Abstract
Little is known about the role of skin color in the forensic sexual assault examination. The purpose of this study was to determine whether anogenital injury prevalence and frequency vary by skin color in women after consensual sexual intercourse. The sample consisted of 120 healthy (63 Black, 57 White) women who underwent a forensic sexual assault examination following consensual sexual intercourse. Experienced sexual assault forensic examiners using visual inspection, colposcopy technique with digital imaging, and toluidine blue application documented the number, type, and location of anogenital injuries. Although 55% of the total sample was observed to have at least one anogenital injury of any type following consensual intercourse, the percentages significantly differed for White (68%) and Black (43%) participants (p 0.02). When the presence of anogenital injury was analyzed by specific anatomical region, a significant difference between White and Black participants was only evident for the external genitalia (White = 56%, Black = 24%, p = .003), but not for the internal genitalia (White = 28%, Black = 19%, p = .20) or anus (White = 9%, Black = 10%, p = 0.99). A one standard deviation-unit increase in L* values (lightness) was related to a 150% to 250% increase in the odds of external genitalia injury prevalence (p < 0.001). While Black and White participants had a significantly different genital injury prevalence, dark skin color rather than race was a strong predictor for decreased injury prevalence. Sexual assault forensic examiners, therefore, may not be able to detect injury in women with dark skin as readily as women with light skin, leading to health disparities for women with dark skin.
Keywords: forensic nursing, forensics, health disparities, Injury, skin color
The role of skin color in the identification of anogenital injury during the forensic sexual assault examination has been largely unexplored. The authors of the national protocol guiding the general physical examination noted that it may be difficult to see anogenital injury in dark-skinned individuals (Department of Justice, 2004), but provided no empirical evidence to support the statement, nor did they make recommendations on ways to enhance assessment when injury is difficult to visualize. If individuals with dark skin are less likely than those with light skin to have injuries identified following sexual assault, a health outcomes disparity exists. Health outcomes disparities are differences in health status that occur despite equal access to health care services. While females with dark skin have equal access for forensic sexual assault programs, they may not have their injuries assessed and treated if injuries are not as easily identified as those in females with light skin. Furthermore, the progression of their sexual assault cases through the criminal justice process may be disadvantaged if their anogenital injuries are not detected and documented. If barriers exist to injury detection in females with dark skin, the forensic examination needs to be changed so that all injuries are identified regardless of the victim's skin color and so that health outcomes disparities are eliminated (Giger et al., 2007; Phillips & Grady, 2002).
Investigators have examined skin color associated with a wide range of phenomena, including the blanching response (Matas et al., 2001), erythema (Riordan, Sprigle, & Linden, 2001), and the effects of ultraviolet exposure (Coelho, Miller, Zmudzka, & Beer, 2006). Skin color also is an important variable when studying wound healing, including the color changes of burn injuries (de Chalain, Tang, & Thomson, 1998), and venous ulcer wound healing (Romanelli, 1997). While skin color is a significant variable of interest for both clinicians and researchers, little is known about the role of skin color in the context of the forensic sexual assault examination. Even though skin color is not mentioned, a careful reading of reports of anogenital injury prevalence in several published articles demonstrates that racial/ethnic differences in skin injury may be present. Investigators have found differences in genital injury following vaginal births, with Whites more likely than Blacks to have third and fourth degree perineal lacerations and tears (Howard, Davies, DeLancey, & Small, 2000; Robinson, Norwitz, Cohen, McElrath, & Lieberman, 1999). In a retrospective review of medical records of sexual assault victims, White women of all ages had almost twice the number of anogenital injuries as Black women (Cartwright, 1987). From a community sample of sexual assault victims, a significant association between race (Black versus White) and anogenital injury (AOR = 4.30; 95% CI = 1.09–25.98, p = 0.03) was found, indicating that the odds for anogenital injury among Whites was more than four times greater than Blacks (Sommers et al., 2006). These investigators suggested that individuals with dark skin may be at a disadvantage for anogenital injury identification with the current forensic examination.
The prevalence of anogenital injury resulting from consensual and nonconsensual intercourse varies by examination type and ranges from 5% on direct visualization (Massey, Garcia, & Emich, 1971) to 87% with colposcopic technique (Slaughter & Brown, 1992). Clinicians and researchers alike have attempted to understand the relevance of anogenital injury related to sexual assault as well as anogenital injury resulting from consensual sexual intercourse (Fraser et al., 1999; Hilden, Schei, & Sidenius, 2005; Jones, Rossman, Hartman, & Alexander, 2003; Jones, Rossman, Wynn, Dunnuck, & Schwartz, 2003; Sommers & Buschur, 2004; Sommers, Fisher, & Karjane, 2005). Surprisingly, investigators have found a genital injury prevalence ranging from 11% to 73% in females following consensual sexual intercourse (Fraser et al., 1999; Jones, Rossman, Hartman, & Alexander, 2003; Norvell, Benrubi, & Thompson, 1984; Slaughter, Brown, Crowley, & Peck, 1997). Few have considered a comprehensive assessment of anogenital injury pattern (frequency, prevalence, location, severity, and type of injury), and only one research team has posited that skin color, rather than race/ethnicity, may be an important variable in anogenital injury detection in the context of the sexual assault forensic examination (Sommers, 2007; Sommers et al., 2006; Sommers et al., 2008). The purpose of this investigation was to determine the role of skin color in the forensic sexual assault examination. The specific aim of the study was to determine whether anogenital injury prevalence and frequency vary by skin color in women after consensual sexual intercourse.
