Abstract
Background
Type 2 diabetes genetic risk testing might motivate at-risk patients to adopt diabetes prevention behaviors. However, the influence of literacy and numeracy on patient response to diabetes genetic risk is unknown.
Objective
The authors investigated the association of health literacy, genetic literacy, and health numeracy with patient responses to diabetes genetic risk.
Design and Measurements
Overweight patients at high phenotypic risk for type 2 diabetes were recruited for a clinical trial of diabetes genetic risk testing. At baseline, participants predicted how their motivation for lifestyle modification to prevent diabetes might change in response to hypothetical scenarios of receiving “high” and “low” genetic risk results. Responses were analyzed according to participants’ health literacy, genetic literacy, and health numeracy.
Results
Two-thirds (67%) of participants (n = 175) reported very high motivation to prevent diabetes. Despite high health literacy (92% at high school level), many participants had limited health numeracy (30%) and genetic literacy (38%). Almost all (98%) reported that high-risk genetic results would increase their motivation for lifestyle modification. In contrast, response to low-risk genetic results varied. Higher levels of health literacy (P = 0.04), genetic literacy (P = 0.02), and health numeracy (P = 0.02) were associated with an anticipated decrease in motivation for lifestyle modification in response to low-risk results.
Conclusions
While patients reported that high-risk genetic results would motivate them to adopt healthy lifestyle changes, response to low-risk results varied by patient numeracy and literacy. However, anticipated responses may not correlate with true behavior change. If future research justifies the clinical use of genetic testing to motivate behavior change, it may be important to assess how patient characteristics modify that motivational effect.
Keywords: genetics, diabetes, internal medicine, risk communication or perception, decision aids and tools, numeracy, individual differences, health literacy, judgment and decision psychology
Genetic testing is increasingly being used as a diagnostic and prognostic tool for medical decision making.1 Genetic risk information might also motivate behavior change,2 including improvement in diet and physical activity,3 smoking cessation,4,5 and acceptance of medical treatment.6 Type 2 diabetes is an increasingly prevalent clinical problem, in part due to the current epidemic of obesity and physical inactivity. Strong evidence from clinical trials has shown that lifestyle modification can delay or prevent the onset of type 2 diabetes,7–9 but adherence to such regimens outside the clinical trial setting has proven difficult.10–12 New approaches to motivate lifestyle change interventions are needed to curb the type 2 diabetes epidemic.
Although not yet part of clinical practice, genetic testing for diabetes risk may become one example of the clinical implementation of “personalized medicine.” Genomewide association studies have identified at least 40 independent single-nucleotide polymorphisms that increase one’s risk of type 2 diabetes.13 Although the addition of genotype information to traditional diabetes prediction models correctly reclassifies less than 10% of individuals into risk categories, having a large number of these variants can more than double one’s risk for type 2 diabetes.13,14 While risk factors such as obesity and family history (a measure of disease inheritance already used in clinical practice) are much stronger predictors of diabetes, it has been proposed that the “personalized” nature of genetic risk information may make it more meaningful to patients than traditional risk factors.2,15
According to the health belief model,16 if genetic risk information changes one’s perceived susceptibility to diabetes, one’s likelihood to adopt health behaviors may also change. For individuals already known to be at high risk for diabetes based on clinical risk factors, a high-risk genetic test result may additionally motivate lifestyle modification if it increases one’s risk perception. However, it will be important that a low-risk genetic result not decrease one’s motivation for lifestyle modification, since weight loss can prevent type 2 diabetes even in those with low-risk genetic profiles.17,18 This directional difference in desired effect—in which high-risk genetic results increase motivation for lifestyle modification but low-risk results do not decrease it—may require of the individual a nuanced understanding of genotypic and phenotypic risk. That is, patients with clinical characteristics such as obesity or metabolic syndrome will need to understand that they are at markedly increased risk for type 2 diabetes regardless of their genetic profiles. In this context, any extra motivation derived from high-risk genetic results may depend more on its “personalized” nature than on its absolute risk reclassification.
The health belief model also theorizes that individual characteristics may modify the behavior change that disease susceptibility perception may affect. A key factor in a patient’s response to test results is his or her understanding of the risk presented, and ample evidence demonstrates that health literacy and numeracy influence that under-standing.19–22 Poor health literacy and numeracy have implications for diabetes prevention. Individuals with limited health literacy use fewer preventive services23,24 and have difficulty estimating food portion sizes.25 Those with poor health numeracy have difficulty interpreting food labels26 and are more likely to be obese.27 Numeracy also influences how individuals interpret medical risk information.19 If new sources of risk information, including genetic testing, are to motivate behavior change, then one must first be able to interpret that information. In addition to health literacy and numeracy, the new domain of genetic literacy may also be important for understanding a patient’s interpretation of genetic risk information.28
Because the desired motivational effects of diabetes genetic testing may require a sophisticated understanding of the relationship between phenotypic and genotypic risk, we hypothesized that health literacy, genetic literacy, and health numeracy may influence the motivational impact derived from genetic risk information. Here we examined the relationship of these 3 domains with anticipated motivation to adopt lifestyle changes in response to “high” and “low” diabetes genetic risk results among prediabetic participants in the Genetic Counseling and Lifestyle Change for Diabetes Prevention (GC/LC) Study.
