Summary
Toxoplasma gondii uses specialized secretory organelles called rhoptries to deliver virulence determinants into the host cell during parasite invasion. One such determinant called rhoptry protein 18 (ROP18) is a polymorphic serine/threonine kinase that phosphorylates host targets to modulate acute virulence. Following secretion into the host cell, ROP18 traffics to the parasitophorous vacuole membrane (PVM) where it is tethered to the cytosolic face of this host-pathogen interface. However, the functional consequences of PVM association are not known. In this report, we show that ROP18 mutants altered in an arginine-rich domain upstream of the kinase domain fail to associate to the PVM following secretion from rhoptries. During infection, host cells up-regulate immunity-related GTPases that localize to and destroy the PVM surrounding the parasites. ROP18 disarms this host innate immune pathway by phosphorylating IRGs in a critical GTPase domain and preventing loading on the PVM. Vacuole-targeting mutants of ROP18 failed to phosphorylate Irga6 and were unable to divert IRGs from the PVM, despite retaining intrinsic kinase activity. As a consequence, these mutants were avirulent in a mouse model of acute toxoplasmosis. Thus, the association of ROP18 with the PVM, mediated by its N-terminal arginine-rich domain, is critical to its function as a virulence determinant.
Introduction
The protozoan Toxoplasma gondii is an obligate intracellular parasite that employs actin-based propulsion to invade host cells (Shen et al., 2012). The invasion process is highly dynamic with fast kinetics, ending in under 20 sec with the formation of the parasitophorous vacuole (PV) within which the parasite resides (Morisaki et al., 1995). Once inside the PV, the parasite is restricted from the host cytosol by the vacuolar membrane (PVM), which is derived by parasite invagination of the host plasma membrane during invasion (Mordue et al., 1999, Suss-Toby et al., 1996). The PV provides a refuge for parasite replication but also provides a foreign structure on which the host can mount an attack. In order to protect its niche, the parasite modifies the PVM by stripping host-derived integral membrane components and decorating this interface with parasite encoded effector proteins (Mordue et al., 1999). To accomplish this feat, Toxoplasma utilizes protein secretion from specialized secretory organelles limited to apicomplexan parasites called rhoptries (Boothroyd et al., 2008, Bradley et al., 2007).
Rhoptries are club-shaped organelles emanating from the apical end of the parasite that discharge their contents directly into the host cell during invasion (Carruthers et al., 1997). Recent forward genetic analysis revealed that rhoptries deploy key virulence determinants, including ROP18, into the host cell to modulate strain-specific parasite virulence (Behnke et al., 2011, Reese et al., 2011, Saeij et al., 2006, Taylor et al., 2006). Although ROPs have various destinations in the host cell, ROP18 traffics back to the nascent PVM following secretion and associates with this membrane for the duration of the intracellular cycle (El Hajj et al., 2007, Taylor et al., 2006). To modulate infection outcomes, ROP18 targets host-expressed immunity related GTPases (IRGs) by phosphorylating key threonine residues in switch region I of the GTPase domain (Fentress et al., 2010, Steinfeldt et al., 2010a). IRGs are key mediators of Toxoplasma resistance that are up-regulated during the course of infection through interferon-γ (IFN-γ) signaling (Taylor et al., 2007). Upon host cell infection by Toxoplasma, IRGs traffic to the PVM in a GTP-bound, oligomerized form (Papic et al., 2008), resulting in vesiculation of the PVM and dissolution of the membrane leaving the parasite exposed to the host cytosol where it undergoes a necrotic-like death (Martens et al., 2005). ROP18 phosphorylation of IRGs is associated with their displacement from the PVM, protection of the vacuolar membrane, and survival of the parasite (Fentress et al., 2010, Steinfeldt et al., 2010a).
Although some ROPs were once thought to mediate membrane binding via a predicted C-terminal transmembrane domain (Beckers et al., 1994); (Sinai et al., 2001), recent molecular modeling (El Hajj et al., 2006) and structural work (Labesse et al., 2009, Qiu et al., 2008) reveals that C-terminus of mature ROPs are instead dominated by a serine/threonine kinase domain, inconsistent with this mode of membrane binding. Analysis of ROP sequences reveals that these proteins lack residues that are predicted to be modified by fatty acid anchors (Reese et al., 2009). Instead, vacuole localized ROPs have three amphipathic alpha helices that are to various degrees necessary and sufficient for membrane binding in a heterologous system of mammalian cell expression (Labesse et al., 2009, Reese et al., 2009). Nuclear magnetic resonance elucidated a structured and partially helical nature of a peptide corresponding to the middle helix for ROP2 in the presence of deuterated lipid (Labesse et al., 2009). These helices have both a basic and hydrophobic characteristic, with hydrophobic residues aligning on one face of the helix to provide an amphipathic nature to the structure (Reese et al., 2009). Basic arginine residues are the most abundant amino acid in this domain, accounting for 14.8% of amino acids in the ROP18 membrane binding domain, and are required for localization to the PVM in a heterologous system (Reese et al., 2009).
The importance of PVM binding for any ROP effector remains to be directly tested. ROP18 provides a prime candidate to elucidate the significance of vacuole membrane association due to its robust phenotypes in modulation of the IRG pathway and in acute virulence. In this study, we report the use of an endogenous parasite expression system to test the cellular targeting and functionality of ROP18 mutants modified in the arginine-rich vacuole-targeting domain.
Results
Expression of ROP18 mutants deficient in arginine-rich helices
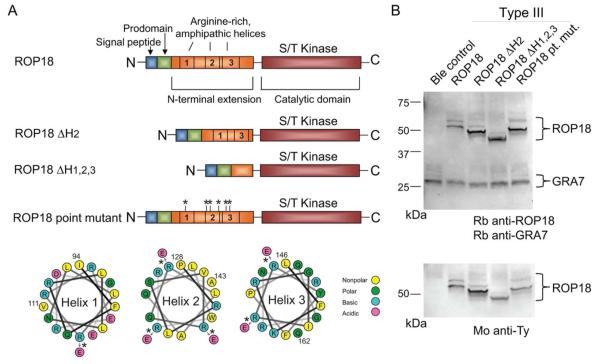
Wild-type ROP18 encodes a protein containing an N-terminal signal peptide and prodomain that directs ROP18 to the rhoptry organelle in the parasite (Fig. 1A). During intracellular trafficking, both the signal peptide and prodomain are subject to proteolytic cleavage and are, therefore, not present in the mature protein (Qiu et al., 2008). The C-terminus of ROP18 is dominated by a highly conserved serine/threonine kinase domain that is required for the virulence-enhancing activity of ROP18 (Fig. 1A). Preceding the kinase domain is an arginine-rich domain containing three predicted helical regions previously described to target the vacuole membrane when expressed in mammalian cells (Labesse et al., 2009, Reese et al., 2009) (Fig. 1A). To ascertain the function of this domain in parasite biology and virulence, we created two deletion mutant constructs and one point mutant construct and expressed these in the type III parasite background that is effectively null for ROP18 (Taylor et al., 2006). The mutant constructs consist of a mutant lacking the region corresponding to helix 2 (ROP18ΔH2; P128 to A143), a mutant lacking all three predicted helices (ROP18ΔH1,2,3; I94 to Q162), and a point mutant construct that substitutes six arginines for glutamate residues (ROP18 point mutant or ROP18 pt. mut.) (Fig. 1A). Stable parasite clonal lines express ROP18 mutants with a C-terminal Ty-1 tag at levels comparable to expression of wild-type ROP18 (Fig. 1B). ROP18 deletion mutants migrate faster in SDS-PAGE likely due to their lower molecular mass as compared to wild-type ROP18 (Fig. 1B).
