study | 115(94%) | 111(93%) | 109(89%) | .65 |
|
| ||||
| Primary ICU Admission Diagnosis Category | Number (%) | Number (%) | Number (%) | |
| Pulmonary | 77(6) | 70(58) | 71(57) | |
| Cardiac-medical | 14 (11) | 9(7) | 9(7) | |
| Sepsis/infection | 10(8) | 7(6( | 6(4) | |
| Gastrointestinal | 6(5) | 8(7) | 7(6) | |
| Neurological or neuromuscular | 2(2) | 6(5) | 7(6) | |
| Oncology | 4(3) | 3(2) | 4(3) | |
| Shock/hypotension | 3(2) | 2(2) | 5(4) | |
| Trauma | 3(2) | 4(3) | 2(2) | |
| Cardiac Surgical | 0 | 3(2) | 3(2) | |
| Surgical | 2(2) | 3(2) | 0 | |
| Vascular | 2(2) | 0 | 2(2) | |
| Other or missing | 3(2) | 7(6) | 9(7) | |
|
| ||||
| Indication for Mechanical Ventilation | Number (%) | Number (%) | Number (%) | |
| Respiratory failure | 63(50) | 63(52) | 61(49) | |
| Respiratory distress | 32(25) | 27(22) | 36(29) | |
| Pneumonia | 7(6) | 5(4) | 7(6) | |
| Respiratory arrest | 3(2) | 4(3) | 4(3) | |
| Airway protection | 2(1) | 5(4) | 4(3) | |
| Surgery-post-operative | 2(1) | 3(3) | 4(3) | |
| COPD | 7(6) | 4(3) | 0 | |
| Hypoxia | 2(1) | 3(3) | 2(2) | |
| ARDS | 1(1) | 1(1) | 0 | |
| Tachypnea | 1(1) | 0 | 1(1) | |
| Cardiac arrest | 4(3) | 2(2) | 5(4) | |
| Pulmonary edema | 1(1) | 0 | 0 | |
| Asthma | 0 | 0 | 1(1) | |
| Other or missing | 1(1) | 5(4) | 0 | |
p<.05
Abbreviations: APACHE III, Acute Physiology, Age and Chronic Health Evaluation III; VAS-A, Visual Analog Scale-Anxiety
Figure 1.
CONSORT Flow Diagram of Study Subjects
PDM patients listened to music for a mean of 79.8 (SD 126) (median (range); 12(0–796) minutes per day. HP patients wore the noise-abating units for a mean of 34.0 (SD 89.6) (median (range); 0(0–916) minutes per day. There was no linear relationship between device use time and anxiety for either PDM (r = .07; p = .14) or HPs (r = −.06; p = .23). More PDM patients were extubated at the end of the study (Table 1).
Anxiety
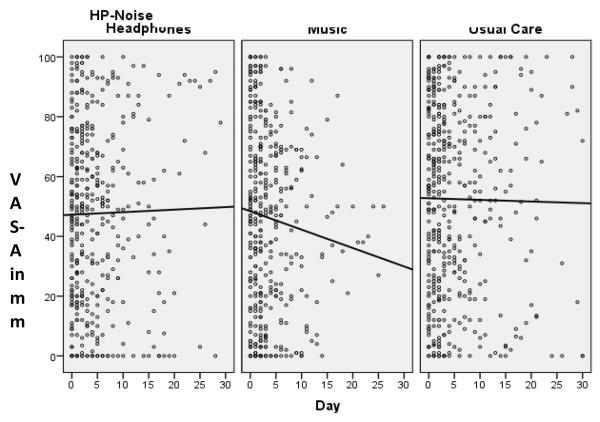
The analysis is from the 241 patients with 2 or more anxiety assessments in order to model change. Not all patients were able to provide anxiety assessments each day due to fatigue, medical condition, sedated, inability or refusal to complete assessments, or were off the unit (Figure 1). Unadjusted mean VAS-A was not significantly different among groups at study entry (Table 1). We did not observe a non-linear pattern or any obvious inflection point in the individual patterns of change; therefore, change was modeled as linear. Both the unconditional means model and the unconditional growth model indicated significant unexplained variance that warranted further modeling. Covariates of interest in the model were APACHE III, VAS-A at enrollment, and sedative exposure. Two final models were produced using either sedation frequency or sedation intensity (Table 2). After the adjustment due to APACHE III and sedation frequency and intensity, the adjusted baseline VAS-A was different between study groups, and the interaction of baseline with treatment group was significant. Pair-wise comparisons indicated that PDM patients had a significantly lower VAS-A score at study entry than UC patients regardless of whether sedation intensity or frequency was used. Sedation intensity (β = .75(.01, 1.5), p = .05) was associated with higher VAS-A scores. After adjusting for these covariates, the final models showed that the main effect of PDM was to lower the VAS-A consistently by more than 19 mm over the study period compared to UC [(β = −19.3(−32, −6.6) sedation intensity, β = −19.5(−32.2, −6.8) sedation frequency, p = .003, both)] (Figure 2).