METHODS
The GC/LC Study29 is an ongoing randomized controlled trial assessing the effects of genetic testing for type 2 diabetes risk plus genetic counseling on participant adherence to a 12-week lifestyle modification curriculum based on the Diabetes Prevention Program.8,30 Study participants were randomized to 2 groups: The intervention arm received a genetic risk score for type 2 diabetes13 plus personalized genetic counseling, while the control group received neither genetic testing nor counseling. Participants underwent a baseline questionnaire that assessed health literacy, numeracy, motivation to prevent diabetes, and perceived risk of diabetes. This report presents the analyses of these baseline characteristics of all GC/ LC Study participants who completed the first baseline visit.
Study Participants
We recruited participants from a large academic hospital and its affiliated primary care practices. Eligible patients were at least 21 years old and had a high risk of developing type 2 diabetes based on features of the metabolic syndrome, as identified in the electronic medical record by a previously validated algorithm.31 Specifically, they were overweight (body mass index ≥ 27.2 kg/m2 in women or ≥ 29.1 kg/m2 in men) and had any 2 of the following additional characteristics: fasting plasma glucose ≥ 100 mg/dL (5.55 mmol/L), blood pressure ≥ 130/85 mmHg or on antihypertensive medication, waist circumference > 35 in. (89 cm) in women or > 40 in. (102 cm) in men, triglycerides ≥ 150 mg/ dL (1.69 mmol/L), and high-density lipoprotein cholesterol < 50 mg/dL (1.3 mmol/L) in women or < 40 mg/dL (1.0 mmol/L) in men. Additional inclusion criteria were physical ability and willingness to participate in the 12-week Diabetes Prevention Program curriculum and proficiency in English. Patients were excluded if they had a diagnosis or treatment for type 1 or 2 diabetes mellitus or cardiovascular disease, including myocardial infarction, heart failure, peripheral vascular disease, or a history of coronary bypass surgery. After permission was obtained from their primary care providers, participants were invited to participate in a study on diabetes prevention. To maximize the participation rate, each eligible person was contacted by letter and at least 1 telephone call. The general information given to the participants at the baseline visit informed them that they were at high risk for developing type 2 diabetes based on the information in their medical records. The Partners Institutional Review Board approved this study, and all patients provided written consent.
Main Measures
The baseline GC/LC Study questionnaire assessed participant characteristics. Race (white/Caucasian, black/African American, American Indian/Alaskan Native, or other) and ethnicity (Hispanic or non-Hispanic) were self-reported and categorized for these analyses as non-Hispanic white or nonwhite. Highest educational attainment was self-reported as “8th grade or less,” “9th–11th grade,” “12th grade or GED,” “1–3 years of college,” or “4 or more years of college or graduate school.” Participants self-reported household income and any family history of diabetes. Participants used a 10-point visual analog scale to rate their current motivation to prevent diabetes. They were also asked to describe their perceived risk of developing diabetes as “almost no chance,” “slight chance,” “moderate chance,” or “high chance.”32
Health literacy was assessed with the validated Rapid Estimate of Adult Literacy in Medicine (REALM).33 We assessed genetic health literacy using the short version of the Rapid Estimate of Adult Literacy in Genetics (REAL-G). This validated instrument was modeled on the REALM and requires respondents to read 8 genetics terms aloud. In the original validation study, this 8-item REAL-G had a Spearman rank coefficient of 0.80 with the REALM and predicted participants’ knowledge after receiving genetic information.28 Health numeracy was assessed with the Schwartz-Woloshin numeracy test, a validated set of 3 questions that ascertains a participant’s comprehension of risks and probabilities. We defined adequate health numeracy as answering 2 or 3 of these questions correctly, because these scores were significantly associated with greater accuracy in applying risk information in the test’s validation study.34
The baseline questionnaire then asked participants to imagine hypothetical situations in which they received genetic test results for diabetes. These questions were modeled on our previous work in a similar population, which used expert review and patient focus groups to develop questions about genetic testing and motivation for lifestyle change.35 Specifically, participants in the present study were given the following scenario:
Imagine that before getting the genetic test, your doctor told you that you were at increased risk for diabetes and recommended lifestyle changes (exercising 30 minutes 5 days a week and changing your diet to lose 7% of your body weight; usually between 10–20 lbs) to prevent diabetes. If your genetic test result indicated that you were at “high” genetic risk for diabetes:
How would this genetic test result change your motivation to make recommended lifestyle changes?
How would this genetic test result change your motivation to take a medicine to prevent diabetes if recommended by your doctor?
Participants were then asked the same questions regarding a hypothetical “low” genetic risk result. The research assistant proctored the survey and was available to answer questions about any of the survey items. Our previous structured interviews have demonstrated that our study population had good comprehension of the scenarios.15 This anticipated motivation change is the primary outcome for the present analyses and was scored on a 5-point Likert scale: “much less motivated,” “somewhat less motivated,” “no change,” “somewhat more motivated,” or “much more motivated.”