Fig. 1.
A. Construct design of ROP18 mutants. Schematics are not drawn to scale. Helical wheel projections were drawn using previously defined algorithms (http://rzlab.ucr.edu/scripts/wheel/wheel.cgi). For the point mutant, six arginines were mutated to glutamate residues as noted by arrows on the helical wheels. B. Western blot analysis of ROP18 mutant expression in transgenic parasites. Parasite lysates were resolved by SDS-PAGE and blotted for rabbit anti-ROP18 (Rb anti-ROP18) and rabbit anti-GRA7 (Rb anti-GRA7) (top) or mAb BB2 against the Ty tag (Mo anti-Ty) expressed at the C-terminus of ROP18 (bottom). Proteins denoted by brackets. In all transgenic lines, ROP18 exists as two species corresponding to full-length protein (higher molecular weight band) and a mature form that is proteolytically cleaved and is devoid of the signal peptide and prodomain (lower molecular weight band).
Mutations in ROP18 do not disrupt trafficking to rhoptries or secretion into the host cell
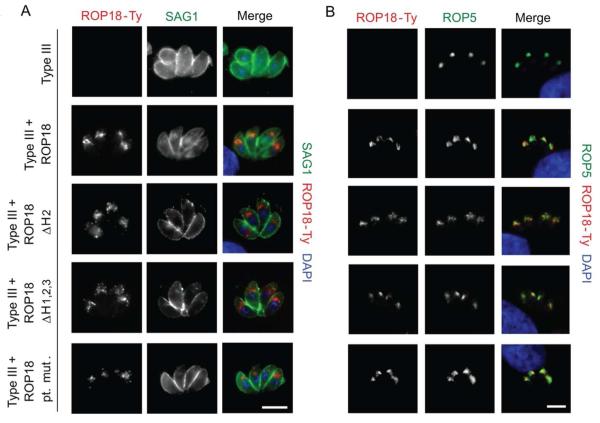
ROP18 enters the secretory pathway through a canonical eukaryotic signal peptide and is directed to the rhoptries via an N-terminal prodomain that is cleaved en route (Bradley et al., 1999). In order to determine if protein trafficking within the parasite is modified by mutations in the arginine-rich domain of ROP18, we performed immunofluorescence analysis on stable lines expressing the various mutants. Consistent with rhoptry localization, staining for ROP18 mutants was observed at the apical region of the parasite (Fig. 2A) and co-stained with a known rhoptry marker, ROP5 (Fig. 2B). In these examples, ROP18 was detected within the rhoptries, but not at the PVM due to extraction with Triton X-100 used to permeabilize the cells. Under more gentle detergent conditions, for example using saponin, it is possible to localize ROP18 in membranous secretions and on the PVM. Rhoptries discharge their contents directly into host cells during invasion into structures termed evacuoles, which still form when invasion is blocked by cytochalasin D (CytD) (Håkansson et al., 2001). The ability of ROP18 mutants to secrete the contents of rhoptries into the host cell during CytD treatment was evaluated by immunofluorescence staining (Fig. 3A). ROP18 was found in evacuoles within the host cell; these structures were proximal to attached, but non-invaded parasites (Fig. 3A, arrows). We conclude, therefore, that mutations in the arginine-rich domain of ROP18 do not disrupt trafficking to the rhoptry or secretion into the host cell.
Fig. 2.
ROP18 mutants traffic properly to rhoptry secretory organelles in the parasite. A. Direct immunofluorescence of parasite infected HFFs stained for a parasite surface marker SAG1 with mAb DG52 and for ROP18-Ty with mAb BB2 directly conjugated to Alexa Fluor 488 and 594, respectively. B. Indirect immunofluorescence of parasite infected HFFs. Cells were stained for the rhoptry marker ROP5 with rabbit anti-ROP5 and for ROP18-Ty with mAb BB2 followed by secondary antibodies conjugated to Alexa Fluor 488 and 594, respectively. Scale = 5μm. Images were acquired using wide field epifluorescent microscopy. ROP18 is detected within the rhoptries, but not at the PVM due to extraction with Triton X-100 used to permeabilize the cells.
Fig. 3.
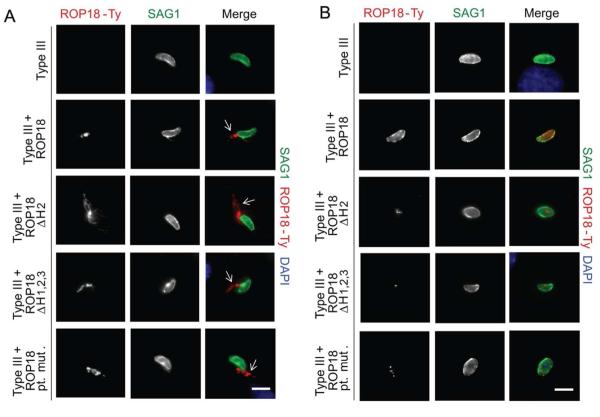
ROP18 mutants are secreted into host cells preceding parasite invasion, but fail to traffic to the PV membrane. A. Localization of ROP18 following secretion into evacuoles. Direct immunofluorescence of parasites pretreated with 1μM Cytochalasin D (CytD) to inhibit parasite invasion into HFFs. Samples were fixed 15 min after infection, permeabilized with saponin and stained for a parasite surface marker SAG1 with mAb DG52 and for ROP18-Ty with mAb BB2 directly conjugated to Alexa Fluor 488 and 594, respectively. White arrows denote secretion of ROP18 into the host cell in “evacuoles”. B. Localization of ROP18 on the surface of the PV membrane shortly after invasion. Samples were fixed 15 min after infection, permeabilized with saponin, and stained for a parasite surface marker SAG1 with mAb DG52 and for ROP18-Ty with mAb BB2 directly conjugated to Alexa Fluor 488 and 594, respectively. Images were acquired using wide field epifluorescent microscopy. Scale = 5μm.