Methods
We used a cross-sectional, descriptive design with a sample 120 females, aged 21 years and older. Sample size determination is described below following an explanation of the study variables. Participants were healthy, English-speaking, community volunteers who identified themselves as either Black or White. We chose these two races/ethnicities as our population of interest because of the desire to enroll participants across the continuum of skin color (Shriver & Parra, 2000). Exclusion criteria included injury to the genitalia treated by a health practitioner in the last month, pregnancy, hysterectomy, menses at the time of examination, treatment for an abnormal pap smear or sexually transmitted infection in the past 6 months, or history of gynecologic cancer. Participants were recruited from an urban health sciences center as well as women's advocacy agencies. Candidates were screened by phone to determine whether or not they met inclusion/exclusion criteria. All examinations were completed in a digital imaging laboratory in a controlled environment at the health sciences center.
Sampling procedures and participant selection
We sought to obtain a sample representative of sexual assault victims in terms of race/ethnicity, age, and the time interval between intercourse and examination. Data from a sexual assault registry (N = 761) housed in a Midwestern level I trauma center and maintained by the principal investigator were used to determine the distributions of race/ethnicity (Black/White), age (21–24, 25–34, 35–44, 45–54, 55–64, 65+ years old), and time interval between sexual intercourse and examination (1–4, 5–8, 9–12, 13–16, 17–20, 21–24 hours) to be sampled among consensual volunteers. A maximum 24-hour time interval between consensual sexual intercourse and the examinations was chosen to reduce the effect of wound healing on the study outcomes.
Procedures
Two female sexual assault forensic examiners (SAFE), who had each completed more than 250 forensic examinations with colposcopy in an affiliated sexual assault program, performed all the examinations. Prior to the start of the study, each examiner completed at least two examinations with a paid model and received feedback from a physician-expert and an expert on digital image capture. Throughout data collection, the examiners were evaluated every six months by a physician-expert for examination consistency. Examiners used the TEARS classification (Slaughter et al., 1997) (Tears, Ecchymoses, Abrasions, Redness, and Swelling) with the following definitions for anogenital injury: Tears were defined as any breaks in tissue integrity including fissures, cracks, lacerations, cuts, gashes, or rips. Ecchymoses were defined as skin or mucous membrane bruising or “black and blue” areas. Abrasions were defined as excoriations caused by the removal of the epidermal layer and with a defined edge. Redness was defined as skin or mucous membrane that is abnormally inflamed due to irritation or injury without a defined edge or border. Swelling was defined as edematous tissues. Examiners were not informed that skin color was a variable in the study to reduce their bias.
The study was approved by the Institutional Review Board affiliated with the principal investigator's university. Study staff reviewed the consent document with the participants, after which all participants provided written informed consent. Participants who qualified for the study were scheduled for a face-to-face interview about their sexual behaviors and then scheduled for an examination. Prior to the examination, study staff requested that they have consensual sexual intercourse with a male partner at an assigned interval prior to the examination. The study staff did not specify the type of consensual sexual encounter, other than to request that the partners have sexual intercourse. On a return visit, self-reported data were collected about the consensual sexual encounter, number of pregnancies and parity, descriptors of sexual behavior during intercourse, use of lubricants and birth control, length of the encounter, partner penile size, and the use of sexual enhancements and/or alcohol and drugs. Participants then underwent a standard forensic sexual assault examination by a trained sexual assault nurse examiner. A pregnancy test and test for sexually transmitted infections were performed on all participants (Sommers et al., 2008).
The examination occurred in three stages: visual inspection, colposcopy with digital image capture, and toluidine blue contrast application. For the colposcopy portion of the examination, a CooperSurgical Leisegang Optik colposcope system (CooperSurgical, Trumbull, CT) was used. For the toluidine blue contrast portion of the study, a 1% aqueous solution of toluidine blue contrast was applied to the external genitalia and anus and removed with cotton balls moistened in water-based lubricant. Twenty six standardized digital images of the skin, external, and internal genitalia were captured with the colposcope-camera system. With the first group of 55 participants, the camera interface from the colposcope was used, and with the second group of 65 participants, a Hitachi KP-D20A high quality, 1 megapixel color video camera (Hitachi, Ltd., Tokyo, Japan) with internal color balance and sensitivity controls was used. Except for an increase in resolution, there were no differences in the digital imaging protocol between the two groups.
Variables and measurement strategies
Anogenital injury frequency was defined as the number of injuries counted by the examiner during each aspect of the examination: visual inspection, colposcopy, and toluidine blue application. Anogenital injury prevalence was the proportion of participants with an occurrence of anogenital injury as calculated by dividing the number of participants with one or more anogenital injuries by the total number of participants. Anogenital injury type was determined by the TEARS classification. Anogenital injury location was the anatomic site of anogenital injury: external genitalia (labia majora, labia minora, periurethral area, perineum, posterior fourchette, and fossa navicularis); internal genitalia (hymen, vagina, cervix); and anus (anus, rectum).
Skin color was defined as the genetically determined, constitutive (untanned), natural color of the human epidermis (Freinkel & Woodley, 2001). Measures of skin color were obtained during digital image analysis (DIA), which included captured images of the untanned skin of the vulvar epidermis or buttocks (constitutive skin color), external genital mucosa at the posterior fourchette (mucous membrane color), and internal genital mucosa at the vaginal wall (vaginal color). Skin color measurements were determined using colorimetric analyses, based on the International Commission on Illumination (CIE, or Commission Internationale de l'Eclairage) system of colorimetry (Colourware, 2000; Fairchild, 2005).