Statistical Analysis
Data are reported as means (standard deviation) or medians (interquartile range) for continuous traits and as percentages for categorical data. We used t tests to compare values of age and body mass index between groups. We used Fisher exact tests to compare categorical data (sex, race/ethnicity, family history, and risk perception) and Mantel-Haenszel exact trend tests with 1 degree of freedom to analyze income and education. Because of its nonnormal distribution, we used Wilcoxon rank–sums to test whether anticipated motivation change to hypothetical genetic test results varied among subgroups of participants. We used Spearman rank–testing for correlations between continuous variables. We considered P < 0.05 to indicate statistical significance. We excluded participants with missing data from the corresponding analyses (see Table 1).
Table 1.
Baseline Characteristics of Participants: Overall and Stratified
| Health Literacy33 |
Genetic Literacy28 |
Health Numeracy34 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All, n = 175 | Low, n = 14 |
High, n = 160 |
Pa | Low, n = 66 |
High, n = 108 |
Pa | Low, n = 53 |
High, n = 122 |
Pa | |
| Age,b years | 58 ± 11 | 58 ± 11 | 58 ± 11 | 0.99 | 60 ± 10 | 57 ± 11 | 0.053 | 61 ± 11 | 57 ± 10 | 0.03 |
| Female | 68 (39) | 2 (14) | 66 (41) | 0.08 | 22 (33) | 46 (43) | 0.26 | 24 (45) | 44 (35) | 0.31 |
| Nonwhite | 22 (13) | 6 (43) | 15 (10) | 0.003 | 11 (17) | 10 (10) | 0.16 | 12 (24) | 10 (8) | 0.01 |
| Body mass index,b kg/m2 | 35 ± 5 | 35 ± 6 | 35 ± 5 | 0.89 | 34 ± 4 | 35 ± 5 | 0.13 | 35 ± 4 | 35 ± 5 | 0.84 |
| Annual household income | ||||||||||
| < $30,000 | 30 (18) | 4 (33) | 26 (17) | 0.051c | 18 (29) | 12 (12) | 0.04c | 18 (37) | 12 (10) | <0.001c |
| $30,000–$49,000 | 19 (12) | 2 (17) | 17 (11) | 5 (8) | 14 (14) | 5 (10) | 14 (12) | |||
| $50,000–$99,000 | 46 (28) | 4 (33) | 41 (27) | 16 (25) | 29 (29) | 12 (24) | 34 (29) | |||
| $100,000–$150,000 | 33 (20) | 1 (8) | 32 (21) | 14 (22) | 19 (19) | 11 (22) | 22 (19) | |||
| > $150,000 | 37 (22) | 1 (8) | 36 (24) | 10 (16) | 27 (27) | 3 (6) | 34 (29) | |||
| Education | ||||||||||
| ≤ 12th grade or GED | 34 (19) | 4 (29) | 29 (18) | 0.04c | 20 (30) | 13 (12) | < 0.001c | 18 (34) | 16 (13) | < 0.001c |
| 1–3 years of college | 48 (27) | 6 (43) | 42 (26) | 27 (41) | 21 (19) | 21 (40) | 27 (22) | |||
| ≥ 4 years of college | 93 (53) | 4 (29) | 89 (56) | 19 (29) | 74 (69) | 14 (26) | 79 (65) | |||
| Family history–yes | 96 (55) | 7 (50) | 88 (55) | 0.78 | 40 (61) | 55 (51) | 0.27 | 32 (60) | 64 (52) | 0.41 |
| Perceived risk–high | 118 (68) | 6 (43) | 111 (70) | 0.07 | 41 (62) | 76 (71) | 0.24 | 35 (66) | 83 (69) | 0.73 |
| Motivation to prevent diabetesd | 10 (9–10) | 10 (10–10) | 10 (9–10) | 0.007e | 10 (9–10) | 10 (9–10) | 0.38e | 10 (10–10) | 10 (9–10) | 0.08e |
Note: Values cited as n (%) unless noted. Observations do not necessarily sum to 175 due to missing values: household income (n = 10), race/ethnicity (n = 4), perceived risk of diabetes (n = 1), health literacy (n = 1), and genetic literacy (n = 1).
P values correspond to t tests (continuous variables) or Fisher tests (categorical variables) unless noted. Bold font indicates statistical significance.
Mean ± standard deviation.
Based on 2 trend test.
Median (interquartile range).
Based on Wilcoxon rank-sum test.
RESULTS
Baseline Characteristics of Participants
We contacted 1463 potentially eligible patients by letter and telephone call. Of these, 83 were found to be ineligible for study participation after telephone screening. Of the remaining 1380, 177 (12.8%) consented to enroll in the GC/LC Study and participated in the baseline research visit. We subsequently excluded 2 patients who had diabetes on further review. Table 1 shows the baseline characteristics of the 175 participants who completed the baseline research visit. The majority were male (61%) and non-Hispanic white (85%), and all were overweight or obese. When asked to rate their current motivation to prevent diabetes on a scale of 0 (lowest) to 10 (highest), the median response was 10 (interquartile range, 9–10). As a whole, the group was well educated, with 141 (81%) having completed at least some college. Only 14 (8%) had health literacy at less than a high school reading level. However, 66 (38%) had inadequate genetic literacy, and 53 (30%) had inadequate health numeracy.
