Abstract
Introduction
Osteoarthritis (OA), the most prevalent form of joint disease, affecting as much as 13% of the world’s population. In the United States, it is the leading cause of disability in people over age 65 and is characterized by progressive cartilage loss, bone remodeling, osteophyte formation and synovial inflammation with resultant joint pain and disability. There are no treatments marketed for structural disease modification; current treatments mainly target symptoms, with >75% of patients reporting need for additional symptomatic treatment.
Areas covered
Drugs in later development (Phase II-III) for osteoarthritis pain and joint structural degeneration are reviewed. Not covered are procedural (e.g. arthrocentesis, physical therapy), behavioral (e.g. weight loss, pain coping techniques) or device (e.g. knee braces, surgical implants) based treatments.
Expert opinion
More in depth understanding of the pathophysiology of the disease, as well as elucidation of the link between clinical symptomatology and structural changes in the joint will likely lead to development of novel target classes with promising efficacy in the future. Efficacy notwithstanding, there remain significant hurdles to overcome in clinical development of these therapeutics, inherent in the progression pattern of the disease as well as challenges with readouts for both pain and structure modification trials.
Keywords: Clinical Trial, Disease Modification, Joint, Osteoarthritis, Pain
1 Background
1.1 Disease Prevalence and Impact
Osteoarthritis (OA), the most prevalent form of joint disease, affects 28 million people in the US, and an estimated 4–13% of the world’s population. 1–3. It is the most common cause of disability in people over age 65, and second only to migraine as a leading cause of chronic pain.4,5 In the US alone, the financial burden has been estimated to be $81 billion in medical costs and $128 billion in total cost, given approximately 21 million people with OA associated limitations, 36 million outpatient visits and 750,000 hospitalizations per year.3
1.2 Disease Characteristics and Causes
Clinically, patients with OA may experience a spectrum of signs and symptoms that can include joint specific pain at rest or while walking/climbing stairs, pain at night, soreness to touch of the affected joint(s), decreased range of motion, and in some cases crepitus and/or bony enlargement. While the characteristics of the disease, including progressive cartilage loss, subchondral bone remodeling, osteophyte formation, and mild to moderate synovial inflammation,6 are well recognized, and often lead to increasing joint pain and disability, the etiopathophysiology of the disease remains poorly understood, despite persistent and laudable efforts on the part of academic, industry, and government researchers. It is generally acknowledged that a number of factors can work independently or in combination to create a final common pathway recognized as OA, including abnormal mechanical stress on a normal joint or relatively normal forces on a compromised/susceptible joint leading to sub-optimal force dissipation through joint tissues. Either of these situations is often present in combination with cross-talking biochemical changes in cartilage and synovium, on a background of poorly characterized genetic factors.
1.3 Target Selection Challenges
Because OA is a chronic disease that early on may exhibit intermittent symptomatology and as the disease progresses may do so at an unpredic` rate or pattern, research into the development of the disease is fraught with “chicken and egg” syndrome, i.e. whether a given finding is causal, an effect of the disease process, or secondary to other unrelated processes, particularly considering that these patients very often have co-morbidities such as obesity, high blood pressure, cardiovascular disease, metabolic syndrome, or even diabetes mellitus.