Table 2.
Final models for Anxiety and Sedative Exposure (≥ 2 days of data)
| Model Results VAS-A Sedation frequency N = 241 | Model Results VAS-A Sedation frequency N = 241 | Model Results Sedation intensity N = 266 | Model Results Sedation frequency N = 266 | |||||
|---|---|---|---|---|---|---|---|---|
| β(95% CI) Change in mm for VAS-A for 1 unit change in predictor | p-value | β(95% CI) Change in mm for VAS-A for 1 unit change in predictor | p-value | β(95% CI) Change in sedation intensity for 1 unit change in predictor | p-value | β(95% CI) Change in sedation frequency for 1 unit change in predictor | p-value | |
| Intercept | 35.6(23.3, 48.0) | <.001 | 34.9(22.6,47.2) | <.001 | 5.3(3.5,7.1) | <.001 | 7.3(5.1,9.5) | <.001 |
| Day | −.50(−1.1, .05) | .08 | −.51(−1.1,.04) | .07 | −.03(−.15,.09) | .65 | −.17(−.27, −.07) | <.001 |
| VAS-A day 0 | .11(−.05, .27) | .18 | .11(−.05,.27) | .18 | ||||
| PDM | −19.5(−32.2, −6.8) | .003* | −19.3(−32.0,−6.6) | .003* | .14(−.92,1.2) | .79 | .69(−.68,2.1) | .33 |
| HP | −8.3(−21.4, 4.8) | .22 | −8.3(−8.7,−7.9) | .21 | 18(−.86, 1.2) | .73 | −.01(−1.3,1.3) | .99 |
| APACHE III | .16(.02, .30) | .02* | .16(.02,.30) | .02* | .003(−.01.02) | .79 | .005(−.01,.02) | .65 |
| Age | −.03(−.05, −.01) | .03* | −.04(−.08,−.008) | .03* | ||||
| sex | 88(.15,1.6) | .02* | .94(.02,1.9) | .047* | ||||
| PDM * day | −.18(−.36,−.004) | .05 | −.21(−.37,−.05) | .01* | ||||
| HP * day | −08(−.24,.08) | .32 | −.03(−.17,.11) | .72 | ||||
| Adj sedation intensity | .75(.01,1.5) | .05 | ||||||
| Adj sedation frequency | .42(−.15, .99) | .14 | ||||||
| PDM*VAS-A day 0 | .25(.03, .47) | .02* | .25(.03,.47) | .02* | ||||
| HP*VAS-A day 0 | .11(−.13, .35) | .33 | .12(−.12,.36) | .32 | ||||
| Pair-wise comparisons | ||||||||
|---|---|---|---|---|---|---|---|---|
| VAS-A day 0 | VAS-A day 0 | Sedation intensity | Sedation Frequency | |||||
| PDM vs HP | .14(−.08,.36) | .24 | .13(−.09,.35) | .24 | −.09(−.27,.09) | .32 | −.18(−.36,−004) | .04 |
| PDM vs UC | .25(.03,.47) | .02* | .25(.03,.47) | .02* | −.18(−.36,−.004) | .05* | −.21(−.37,−.05) | .01* |
| HP vs UC | .11(−.13,.35) | .33 | .12(−.12,.36) | .32 | −.08(−.24,.08) | .32 | −.03(−.17, .11) | .72 |