Associations With Health Literacy, Genetic Literacy, and Health Numeracy
REALM and REAL-G scores had a Spearman correlation coefficient of 0.60 (P < 0.001), while the numeracy instrument had a correlation coefficient 0.26 with REALM and 0.31 with REAL-G (both P < 0.001). We examined the association between health literacy, genetic literacy, and health numeracy and other patient characteristics (Table 1). Even in this well-educated population, lower educational attainment was associated with lower health literacy (P = 0.04), lower genetic literacy (P < 0.001), and lower health numeracy (P < 0.001). The same associations were evident for lower household income, which was modestly correlated with educational attainment (Spearman coefficient, 0.37; P < 0.001). Participants with low health numeracy were older (P = 0.03) and more likely to be nonwhite (P = 0.01). Health literacy varied significantly by race/ethnicity; although nonwhites composed only 13% of the total sample, they constituted 43% of those with low health literacy (P = 0.003). Health literacy, genetic literacy, and health numeracy did not differ significantly by sex.
Associations of Health Literacy and Numeracy With Motivation Change
Most participants reported that high-risk genetic results would increase their motivation for lifestyle modification (median response corresponding to “much more motivated”) and for medication use to prevent diabetes (median response corresponding to “somewhat more motivated”). Only 3 (2%) reported that high-risk results would decrease or have no effect on their motivation for lifestyle changes, while 39 (23%) said that high-risk results would decrease or have no effect on their motivation to take medication. Despite these apparent ceiling effects, those reporting higher baseline motivation to prevent diabetes and those with at least some college education were more likely to anticipate greater motivation change for lifestyle modification (P = 0.04 and P = 0.003, respectively). Responses to hypothetical high-risk results did not differ by participant health literacy, genetic literacy, or health numeracy.
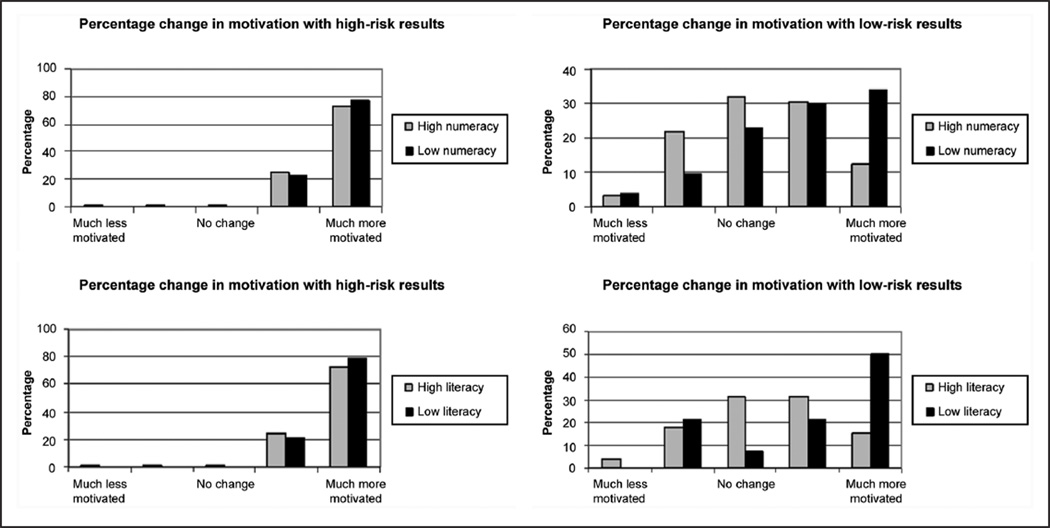
In contrast to the high-risk results, we observed significant variation in responses to low-risk results (Table 2). Thirty-eight participants (22%) anticipated that their motivation for lifestyle modification would decrease, and 73 (42%) anticipated decreased motivation to take medication to prevent diabetes. An increase in motivation for lifestyle modification was more likely to be anticipated by patients who were nonwhite; were ≥ 60 years; had low baseline motivation to prevent diabetes; had low baseline risk perception for diabetes; and had low health literacy, genetic literacy, and health numeracy. Of these patient characteristics, only age ≥ 60 years, low genetic literacy, and low health numeracy were significantly associated with greater motivation for medication use to prevent diabetes in response to low-risk diabetes genetic test results. Lower household income predicted a higher distribution of motivation scores for medication use but not lifestyle modification. Although the median anticipated motivation changes for lifestyle modification and medication use were higher among those with lower educational attainment, the results did not reach statistical significance. Figure 1 illustrates the distribution of anticipated motivation changes for lifestyle modification in response to hypothetical high-risk and low-risk genetic results, stratified by health literacy and health numeracy. The results were similar when stratified by genetic literacy.
Table 2.