Two areas of inadequately addressed medical need, OA pain relief and arresting or impeding structural joint degeneration (referred to here as structure modification), co-exist but do not necessarily coincide. Agents that relieve pain rarely have structure modification benefits, and in fact can in some cases be detrimental to the joint,7–9 whereas potentially successful structure modifying agents (e.g. doxycycline) often fail to provide pain relief.10,11 Our inability as a research community to fully understand the disease and its causes has resulted in challenges with respect to the generation of a valid list of targets that in turn demonstrate success in well designed clinical trials. On the pain front, scholars in the field cannot agree on how best to classify OA pain or the relative contributions of inflammatory, neuropathic, and/or nociceptive pain components. The specific source(s) of OA pain within the joint remain elusive as well, although as OA is a disease of the whole joint, the pain is likely to have multiple sources, including bone, subchondral bone marrow lesions, synovium, extra-articular bursae and soft tissues surrounding the joint, including periosteum and the infrapatellar fat pad 12–14
Structurally, the historical utility of inflammatory cytokines such as interleukin-1beta (IL-1β) for inducing osteoarthritic phenotypes in joint cells and joint tissue explants in culture led over several decades to the generation of a plethora of anti-cytokine strategies (anti-IL-1, -6, -7, -17, -18, tumor necrosis factor alpha (TNFα)), often used to treat other inflammatory diseases that were then translated to OA, most of which have largely failed to produce an effective pain or structure modifying therapeutic.15,16 Some potential flaws in the logic of treating with inflammatory cytokine inhibitors stem from the fact that while cytokines in culture such as IL-1 reliably induce cartilage degradation through activation of multiple catabolic signaling pathways, resulting in generation of matrix degrading proteinases, a review of the relatively scant literature on actual cytokine levels in joints generally reveals very low levels of these inflammatory mediators in joint fluid and tissues from OA patients (e.g. IL-1 levels < 2–28 pg/ml; in some cases undetectable),17,18 and little correlation of expression levels with disease signs and symptoms has been identified.19 While IL-1 and other cytokines, such as TNFα and oncostatin M continue to have utility in culture systems that study disease mechanisms and potentially screen candidate therapeutics prior to in vivo application in preclinical models, it is becoming evident from both preclinical and clinical studies performed with agents targeting cytokines and the factors they induce (e.g. matrix metalloproteinases, p38 MAP kinases) that these are not likely to be effective therapeutic targets. Other strategies that intuitively should have value including directly inhibiting catabolic enzymes (matrix metalloproteinases, aggrecanases, cathepsins) that degrade the critical structural matrix components collagen and aggrecan have suffered from inadequate efficacy or unacceptable side effects. Within the past two years, multiple clinical programs for products that inhibit each of the catabolic enzymes listed have been discontinued. This may be due to these safety and/or efficacy failures, or to some of the development challenges that are discussed later in this review.
2. Medical Need
The current treatment paradigm for OA20,21 consists initially of behavioral interventions including patient education, physical and occupational therapy, weight reduction, quadriceps strengthening, exercise, and use of assistive devices. Patient compliance with these measures is often poor, thus treatment with a simple analgesic such as acetaminophen (paracetamol) is frequently initiated. Worsening pain or lack of persistent efficacy with these products may call for the addition of over the counter or prescription nonsteroidal anti-inflammatories, either specific or non-specific for inhibition of COX-2. These too may suffer from inadequate efficacy and carry risks of gastrointestinal, renal, and cardiovascular toxicities. As the disease progresses, the analgesic benefits of this regimen frequently prove insufficient, thus therapy may be supplemented with intraarticular injections of hyaluronic acid and/or corticosteroid. Once these treatment regimens fail, surgical joint replacement, an invasive procedure with a fairly lengthy recuperative period and modest options for revision when needed, is the main option left for patients. Market research indicates that a mere 19% of knee OA patients are satisfied with their current treatment, 91% are not ready for total joint replacement, and 59% would try “almost anything” to avoid joint surgery.22 A 2005 health survey23 indicates that 75% of patients want more effective treatment for their existing OA. Unsatisfactory efficacy of currently available treatment options combined with a recent exodus of pharmaceutical companies willing to undertake the challenges of identifying a suitable osteoarthritis treatment and proving it effective, have left a large gap in the therapeutic pipeline for this disease, causing an ongoing and increasingly unmet medical need.
3. Current Research Goals
There have been several excellent reviews recently published that cover current, theoretical, and emerging OA treatments for pain and structure modification, including target rationale.24–26 The intention of this review is not to reproduce the work from those reviews but to provide an update on those product concepts currently in mid to late stage clinical development, reiterate their rationale, and reflect briefly on those that have fallen from the pipeline.