Abbreviations: PDM, patient-directed music; HP, headphones; UC, usual care; Adj, adjusted ; APACHE III, Acute physiology, age and chronic health evaluation. Example of interpretation of table: formula to predict sedation frequency for PDM group at any time point:
Sedation Frequency = 7.3 −.17(day in study) −.21(day if in PDM group) + .69(if in PDM group) + .005(APACHE III score) −.04(age) + .94(if female). The intercept represents the overall average of frequency sedation at baseline, 7.3 doses. Every patient goes down an average of .17 doses per day, if the patient is in the PDM group, for each day, the dose frequency goes down another .21 points for (.17 + .21 = .38) decrease per day. If the patient was in the PDM group, the baseline average was .69 higher (7.3+.69 = 7.99), every increase of 1 point in the APACHE III score raises the total daily dose frequency another .005. For every 1 year older a patient is, their sedation frequency goes down .04 pts. If the patient is female, the dose frequency goes up .94.
Figure 2.
Visual Analog Scale-Anxiety (VAS-A) Scatterplots by Group
Sedative Exposure
The analysis is from the 266 patients who were on protocol for 48+ hours. A linear pattern of change was supported by graphs of sedation intensity and frequency over time. Sedation frequency and intensity were not significantly different among groups 24-hours prior to study entry (Table 1).
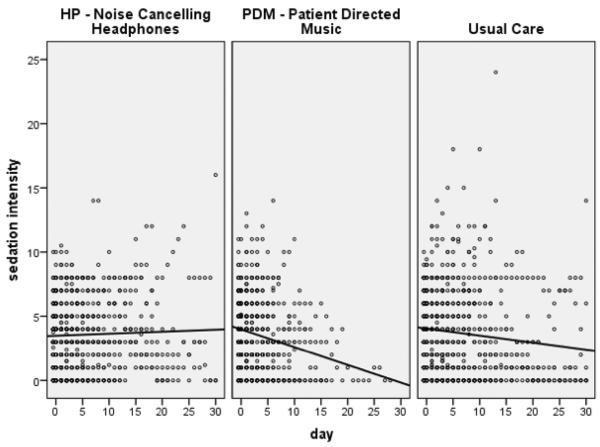
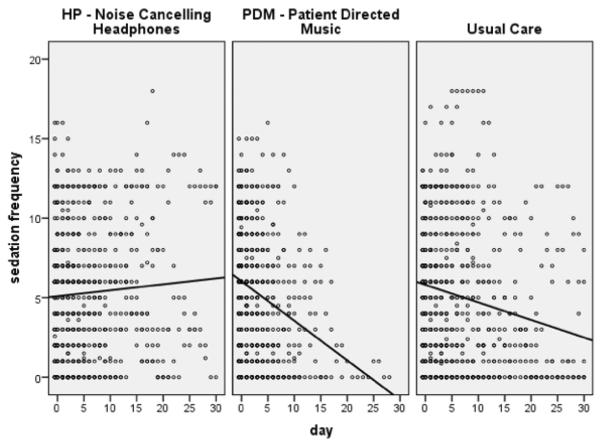
Covariates associated with sedation intensity and sedation frequency were age, gender, and APACHE III scores. Age was significant in both models; the higher the age, the lower the sedation intensity or sedation frequency. Models showed a significant interaction of the PDM group and time, showing a decrease in sedation intensity and sedation frequency over time (per day) for the PDM group only (Table 2). In post hoc pair wise comparisons, the PDM group had a greater decrease in the change over time of the sedation intensity score compared to UC (β = −.18(−.36, −.004), p = .05). Using the sedation frequency measure, the PDM group had a greater decrease in the change over time compared to UC (β = −.21(−.37, −.05), p = .01) and the HP group (β = −.18(−.36, −.004), p = .04) (Figures 3 and 4).