ROP18 mutants fail to localize to early and late stage parasitophorous vacuole membranes
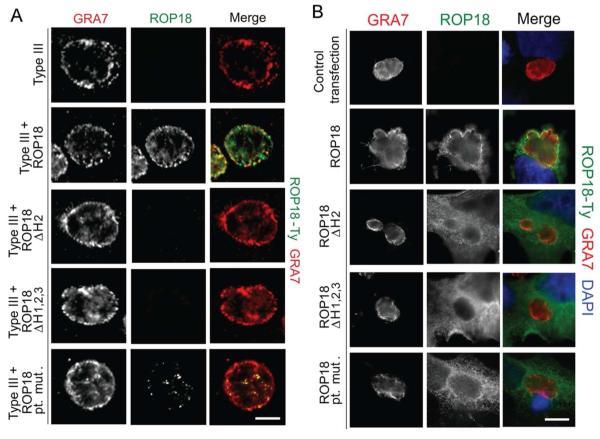
To test whether the arginine-rich domain of ROP18 was necessary for targeting ROP18 to newly formed PVMs, immunofluorescence analysis was employed to examine infected cells shortly after invasion. Although wild-type ROP18 localized to the early stage vacuole, all three of the ROP18 mutants fail to localize to this interface but instead showed residual internal staining in the rhoptries (Fig. 3B). This phenotype could be explained by a failure of these mutant proteins to tether to the PV membrane or by a delay in trafficking to the nascent vacuole. To delineate these possibilities, late stage vacuoles were also processed for immunofluorescence analysis. Both widefield epifluorescence (Fig. S1) and confocal imaging (Fig. 4A) demonstrated a failure of ROP18 mutants to localize to late stage PVMs. Interestingly, the ROP18 point mutant displays some internal vacuole staining in late stage vacuoles (Fig. S1 and 4A), similar to wild-type ROP18 (Fig. S1 and 4A). This pattern is consistent with staining of the tubulo-vesicular network, as reported previously (Reese et al., 2009). Both deletion mutants failed to localize at the membrane of late stage vacuoles and did not stain any structures within the host cell (Fig. S1 and 4A). Because the level of staining of ROP18 mutants was quite low following endogenous secretion, we also examined the distribution of these proteins following transient transfection in COS-7 cells, followed by infection with a type III strain that does not express detectable levels of ROP18 (Fig. 4B). Transient expression of wild-type ROP18 resulted in high level of expression in the cytosol of COS-7 cells and prominent recruitment to the PVM, as shown by costaining with the vacuole membrane marker GRA7 (Fig. 4B). In contrast, none of the mutant forms of ROP18 were recruited to the PVM surrounding type III parasites, despite showing high level of expression in COS-7 cells (Fig. 4B). Collectively, these data demonstrate that the arginine-rich helical region of ROP18 is a vacuole-targeting domain necessary for PVM localization following rhoptry secretion.
Fig. 4.
Localization of ROP18 in the PV membrane of mature vacuoles and following transient transfection of host cells. A. Vacuole localization of ROP18 mutants was determined with laser scanning confocal microscopy of indirect immunofluorescence. Infected HFFs were fixed 24 hpi and permeabilized with digitonin. Samples were stained for a parasite vacuole marker GRA7 with rabbit anti-GRA7 and for ROP18-Ty with mAb BB2 followed by secondary antibodies conjugated to Alexa Fluor 594 and 488, respectively. B. Trafficking of ROP18 following transient transfection of COS-7 cells. Cells transiently expression variants of ROP18 were infected with Type III parasites that naturally lack ROP18 expression, followed by culture for an additional 24 hr. Samples were fixed, permeabilized with saponin, and stained for a parasite vacuole marker GRA7 with rabbit anti-GRA7 and for ROP18-Ty with mAb BB2 followed by secondary antibodies conjugated to Alexa Fluor 594 and 488, respectively. Images were deconvolved in Axiovision v4.5 (Carl Zeiss) using the nearest neighbor algorithm and a single Z-slice is shown. Scale = 5μm.
Kinase activity of ROP18 mutants
Kinase activity of ROP18 is critically important to functional virulence enhancement by this enzyme (Fentress et al., 2010, Taylor et al., 2006). Mutations in the arginine-rich domain fall outside of the kinase domain of ROP18; therefore, we did not anticipate that these ROP18 mutants would display altered kinase activity. To directly test this, we immunoprecipitated wild-type or mutant ROP18 from parasites (Fig. 5A) and tested in vitro phosphorylation of the heterologous substrate dephosphorylated myelin basic protein (dMBP) (Fig. 5B). Although the helix 2 mutant (ROP18ΔH2) and the point mutant (ROP18 pt. mut.) demonstrated comparable activity to wild type enzyme (ROP18), the mutant deleted for all three helices (ROP18ΔH1,2,3) exhibited substantially diminished activity (Fig. 5B).
Fig. 5.
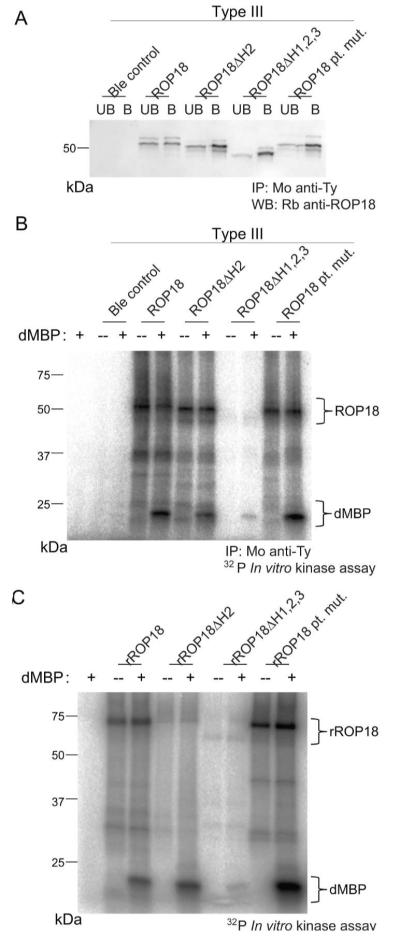
Kinase activity remains at wild-type levels in select ROP18 mutants. A. Western blot analysis of ROP18-Ty immunoprecipitation with mAb BB2 (Mo anti-Ty). Unbound (denoted as UB) and bound (denoted as B) fractions were resolved by SDS-PAGE and blotted with rabbit anti-ROP18 (Rb anti-ROP18). B. In vitro kinase assay of immunoprecipitated ROP18-Ty from (A) in the presence or absence of the heterologous substrate dMBP (5μg per reaction) indicated by tracking 32P radiolabeled ATP. C. In vitro kinase assay of recombinant ROP18 in the presence or absence of the heterologous substrate dephosphorylated myelin basic protein (dMBP) (5μg per reaction) indicated by tracking 32P radiolabeled ATP. Brackets denote autophosphorylated ROP18 and phosphorylated dMBP in (B) and (C).
To determine if the ROP18ΔH1,2,3 mutant displayed lower activity due to a regulatory failure or an intrinsic defect in enzyme, recombinant proteins were produced in E. coli for all versions of ROP18 and used to phosphorylate dMBP (Fig. 5C). Recombinant ROP18ΔH1,2,3 (rROP18ΔH1,2,3) demonstrated reduced activity compared to wild-type enzyme (rROP18), while the helix 2 deletion mutant (rROP18ΔH2), and the ROP18 point mutant (rROP18 pt. mut.) were normal (Fig. 5C). The reason for decreased autophosphorylation in the ROP18ΔH2 mutant is unclear, although this mutant retained normal activity against the heterologous substrate dMBP. The differential targeting and activity phenotypes of these mutants provided the potential to separately test the importance of kinase activity and vacuole targeting during infection.