Pain
In light of the predominant symptom of OA being pain and our current inability to meet expectations of patients with regards its amelioration, there is a great need to develop agents capable of alleviating pain. In addition to modest efficacy, available therapeutics also have toxicity profiles that are ill suited to long term administration for this chronic disease. Most of the current research in the area of osteoarthritis pain arises from broader pain research efforts that eventually come to bear on OA treatment. Until recently, there have been no potentially transformative pain therapeutics in the OA field for nearly half a century. A review of open and enrolling clinical trials (http://www.clinicaltrials.gov) reveals that the majority of current development efforts include re-formulations, alternate dosing, and specificity changes of nonsteroidal anti-inflammatory drugs (NSAIDs)/opioids and development of single injection hyaluronic acid products to co-exist with multi-injection products already marketed. Cardiovascular safety concerns with highly potent cyclo-oxygenase-2 specific (COX-2) inhibitors (e.g. Vioxx®, Bextra®, Celebrex®) that have led to drug retraction from the market or black box labeling, have prompted development of combinations of less selective NSAIDs combined with agents intended to ameliorate cardiovascular risk factors such as increased blood pressure (e.g. naproxcinod, naproxen combined with a nitric oxide donor).27,28 Changes in opioid drugs seek to maintain efficacy while minimizing side effect profiles.29 One injection hyaluronic acid products may give patients receiving benefit from these formulations a less invasive, more convenient, and potentially safer option, but this strategy does not enhance efficacy. Some drug developers have repurposed drugs from other indications to OA. A good recent example of this is duloxetine, a selective serotonin and norepinephrine reuptake inhibitor with central nervous system activity,30,31 developed as an antidepressant and used to treat select chronic pain conditions, including fibromyalgia, diabetic peripheral neuropathy, and chronic low back pain.32 In a recently completed clinical study,32 duloxetine showed significant pain relief over placebo over the 13 week trial period. Path analysis demonstrated that this was a primary analgesic effect, not due to elevation in mood or changes in anxiety or depression symptoms.
With regard to more novel therapeutics, a great deal of investigation has been performed on inhibitors of ion channels and pain signaling neurotrophins. Transient receptor potential vanilloid (TRPV1, TRPV3, and TRPV4) and sodium (Nav1.3, Nav1.7, Nav1.8, Nav1.9) channel blockade have been the subject of early discovery and preclinical research programs, but have not yet been in late stage trials for OA pain. TRPV1 inhibition has recently been tested as a treatment for migraine pain, with trial results pending. Most significantly in the pain arena is the development of inhibitors of the neurotrophin nerve growth factor (NGF), which although currently challenged by potential safety issues, may help set in motion revolutionary changes in the treatment of chronic pain, including that resulting from OA. The scientific rationale for these targets, as well as for the structure modification targets described below, is presented in the next section.
Structure modification
Once a popular area for drug development, with a multitude of discovery and preclinical programs at major pharmaceutical and biotech companies, and dozens of compounds moving through pharmaceutical pipelines toward pivotal clinical trials, today research on slowing or stopping progression of cartilage loss and other structural changes in the joint has been significantly scaled back, due in large part to the challenges with target identification and clinical development referred to above and described in more detail below. Indeed as little as two years ago, agents targeting catabolic cytokines and proteases (e.g. inhibitors of IL-1β, MMPs, aggrecanases, cathepsins), and pathways common to inflammation (e.g. components of the NFκB and more specifically the MAP kinase pathway) were actively being investigated in the clinic (see Ref. 25 for a list of recently completed structure modification trials). Other approaches, such as the use of risedronate,33 have focused on maintaining bone integrity to support the joint surface and thus help keep the cartilage surface healthy and intact. Currently, clinical research efforts have dropped to only a small number of candidates still in later stage trials (Table 1). These most notably include intraarticular administration of the pro-anabolic agents osteogenic protein-1 (OP-1) and fibroblast growth factor-18 (FGF-18), in addition to anti-catabolic inducible nitric oxide synthase (iNOS) inhibitor. The results of recently completed clinical trials evaluating oral salmon calcitonin are pending. There are also trials ongoing or in planning evaluating cell therapy (e.g. autologous mesenchymal stem cells) for structure modification effects. Publicly available details of these trials can be found at http://www.clinicaltrials.gov/.