Figure 3.
Sedation Intensity Scatterplots by Group
Figure 4.
Sedation Frequency Scatterplots by Group
For an average patient on the fifth study day (the average time patients were enrolled), a control patient received 5 doses of any one of the 8 study-defined sedative medications. An equivalent PDM patient received just 3 doses on the fifth day, a relative reduction of 38%. By the end of the fifth day, an average control patient had a sedation intensity score of 4.4. An equivalent PDM patient had a sedation intensity score of 2.8, a relative reduction of 36%. By the end of the fifth day, an average control patient had an anxiety score of 52. An equivalent PDM patient had an anxiety score of 33 which is an absolute difference of 19 on a 100 point scale and a relative reduction of 36.5%. Refer to Table 3 for additional details.
Table 3.
Illustration of Anxiety (VAS-A) and Sedative Exposure (dose frequency and sedation intensity) using Mixed Models Results
| Estimated VAS-A | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case | APACHE III | Sedation intensity | Sedation frequency | Baseline VAS-A | Using sedation intensity | Using sedation frequency | ||||
| PDM | HP | control | PDM | HP | control | |||||
| Average/median | 64 | 4 | 7 | 52mm | 33mm | 44mm | 52mm | 32mm | 43mm | 51mm |
| By day 5,PDM reduces VAS-A by 37% compared to control | By day 5, PDM reduces VAS-A by 37% compared to control | |||||||||
| Sedation Measures | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gender | Estimated Sedation intensity | Estimated Sedation Frequency | ||||||||
| PDM | HP | control | PDM | HP | control | |||||
| Average/median | 64 | 4 | 7 | Female | 2.8 | 4.2 | 4.4 | 3.3 | 5.2 | 5.3 |
| By day 5, PDM reduces sedation intensity by 36% compared to control | By day 5, PDM reduces sedation frequency by 38% compared to control | |||||||||
Note: Patient age of 60 years, APACHE III scores, and study day 5 kept constant throughout.
Using sedation intensity to predict estimated Anxiety Scores it would be estimated that the usual care group would have an anxiety rating of 52mm at 5 days into the study. For the HP group, they would have an anxiety rating of 44mm, a decrease of 8 points (mm). For the PDM group, they would have an anxiety rating of 33mm, a drop of 19mm from usual care at 5 days into the study.
Using sedation frequency to predict estimated anxiety scores, usual care would have an anxiety rating of 51 mm at 5 days into the study. HP group would have an anxiety rating of 43mm, a decrease of 8 points (mm). The PDM group would have an anxiety rating of 32mm, a drop of 19mm from usual care.
Estimated sedation intensity (The sum of the dose quartiles over 8 medications) For an average patient with usual care, their sedation intensity score would be 4.4. at 5 days into the study. The HP group would be 4.2, a decrease of .2. The PDM group would be 2.8, a decrease of 1.6 from usual care.
Estimated sedation frequency for an average patient in usual care, their sedation frequency score would be 5.3 at 5 days into the study. The HP group would be 5.2, a decrease of .1. The PDM group would be 3.3, a decrease of 2.0 from usual care at 5 days.
Environmental Scan. Nurses caring for patients had a median of 5.9 years (range .25–44 years) ICU experience. When asked to appraise the shift activity in the patient's room, 21% of the nurses said quiet, 49% said it was at a usual pace, 24% said it was busy, 6% said it was very busy to hectic. Comments included the nurses' efforts offering PDM or headphones to patients and their observations of the protocol. Table 4 summarizes these positive comments about the PDM intervention or headphones use.