Participant-Predicted Change in Motivation for Lifestyle Modification and Medication Initiation in Response to Hypothetical Low-Risk Genetic Testing Results for Diabetes
| Lifestyle Modification |
Medication Use to Prevent Diabetes |
||||
|---|---|---|---|---|---|
| n | Pa | Pa | |||
| Age | |||||
| < 60 | 95 | 0 | 0.02 | −1 | < 0.001 |
| ≥ 60 | 80 | 1 | 0 | ||
| Sex | |||||
| Male | 107 | 0 | 0.95 | 0 | 0.62 |
| Female | 68 | 0.5 | 0 | ||
| Race/ethnicity | |||||
| Non-Hispanic white | 149 | 0 | < 0.001 | 0 | 0.73 |
| Nonwhite | 22 | 1.5 | 0 | ||
| Education | |||||
| ≤ 12th grade or GED | 34 | 1 | 0.40 | 0b | 0.09 |
| At least some college | 141 | 0 | 0 | ||
| Body mass index | |||||
| < 35 | 105 | 0 | 0.61 | 0 | 0.75 |
| ≥ 35 | 70 | 0.5 | 0 | ||
| Income | |||||
| < $50,000 | 59 | 1 | 0.15 | 0b | 0.04 |
| ≥ $50,000 | 116 | 0 | 0 | ||
| Family history | |||||
| No | 79 | 0 | 0.31 | 0 | 0.13 |
| Yes | 96 | 1 | 0 | ||
| Risk perception | |||||
| Low | 56 | 1 | 0.047 | 0 | 0.69 |
| High | 118 | 0 | 0 | ||
| Motivation to prevent diabetes | |||||
| Low | 58 | 0 | 0.01 | 0 | 0.64 |
| High | 117 | 1 | 0 | ||
| Health literacy | |||||
| High | 160 | 0 | 0.04 | 0 | 0.11 |
| Low | 14 | 1.5 | 0 | ||
| Genetic literacy | |||||
| High | 108 | 0 | 0.02 | −0.5 | 0.003 |
| Low | 66 | 1 | 0 | ||
| Health numeracy | |||||
| High | 118 | 0 | 0.02 | 0 | 0.04 |
| Low | 57 | 1 | 0b | ||
Note: Data presented are median change in motivation for each group: –2, much less motivated; –1, somewhat less motivated; 0, no change in motivation; 1, somewhat more motivated; 2, much more motivated. Bold font indicates statistical significance.
P values correspond to Wilcoxon rank sum tests.
Group with higher distribution of responses.
Figure 1.
Distribution of responses to how motivation for lifestyle modification would change in hypothetical scenarios of high- and low-risk genetic results for type 2 diabetes, stratified by health literacy and health numeracy.
DISCUSSION
Our survey of overweight patients at high risk for diabetes yielded 3 important results regarding the anticipated motivational impact of diabetes genetic risk testing. First, our cohort uniformly (98%) anticipated that high-risk genetic results would increase their motivation to make lifestyle changes to prevent diabetes. Thus, we found no evidence that high-risk results brought a sense of fatalism to the participants, since almost no patients reported that such results would decrease their motivation for behavior change. These results are consistent with our previous survey of nondiabetic primary care patients, of whom 71% anticipated that high-risk genetic results would increase their motivation to prevent diabetes35 and with qualitative interviews with individuals at high risk for diabetes.15 Whether these anticipated reactions translate into true behavior change will determine the degree to which the concerns about genetic fatalism can be allayed. We did observe that fewer respondents (77%) anticipated that high-risk results would increase their motivation to take medications to prevent diabetes. A qualitative study of primary care patients in the United Kingdom has similarly demonstrated a tendency for patients to prefer lifestyle modification over medication use for cardiovascular disease prevention. In that study, respondents frequently cited concern for adverse effects as the reason for this preference.36
Second, we observed a greater range of responses to hypothetical low-risk results, including sizable proportions anticipating decreased motivation for lifestyle change and motivation to take a medication to prevent diabetes. This decreased motivation is concerning if it indeed results in poorer adherence to the recommendations for lifestyle modification necessary to prevent diabetes. Even more so than in healthy populations, motivational genetic testing in phenotypically at-risk individuals should have a greater responsibility not to decrease motivation for clinically relevant behavior change. Given their clinical risk factors, all participants already had high risk for diabetes, regardless of their genetic risk. Moreover, the Diabetes Prevention Program has shown that they would all benefit from lifestyle modification, independently of their genetic profiles.17,18,37 Our findings raise the concern that low-risk genetic results might decrease the motivation for some at-risk individuals to adopt preventive health behaviors. Prospective studies are needed to assess whether this is true. If so, it would be consistent with the health belief model, in which lower perception of disease susceptibility might translate to a lower likelihood of adopting healthy behaviors.