To understand the colorimetric theories, further information about the science of color measurement (colorimetry) is needed. A color space is a geometric representation of colors that can be produced by a particular color model (Fraser, 2005). The color space that most appropriately met the needs of the study was the CIELAB (Commission Internationale de l'Eclairage L* value, a* value, b*value) color space, which is designed to approximate and linearly correlate with the response of the human eye (Fairchild, 2005). The rationale for its choice was that it is commonly used in situations where the closeness of color must be quantified scientifically (Coelho et al., 2006; Takiwaki, 1998). An individual color can be graphed on the CIELAB color space by providing its three coordinates (L*, a*, b*; see below), which are unique to each color (Fairchild, 2005; Howard et al., 2000). Three color values comprise this model: a measure of lightness/darkness (known as the L* value), a measure of redness/greenness (known as the a* value), and a measure of yellowness/blueness (known as the b* value). The L* value ranges from 0 (black) to 100 (white). For the a* value (redness/greenness), a positive value is red and a negative value is green, and for the b* value (yellowness/blueness), a positive value of b* is yellow and a negative value is blue (Chardon, Cretois, & Hourseau, 1991; Fairchild, 2005).
Two trained technicians determined measures of skin color (L*, a*, and b* values) using the colorimetry functions within Adobe PhotoShop CS2© with procedures that are standard for DIA of skin color (Coelho et al., 2006; Mattsson et al., 1999). For each participant, both technicians recorded at least nine measurements of L* (lightness/darkness), a* (redness/greenness), and b* (yellowness/blueness) values for each tissue location (vulvar or buttocks epidermis, genital mucosa, and vaginal wall).
Sample size analysis, data management, and primary data analysis
A sample size analysis (Hintze, 2001) indicated that 120 participants were necessary to achieve at least 80% power to detect (α = 0.05): 1) as small as a 5% change in the odds of anogenital injury occurrence (prevalence) associated with a 10-unit change in L* values (lightness/darkness) and 2) as small as a 5% increase in the number of observed injuries (frequency) associated with a 10-unit change in L* values. L* values were selected as they are the most analogous to White and Black skin color and their values are constrained to fall between 0 and 100, while a* and b* values are boundless (Sommers et al., 2008). All data were double entered and all discrepancies were rectified by consultation with the principal investigator. Statistical analyses were conducted using either the R statistical environment (R Development Core Team, 2006) or StatXact-5 (Cytel Software Corporation, 2001).
Two-way mixed-effects intraclass correlation coefficients (ICCs) were computed to evaluate the intra-rater reliability (consistency or agreement across each technician's own measurements) and inter-rater reliability (consistency or agreement between the two technicians) of the color measurements for each tissue location. Measurements were averaged: (1) within technicians following establishment of acceptable intrarater agreement (ICC ≥0.90); and (2) between technicians following establishment of acceptable interrater agreement (average ICC ≥0.90) in order to yield a single value for each skin color and tissue type. Due to their nonnormal distributions, skin color variables were compared across racial/ethnic groups by use of a series of independent-samples permutation tests, which do not require a normality of sampling distributions. Simple odds ratios (OR) and rate ratios (RR) were computed to assess the association between skin color measurements (i.e., L*, a*, and b* values) and anogenital injury prevalence and frequency, respectively. ORs were computed from simple (single predictor) logistic regression models for binary outcomes and RRs were computed from simple negative binomial regression models for overdispersed count outcomes or frequencies. Skin color values were measured from the (1) mucous and epidermal membranes for both the external genitalia and anus and (2) mucous and vaginal membranes for the internal genitalia.
Results
Of the 253 women who were screened, 132 met study inclusion criteria and were enrolled in the study. The sample that was used for statistical analysis included 120 women, because twelve participants either did not complete the entire protocol or did not have usable data (See Table 1 for demographic characteristics). Four participants did not return for the examination; data from eight additional participants were excluded from the study because of pilot testing the instruments (n = 2), pregnancy (n = 3), and instrument error (n = 3).
Table 1.