Table 1.
| Compound/Product | Company | Primary Indication |
Stage of Development |
Mechanism of Action (Route) |
|---|---|---|---|---|
| Tanezumab | Pfizer | Pain | Phase 3, trial halted due to FDA concerns with safety |
Anti-NGF antibody (Ab)(IV) |
| JNJ-42160443 | J&J | Pain | Phase 2, on clinical hold |
Anti-NGF Ab (SC) |
| REGN-475 | Sanofi/Regener on |
Pain | Phase 2, on clinical hold |
Anti-NGF Ab (IV) |
| MEDI-578 | Astra-Zeneca | Pain | Phase 1, on clinical hold |
Anti -NGF Ab (IV) |
| PG110 | Abbott | Pain | Phase 1 | Anti-NGF Ab (IV) |
| PF-04191834 | Pfizer | Pain? | Phase 2 | 5-LOX inhibitor (oral) |
| NSAID reformulation/dose changes |
multiple | Pain | Phase 2/3 | Prostaglandin inhibition; anti- inflammatory |
| Hyaluronic acid (HA) reformulation/dose changes |
Anika, Ferring, Seikagaku, Q- Med |
Pain | Most Phase 3 or at FDA post-Phase 3 |
synovial fluid HA replacement (IA) |
| Hydros/Hydros TA | Carbylan Biosurgery |
Pain | Phase 2 ex-US, results expected late 2011 |
HA + steroid combination (IA) |
| BMP-7 (OP-1) | Stryker | Structure modification |
Phase 1/2 | Pro-anabolic growth factor (IA) |
| Salmon Calcitonin | Novartis | Structure modification |
Phase 3 | Bone and articular surface contour preservation (oral) |
| PH-797804 | Pfizer | Structure modification |
Phase 2 | MAP kinase inhibitor |
| SD-6010 | Pfizer | Structure modification |
Phase 2; Joint-space narrowing over 24 months |
iNOSinhibitor |
| FGF-18 | Merck-Serono | Structure modification |
Phase 1; Change in cartilage thickness by MRI |
Pro-anabolic growth factor (IA) |
| Autologous Bone Marrow Stem Cells |
Internation Stemcell Services and others |
Structure modification |
Phase 1/2; Pain and cartilage mapping by MRI |
Regenerative, pro- anabolic, anti- inflammatory |
| Canakinumab | Novartis | Structure modification? |
Phase 2 | Anti-IL-1 Ab (IA) |
| SAR-113945 | Sanofi | Unknown | Phase 1 | IKK-beta kinase inhibitor (IA) |
| ABT-652 | Abbott | Unknown | Phase 2 | Unknown |
4. Scientific Rationale
4.1 Pain – novel targets
4.1.1 Ion channel inhibition
Of the ion channels known to participate in pain signaling, perhaps the most targeted for pain relief currently is the cation channel TRPv1, a polymodal nociceptor most commonly known as the capsaicin receptor. Capsaicin is only one of a number of activators of this channel, however, others including heat, acid, and of most interest to the OA pain field, inflammatory acute phase proteins and later phase mediators such as bradykinin, serotonin, histamine, and prostaglandins.34 Simulation of TRPV1 activity by these mediators can be via several different mechanisms, including activation of protein kinase C35–37 or protein kinase A pathways,38 reversal of phosphatidylinositol 4,5-bisphosphate-dependent inhibition,39,40 or by formation of 12-hydroperoxyeicosatetraenoic acid (12-HPETE).41 (See Ref. 34 for more in depth review).
Activation of TRPv1 by any of these mechanisms results in channel opening and influx of calcium, driving action potential formation and sensitizing sensory nerves. TRPv1 inhibitors (SB-705498 and AMG 517) have recently been investigated in clinical trials for relief of chronic pain in conditions such as migraine and inflammatory hyperalgesia, which seems a sound approach based on the multi-faceted role of these channels in pain signaling. However, earlier trials using TRPv1 antagonists identified a negative impact on thermoregulation resulting in fever; thus development was discontinued.42,43 While preclinical studies are underway using TRPv1 channel inhibitors to treat OA pain, to date no human clinical trials have been initiated for this specific indication.