Our third major finding is the identification of patient characteristics associated with different responses to the hypothetical situation of low-risk genetic results for diabetes. We found that participants who anticipated decreased motivation for lifestyle modification in response to low-risk results were more likely to be younger than 60, be non-Hispanic white, and have high health literacy, genetic literacy, and health numeracy. They were also more likely to have low baseline motivation to prevent diabetes but high diabetes risk perception. Many of the risk factors for an anticipated decrease in motivation were correlated: Adequate health numeracy was associated with younger age and non-Hispanic white race/ethnicity. Educational attainment was not associated with anticipated motivation change from low-risk results, but it was significantly associated with health literacy, genetic literacy, and health numeracy. These 3 domains are attractive candidates for the mechanisms through which participant characteristics might modify the motivational effect of low-risk genetic results. It could be that numerate participants approached this risk scenario more mathematically and reasoned that low-risk results should lower, or at least not change one’s motivation for lifestyle modification. However, less numerate individuals, who tend to overestimate their risk and the benefit of prevention or treatment when presented with quantitative risk information,38 may have had more difficulty with the scenario and approached it with more of an emotional response.39
This is the first study to examine the association between health literacy, genetic literacy, and health numeracy and anticipated motivation change for diabetes prevention from genetic testing. In our sample, the 8-item REAL-G had a Spearman correlation coefficient of 0.60 with the REALM, compared with 0.80 in the original validation study.28 It is possible that the age difference between the participants of the 2 studies explains the discrepant correlation coefficients: Our participants had a mean age of 58 years, while the validation study participants had a mean age of 40 years. A recent survey of 971 racially diverse participants explored health beliefs about the behavioral and genetic components of risk for obesity and diabetes.40 This study found that older age groups (particularly older than 50 years) had lower health literacy and genetic knowledge, as measured with 5 questions that assess understanding of basic genetics concepts. After adjustment for covariates, including education, age, and race, lower genetic knowledge was associated with a greater likelihood to attribute one’s body weight, but not one’s risk of diabetes, to genetic rather than behavioral factors. The authors of this study concluded that patients with lower genetic knowledge might therefore be less motivated to lose weight, since they are more likely to consider weight genetically determined. Our results suggest the opposite may be true. In a highly motivated population, we found that lower health literacy, genetic literacy, and health numeracy were in fact associated with higher motivation for lifestyle modification and medication use to prevent diabetes in the hypothetical situation of low-risk genetic results. It is important to note, however, that the REAL-G used in the present study was designed to measure genetic literacy and not genetic knowledge, although its validation study demonstrated that it did predict performance on a genetics learning task.28
The present analysis must be interpreted in the context of the study design. Although it permits the examination of the participants’ baseline approach to genetic risk information, the cross-sectional design tempers the conclusions that one can draw about how well anticipated motivation change from genetic results will correlate with actual health behavior. The degree to which survey demand characteristics positively or negatively influenced self-reported anticipated motivation change is unknown.41,42 It is also possible that because the hypothetical high-risk scenario was always presented before the low-risk scenario, a framing bias may have influenced how participants responded to the low-risk results. We do not know whether the use of a 5-point scale to measure baseline motivation and a 10-point scale to measure anticipated motivation change affected participant responses.
Our analyses cannot disentangle the interplay among the many patient characteristics that may influence the motivational impact of genetic results. While the domains of literacy and numeracy have face validity as important effect modifiers in the response to genetic risk information, we have demonstrated that they covary with other patient characteristics, including age, education, and race/ ethnicity. Moreover, our study population is not representative of the average patient at high risk for diabetes. Our participants had relatively high educational attainment and household incomes. They also self-described as already extremely motivated to prevent diabetes; thus, they may not be the ultimate target population for genetic testing for behavior change motivation. These characteristics, in addition to our low recruitment rate, limit the generalizability of our findings. Even within this relatively homogeneous sample, we still observed significant differences in the way that participants responded to hypothetical low-risk genetic results. Studies in more diverse populations will likely uncover even greater differences. It will be particularly important to include participants with lower baseline motivation to prevent diabetes, because genetic testing may have the greatest motivational impact for such individuals.
The limitations of the present study suggest areas for future research. Prospective studies should assess whether genetic risk information changes motivation and whether motivation change translates into actual behavior change. If such changes are indeed observed, it will be important to elucidate the specific mechanisms by which an individual’s ability to interpret genetic risk information might mediate those changes. Our results suggest that health literacy, health numeracy, and genetic literacy influence one’s response to genetic risk information, but larger studies will be able to adjust for potential confounders of these apparent associations. Moreover, the REAL-G may not be the best measure of one’s understanding of genetic concepts and their relevance to health risk. Studies should further assess the relationship between genetic literacy and genetic knowledge as they pertain to the interpretation of risk information. In addition, the communication of absolute risk information in future research (as opposed to the relative nonquantitative “high” and “low” results used in our hypothetical scenarios) will allow a better understanding of how respondents perceive and valuate risk.
Our findings may have critical implications for the delivery of genetic risk information and its use in medical decision making. At present, diabetes genetic risk information cannot be recommended for risk reclassification over routine clinical factors, such as family history and obesity. However, whether the “personalized” nature of genetic information has greater value for motivating behavior change than other clinical risk factors is an area of active research. If future studies confirm that certain patient characteristics modify the potential motivational impact of genetic testing, then clinicians might take these characteristics into account before offering genetic testing and when delivering results. Baseline assessment of patient characteristics including genetic literacy and health numeracy may be prudent to help frame the delivery of genetic risk information. For example, our results suggest that patients with greater genetic literacy and numeracy who receive low-risk genetic results may require more emphasis on their phenotypic risk to motivate them for behavior change. Although our results give cause for optimism that those with low health literacy, genetic literacy, and health numeracy may be motivated for health behavior change by genetic risk results, it is noteworthy that this is a vulnerable and prevalent group. About 36% of the US adult population has limited health literacy, and 55% have basic or below-basic numeracy skills.43 Poor health literacy is associated with higher incidence of chronic disease and lower utilization of preventive health services.23 It will be important to examine whether the increased motivation that these groups reported in our study translates into the behavior changes that will improve their health.
ACKNOWLEDGMENTS
We thank Katherine Stember for her assistance with data entry and figure design. Dr. Florez has received consulting honoraria from Daiichi-Sankyo and AstraZeneca. Dr. Meigs has a consulting agreement with Interleukin Genetics, Inc. This work was funded by the National Institutes of Health and the National Institute of Diabetes and Digestive and Kidney (R21 DK84527). Dr. Vassy is supported by an National Institutes of Health T32 National Research Service Award (HP12706-02-00) from the Health Resources and Services Administration and the National Institutes of Health’s Loan Repayment Program (per the National Institute of Diabetes and Digestive and Kidney). Dr. Meigs is supported by the National Institute of Diabetes and Digestive and Kidney (K24 DK080140). The funding agreements ensured our independence in designing the study, interpreting the data, writing, and publishing the report.