Demographic and sexual intercourse-related characteristics
| Characteristic | Black (n = 63) | White (n = 57) | Total Sample (N = 120) | |
|---|---|---|---|---|
| Age, mean (SD), y | 33.3 (9.7) | 31.7 (8.1) | 32.5 (9.0) | |
| Marital status†, No.,% | ||||
| Single | 40 (63.5) | 26 (45.6) | 66 (55.0) | |
| Married | 22 (34.9) | 30 (52.6) | 52 (43.3) | |
| Other | 1 (1.6) | 1 (1.8) | 2 (1.7) | |
| Parity†, No.,% | ||||
| 0 | 8 (12.7) | 26 (45.6) | 34 (28.3) | |
| 1 | 11 (17.5) | 7 (12.3) | 18 (15.0) | |
| 2 | 28 (44.4) | 17 (29.8) | 45 (37.5) | |
| 3 | 9 (14.3) | 6 (10.5) | 15 (12.5) | |
| 4+ | 7 (11.2) | 1 (1.8) | 8 (6.7) | |
| Number of sexual partners, No.,% | ||||
| 1 | 2 (3.2) | 8 (14.0) | 10 (8.3) | |
| 2 | 1 (1.6) | 3 (5.3) | 4 (3.3) | |
| 3–5 | 14 (22.2) | 19 (33.3) | 33 (27.5) | |
| 6–10 | 21 (33.3) | 18 (31.6) | 39 (32.5) | |
| 11–15 | 8 (12.7) | 3 (5.3) | 11 (9.2) | |
| 16–20 | 6 (9.5) | 2 (3.5) | 8 (6.7) | |
| 21–30 | 7 (11.1) | 1 (1.8) | 8 (6.7) | |
| 31+ | 3 (3.2) | 3 (5.3) | 5 (4.1) | |
| Refused | 2 (3.2) | 0 (0) | 2 (1.7) | |
| Birth control use†, No.,% | ||||
| None | 22 (34.9) | 5 (8.8) | 27 (22.5) | |
| Some of the time | 6 (7.5) | 3 (5.3) | 9 (7.5) | |
| Always | 35 (55.6) | 49 (86.0) | 84 (70.0) | |
| Frequency of sexual intercourse†, No.,% | ||||
| <1/month | 1 (1.6) | 0 (0.0) | 1 (0.8) | |
| 1/month | 2 (3.2) | 4 (7.0) | 6 (5.0) | |
| >1/month | 11 (17.5) | 5 (8.8) | 16 (13.3) | |
| 1–2/week | 22 (34.9) | 31 (54.4) | 53 (44.2) | |
| 3–5/week | 27 (42.9) | 14 (24.6) | 41 (34.2) | |
| 1/day | 0 (0.0) | 3 (5.3) | 3 (2.5) | |
| Time since last episode of sexual intercourse, mean (SD), hrs | 8.2 (6.3) | 8.5 (6.5) | 8.2 (6.3) | |
| Partner's penis size, mean (SD), 1 (very small) to 10 (very large) | 6.4 (1.8) | 6.5 (1.4) | 6.5 (1.4) | |
| Degree of lubrication†, mean (SD), 1 (dry) to 10 (very lubricated) | 7.5 (2.3) | 5.9 (2.2) | 6.7 (2.4) | |
| Duration of penetration†, mean (SD), min | 19.3 (16.8) | 12.9 (8.4) | 16.2 (13.8) |
Significant differences (p < 0.05) between Black and White subgroup means or proportions following independent-samples t- or χ2 tests. Abbreviation: min, minutes; hrs, hours; mnth, months; y, years.
Anogenital injury
Results from the three examination techniques were pooled to obtain single estimates of anogenital injury prevalence and frequency. The presence of an anogenital injury was noted if it was observed during any of the three examination techniques. Descriptive indices of anogenital injury are presented as prevalence in Table 2 and frequency in Table 3, stratified by anogenital region, anogenital injury type, and race/ethnicity. Although 55% of the total sample was observed to have at least one anogenital injury of any type following consensual intercourse, the percentages significantly differed for White (68%) and Black (43%) participants (p = 0.02) (raw data not presented in tables). However, when the presence of anogenital injury was analyzed by specific anatomical region, a significant difference between White and Black participants was only evident for the external genitalia (White = 56%, Black = 24%, p = 0.003), but the not for the internal genitalia (White = 28%, Black = 19%, p 0.20) or anus (White = 9%, Black = 10%, p = 0.99). Stated differently, the odds of at least one external genital injury were nearly four times greater for White as compared to Black consensual participants (OR = 3.98, 95% CI = 1.09–17.66, p = 0.04). Further, the odds of two or more external genital injuries were more than three times greater for White as compared to Black participants (OR = 3.20, 95% CI = 1.29–8.19, p = 0.001).
Table 2.
Injury prevalence (Occurrence) by anogenital region, anogenital injury type, and race/ethnicity for consensual intercourse participants
| Anogenital Injury Type |
|||||||
|---|---|---|---|---|---|---|---|
| Anogenital Region | Race/Ethnicity | Tears | Ecchymoses | Abrasions | Redness | Swelling | Any |
| External Genitalia | Total Sample | 31 (26%) | 0 (0%) | 17 (14%) | 11 (9%) | 0 (0%) | 47 (39%) |
| Black | 11 (18%) | 0 (0%) | 4 (6%) | 1 (2%) | 0 (0%) | 15 (24%) | |
| White | 2 (35%) | 0 (0%) | 13 (23%) | 10 (18%) | 0 (0%) | 32 (56%) | |
| Internal Genitalia | Total Sample | 3 (3%) | 10 (8%) | 5 (4%) | 16 (13%) | 0 (0%) | 28 (23%) |
| Black | 1 (2%) | 6 (10%) | 2 (3%) | 6 (10%) | 0 (0%) | 12 (19%) | |
| White | 2 (4%) | 4 (7%) | 3 (5%) | 10 (18%) | 0 (0%) | 16 (28%) | |
| Anus | Total Sample | 5 (4%) | 0 (0%) | 1 (1%) | 5 (4%) | 1 (1%) | 11 (9%) |
| Black | 4 (6%) | 0 (0%) | 0 (0%) | 2 (3%) | 0 (0%) | 6 (10%) | |
| White | 1 (2%) | 0 (0%) | 1 (2%) | 3 (5%) | 1 (2%) | 5 (9%) | |
Note. Prevalence values represent No. and (%) of participants with at least one injury by anogenital region and anogenital injury type for total sample (N = 120) and within each racial/ethnic group (White, n = 57; Black, n = 63).
Table 3.