Vacuole-targeting domain of ROP18 is required to subvert the IRG pathway in host cells and to enhance virulence
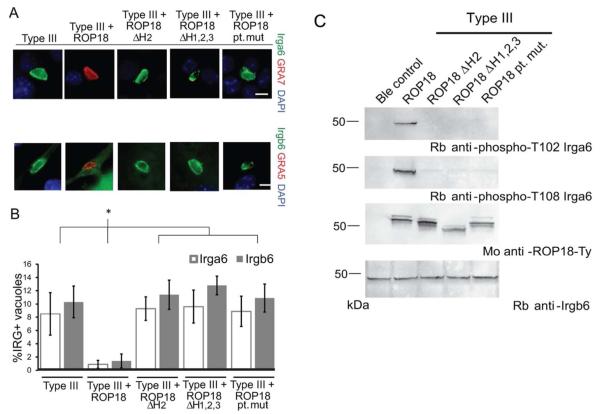
In order to determine if vacuole localization of ROP18 was important for function, we tested whether vacuole-targeting mutants of ROP18 were sufficient to displace Irgb6 from the vacuolar membrane in IFN-γ activated macrophages (Fig. 6A and B). Type III parasites expressing the vacuole-targeting ROP18 mutants accumulated Irgb6 on the vacuole membrane unlike those expressing wild-type ROP18, which avoided recruitment (Fig. 6A and B). Similarly, type III parasites expressing the vacuole-targeting ROP18 mutants failed to block accumulation of Irga6 onto the PVM, in contrast to parasites expressing wild-type ROP18 (Fig. 6A,B). Parasite-containing vacuoles that stained strongly for Irga6 or Irgb6 also stained weakly for GRA5 or GRA7 (Fig. 6A,B); this result likely reflects loss of staining due to damage of the vacuole membrane that occurs coincident with IRG recruitment. Previous studies have shown that ROP18 phosphorylates key Thr residues in Irga6, and that this is associated with disruption of GTPase activity and failure to properly assemble onto the PVM surrounding susceptible parasites (Fentress et al., 2010, Steinfeldt et al., 2010a). To determine if Irga6 accumulation was associated with an inability to phosphorylate this substrate, we took advantage of previously characterized antibodies that recognize the phosphorylated Thr102 and Thr108 residues that are targeted by ROP18 (Steinfeldt et al., 2010a). Infection of IFN-γ activated L929 cells with wild type ROP18 led to robust phosphorylation of Thr 102 and Thr108 in Irga6, as detected using phospho-specific antibodies (Fig. 6C). In contrast, infection with the vacuole-targeting mutants of ROP18 did not lead to detectable phosphorylation of Irga6 (Fig. 6C). Taken together, these findings suggest that the failure of vacuole-targeting mutants to avoid IRG recruitment is due to their inability to phosphorylate Irga6 and likely other IRGs that share a similar motif.
Fig. 6.
Function of ROP18 vacuole-targeting mutants in subversion of the host IRG pathway. A. Indirect immunofluorescence of IFN-γ-activated RAW 264.7 30 min postinfection. Samples were permeabilized with saponin and stained for a member of the host IRG pathway, either Irgb6 with rabbit anti-Irgb6 or Irga6 with mAb 10D7, and a parasite vacuole marker, either GRA5 with mAb Tg17-113 or GRA7 with rabbit anti-GRA7, followed by secondary antibodies conjugated to Alexa Fluor 488 and 59. Scale = 5μm. IRG positive vacuoles tend to stain weakly for GRA5 or GRA77, hence they appear negative in the composite image. B. Quantification of Irgb6 and Irga6 localization to the vacuolar membrane surrounding ROP18 mutants. Means ± S.D. n = 6 samples from 2 combined experiments. Student’s t test, *P < 0.005. C. Western blot analysis of the phosphorylation state of Irga6 during infection with parasites expressing ROP18 mutants. Infected IFN-γ-activated L929 cells were harvested 2 hr postinfection, resolved by SDS-PAGE, and blotted for phosphorylated T102 with rabbit anti-phospho-Irga6 T102 (555), phosphorylated T108 with rabbit anti-phospho-Irga6 T108 (558), ROP18-Ty1 expression with mAb BB2, or total Irgb6 expression with rabbit anti-Irgb6.
To determine if vacuole targeting was required for parasite virulence, we challenged mice with an interperitoneal inoculation of parasites and monitored survival over 30 days (Table 1). Mice infected with parasites expressing wild-type ROP18 (Type III + ROP18) succumbed to infection at a low dose inoculum (Table 1). In contrast, ROP18-deficient parasites (Type III) and parasites expressing vacuole-targeting ROP18 mutants (Type III + ROP18ΔH2, Type III + ROP18ΔH1,2,3, and Type III + ROP18 pt. mut.) caused no mortality in mice (Table 1). These results were observed consistently for two independent transgenic parasite clones of each targeting mutant (Table 1). Collectively, these findings indicate that correct targeting of ROP18 to the PVM is critically important for the function of this virulence determinant in both subversion of the IRG pathway and in enhancing acute disease.
Table 1.
Survival of mice challenged with ROP18 vacuole-targeting mutants.
| Parasite line | Clone a | % Survivalb | |||
|---|---|---|---|---|---|
| 102 | 103 | 104 | 105 | ||
| Type III | Ble | 100 | n.d. | n.d. | 100 |
| Type III + ROP18 | V1 | 0 | n.d. | n.d. | 0 |
| Type III + ROP18ΔH2 | G4 | 100 | 100 | 100 | 100 |
| E11 | 100 | 100 | 100 | 100 | |
| Type III + ROP18ΔH1,2,3 | G5 | 100 | 100 | 100 | 100 |
| C5 | 100 | 100 | 100 | 100 | |
| Type III + ROP18 pt. mut. | A12 | 100 | 100 | 100 | 100 |
| B5 | 100 | 100 | 100 | 100 | |
Independent transgenic clones as defined in Table S1
bCumulative results from 2 independent experiments. N=5 mice per group. n.d. Not done
Discussion
We have described the functional requirement of vacuole-targeting for a secreted ROP virulence determinant. Mutational analysis of an arginine-rich amphipathic domain of ROP18 in an endogenous parasite expression system revealed a requirement for predicted α–helices, particularly that of helix 2, and of specific arginine residues in targeting this effector to the PVM. vacuole-targeting mutants fail to phosphorylate and disrupt loading of host IRGs, even though kinase activity was retained by all but one of these mutants. Perhaps the most rigorous functional phenotype described for ROP18 is the gain of function in virulence as a result of expression of a kinase active allele of ROP18 in an avirulent type III parasite background. vacuole-targeting mutants failed to enhance lethality in mice, thus elucidating the importance of membrane-targeting for the virulence determinant ROP18.
Prior studies have noted that the arginine-rich membrane targeting domain is conserved among the ROP2-family of proteins (Reese et al., 2009), of which ROP18 is a member (Peixoto et al., 2010), and among related ROPs such as ROP17 (Reese et al., 2009). Amphipathic αhelices have also been implicated in the membrane association of dense granule proteins in Toxoplasma, such as GRA2 (Mercier et al., 1998), which traffics only to the TVN. Secretory dense granules organelles establish a second wave of protein secretion following rhoptry discharge and parasite invasion that delivers constituent proteins to the forming vacuole. Amphipathic domains may, therefore, constitute a general mechanism for membrane-targeting by vacuolar-localized secreted proteins in Toxoplasma. This would be an energetically advantageous mechanism to deliver proteins to the PV, as amphipathic domains do not require ATP for lipid bilayer integration, and would not constrain evolution of the these proteins, as amphipathic domains have low sequence complexity. In fact, amphipathic helices are responsible for membrane-targeting of many virus-encoded proteins (Rossman et al., 2010, Gibbs et al., 2003, Liu et al., 2009, Min et al., 2008). Additionally, Legionella pneumophila encodes the secretion translocase-associated IcmQ that binds/permeablizes membranes via amphipathic helices that are enriched in lysines and arginines (Raychaudhury et al., 2009).