Table 4.
Summary of ICU Nurse Comments and Observations Concerning Study Protocol
| Randomized Group | Written Nurse Comments and Observations |
|---|---|
| Patient-directed music | Patient's wife says he listens to the music all of the time and it has been working well. Patient was sleeping with headphones on with his wife sleeping next to him in a chair. |
| Patient looks very peaceful and states she likes the music. | |
| Patient was tapping fingers to some of the music provided to him by the music therapist. | |
| Patient listened to music most of yesterday (about 10 hours). Tends to be anxious and her blood pressure is lower when she is listening to music. | |
| Patient likes music and always nods head “yes” to have headphones in place when asked. | |
| After putting headphones on, patient appears less anxious. | |
| Patient wears headphones very often and rests well with them in place. Always nods `yes' to wearing headphones. | |
| Patient has been tapping feet to the music and listens for a couple of hours each night; seems happy with it! | |
| After putting headphones on, patient appears less anxious. | |
| Able to decrease propofol slightly. | |
| Evening was quieter. Patient put headset on which seemed to help a lot. | |
| Family visited for 1 hour. Patient had difficulty sleeping; tried reading and quiet time before using headphones. | |
| Patient calm and resting on headphones. | |
| Patient was relaxing with music on for 3 hours. | |
| Patient slept well, headphones for 3 hours. | |
| Music was on entire night (8 hour shift) | |
| Headphones Only | Patient really benefited from headphones! |
| Patient relaxed with headphones | |
| I'm glad he's participating. I think the headphones will help him rest. | |
| The headphones would help her get more rest (due to the commotion on the other side of the curtain with roommate). | |
| The patient wanted to wear the headphones most of the day yesterday and communicated that they helped her rest. | |
| Patient put headphones on without prompting. | |
| Headphones helped patient sleep during dialysis. | |
| Patient wanted to wear headphones all night. | |
| Patient had earphones on about 1 hour early in the night, then declined to use them rest of night. | |
| Headphones decrease nerves per patient and patient's wife. | |
| Patient appeared calmer with headphones on. |
COMMENT
The two primary study aims were to determine if patient-directed music reduced anxiety and sedative exposure in a sample of patients receiving mechanical-ventilatory support. PDM decreased anxiety and sedative exposure over time more effectively than the control conditions. To our knowledge these findings are from the first-ever randomized controlled trial to test an integrative therapy for self-management of anxiety in ventilated ICU patients that does not rely solely on medications. The unique approach involving patients themselves in self-management of anxiety launches a novel area of ICU clinical research.
The PDM protocol was modeled after the patient-controlled analgesia intervention whereby patients report better pain control and are more satisfied when they self-administer analgesic therapy.8,23,24 Music provides patients with a comforting and familiar stimulus and the PDM intervention empowers patients in their own anxiety management; it is an inexpensive, easily implemented non-pharmacologic intervention that can reduce anxiety, reduce sedative medication exposure and potentially associated adverse effects.25–29 PDM patients received less frequent and less intense sedative regimens while reporting decreased anxiety levels.
We report a reduction in sedative exposure with PDM using a method to aggregate medications from disparate drug classes. This is a significant finding in that strategies are needed to reduce the amount and frequency sedative medications are administered to mechanically ventilated ICU patients. Appropriately tailored music intervention holds great promise for use in clinical practice as a method to potentially avoid or reduce the cumulative adverse side effects of these potent medications, but requires further study..
As more clinicians are advocating to minimize sedative administration,30,31 our data suggest that patients still experience moderate levels of anxiety. Patients in this study with higher sedation intensity scores had higher VAS-A scores. This finding is consistent with previous investigations that demonstrate ICU patients report moderate anxiety levels throughout the course of ventilatory support, despite receiving sedative medications.7 Given the detrimental physiological and psychological effects of sustained anxiety, it is important that this symptom be effectively managed. As clinicians seek “lighter” sedative regimens in the ICU, PDM may be an appropriate adjunctive intervention by which patients can self-manage anxiety. There were no comments from nurses that would suggest the study protocol was burdensome to their patient care practices.