Injury frequency (count) by anogenital region, anogenital injury type, and race/ethnicity for consensual intercourse participants
| Injury Type |
|||||||
|---|---|---|---|---|---|---|---|
| Anogenital Region | Race/Ethnicity | Tears | Ecchymoses | Abrasions | Redness | Swelling | Any |
| External Genitalia | Total Sample | 59 | 0 | 22 | 17 | 0 | 98 |
| Black | 19 | 0 | 4 | 2 | 0 | 25 | |
| White | 40 | 0 | 18 | 15 | 0 | 73 | |
| Internal Genitalia | Total Sample | 6 | 12 | 5 | 18 | 0 | 41 |
| Black | 1 | 8 | 2 | 6 | 0 | 17 | |
| White | 5 | 4 | 3 | 12 | 0 | 24 | |
| Anus | Total Sample | 11 | 0 | 1 | 5 | 1 | 18 |
| Black | 9 | 0 | 0 | 2 | 0 | 11 | |
| White | 2 | 0 | 1 | 3 | 1 | 7 | |
Note. Frequency values represent total injury count by anogenital region and anogenital injury type for total sample (N = 120) and within each racial/ethnic group (White, n = 57; Black, n = 63).
Skin color measurements
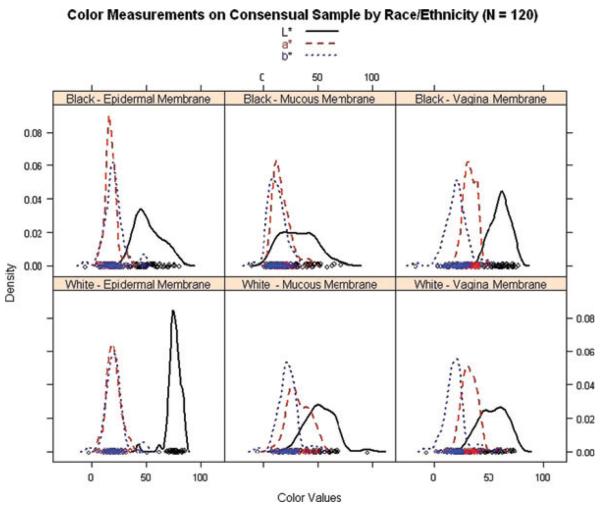
Skin color measurements for the three membrane types (epidermal, mucous, vaginal) are summarized in Table 4. Both intrarater and interrater reliability of skin color measurements from each membrane type were acceptably high (ICCs >0.92) to warrant averaging. Independent-samples permutation tests indicated significant differences between White and Black participants in terms of the magnitude of (1) L* color values on epidermal, mucous, and vaginal membranes; (2) a* color values on epidermal and mucous membranes; and (3) b* color values only on the mucous membrane. The distributions of each skin color variable are presented in Figure 1, stratified by membrane type and race/ethnicity. Although many significant differences were detected, note the overlap among skin color values distributions between Black and White participants.
Table 4.
Descriptive statistics and inter-rater reliability of L*, a*, and b* values
| Black (n = 63) |
White (n = 57) |
Total Sample (N = 120) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Membrane | ICC | M | Mdn | SD | Min | Max | M | Mdn | SD | Min | Max | M | Mdn | SD | Min | Max |
| Epidermal | ||||||||||||||||
| †L* | 0.99 | 51.9 | 49.9 | 12.1 | 31.8 | 82.2 | 75.3 | 75.1 | 6.4 | 42.5 | 84.9 | 62.8 | 69.4 | 15.3 | 31.8 | 84.9 |
| †a* | 0.99 | 17.7 | 16.9 | 5.4 | 6.5 | 36.6 | 19.9 | 19.3 | 6.4 | 6.5 | 35.8 | 18.7 | 17.8 | 6.0 | 6.5 | 36.6 |
| b* | 0.99 | 20.2 | 19.6 | 9.3 | −6.5 | 49.9 | 20.7 | 20.2 | 8.9 | −3.0 | 50.6 | 20.5 | 19.8 | 9.1 | −6.5 | 50.6 |
| Mucous | ||||||||||||||||
| †L* | 0.92 | 32.2 | 45.6 | 15.7 | 15.5 | 74.7 | 52.0 | 53.1 | 13.2 | 74.7 | 74.7 | 50.6 | 49.4 | 10.8 | 15.5 | 74.7 |
| †a* | 0.95 | 16.7 | 29.6 | 7.6 | 11.7 | 46.6 | 32.7 | 35.4 | 10.0 | 11.0 | 57.6 | 32.3 | 31.8 | 8.2 | 11.0 | 57.6 |
| †b* | 0.94 | 12.3 | 21.2 | 7.4 | −11.4 | 47.4 | 22.6 | 24.8 | 9.2 | −1.2 | 56.2 | 22.3 | 22.5 | 9.0 | −11.4 | 56.2 |
| Vaginal | ||||||||||||||||
| †L* | 0.95 | 60.3 | 61.6 | 8.6 | 40.2 | 76.7 | 53.6 | 54.3 | 12.1 | 28.8 | 73.7 | 57.2 | 59.2 | 10.8 | 28.8 | 76.7 |
| a* | 0.93 | 32.6 | 32.7 | 5.6 | 20.9 | 46.6 | 32.0 | 30.7 | 7.7 | 10.1 | 59.4 | 32.3 | 31.8 | 6.6 | 10.1 | 59.4 |
| b* | 0.98 | 20.1 | 20.7 | 10.2 | −16.9 | 50.8 | 18.7 | 18.2 | 10.0 | −7.5 | 58.9 | 19.5 | 19.8 | 10.1 | −16.9 | 58.9 |
Mean difference between Black and White subgroups, p < 0.05; ICC = Intraclass correlation coefficient, estimate of interrater reliability of skin color measurements.