Amphipathic helical structures are thought to mediate membrane binding by embedding the hydrophobic face of the helix into the lipid bilayer (Drin et al., 2007). The amphipathic domains of ROP18 and GRA2 differ in the enrichment of arginine-residues, found only in ROPs, hence this signal may delineate PVM localization from TVN localization. Indeed, while arginine mutants of ROP18 retain the amphipathic nature of the domain (Fig. 1A) and displace ROP18 from the PVM (Fig. 5 and S2), partial TVN localization was still evident (Fig. 5 and S2). Although the significance of arginine residues in this domain is not known, they may mediate association with negatively charged phospholipids in the PVM. Further biochemical analysis will be required to determine the specificity for this domain for membrane targeting.
Kinase activity of ROP18 was previously characterized as being necessary for IRG avoidance (Fentress et al., 2010) and virulence (Taylor et al., 2006). We now report that kinase activity, while necessary, is not sufficient for ROP18-mediated pathogenesis. Both ROP18ΔH2 and ROP18 point mutants retain kinase activity (Fig. 4) but fail to form a stable association with the PVM. These mutants were unable to avoid recruitment of Irga6 and Irgb6 to susceptible parasite-containing vacuoles. In the case of Irga6, this defect as associated with an inability to phosphorylate the substrate at key Thr residues that have previously been implicated in function (Steinfeldt et al., 2010a). Although we have not tested all of the known substrates of ROP18, it is likely that lack of membrane targeting of the kinase to the PVM also disrupts the ability to phosphorylate these other targets. The vacuole targeting mutants were avirulent, likely due to a spatial and temporal failure to phosphorylate their substrates in the infected cell. Because kinase activity is diminished for ROP18ΔH1,2,3 (Fig. 4), interpretation of functional assays for parasites expressing this vacuole targeting mutant could result both from failure to associate to the PVM and reduced kinase activity. These results also point to a yet undescribed role for the N-terminus of ROP18 in catalytic activity of the enzyme.
Recent studies have implicated ROP18 in modulation of a second host cell factor, a transcription factor called ATF6β, which resides on the endoplasmic reticulum (ER) (Yamamoto et al., 2011). During infection, the host ER and the PVM share intimate contact (Sinai et al., 1997), and, therefore, it is plausible that these two factors could interact in an infected cell. Although the prior study did not demonstrate that a ROP18-dependent phosphorylation of ATF6β occurs in vivo, our current study would suggest that ROP18 vacuole targeting mutants would not be properly localized to modulate this host transcription factor or any other putative substrate on the PVM. In a previous report describing the ATF6β interaction, ROP18 mutants devoid of helix 2 were used to genetically complement a type I strain knocked-out for the endogenous ROP18 allele (Yamamoto et al., 2011). Although vacuole targeting was not examined for this mutant, mouse virulence was not fully restored, in line with results described here.
Genetic analysis and mapping have identified two additional kinase domain-containing ROPs that alter host function: ROP16 (Saeij et al., 2006) and ROP5 (Behnke et al., 2011, Reese et al., 2011). ROP16 is an active kinase secreted from the rhoptries but does not contain an arginine-rich, vacuole-targeting domain. Instead, ROP16 contains a nuclear-targeting sequence rich in lysines that target this effector to the host nucleus where it modulates STAT signaling and transcription (Ong et al., 2010, Saeij et al., 2007, Yamamoto et al., 2009). ROP5, however, is a polymorphic pseudokinase that localizes to the PVM and contains an arginine-rich, vacuole-targeting domain (Reese et al., 2009). At present, the requirement of PVM binding by this effector in its role in virulence-enhancement has not been directly tested. However, based on our findings here, we would predict the N-terminal amphipathic region will also prove essential for ROP5 function.
IRG resistance to Toxoplasma is mediated by a family of ~20 proteins in the murine system that is up-regulated by IFN-γ and abundantly expressed in the host cytosol (Martens et al., 2006). Four members are known to accumulate at the PVM during the first 90 min after invasion, beginning with loading of early IRGs, Irgb6 and Irgb10, and followed by Irga6 and Irgd (Khaminets et al., 2010). ROP18 phosphorylates Irgb6, Irgb10 and Irga6 on conserved threonine residues and deflects these members from the PVM (Fentress et al., 2010); (Steinfeldt et al., 2010b). In this study, we have analyzed the significance of ROP18 vacuole-targeting in disruption of the IRG pathway using Irgb6, which is an early sentinel of downstream events, and Irga6, which arrives later. Although it was conceivable that ROP18 might disrupt the IRG pathway by acting in the host cytosol, this model is not consistent with the finding that targeting mutants were unable to phosphorylate Irga6 and did not block Irga6 or Irgb6 loading. Instead, these findings indicate that proper ROP18 vacuole-targeting is vital to it function in disarming IRGs and in modulating acute virulence. These results also imply that IRG proteins must first engage the PVM prior to being phosphorylated by ROP18. Collectively, these findings indicate that ROP18 blocks a key step in assembly of IRG effectors and that its localization to the PVM is critical for this function.
In summary, we have determined a critical role for vacuolar membrane association for the Toxoplasma virulence determinant ROP18. Protein secretion and modification of vacuoles is a common theme among vacuole-enclosed pathogens, such as with Plasmodium (Cesbron-Delauw et al., 2008), Chlamydia (Peters et al., 2007, Betts et al., 2009), and Legionella (Ge et al., 2011, Hubber et al., 2010). Toxoplasma ROP kinase effectors have evolved a low complexity and efficient means to target secreted factors to the host-pathogen interface. Future work to define the functionality of PVM association for other ROPs will provide insight into parasite biology and mechanism of action for these effectors.
Experimental Procedures
Parasite propagation, cloning, and transfection
Type III parasites (either CTG WT or CTG Ble) and type III + ROP18 parasites (CTG V1) were passaged in human foreskin fibroblasts (HFFs) as described previously (Fentress et al., 2010). Helix deletions in ROP18 were made by PCR (Table S2) of the template pROP18-I-Ty (Taylor et al., 2006) and blunt end ligation. Point mutations in the pROP18-I-Ty template were made with the QuikChange Multi site-directed mutagenesis kit (Stratagene) (Table S2). Transgenic type III parasites (Table S1) were made by electroporating CTG WT parasites with 30μg of ROP18-expression plasmid and 6μg of pTUB/ble/SAG in cytomix as described previously (Taylor et al., 2006). Parasites were subjected to two rounds of phleomycin selection (Messina et al., 1995) and single cell cloned in 96 well plates. Individual clones were assessed for ROP18 transgene expression by immunofluorescence and western blotting. Two clones were selected for each mutation based on equal expression as compared to wild-type ROP18 (CTG V1): for type III + ROP18ΔH2 (G4 and E11), for type III + ROP18ΔH1,2,3 (G5 and C5), and for type III + ROP18 point mutant (A12 and B5) (Table S1). The first clone of each set was used for functional assays, except in the case of mouse virulence assays where both clones were tested.
Western blot analysis of expression
Parasites were solubilized in lysis buffer (1% NP-40, 50 mM Tris–HCl, 150 mM NaCl, pH 8.8 plus protease inhibitors), centrifuged at 1,000g 4°C to remove large debris, and boiled in Laemmeli sample buffer. Proteins were resolved on 12% polyacrylamide gels, blotted on nitrocellulose membranes, and blocked with 5% nonfat dry milk in PBS with 0.05% Tween-20. Blots were probed with rabbit anti-ROP18 (Taylor et al., 2006), rabbit anti-GRA7 (Dunn et al., 2008), and/or mAb BB2 against the Ty-1 tag (Bastin et al., 1996) followed by secondary antibodies conjugated to HRP. Detection occurred with ECL Plus (GE Healthcare) and FLA5000 phosphorimager analysis (Fuji).
