Abstract
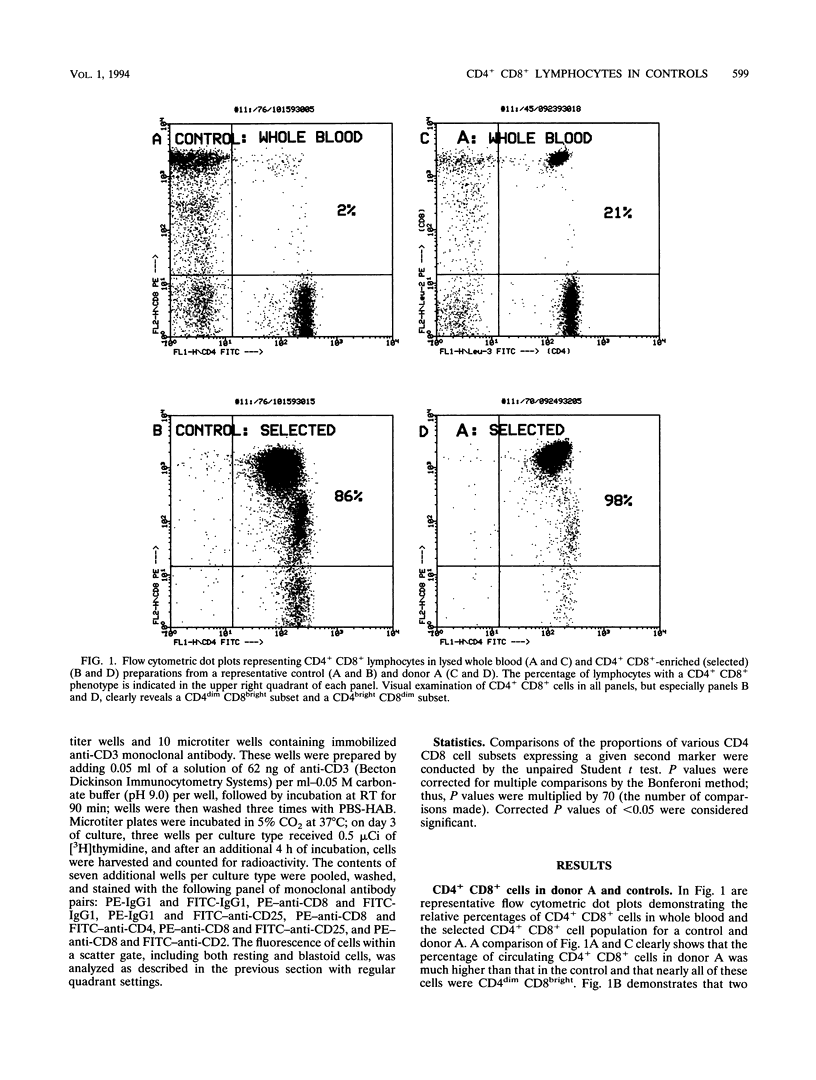
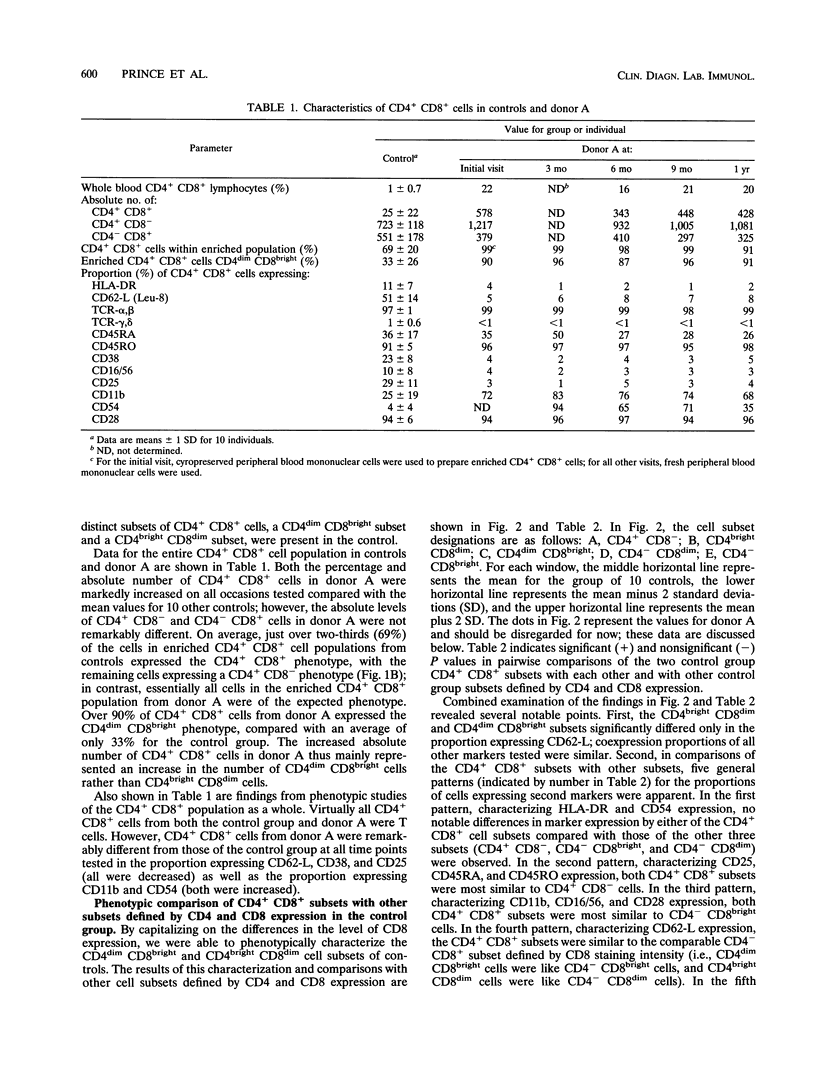
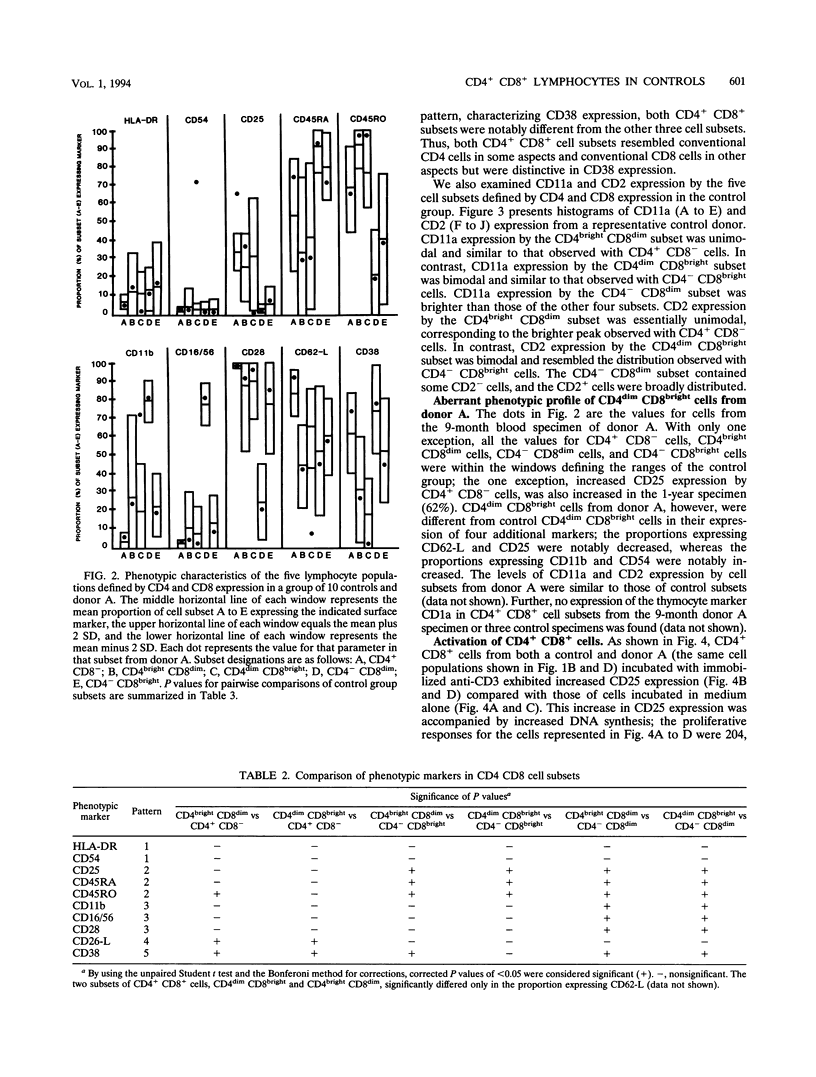
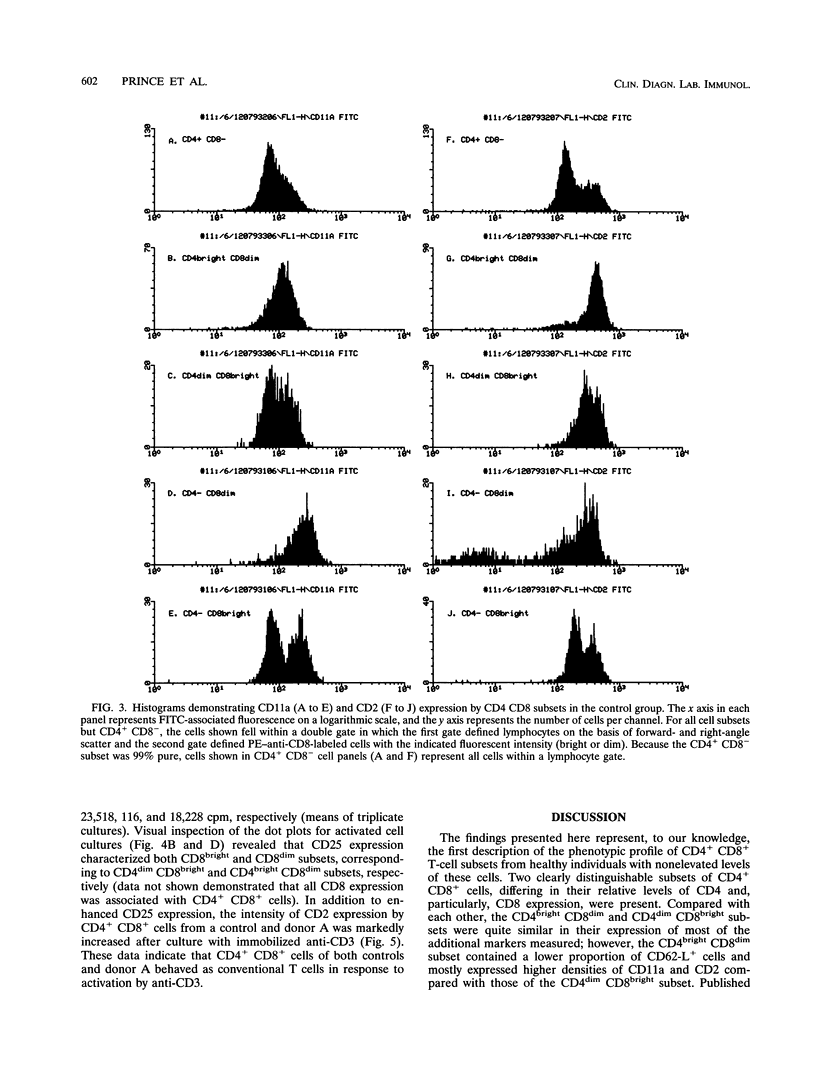
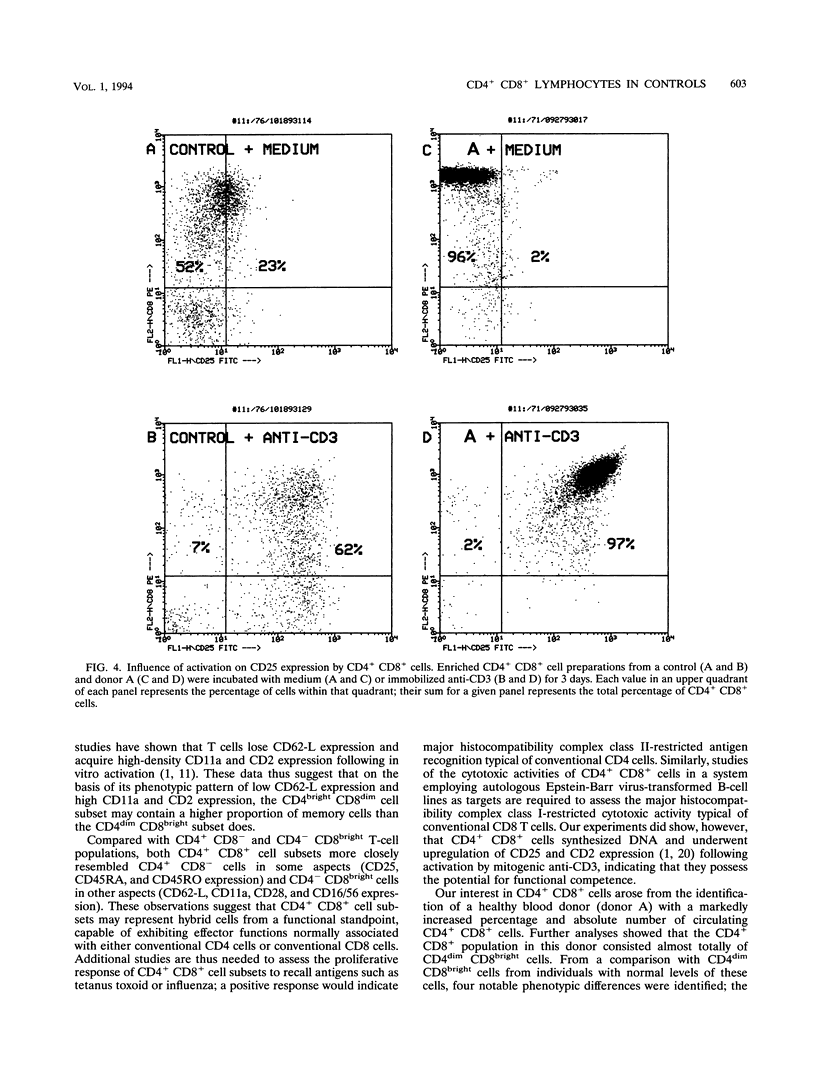
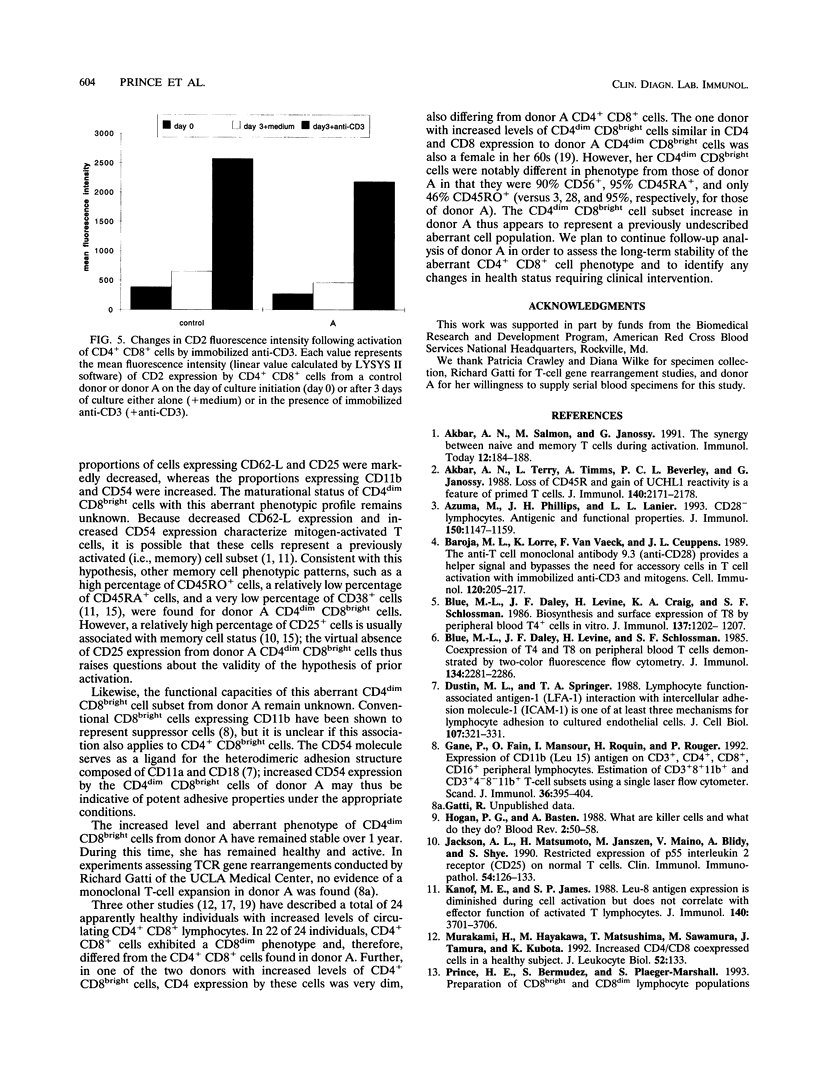
During flow cytometric analysis of lymphocytes from healthy donors, we identified a donor (donor A) with 22% CD4+ CD8+ cells (versus values of < 4% for 65 other controls). To determine if CD4+ CD8+ cells from donor A and other controls were similar, we first defined the phenotypic profile of control CD4+ CD8+ cells. Enriched CD4+ CD8+ cell populations for 10 controls were prepared by a two-step positive selection scheme with anti-CD4-coated magnetic beads and anti-CD8-coated culture flasks; the selected population averaged 69% CD4+ CD8+ cells and 31% CD4+ CD8- cells. For all 10 controls, two subsets of CD4+ CD8+ cells, CD4dim CD8bright and CD4bright CD8dim, were observed. Phenotypic profiles of these two CD4+ CD8+ subsets were defined by pairing anti-CD8 with other monoclonal antibodies, and the profiles were compared with each other and with those of CD4+ CD8-, CD4- CD8bright, and CD4- CD8dim cells. CD8bright and CD4bright CD8dim cells differed in their proportions of CD62-L+ cells and in their levels of CD11a and CD2 expression. Both CD4+ CD8+ subsets resembled CD4+ CD8- cells in CD45RA, CD45RO, and CD25 expression; the comparable CD- CD8+ cells in CD62-L expression; and CD4- CD8bright cells in CD11b, CD11b, CD16/56, and CD28 expression. CD38 expression in both CD4+ CD8+ subsets was decreased compared with those of other cell subsets. Whereas control CD4+ CD8+ cells averaged 33% CD4dim CD8bright, CD4+ CD8+ cells from donor A were > 90% CD4dim CD8bright. Donor A CD4dim CD8bright cells exhibited proportional decreases in CD25 and CD62-L expression and increases in CD11b and CD54 expression compared with those of control CD4dim CD8bright cells.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar A. N., Salmon M., Janossy G. The synergy between naive and memory T cells during activation. Immunol Today. 1991 Jun;12(6):184–188. doi: 10.1016/0167-5699(91)90050-4. [DOI] [PubMed] [Google Scholar]
- Akbar A. N., Terry L., Timms A., Beverley P. C., Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988 Apr 1;140(7):2171–2178. [PubMed] [Google Scholar]
- Azuma M., Phillips J. H., Lanier L. L. CD28- T lymphocytes. Antigenic and functional properties. J Immunol. 1993 Feb 15;150(4):1147–1159. [PubMed] [Google Scholar]
- Baroja M. L., Lorre K., Van Vaeck F., Ceuppens J. L. The anti-T cell monoclonal antibody 9.3 (anti-CD28) provides a helper signal and bypasses the need for accessory cells in T cell activation with immobilized anti-CD3 and mitogens. Cell Immunol. 1989 Apr 15;120(1):205–217. doi: 10.1016/0008-8749(89)90188-3. [DOI] [PubMed] [Google Scholar]
- Blue M. L., Daley J. F., Levine H., Craig K. A., Schlossman S. F. Biosynthesis and surface expression of T8 by peripheral blood T4+ cells in vitro. J Immunol. 1986 Aug 15;137(4):1202–1207. [PubMed] [Google Scholar]
- Blue M. L., Daley J. F., Levine H., Schlossman S. F. Coexpression of T4 and T8 on peripheral blood T cells demonstrated by two-color fluorescence flow cytometry. J Immunol. 1985 Apr;134(4):2281–2286. [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J Cell Biol. 1988 Jul;107(1):321–331. doi: 10.1083/jcb.107.