Figure 1.
Distributions of skin color (density plots) by membrane type and race/ethnicity.
Association between skin color values and anogenital injury prevalence and frequency
With the reliability of L*, a*, and b* values from digital image analysis established, we next explored relationships between quantified measures of skin color and anogenital injury by computing a series of simple ORs and RRs ratios for anogenital injury prevalence and frequency, respectively. No significant associations were observed between skin color values and injury prevalence or frequency to the internal genitalia or anus. Significant associations emerged between epidermal and mucous L* values and external genital injury. For instance, a one SD-unit increase in L* values (lightness) was approximately related to a 150% to 250% increase in the odds of anogenital injury prevalence (respectively for mucous and epidermal membranes, OR = 1.62, 95% CI = 1.24–2.16, p < 0.001 and OR = 2.50, 95% CI = 1.63–4.07, p < 0.001) and expected anogenital injury frequencies (respectively for mucous and epidermal membranes, RR = 1.49, 95% CI = 1.24–1.83, p < 0.05 and RR = 2.35, 95% CI = 1.68–3.40, p < 0.001). Only mucous a* values were significantly associated with an increased prevalence of external genitalia injury, such that a one SD-unit increase in a* values (more redness) was associated with a 169% increase in odds of anogenital injury prevalence (OR = 1.69, 95% CI = 1.28–2.31, p < 0.001). However, both epidermal and mucous a* values were associated with increased external genitalia injury frequency, such that the expected anogenital injury count increased by approximately 150% for each one SD-unit increase in a* values (more redness) (respectively for epidermal and mucous membranes, RR = 1.45, 95% CI = 1.02–2.11, p < 0.05 and RR = 1.62, 95% CI = 1.31–2.03, p < 0.001).
Discussion
We found that White women have a higher prevalence and frequency of anogenital injury than Black women. However, the differences in external anogenital injury prevalence were explained more fully by skin color than by race/ethnicity. The most likely reason for the significant difference in injury prevalence in females with light skin as compared to dark skin is that, with current forensic techniques, external anogenital injury is more difficult to detect in females with dark skin than in females with light skin.
Health outcomes disparities are those health inequalities that occur despite equal access to health care services. While individuals across the continuum of skin color likely have equal access to treatment by sexual assault forensic examiners, the findings of this study indicate that women with dark skin may be at a health care disadvantage because their external anogenital injuries are difficult to visualize with current forensic techniques. We also found that women with constitutive (natural) skin color with more redness were more likely to have anogenital injuries detected than women with less redness in their natural skin color.
Clinical considerations about skin color
A patient's skin has been described as the “ideal interface” between nurses and their patients (Lott & Hoath, 1998) because skin assessment plays an important role in evaluating a patient's overall health. In contrast, skin color also can be described as a socially charged concept, with the analysis of skin color raising important questions about stereotyping. Sexual assault forensic examiners cannot be “color blind.” Skin color most likely will need to change examination techniques in the same way that gender changes the examination following sexual assault. More importantly, clinicians and scientists together need to develop examination procedures grounded in research so that all injuries are detected and documented, regardless of skin color and regardless of injury location. Indeed, new techniques are also likely needed for injury detection and measurement in the extragenital regions of the body such as the face and neck, extremities, and trunk for victims of sexual assault and intimate partner violence.
The research methods used in this study to quantify skin color were fairly complex and time intensive, and not readily extrapolated to the clinical setting. A number of hand-held instruments such as colorimeters and spectrophotometers could be purchased by sexual assault programs to be used for skin color determination, but the usefulness of those measurements clinically is questionable. One might then question why the investigators chose to study skin color in the setting of the forensic examination. The answer is that the novel findings of this study are the first empirical support for the statement about dark skin that the authors wrote in the national protocol guiding the sexual assault forensic examination (Department of Justice, 2004). Investigators can now build on these findings to explore innovative techniques to ensure that all anogenital injuries are detected and documented, and that health disparities are eliminated (Phillips & Grady, 2002).
Criminal justice considerations
From a criminal justice perspective, evidence of anogenital injury influences decision making and legal outcomes throughout the criminal justice process. Forensic documentation of anogenital injury of women who seek health care post-assault is critical to the successful prosecution of sexual assault cases (DuMont & White, 2007) For example, Wiley, Sugar, Fine, Eckard (2003) reported that the presence of anogenital trauma was significantly related to charges against a suspect being filed by the prosecutor. Lindsay (1998) reported evidence of more than one site of anogenital injury was significant related with the suspect being charged, and this work was replicated by others (McGregor, DuMont, & Myhr, 2002; Mc-Gregor, Le, Marion, & Wiebe, 1999).
Evidence of anogenital injury in sexual assault cases is part of a constellation of evidentiary factors that are associated with, but not a prerequisite to, a successful legal outcome. Further research into the role of skin color using colorimetry techniques may improve the validity and reliability of the identification and documentation of anogenital injury, thereby affecting the quality of forensic evidence proffered and decisions made throughout the criminal justice process. Forensic evidence based on such improved measurement techniques has the potential to corroborate other physical evidence and the victim's testimony, influence more objective decision making, and ultimately enhance the quality of justice for sexual assault victims of all skin color, regardless of their race/ethnicity (Phillips & Grady, 2002).