Immunofluorescence staining and microscopy for rhoptry and vacuole localization
For rhoptry localization, HFF monolayers were challenged with parasites for 24 hr, fixed with 4% formaldehyde in PBS for 10 min, permeabilized with 0.1% Triton X-100 in PBS for 5 min, and blocked in 10% fetal bovine serum for 20 min. Samples were incubated with mAb DG52 against SAG1 to stain the surface of the parasite directly conjugated to Alexa Fluor 488 and/or mAb BB2 against the Ty-1 tag to stain for ROP18 directly conjugated to Alexa Fluor 594. Antibody labeling was performed according to the manufacturer’s specifications (Molecular Probes). Samples were incubated with rabbit polyclonal anti-ROP5 (Behnke et al., 2011) to stain for ROP5 followed by secondary antibodies conjugated to Alexa Fluor 488.
To examine the secretion of ROP18 into host cells, freshly egressed parasites were treated with 1μM Cytochalasin D (CytD) for 5 min and used to challenge HFF monolayers for 15 min as described previously (Håkansson et al., 2001). Samples were fixed with 4% formaldehyde in PBS for 10 min, and permeabilized with 0.05% saponin. Samples were incubated with mAb BB2 against the Ty-1 tag and rabbit polyclonal anti-GRA7 (Dunn et al., 2008) to stain for the parasitophorous vacuole followed by secondary antibodies conjugated to Alexa Fluors. For late stage vacuole localization, infected samples were fixed 24 hr after infection, permeabilized with 0.02% digitonin in PBS, and stained as described above. Antibody incubation was following by washing in PBS and mounting in Prolong Gold with DAPI. Samples were visualized with either a Zeiss Axioskop 2 MOT Plus microscope equipped for epifluorescence or a Zeiss LSM 510 equipped for confocal imaging (Carl Zeiss, Inc.) as indicated. Acquired images were processed with Photoshop CS4.
Transient expression and trafficking of ROP18 in COS-7 cells
For ROP18 expression in mammalian cells, ROP18 was amplified by PCR using the various mutant alleles described above and cloned into a pcDNA3.1/V5-His TOPO vector (Invitrogen). Transient transfection of Cos-7 cells (ATCC CRL-1651) was facilitated by complexing expression plasmids with X-tremeGENE 9 DNA transfection reagent (Roche) according to the manufacturer’s specifications. After 24 hr of transient transfection, cells were infected with Type III parasites that naturally lack ROP18 expression, followed by culture for an additional 24 hr. Samples were fixed, permeabilized with saponin, and stained for a parasite vacuole marker GRA7 with rabbit anti-GRA7 and for ROP18-Ty with mAb BB2 followed by secondary antibodies conjugated to Alexa Fluor 594 and 488, respectively. Z-stacks were automatically acquired using wide field epifluorescence microscopy and deconvolved in Axiovision v4.5 (Carl Zeiss) using the nearest neighbor algorithm.
Immunoprecipitation and recombinant protein expression
Immunoprecipitations were performed as described previously with mAb BB2 against the Ty-1 tag (Fentress et al., 2010). Efficiency of ROP18 precipitation was assessed by western blotting. For expression of recombinant protein, genomic DNA from the type I RH strain of T. gondii was used to amplify the genes encoding full length wild-type ROP18 (starting from Glu83 based on the second ATG of GenBank protein CAJ27113) and mutants were amplified from parasites expression plasmids described above using iProof high-fidelity polymerase (Bio-Rad) (Table S2). Products were cloned into pGEX-6P-1 using primers that introduced a C-terminal His6 tag (Table S2). ROP18 was expressed in BL21 (DE3)-V2R-pACYC LamP, as described previously (Qiu et al., 2008). Cells were induced with 1mM IPTG, grown overnight at 15 °C, and soluble proteins were purified using Glutathione Sepharose 4B (GE Healthcare) according to the manufacturer’s recommendations. Protein purity and concentration were assessed by SDS-PAGE and SYPRO Ruby staining (Invitrogen).
In vitro kinase assay
Either immunoprecipitated (~1-5ng/reaction) or recombinant ROP18 kinase (500ng/reaction) was incubated with the heterologous substrate dMBP (5μg/reaction) (Millipore) in kinase buffer (25 mM Tris–HCl pH 7.5, 15 mM MgCl2 and 2 mM MnCl2) containing 10μCi of32P γ-ATP (specific activity: 3000 Ci/mmol) (Perkin Elmer, Inc) in addition to 33μM unlabelled ATP (Sigma-Aldrich). Reactions were incubated for 15-30 min at 30°C, samples were heated to 95°C in Laemmli sample buffer, resolved on 12% SDS–PAGE gels, dried, and imaged using a FLA5000 phosphorimager (Fuji).
IRG vacuole accumulation
RAW 264.7 cells were cultured in DMEM as described previously (Fentress et al., 2010). Prior to experimentation, cells were activated by treatment with 10U/mL murine rIFN-γ (R & D Systems) and 0.1ng/mL LPS (Sigma-Aldrich, E. coli O55:B5) for 24 hr. Activated cells were challenged with parasites for 30 min, achieving 30-40% infection rates, and fixed for immunofluorescence analysis. The parasitophorous vacuole was imaged by staining for GRA5 (Mo anti-GRA5, Tg17-113) (Charif et al., 1990), or for GRA7 (Rb anti-GRA7) (Dunn et al., 2008), and host IRG localization was tracked by staining for Irgb6 (Rb anti-Irgb6) (Henry et al., 2009), or for Irga6 (Mo anti-Irga6, 10D7) (Papic et al., 2008), followed by secondary antibodies conjugated to Alexa Fluor dyes (Molecular probes). The number of Irgb6 positive parasites containing vacuoles was determined by counting 10 fields at 40X for three technical replicates in two biological experiments.
Irga6 phosphorylation during infection
L929 cells (ATCC CCL-1) were activated by treatment with 200 U/mL murine rIFN-γ (R & D Systems) for 24 hr. Activated cells were challenged with parasites for 2 hr, achieving 70-80% infection rates. Cells were solubilized in lysis buffer (1% NP-40, 50 mM Tris–HCl, 150 mM NaCl, pH 8.8 plus protease inhibitors (Roche) and phosphatase inhibitors (PhosSTOP, Roche), centrifuged at 1,000g 4°C to remove large debris, and boiled in Laemmeli sample buffer. Proteins were resolved on 12% polyacrylamide gels, blotted on nitrocellulose membranes, and blocked with 5% BSA in PBS with 0.05% Tween-20. Blots were probed with rabbit anti-phospho-Irga6 T102 (555) or rabbit anti-phospho-Irga6 T108 (558) (Steinfeldt et al., 2010a) followed by secondary antibodies conjugated to HRP. Detection occurred with ECL Plus (GE Healthcare) and FLA5000 phosphorimager analysis (Fuji).
In vivo infection
CD-1 mice (Charles River Laboratories) were maintained in an AAALAC-approved facility and all protocols were approved by the Institutional Care Committee (School of Medicine, Washington University in St. Louis). Female mice between 8-9 weeks of age were challenged with parasites through i.p. injection and monitored for 30 days as described previously (Fentress et al., 2010). Parasite viability for each clone was tested by plaque assay following mouse inoculation. For each mutant transgenic parasite tested, two clones were administered at four doses with groups of five mice per dose. Serum was collected from surviving mice and tested for reactivity to Toxoplasma antigens to verify that each animal was infected. Two independent challenge experiments were performed for each clone.