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gane P., Fain O., Mansour I., Roquin H., Rouger P. Expression of CD11b (Leu15) antigen on CD3+, CD4+, CD8+, CD16+ peripheral lymphocytes. Estimation of CD3+8+11b+ and CD3+4-8-11b+ T-cell subsets using a single laser flow cytometer. Scand J Immunol. 1992 Sep;36(3):395–404. doi: 10.1111/j.1365-3083.1992.tb02953.x. [DOI] [PubMed] [Google Scholar]
- Hogan P. G., Basten A. What are killer cells and what do they do? Blood Rev. 1988 Mar;2(1):50–58. doi: 10.1016/0268-960x(88)90008-2. [DOI] [PubMed] [Google Scholar]
- Jackson A. L., Matsumoto H., Janszen M., Maino V., Blidy A., Shye S. Restricted expression of p55 interleukin 2 receptor (CD25) on normal T cells. Clin Immunol Immunopathol. 1990 Jan;54(1):126–133. doi: 10.1016/0090-1229(90)90012-f. [DOI] [PubMed] [Google Scholar]
- Kanof M. E., James S. P. Leu-8 antigen expression is diminished during cell activation but does not correlate with effector function of activated T lymphocytes. J Immunol. 1988 Jun 1;140(11):3701–3706. [PubMed] [Google Scholar]