Because patients were enrolled when they were not receiving high levels of sedative medications (otherwise they would have been too sedated for consenting to enrollment) it is difficult to interpret the pharmacological or cost significance of a reduction in sedative exposure in the days after enrollment compared to the higher doses patients likely received earlier in their episode of respiratory failure. However, even with a modest reduction in sedative exposure, patients assigned to PDM also experienced less anxiety compared to usual care.
There are a number of limitations to this study. Because research nurses completed the anxiety assessments (to ensure consistent administration and minimize influence on the bedside nurse's practice), only one anxiety assessment was performed daily. For some patients the assessment was not performed in relation to use of the PDM intervention, and if the patient was not available or the patient deferred due to fatigue, medical condition or was sedated, the assessment was not completed (Figure 1).
Because the intervention was patient-initiated, not all patients randomized to PDM actually used music twice daily. Some patients may have relied on the bedside nurse to assist with the equipment. This may have affected the length or frequency of music listening by patients or the non-significant relationship between music listening time and anxiety. However, even having the option and availability of PDM may provide patients a sense of control over one aspect of their ICU care. Given that anxiety is an individually perceived symptom, self-initiation of treatment with music whenever desired and for as long as desired is the preferred method of music listening much in the same manner as PCA for pain relief.
Only a small number of nurses provided written comments about the protocol. While positive, it is unknown if the ICU nurses were reluctant to write negative comments, despite them being anonymous. We did not query nurses for reasons that they administered sedative medications to study patients. ICU nurses were not blinded to the assigned group, which may have introduced bias into the study. Further, we did not collect data from patients after they were extubated or transferred from the ICU. It is recommended that future research should include replication of the study with the inclusion of post-ICU outcomes and other clinically important outcomes.
CONCLUSIONS
Music is an intervention that reduces anxiety and sedative exposure in mechanically ventilated patients. Because music is an inexpensive non-pharmacological adjunct that critically ill patients can self-initiate, clinicians should implement music therapy programs in their ICUs. Further research is needed to determine if music, and other integrative therapies, can reduce ventilator time, ICU stay, medication costs, and improve post-ICU outcomes.
Acknowledgments
Grant support from the National Institutes of Health, National Institute of Nursing Research grant #R01-NR009295. The full trial protocol can be obtained from the Dr. Chlan.
Trial registration: www.clinicaltrials.gov. Identifier: NCT00440700
Conflicts of Interest and Financial Disclosures:
Dr. Linda L. Chlan was the Principal Investigator of the grant that funded this study (National Institutes of Health, National Institute of Nursing Research grant #R01-NR009295). A portion of her salary was paid by the National Institutes of Health (NIH) to her institution for the work performed. Dr. Chlan receives payment for her editorial contributions to Critical Care Alert and received royalties from Springer Publishing for editing a textbook
Drs. Weinert, Tracy, Skaar, and Ms. Savik had a portion of their salaries paid by NIH to their institutions for work performed on the grant.
Dr. Heiderscheit had a portion of her salary paid by NIH to her institution for work performed on the grant, and she maintains a private music therapy practice (Music Medicine) for which she receives payment.
Dr. Jill L. Guttormson was a paid project coordinator on this study funded by the National Institutes of Health.
The funding sponsor of this study (NINR, NIH) had no role in: the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review or approval of the manuscript.
Footnotes
Data access and responsibility: Dr. Chlan, principal investigator, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author Contributions: Study concept and design: Chlan, Weinert, Heiderscheit, Skaar, Tracy
Acquisitions of data: Chlan, Guttormson, Heiderscheit, Tracy
Analysis and interpretation of the data: Chlan, Savik, Guttormson
Drafting of the manuscript: Chlan, Weinert, Heiderscheit, Skaar, Tracy, Guttormson, Savik
Critical revisions of the manuscript for important intellectual content: Chlan, Weinert, Skaar, Tracy, Guttormson, Savik
Statistical analysis: Savik
Study supervision: Chlan, Weinert, Tracy, Guttormson
Linda Raab Communications LLC provided paid editing assistance on the final version of the first submitted manuscript.