Limitations and methodological considerations
Several variables such as length of the sexual encounter and degree of lubrication were measured by the participants' self-report, which may be limited by recall bias. The sample was also composed of community volunteers, leading to biases associated with participant self selection. The internal validity of the study was limited because the TEARS classification, while in standard use in clinical practice, has not undergone extensive testing for interob-server reliability. In addition, two individuals performed all the examinations, and their findings may not be generalizable to other examiners, or they may have misidentified, missed, or misclassified anogenital injuries. Finally, the differences in anogenital injury prevalence and frequency may not be a function of anogenital injury detection based on skin color, but rather could occur because of innate differences in the properties of the skin.
Summary
Sexual assault forensic examiners are in a unique position to provide medico-legal evidence that affects both health care and criminal justice outcomes for their patients. From a healthcare perspective, it is important that all injuries are detected and treated appropriately. From a criminal justice perspective, the documentation of anogenital and other injuries has implications during decisions to report, prosecute, and convict. Based on the results of this study, women with light skin have significantly more external anogenital injuries than women with dark skin, and that difference is likely explained by problems with anogenital injury detection on dark skin. Further research is warranted to investigate the current procedures used in the forensic sexual assault examination and to investigate alternative techniques that would be optimal in patients of all skin colors.
Acknowledgment
Funded by the National Institute of Nursing Research, R01NR05352, Marilyn S. Sommers, Principal Investigator.
References
- Cartwright P. Factors that correlate with injury sustained by survivors of sexual assault. Obstetrics and Gynecology. 1987;70:44–46. [PubMed] [Google Scholar]
- Chardon A, Cretois I, Hourseau C. Skin colour typology and suntanning pathways. International Journal of Cosmetic Science. 1991;13:191–208. doi: 10.1111/j.1467-2494.1991.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Coelho SG, Miller SA, Zmudzka BZ, Beer JZ. Quantification of UV-induced erythema and pigmentation using computer-assisted digital image evaluation. Photochemistry & Photobiology. 2006;82(3):651–655. doi: 10.1562/2005-08-02-TSN-635. [DOI] [PubMed] [Google Scholar]
- Colourware Frequently asked questions about colour physics. 2000 Retrieved September 1, 2008 from http://www.colourware.co.uk/cpfaq.htm.
- Cytel Software Corporation . StatXact-5 for windows user manual. Cytel Software Corporation; Cambridge, MA: 2001. [Google Scholar]
- de Chalain TMB, Tang C, Thomson HG. Burn area color changes after superficial burns in childhood: Can they be predicted. Journal of Burn Care and Rehabilitation. 1998;19:39–49. doi: 10.1097/00004630-199801000-00010. [DOI] [PubMed] [Google Scholar]
- Department of Justice A national protocol for sexual assault medical forensic examinations (adults/adolescents) 2004 Retrieved October 5, 2008 from http://www.ncjrs.org/pdffiles1/ovw/206554.pdf.
- DuMont J, White D. The uses and impacts of medico-legal evidence in sexual assault cases: A global review. World Health Organization; Geneva, Switzerland: 2007. [Google Scholar]
- Fairchild M. Color appearance models. Wiley; Hoboken, NJ: 2005. [Google Scholar]
- Fraser B. Out of gamut: Color-correct vocabulary. 2005 Retrieved October 5, 2008, from http://www.creativepro.com/story/feature/11132.html?origin=story.
- Fraser I, Lahteenmaki P, Elomaa K, Lacarra M, Mishell D, Alvarez F, et al. Variations in vaginal epithelial surface appearance determined by colposcopic inspection in healthy, sexually active women. Human Reproduction. 1999;14:1974–1978. doi: 10.1093/humrep/14.8.1974. [DOI] [PubMed] [Google Scholar]
- Freinkel RK, Woodley DT. Biology of the skin. The Parthenon Publishing Group; New York: 2001. [Google Scholar]
- Giger J, Davidhizar RE, Purnell L, Harden JT, Phillips J, Strickland O. American Academy of Nursing Expert Panel Report: Developing cultural competence to eliminate health disparities in ethnic minorities and other vulnerable populations. Journal of Transcultural Nursing. 2007;18(2):95–102. doi: 10.1177/1043659606298618. [DOI] [PubMed] [Google Scholar]
- Hilden M, Schei B, Sidenius K. Genitoanal injury in adult female victims of sexual assault. Forensic Science International. 2005;154(2–3):200–205. doi: 10.1016/j.forsciint.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Hintze J. NCSS and PASS. Number Cruncher Statistical Systems; Kaysville, UT: 2001. [Google Scholar]
- Howard D, Davies P, DeLancey J, Small Y. Differences in perineal lacerations in black and white primiparas. Obstetrics and Gynecology. 2000;96:622–624. doi: 10.1016/s0029-7844(00)00956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J, Rossman L, Hartman M, Alexander C. Anogenital injuries in adolescents after consensual sexual intercourse. Academic Emergency Medicine. 2003;10:1378–1383. doi: 10.1111/j.1553-2712.2003.tb00013.x. [DOI] [PubMed] [Google Scholar]