Statistics
Statistical calculations were performed with Excel (Microsoft). Student’s t tests were performed under the assumption of equal variance and using a two-tailed test, where P ≤ 0.05 was considered significant.
Supplementary Material
Acknowledgements
We thank Keli Tang for cloning of the ROP18 deletion mutants and for recombinant protein expression, Wandy Beatty for assistance with confocal imaging, Jennifer Barks for expert technical assistance, past and present members of the Sibley lab for discussions and advice, and Greg Taylor, Marie France-Cesbron Delauw, and John Boothroyd for kind gifts of antibodies. This work was supported by the NIH (A1036629 and A1084243 to L.D.S.), the Deutsche Forschungsgemeinschaft (SFB635 and SFB670 to J.C.H.), and a Morse Berg Predoctoral Fellowship (Washington University, St. Louis to S.J.F.).
References
- Bastin P, Bagherzadeh Z, Matthews KR, Gull K. A novel epitope tag system to study protein targeting and organelle biogenesis in Trypanosoma brucei. Molec. Biochem. Parasitol. 1996;77:235–239. doi: 10.1016/0166-6851(96)02598-4. [DOI] [PubMed] [Google Scholar]
- Beckers CJM, Dubremetz JF, Mercereau-Puijalon O, Joiner KA. The Toxoplasma gondii rhoptry protein ROP2 is inserted into the parasitophorous vacuole membrane, surrounding the intracellular parasite, and is exposed to the host cell cytoplasm. Journal of Cell Biology. 1994;127:947–961. doi: 10.1083/jcb.127.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke MS, Khan A, Wootton JC, Dubey JP, Tang K, Sibley LD. Virulence differences in Toxoplasma mediated by amplification of a family of polymorphic pseuodokinases. Proc Natl Acad Sci (USA) 2011;108:9631–9636. doi: 10.1073/pnas.1015338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts HJ, Wolf K, Fields KA. Effector protein modulation of host cells: examples in the Chlamydia spp. arsenal. Curr Opin Microbiol. 2009;12:81–87. doi: 10.1016/j.mib.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Boothroyd JC, Dubremetz JF. Kiss and spit: the dual roles of Toxoplasma rhoptries. Nat. Rev. Microbiol. 2008;6:79–88. doi: 10.1038/nrmicro1800. [DOI] [PubMed] [Google Scholar]
- Bradley PJ, Boothroyd JC. Identification of the pro-mature processing site of Toxoplasma ROP1 by mass spectrometry. Mol Biochem Parasitol. 1999;100:103–109. doi: 10.1016/s0166-6851(99)00035-3. [DOI] [PubMed] [Google Scholar]
- Bradley PJ, Sibley LD. Rhoptries: an arsenal of secreted virulence factors. Curr. Opin. Microbiol. 2007;10:582–587. doi: 10.1016/j.mib.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers VB, Sibley LD. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol. 1997;73:114–123. [PubMed] [Google Scholar]
- Cesbron-Delauw MF, Gendrin C, Travier L, Ruffiot P, Mercier C. Apicomplexa in mammalian cells: trafficking to the parasitophorous vacuole. Traffic. 2008;9:657–664. doi: 10.1111/j.1600-0854.2008.00728.x. [DOI] [PubMed] [Google Scholar]
- Charif H, Darcy F, Torpier G, Cesbron-Delauw MF, Capron A. Toxoplasma gondii: characterization and localization of antigens secreted from tachyzoites. Exp. Parasitol. 1990;71:114–124. doi: 10.1016/0014-4894(90)90014-4. [DOI] [PubMed] [Google Scholar]
- Drin G, Casella JF, Gautier R, Boehmer T, Schwartz TU, Antonny B. A general amphipathic alpha-helical motif for sensing membrane curvature. Nat Struct Mol Biol. 2007;14:138–146. doi: 10.1038/nsmb1194. [DOI] [PubMed] [Google Scholar]
- Dunn JD, Ravindran S, Kim SK, Boothroyd JC. The Toxoplasma gondii dense granule protein GRA7 is phosphorylated upon invasion and forms an unexpected association with the rhoptry proteins ROP2 and ROP4. Infect Immun. 2008;76:5853–5861. doi: 10.1128/IAI.01667-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hajj H, Demey E, Poncet J, Lebrun M, Wu B, Galeotti N, et al. The ROP2 family of Toxoplasma gondii rhoptry proteins: proteomic and genomic characterization and molecular modeling. Proteomics. 2006;6:5773–5784. doi: 10.1002/pmic.200600187. [DOI] [PubMed] [Google Scholar]
- El Hajj H, Lebrun M, Arold ST, Vial H, Labesse G, Dubremetz JF. ROP18 is a rhoptry kinase controlling the intracellular proliferation of Toxoplasma gondii. PLoS Pathogens. 2007;3:e14. doi: 10.1371/journal.ppat.0030014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fentress SJ, Behnke MS, Dunay IR, Moashayekhi M, Rommereim LM, Fox BA, et al. Phosphorylation of immunity-related GTPases by a parasite secretory kinase promotes macrophage survival and virulence. Cell Host Microbe. 2010;16:484–495. doi: 10.1016/j.chom.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge J, Shao F. Manipulation of host vesicular trafficking and innate immune defence by Legionella Dot/Icm effectors. Cell Microbiol. 2011;13:1870–1880. doi: 10.1111/j.1462-5822.2011.01710.x. [DOI] [PubMed] [Google Scholar]
- Gibbs JS, Malide D, Hornung F, Bennink JR, Yewdell JW. The influenza A virus PB1-F2 protein targets the inner mitochondrial membrane via a predicted basic amphipathic helix that disrupts mitochondrial function. J Virol. 2003;77:7214–7224. doi: 10.1128/JVI.77.13.7214-7224.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håkansson S, Charron AJ, Sibley LD. Toxoplasma evacuoles: a two-step process of secretion and fusion forms the parasitophorous vacuole. Embo J. 2001;20:3132–3144. doi: 10.1093/emboj/20.12.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry SC, Daniell XG, Burroughs AR, Indaram M, Howell DN, Coers J, et al. Balance of Irgm protein activities determines IFN-gamma-induced host defense. J Leukoc Biol. 2009;85:877–885. doi: 10.1189/jlb.1008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubber A, Roy CR. Modulation of host cell function by Legionella pneumophila type IV effectors. Annu Rev Cell Dev Biol. 2010;26:261–283. doi: 10.1146/annurev-cellbio-100109-104034. [DOI] [PubMed] [Google Scholar]
- Khaminets A, Hunn JP, Könen-Waisman S, Zhao YO, Preukschat D, Coers J, et al. Coordinated loading of IRG resistance GTPases on to the Toxoplasma gondii parasitophorous vacuole. Cell Microbiol. 2010;12:939–961. doi: 10.1111/j.1462-5822.2010.01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labesse G, Gelin M, Bessin Y, Lebrun M, Papoin J, Cerdan R, et al. ROP2 from Toxoplasma gondii: a virulence factor with a protein-kinase fold and no enzymatic activity. Structure. 2009;17:139–146. doi: 10.1016/j.str.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Liu L, Westler WM, den Boon JA, Wang X, Diaz A, Steinberg HA, Ahlquist P. An amphipathic alpha-helix controls multiple roles of brome mosaic virus protein 1a in RNA replication complex assembly and function. PLoS Pathog. 2009;5:e1000351. doi: 10.1371/journal.ppat.1000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens S, Howard JC. The interferon-inducible GTPases. Annu. Rev. Cell. Dev. Biol. 2006;22:559–589. doi: 10.1146/annurev.cellbio.22.010305.104619. [DOI] [PubMed] [Google Scholar]