Previous presentation of information: Anxiety data was presented at the Society of Critical Care Medicine's annual congress on February 5, 2012. The presentation did not address the sedative-exposure aim. The accepted abstract for the SCCM meeting was submitted with the original version of this manuscript.
Anxiety data from the control usual care group only has been previously published in Nursing Research (2011). A copy of the published paper was submitted with the original version of this manuscript.
References
- 1.Arroliga A, Thompson T, Ancukiewicz M, et al. Use of sedatives, opioids, and neuromuscular blocking agents in patients with acute lung injury and acute respiratory distress syndrome. Crit Care Med. 2008;36(4):1083–1088. doi: 10.1097/CCM.0B013E3181653895. [DOI] [PubMed] [Google Scholar]
- 2.Mehta S, Burry L, Fischer S, et al. Canadian survey of the use of sedatives, analgesics, and neuromuscular blocking agents in critically ill patients. Crit Care Med. 2006;34:374–380. doi: 10.1097/01.ccm.0000196830.61965.f1. [DOI] [PubMed] [Google Scholar]
- 3.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthiol. 2006;104(1):21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Chlan L, Savik K. Patterns of anxiety in critically ill patients receiving mechanical ventilatory support. Nurs Res. 2011;60(3S):S50–S57. doi: 10.1097/NNR.0b013e318216009c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li D, Puntillo K. A pilot study on coexisting symptoms in intensive care patients. Applied Nurs Res. 2006;19:216–219. doi: 10.1016/j.apnr.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Johnson M, Sexton D. Distress during mechanical ventilation: patients' perceptions. Crit Care Nurse. 1990;10(7):48–52. [PubMed] [Google Scholar]
- 7.Sikes P. Endocrine responses to the stress of critical illness. AACN Clin Issues. 1992;3(2):379–391. doi: 10.4037/15597768-1992-2010. [DOI] [PubMed] [Google Scholar]
- 8.Lee D, Chan K, Poon C, et al. Relaxation music decreases the dose of patient-controlled sedation during colonoscopy: A prospective randomized controlled trial. Gastrointestin Endosc. 2002;55(1):33–36. doi: 10.1067/mge.2002.120387. [DOI] [PubMed] [Google Scholar]
- 9.Lepage C, Drolet P, Girard M, Grenier Y, DeGagne R. Music decreases sedative requirements during spinal anesthesia. Anesth Analg. 2001;93:912–916. doi: 10.1097/00000539-200110000-00022. [DOI] [PubMed] [Google Scholar]
- 10.Maranto C. Applications of music in medicine. In: Heal M, Wigram T, editors. Music therapy in Health and Education. J. Kingsley; London: 1993. pp. 153–174. [Google Scholar]
- 11.Thaut M, Davis W. The influence of subject-selected versus experimenter-chosen music on affect, anxiety, and relaxation. J Music Ther. 1993;30(4):210–223. [Google Scholar]
- 12.Chlan L. Psychophysiologic responses of mechanically ventilated patients to music: A pilot study. Amer J Crit Care. 1995;4(3):233–238. [PubMed] [Google Scholar]
- 13.Chlan L. Effectiveness of a music therapy intervention on relaxation and anxiety for patients receiving ventilatory assistance. Heart Lung. 1998;27(3):169–176. doi: 10.1016/s0147-9563(98)90004-8. [DOI] [PubMed] [Google Scholar]