- Murakami H., Hayakawa M., Matsushima T., Sawamura M., Tamura J., Kubota K. Increased CD4/CD8 coexpressed cells in a healthy subject. J Leukoc Biol. 1992 Jul;52(1):133–133. doi: 10.1002/jlb.52.1.133. [DOI] [PubMed] [Google Scholar]
- Prince H. E., Bermudez S., Plaeger-Marshall S. Preparation of CD8bright and CD8dim lymphocyte populations using two positive selection methods in tandem. J Immunol Methods. 1993 Oct 15;165(2):139–148. doi: 10.1016/0022-1759(93)90339-9. [DOI] [PubMed] [Google Scholar]
- Prince H. E., Lesar W. J. Simultaneous determination of absolute total lymphocyte and CD4+ lymphocyte levels in peripheral blood by flow cytometry. Am J Clin Pathol. 1989 Aug;92(2):206–209. doi: 10.1093/ajcp/92.2.206. [DOI] [PubMed] [Google Scholar]
- Prince H. E., York J., Jensen E. R. Phenotypic comparison of the three populations of human lymphocytes defined by CD45RO and CD45RA expression. Cell Immunol. 1992 Dec;145(2):254–262. doi: 10.1016/0008-8749(92)90329-n. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S. J., Sivakumaran M., Parapia L. A., Balfour I., Norfolk D. R., Kaeda J., Scott C. S. A distinct large granular lymphocyte (LGL)/NK-associated (NKa) abnormality characterized by membrane CD4 and CD8 coexpression. The Yorkshire Leukaemia Group. Br J Haematol. 