Other investigators have taken the reverse approach and treated with TRPv1 agonists (e.g. capsaicin or its analogues) in order to bind the receptor, open the channel, and provide long term channel inactivation, resulting in transient burning discomfort followed by long term pain relief. Both topical and intraarticular delivery of capsaicin has been evaluated for the treatment of OA pain with some success. The pain relief provided in a Phase II trial in OA patients by locally injected capsaicin (ALGRX-4975) is worth noting44,45 although the sustained burning sensation experienced in the hours to days following the injections reported by some patients may hinder commercial acceptability of this approach, and it is unclear whether development of this product concept will continue. Clinical trial results of a capsaicin patch (NGX-4010)46 showed some promise for treating post-herpetic neuralgia, while results treating interstitial cystitis with resiniferatoxin, a capsaicin analogue47 were less compelling.48
Sodium channels (Nav1.3, Nav1.7, Nav1.8, Nav1.9), another sub-class of voltage gated ion channels critical for initiation and propagation of action potentials in peripheral sensory neurons49 have also been targeted with some preclinical success. Sodium channel inhibitors have been tested in humans for inflammatory and neuropathic pain, although to the author’s knowledge these have not been in trials for OA. Early data suggests that inhibiting these ion channels carry some promise for relieving pain, and this may be an area to watch as development plans and outcomes materialize in the near future.
4.2 Neurotrophin inhibition
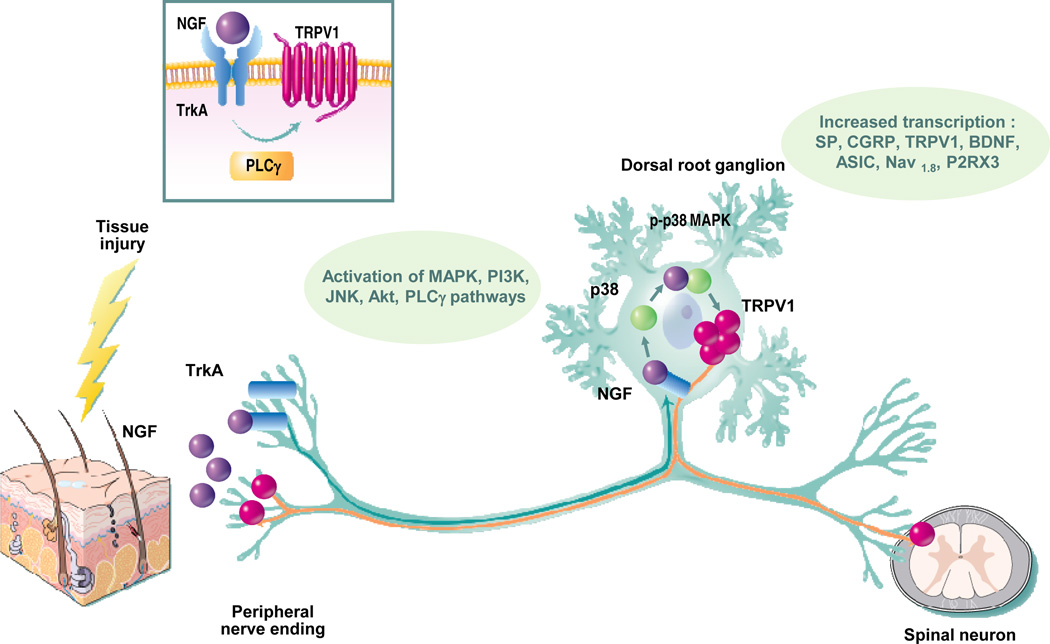
One of the most exciting pain relief targets to hit the pipeline in decades is nerve growth factor (NGF). As NGF signaling is common to multiple pain signaling pathways and post-natal expression of NGF is not reportedly required for nerve maintenance or repair following injury, this target represents a highly valid approach to pain relief. NGF is produced by multiple structural, inflammatory and immune cells types (keratinocytes, Schwann cells, macrophages, etc.) in response to injury, inflammation, or disease.50 Binding of NGF to its high affinity TrkA receptor and/or possibly its low affinity p75 receptor, expressed on peripheral sensory nerves, can initiate pain signaling cascades and receptor-ligand trafficking activity to the nerve cell body to produce gene expression changes that result in enhanced excitability of sensory neurons,51 although the precise mechanism remains unclear. Some studies have provided evidence that NGF can drive signaling through both PKA52 and PKC53 (Figure 1). NGF binding to TrkA is known to enhance TRPv1 signaling, and there is evidence to support potentially dual mechanisms (Figure 1). One mechanism may involve formation of a signaling complex of TrkA, PLCγ, and TRPv1 that increases TRPv1 activity in response to NGF.40 A second mechanism may result from NGF-induced rapid insertion of Trpv1 channels into the plasma membrane of the neuron.54 The increase in TRPv1 activity also appears to be driven by stimulation of p38 MAP kinase.55 Receptor binding by NGF induces expression of neuronal sensitizing factors calcitonin gene related peptide (CGRP) and substance P, with signaling also occurring through PI3 kinase and PLCγ.56,57 These multi-dimensional activities mediated by NGF all point to this factor as a convergence point for many pain signaling pathways,57 and this may make it an ideal target for pain relief strategies.