- Jones J, Rossman L, Wynn B, Dunnuck C, Schwartz N. Comparative analysis of adult versus adolescent sexual assault: epidemiology and patterns of anogenital injury. Academic Emergency Medicine. 2003;10:872–877. doi: 10.1111/j.1553-2712.2003.tb00631.x. [DOI] [PubMed] [Google Scholar]
- Lindsay SP. Doctoral dissertation. University of California; San Diego State University; San Diego: 1998. An epidemiologic study of the influence of victim age and relationship to the suspect on the results of evidentiary examinations and law enforcement outcomes in cases of reported sexual assault. 1998. [Google Scholar]
- Lott JW, Hoath SB. Neonatal skin: The ideal nursing interface. Journal of Pediatric Nursing. 1998;13:302–306. doi: 10.1016/S0882-5963(98)80016-X. [DOI] [PubMed] [Google Scholar]
- Massey J, Garcia C, Emich J. Management of sexually assaulted females. Obstetrics and Gynecology. 1971;38:29–36. [PubMed] [Google Scholar]
- Matas A, Sowa MG, Taylor V, Taylor G, Schattka BJ, Mantsch HH. Elimination the issue of skin color in assessment of the blanch response. Advances in Skin and Wound Care. 2001;14:180–188. doi: 10.1097/00129334-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Mattsson U, Cassuto J, Jontell M, Jonsson A, Sinclair R, Tarnow P. Digital image analysis of erythema development after experimental thermal injury to human skin: effect of postburn topical local anesthetics. Anesthesia and Analgesia. 1999;88:1131–1136. doi: 10.1097/00000539-199905000-00031. [DOI] [PubMed] [Google Scholar]
- McGregor M, Du Mont J, Myhr T. Sexual assault forensic medical examination: is evidence related to successful prosecution? Annals of Emergency Medicine. 2002;39:639–647. doi: 10.1067/mem.2002.123694. [DOI] [PubMed] [Google Scholar]
- McGregor M, Le G, Marion S, Wiebe E. Examination for sexual assault: is the documentation of physical injury associated with the laying of charges? A retrospective cohort study. Canadian Medical Association Journal. 1999;160:1565–1569. [PMC free article] [PubMed] [Google Scholar]
- Norvell M, Benrubi G, Thompson R. Investigation of microtrauma after sexual intercourse. Journal of Reproductive Medicine. 1984;29:269–271. [PubMed] [Google Scholar]
- Phillips J, Grady PA. Reducing health disparities in the twenty-first century: opportunities for nursing research. Nursing Outlook. 2002;50:117–120. doi: 10.1067/mno.2002.123529. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2006. [Google Scholar]
- Riordan B, Sprigle S, Linden M. Testing the validity of erythema detection algorithms. Journal of Rehabilitation Research and Development. 2001;38(1):13–22. [PubMed] [Google Scholar]
- Robinson J, Norwitz E, Cohen A, McElrath T, Lieberman E. Epidural analgesia and third- or fourth-degree lacerations in nulliparas. Obstetrics and Gynecology. 1999;94:259–262. doi: 10.1016/s0029-7844(99)00259-8. [DOI] [PubMed] [Google Scholar]
- Romanelli M. Objective measurement of venous ulcers debridement and granulation with a skin color reflectance analyzer. Wounds: A Compendium of Clinical Research and Practice. 1997;9:122–126. [Google Scholar]
- Shriver M, Parra E. Comparison of narrow-band reflectance spectroscopy and tristimulus for measurements of skin and hair in persons of different biologic ancestry. American Journal of Physical Anthropology. 2000;112:17–27. doi: 10.1002/(SICI)1096-8644(200005)112:1<17::AID-AJPA3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Slaughter L, Brown C. Colposcopy to establish physical findings in rape victims. American Journal of Obstetrics and Gynecology. 1992;166:83–86. doi: 10.1016/0002-9378(92)91834-w. [DOI] [PubMed] [Google Scholar]
- Slaughter L, Brown C, Crowley S, Peck R. Patterns of genital injury in female sexual assault victims. American Journal of Obstetrics and Gynecology. 1997;176:609–616. doi: 10.1016/s0002-9378(97)70556-8. [DOI] [PubMed] [Google Scholar]
- Sommers M. Defining patterns of genital Injury from rape and sexual assault: a review. Trauma, Violence, and Abuse. 2007;8:270–280. doi: 10.1177/1524838007303194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommers M, Buschur C. Injury in women who are raped: what every critical care nurse needs to know. Dimensions of Critical Care Nursing. 2004;23(2):62–68. doi: 10.1097/00003465-200403000-00003. [DOI] [PubMed] [Google Scholar]
- Sommers M, Fisher B, Karjane H. ED rape exam using colposcopy: Issues in health care, forensics, and criminal justice. Journal of Forensic Nursing. 2005;1:28–34. 19. doi: 10.1111/j.1939-3938.2005.tb00008.x. [DOI] [PubMed] [Google Scholar]
- Sommers M, Zink T, Baker R, Fargo J, Porter J, Weybright D, Porter J, Schafer J. Effects of age and ethnicity on physical injury from rape. Journal of Obstetric, Gynecological, and Neonatal Nursing. 2006;35:199–207. doi: 10.1111/j.1552-6909.2006.00026.x. [DOI] [PubMed] [Google Scholar]
- Sommers MS, Zink TM, Fargo JD, Baker RB, Buschur C, Shambley-Ebron D, Fisher BS. Forensic sexual assault examination and genital injury: Is skin color a source of health disparity? American Journal of Emergency Medicine. 2008;26:857–866. doi: 10.1016/j.ajem.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiwaki H. Measurement of skin color: Practical application and theoretical considerations. Journal of Medical Investigation. 1998;44(3,4):121–126. [PubMed] [Google Scholar]
- Wiley J, Sugar N, Fine D, Eckard LO. Legal outcomes of sexual assault. American Journal of Obstetrics and Gynecology. 2003;188:1638–1641. doi: 10.1067/mob.2003.396. [DOI] [PubMed] [Google Scholar]