REFERENCES
- 1.Brower V. FDA to regulate direct-to-consumer genetic tests. J Natl Cancer Inst. 2010;102(21):1610–1617. doi: 10.1093/jnci/djq446. [DOI] [PubMed] [Google Scholar]
- 2.McBride CM, Koehly LM, Sanderson SC, Kaphingst KA. The behavioral response to personalized genetic information: will genetic risk profiles motivate individuals and families to choose more healthful behaviors? Annu Rev Public Health. 2010;31:89–103. doi: 10.1146/annurev.publhealth.012809.103532. [DOI] [PubMed] [Google Scholar]
- 3.Chao S, Roberts JS, Marteau TM, Silliman R, Cupples LA, Green RC. Health behavior changes after genetic risk assessment for Alzheimer disease: the REVEAL Study. Alzheimer Dis Assoc Disord. 2008;22(1):94–97. doi: 10.1097/WAD.0b013e31815a9dcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanderson SC, Michie S. Genetic testing for heart disease susceptibility: potential impact on motivation to quit smoking. Clin Genet. 2007;71(6):501–510. doi: 10.1111/j.1399-0004.2007.00810.x. [DOI] [PubMed] [Google Scholar]
- 5.Hamajima N, Suzuki K, Ito Y, Kondo T. Genotype announcement to Japanese smokers who attended a health checkup examination. J Epidemiol. 2006;16(1):45–47. doi: 10.2188/jea.16.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marteau T, Senior V, Humphries SE, et al. Psychological impact of genetic testing for familial hypercholesterolemia within a previously aware population: a randomized controlled trial. Am J Med Genet A. 2004;128(3):285–293. doi: 10.1002/ajmg.a.30102. [DOI] [PubMed] [Google Scholar]
- 7.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: the Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 8.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindstro¨m J, Louheranta A, Mannelin M, et al. The Finnish Diabetes Prevention Study (DPS) Diabetes Care. 2003;26(12):3230–3236. doi: 10.2337/diacare.26.12.3230. [DOI] [PubMed] [Google Scholar]
- 10.Zhao G, Ford ES, Li C, Mokdad AH. Compliance with physical activity recommendations in US adults with diabetes. Diabet Med. 2008;25(2):221–227. doi: 10.1111/j.1464-5491.2007.02332.x. [DOI] [PubMed] [Google Scholar]
- 11.Nelson KM, Reiber G, Boyko EJ. Diet and exercise among adults with type 2 diabetes: findings from the third national health and nutrition examination survey (NHANES III) Diabetes Care. 2002;25(10):1722–1728. doi: 10.2337/diacare.25.10.1722. [DOI] [PubMed] [Google Scholar]
- 12.Prevalence of self-reported physically active adults: United States, 2007. MMWR Morb Mortal Wkly Rep. 2008;57(48):1297–1300. [PubMed] [Google Scholar]
- 13.de Miguel-Yanes JM, Shrader P, Pencina MJ, et al. Genetic risk reclassification for type 2 diabetes by age below or above 50 years using 40 type 2 diabetes risk single nucleotide polymorphisms. Diabetes Care. 2011;34(1):121–125. doi: 10.2337/dc10-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meigs JB, Shrader P, Sullivan LM, et al. Genotype score in addition to common risk factors for prediction of type 2 diabetes. N Engl J Med. 2008;359(21):2208–2219. doi: 10.1056/NEJMoa0804742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Markowitz SM, Park ER, Delahanty LM, O’Brien KE, Grant RW. Perceived impact of diabetes genetic risk testing among patients at high phenotypic risk for type 2 diabetes. Diabetes Care. 2011;34(3):568–573. doi: 10.2337/dc10-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker MH. The health belief model and sick role behavior. Health Educ Monogr. 1972;2:409–419. [Google Scholar]
- 17.Jablonski KA, McAteer JB, de Bakker PI, et al. Common variants in 40 genes assessed for diabetes incidence and response to metformin and lifestyle intervention in the diabetes prevention program. Diabetes. 2010;59(10):2672–2681. doi: 10.2337/db10-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hivert MF, Jablonski KA, Perreault L, et al. An updated genetic score based on 34 confirmed type 2 diabetes loci is associated with diabetes incidence and regression to normoglycemia in the Diabetes Prevention Program. Diabetes. 2011;60(4):1340–1348. doi: 10.2337/db10-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters E, Hart PS, Fraenkel L. Informing patients: the influence of numeracy, framing, and format of side effect information on risk perceptions. Med Decis Making. 2011;31(3):432–436. doi: 10.1177/0272989X10391672. [DOI] [PubMed] [Google Scholar]
- 20.Brewer NT, Tzeng JP, Lillie SE, Edwards AS, Peppercorn JM, Rimer BK. Health literacy and cancer risk perception: implications for genomic risk communication. Med Decis Making. 2009;29(2):157–166. doi: 10.1177/0272989X08327111. [DOI] [PubMed] [Google Scholar]
- 21.Brown SM, Culver JO, Osann KE, et al. Health literacy, numeracy, and interpretation of graphical breast cancer risk estimates. Patient Educ Couns. 2011;83(1):92–98. doi: 10.1016/j.pec.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tait AR, Voepel-Lewis T, Zikmund-Fisher BJ, Fagerlin A. Presenting research risks and benefits to parents: does format matter? Anesth Analg. 2010;111(3):718–723. doi: 10.1213/ANE.0b013e3181e8570a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berkman N, DeWalt D, Pignone M, et al. Health Literacy: Impact on Health Outcomes. Rockville, MD: Agency for Healthcare Research and Quality; 2004. [Google Scholar]