- 14.Chlan L. Music therapy as a nursing intervention for patients supported by mechanical ventilation. AACN Clin Issues. 2000;11(1):128–138. doi: 10.1097/00044067-200002000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Wong H, Lopez-Nahas V, Molassiotis A. Effects of music therapy on anxiety in ventilator-dependent patients. Heart Lung. 2001;30(5):376–387. doi: 10.1067/mhl.2001.118302. [DOI] [PubMed] [Google Scholar]
- 16.Chlan L. Relationship between two anxiety instruments in patients receiving mechanical ventilatory support. J Adv Nurs. 2004;48(5):493–499. doi: 10.1111/j.1365-2648.2004.03231.x. [DOI] [PubMed] [Google Scholar]
- 17.Weinert C, Calvin A. Epidemiology of sedation for mechanically ventilated patients. Crit Care Med. 2007;35:393–401. doi: 10.1097/01.CCM.0000254339.18639.1D. [DOI] [PubMed] [Google Scholar]
- 18.McCartney JR, Boland RJ. Anxiety and delirium in the intensive care unit. Crit Care Clin. 1994;10(4):673–680. [PubMed] [Google Scholar]
- 19.Vogelsang J. The Visual Analog Scale: an accurate and sensitive method for self-reporting preoperative anxiety. J PostAnesthesia Nurs. 1988;3(4):235–239. [PubMed] [Google Scholar]
- 20.Chlan L, Heiderscheit A. A tool for music preference assessment with critically ill patients receiving mechanical ventilatory support. Music Ther Persp. 2009;27(1):42–47. doi: 10.1093/mtp/27.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chlan L, Patterson R, Heiderscheit A. Data acquisition for a patient-directed intervention protocol in the dynamic intensive care unit setting. Cont Clin Trials. 2011;32:544–546. doi: 10.1016/j.cct.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakraborty H, Gu H. A mixed models approach for intent-to-treat analysis in longitudinal clinical trials with missing data. RTI international; Research Trinagle Park, NC: RTI Press publication No. MR-0009-0903; 2009. Retrieved 5/2/12 http://www.rti.org/rtipress. [PubMed] [Google Scholar]
- 23.Hwang J, Jeon Y, Park H, Lim Y, Oh Y. Comparison of alfentail and ketamine in combination with propofol for patient-controlled sedation during fiberoptic bronchoscopy. Acta Anaesthesiol Scand. 2005;49:1334–1338. doi: 10.1111/j.1399-6576.2005.00842.x. [DOI] [PubMed] [Google Scholar]
- 24.Momeni M, Crucitti M, De Kock M. Patient-controlled analgesia in the management of postoperative pain. Drugs. 2006;66(18):2321–2337. doi: 10.2165/00003495-200666180-00005. [DOI] [PubMed] [Google Scholar]
- 25.Conti G, Iacobone M, Auricchio D, Liberati Q. Sedation in the intensive care unit: The basis of the problem. Minerva Anestesiol. 2002;68(4):240–244. [PubMed] [Google Scholar]
- 26.Mazzeo AJ. Sedation for the mechanically ventilated patient. Crit Care Clin. 1995;11(4):937–955. [PubMed] [Google Scholar]
- 27.Barr J, Donner A. Optimal intravenous dosing strategies for sedatives and analgesics in the intensive care unit. Crit Care Clin. 1995;11(4):827–847. [PubMed] [Google Scholar]
- 28.Stoltzfus DP. Advantages and disadvantages of combining sedative agents. Crit Care Clin. 1995;11(4):903–912. [PubMed] [Google Scholar]
- 29.Tung A, Rosenthal M. Patients requiring sedation. Crit Care Clin. 1995;11(4):791–802. [PubMed] [Google Scholar]
- 30.Riker R, Fraser G. Altering intensive care sedation paradigms to improve patient outcomes. Crit Care Clin. 2009;25(3):527–538. doi: 10.1016/j.ccc.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Strom T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: A randomised trial. Lancet. 2010;375(9713):475–480. doi: 10.1016/S0140-6736(09)62072-9. [DOI] [PubMed] [Google Scholar]