1992 Nov;82(3):494–501. doi: 10.1111/j.1365-2141.1992.tb06458.x. [DOI] [PubMed] [Google Scholar]
- Rubin B., Geisler G., Caspar S., Arnaud J. The indispensable CD2-CD3 molecules: a key to T-cell differentiation and functional activation. Scand J Immunol. 1992 Jul;36(1):1–6. doi: 10.1111/j.1365-3083.1992.tb02933.x. [DOI] [PubMed] [Google Scholar]
- Sala P., Tonutti E., Feruglio C., Florian F., Colombatti A. Persistent expansions of CD4+ CD8+ peripheral blood T cells. Blood. 1993 Sep 1;82(5):1546–1552. [PubMed] [Google Scholar]
- Waldmann T. A. The structure, function, and expression of interleukin-2 receptors on normal and malignant lymphocytes. Science. 1986 May 9;232(4751):727–732. doi: 10.1126/science.3008337. [DOI] [PubMed] [Google Scholar]
- Zola H., Flego L., Macardle P. J., Donohoe P. J., Ranford J., Roberton D. The CD45RO (p180, UCHL1) marker: complexity of expression in peripheral blood. Cell Immunol. 1992 Nov;145(1):175–186. doi: 10.1016/0008-8749(92)90321-f. [DOI] [PubMed] [Google Scholar]