- 24.Scott TL, Gazmararian JA, Williams MV, Baker DW. Health literacy and preventive health care use among Medicare enrollees in a managed care organization. Med Care. 2002;40(5):395–404. doi: 10.1097/00005650-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Huizinga MM, Carlisle AJ, Cavanaugh KL, et al. Literacy, numeracy, and portion-size estimation skills. Am J Prev Med. 2009;36(4):324–328. doi: 10.1016/j.amepre.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothman RL, Housam R, Weiss H, et al. Patient understanding of food labels: the role of literacy and numeracy. Am J Prev Med. 2006;31(5):391–398. doi: 10.1016/j.amepre.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 27.Huizinga MM, Beech BM, Cavanaugh KL, Elasy TA, Rothman RL. Low numeracy skills are associated with higher BMI. Obesity. 2008;16(8):1966–1968. doi: 10.1038/oby.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erby LH, Roter D, Larson S, Cho J. The rapid estimate of adult literacy in genetics (REAL-G): a means to assess literacy deficits in the context of genetics. Am J Med Genet A. 2008;146(2):174–181. doi: 10.1002/ajmg.a.32068. [DOI] [PubMed] [Google Scholar]
- 29.Grant RW, Meigs JB, Florez JC, et al. Design of a randomized trial of diabetes genetic risk testing to motivate behavior change: the Genetic Counseling/Lifestyle Change (GC/LC) Study to Prevent Diabetes. Clin Trials. 2011;8(5):609–615. doi: 10.1177/1740774511414159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25(12):2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hivert MF, Grant RW, Shrader P, Meigs JB. Identifying primary care patients at risk for future diabetes and cardiovascular disease using electronic health records. BMC Health Serv Res. 2009;9:170. doi: 10.1186/1472-6963-9-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker EA, Mertz CK, Kalten MR, Flynn J. Risk perception for developing diabetes. Diabetes Care. 2003;26(9):2543–2548. doi: 10.2337/diacare.26.9.2543. [DOI] [PubMed] [Google Scholar]
- 33.Davis TC, Long SW, Jackson RH, et al. Rapid estimate of adult literacy in medicine: a shortened screening instrument. Fam Med. 1993;25(6):391–395. [PubMed] [Google Scholar]
- 34.Schwartz LM, Woloshin S, Black WC, Welch HG. The role of numeracy in understanding the benefit of screening mammography. Ann Intern Med. 1997;127(11):966–972. doi: 10.7326/0003-4819-127-11-199712010-00003. [DOI] [PubMed] [Google Scholar]
- 35.Grant RW, Hivert M, Pandiscio JC, Florez JC, Nathan DM, Meigs JB. The clinical application of genetic testing in type 2 diabetes: a patient and physician survey. Diabetologia. 2009;52(11):2299–2305. doi: 10.1007/s00125-009-1512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gale N, Greenfield S, Gill P, Gutridge K, Marshall T. Patient and general practitioner attitudes to taking medication to prevent cardiovascular disease after receiving detailed information on risks and benefits of treatment: a qualitative study. BMC Fam Pract. 2011;12(1):59. doi: 10.1186/1471-2296-12-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Florez JC, Jablonski KA, Bayley N, et al. TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N Engl J Med. 2006;355(3):241–250. doi: 10.1056/NEJMoa062418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reyna VF, Nelson WL, Han PK, Dieckmann NF. How numeracy influences risk comprehension and medical decision making. Psychol Bull. 2009;135(6):943–973. doi: 10.1037/a0017327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schapira MM, Fletcher KE, Gilligan MA, et al. A framework for health numeracy: how patients use quantitative skills in health care. J Health Commun. 2008;13(5):501–517. doi: 10.1080/10810730802202169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ashida S, Goodman M, Pandya C, et al. Age differences in genetic knowledge, health literacy and causal beliefs for health conditions. Public Health Genomics. 2011;14(4–5):307–316. doi: 10.1159/000316234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orne MT. On the social psychology of the psychological experiment: with particular reference to demand characteristics and their implications. Am Psychol. 1962;17:776–783. [Google Scholar]
- 42.Nichols AL, Maner JK. The good-subject effect: investigating participant demand characteristics. J Gen Psychol. 2008;135(2):151–165. doi: 10.3200/GENP.135.2.151-166. [DOI] [PubMed] [Google Scholar]
- 43.Kutner M, Greenberg E, Jin Y, Paulsen C. The Health Literacy of America’s Adults: Results From the 2003 National Assessment of Adult Literacy. Washington, DC: US Department of Education, National Center for Education Statistics; 2006. [Google Scholar]