- Martens S, Parvanova I, Zerrahn J, Griffiths G, Schell G, Reichmann G, Howard JC. Disruption of Toxoplasma gondii parasitophorous vacuoles by the mouse p47-resistance GTPases. Plos Pathogens. 2005;1:e24. doi: 10.1371/journal.ppat.0010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier CM, Cesbron-Delauw MF, Sibley LD. The amphipathic alpha helices of the Toxoplasma protein GRA2 mediate post-secretory membrane association. Journal of Cell Science. 1998;111:2171–2180. doi: 10.1242/jcs.111.15.2171. [DOI] [PubMed] [Google Scholar]
- Messina M, Niesman IR, Mercier C, Sibley LD. Stable DNA transformation of Toxoplasma gondii using phleomycin selection. Gene. 1995;165:213–217. doi: 10.1016/0378-1119(95)00548-k. [DOI] [PubMed] [Google Scholar]
- Min CK, Bang SY, Cho BA, Choi YH, Yang JS, Lee SH, et al. Role of amphipathic helix of a herpesviral protein in membrane deformation and T cell receptor downregulation. PLoS Pathog. 2008;4:e1000209. doi: 10.1371/journal.ppat.1000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordue DG, Desai N, Dustin M, Sibley LD. Invasion by Toxoplasma gondii establishes a moving junction that selectively excludes host cell plasma membrane proteins on the basis of their membrane anchoring. Journal of Experimental Medicine. 1999;190:1783–1792. doi: 10.1084/jem.190.12.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisaki JH, Heuser JE, Sibley LD. Invasion of Toxoplasma gondii occurs by active penetration of the host cell. J Cell Sci. 1995;108:2457–2464. doi: 10.1242/jcs.108.6.2457. [DOI] [PubMed] [Google Scholar]
- Ong YC, Reese ML, Boothroyd JC. Toxoplasma rhoptry protein 16 (ROP16) subverts host function by direct tyrosine phosphorylation of STAT6. J Biol Chem. 2010;285:28731–28740. doi: 10.1074/jbc.M110.112359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papic N, Hunn JP, Pawlowski N, Zerrahn J, Howard JC. Inactive and active states of the interferon-inducible resistance GTPase, Irga6, in vivo. J Biol Chem. 2008;283:32143–32151. doi: 10.1074/jbc.M804846200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto L, Chen F, Harb OS, Davis PH, Beiting DP, Brownback CS, et al. Integrative genomics approaches highlight a family of parasite-specific kinases that regulate host responses. Cell Host Microbe. 2010;8:208–218. doi: 10.1016/j.chom.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Wilson DP, Myers G, Timms P, Bavoil PM. Type III secretion a la Chlamydia. Trends Microbiol. 2007;15:241–251. doi: 10.1016/j.tim.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Qiu W, Wernimont A, Tang K, Taylor S, Lunin V, Schapira M, et al. Novel structural and regulatory features of rhoptry secretory kinases in Toxoplasma gondii. EMBO J. 2008;28:969–979. doi: 10.1038/emboj.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhury S, Farelli JD, Montminy TP, Matthews M, Menetret JF, Dumenil G, et al. Structure and function of interacting IcmR-IcmQ domains from a type IVb secretion system in Legionella pneumophila. Structure. 2009;17:590–601. doi: 10.1016/j.str.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese ML, Boothroyd JC. A helical membrane-binding domain targets the Toxoplasma ROP2 family to the parasitophorous vacuole. Traffic. 2009;10:1458–1470. doi: 10.1111/j.1600-0854.2009.00958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese ML, Zeiner GM, Saeij JP, Boothroyd JC, Boyle JP. Polymorphic family of injected pseudokinases is paramount in Toxoplasma virulence. Proc Natl Acad Sci U S A. 2011;108:9625–9630. doi: 10.1073/pnas.1015980108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman JS, Jing X, Leser GP, Lamb RA. Influenza virus M2 protein mediates ESCRT-independent membrane scission. Cell. 2010;142:902–913. doi: 10.1016/j.cell.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeij JPJ, Boyle JP, Coller S, Taylor S, Sibley LD, Brooke-Powell ET, et al. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science. 2006;314:1780–1783. doi: 10.1126/science.1133690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeij JPJ, Coller S, Boyle JP, Jerome ME, White ME, Boothroyd JC. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature. 2007;445:324–327. doi: 10.1038/nature05395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Sibley LD. The moving junction, a key portal to host cell invasion by apicomplexan parasites. Curr Opin Microbiol. 2012 doi: 10.1016/j.mib.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinai AP, Joiner KA. The Toxoplasma gondii protein ROP2 mediates host organelle association with the parasitophorous vacuole membrane. J Cell Biol. 2001;154:95–108. doi: 10.1083/jcb.200101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinai AP, Webster P, Joiner KA. Association of host cell endoplasmic reticulum and mitochondria with the Toxoplasma gondii parasitophorous vacuole membrane: a high affinity interaction. Journal of Cell Science. 1997;110:2117–2128. doi: 10.1242/jcs.110.17.2117. [DOI] [PubMed] [Google Scholar]
- Steinfeldt T, Konen-Waisman S, Tong L, Pawlowski N, Lamkemeyer T, Sibley LD, et al. Phosphorylation of mouse immunity-related GTPase ( IRG) resistance proteins is an evasion strategy for virulent Toxoplasma gondii. PLoS Biol. 2010a;8:e1000576. doi: 10.1371/journal.pbio.1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinfeldt T, Könen-Waisman S, Tong L, Pawlowski N, Lamkemeyer T, Sibley LD, et al. Phosphorylation of mouse immunity-related GTPase (IRG) resistance proteins is an evasion strategy for virulent Toxoplasma gondii. PLoS Biol. 2010b;8:e1000576. doi: 10.1371/journal.pbio.1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suss-Toby E, Zimmerberg J, Ward GE. Toxoplasma invasion: The parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fusion pore. Proc. Natl. Acad. Sci. USA. 1996;93:8413–8418. doi: 10.1073/pnas.93.16.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GA, Feng CG, Sher A. Control of IFN-gamma-mediated host resistance to intracellular pathogens by immunity-related GTPases (p47 GTPases) Microb. Infect. 2007;9:1644–1651. doi: 10.1016/j.micinf.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Taylor S, Barragan A, Su C, Fux B, Fentress SJ, Tang K, et al. A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science. 2006;314:1776–1780. doi: 10.1126/science.1133643. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Ma JS, Mueller C, Kamiyama N, Saiga H, Kubo E, et al. ATF6-beta is a host cellular target of the Toxoplasma gondii virulence factor ROP18. J Exp Med. 2011;208:1533–1546. doi: 10.1084/jem.20101660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Standley DM, Takashima S, Saiga H, Okuyama M, Kayama H, et al. A single polymorphic amino acid on Toxoplasma gondii kinase ROP16 determines the direct and strain-specific activation of Stat3. J Exp Med. 2009;206:2747–2760. doi: 10.1084/jem.20091703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.