Figure 1. Mechanism of nerve growth factor induced hyperalgesia.
NGF binds its high affinity receptor TrkA causing phosphorylation and increased activity of TRPv1. NGF undergoes retrograde transport to the dorsal root ganglion, increasing expression of pain signaling factors including substance P, calcitonin gene related peptide(CGRP), TRPV1, brain-derived neurotrophic factor (BDNF) etc., some of which provide central pain perception via release into spinal cord synapses. Increased peripheral hypersensitivity can also be exacerbated by anteriograde transport of TRPV1.
Further validation of NGF as a target comes from mounting preclinical and clinical evidence. In preclinical studies, both NGF58 and TrkA59 knockout mice are hypoalgesic, failing to respond to noxious stimuli. Transgenic mice overexpressing NGF demonstrate hyperalgesic pain behaviors.60,61 Administration of NGF to animals provokes both rapid and long-lasting hyperalgesia,62,63 and finally, NGF antibodies reverse established allodynia in inflammatory/neuropathic pain models.64
In humans, mutations in TrkA and/or NGF genes are linked to congenital insensitivity to pain.65 Increased NGF levels have been reported in painful conditions such as pancreatitis66 and chronic daily headache,67 and administration of rhNGF induces hyperalgesia and allodynia.68,69 Finally, and most convincingly, intravenous administration of the anti-NGF monoclonal antibody tanezumab to OA patients demonstrated marked pain relief versus placebo in multiple clinical trials which included late stage patients with fairly severe chronic pain.9.
At least five pharmaceutical companies have anti-NGF development programs for treating OA pain, and unfortunately all of them are currently on hold as 16 patients treated with high dose tanezumab in one Phase III trial experienced acceleration of their OA disease process that resulted in the need for surgical intervention (i.e. joint replacement). Curiously, in approximately half of the cases, the toxicity was not in the index joint, but in either the contralateral knee, a hip, or a shoulder.9 Given the high potential of this approach to provide effective pain relief in a multitude of painful conditions, including OA, understanding the basis for these infrequent but important safety events and developing a strategy for mitigating the risk seems a crucial part of the research agenda in the realm of OA pain.
4.3 Structure Modification
4.3.1 Osteogenic protein-1
Bone morphogenetic proteins (BMPs) were isolated and characterized nearly 30 years ago from protein extracts that could drive bone and cartilage formation.70 Since that time, various members of this subgroup of the transforming growth factor beta (TGFβ) superfamily have been used successfully in research and clinical orthopaedic applications to stimulate bone and/or cartilage regeneration. Osteogenic protein-1 (OP-1), also known as bone morphogenetic protein-7 (BMP-7), is expressed by adult human chondrocytes71 and stimulates their matrix anabolic activity,72,73 increasing synthesis of key matrix proteins aggrecan and collagen. OP-1 has also been reported to inhibit the catabolic effects of IL-1β,72,73 although recent evidence has demonstrated significant cross-talk between OP-1 and IL-1β that may keep some of this inhibition in check. In cultured human chondrocytes, IL-1 has been shown to counteract OP-1 signaling by decreasing both OP-1 signaling receptors (activin receptor-like kinase 2 (ALK-2) and ALK-3) and key signaling molecules (Smads 1 and 6).74
Up to a four fold decrease in OP-1 levels in joints has been detected with aging,75 potentially driven by methylation of the OP-1 promoter,76 but it is not clear whether this decline is pathologic or incidental. If it is indeed pathologic, there is strong logic in slowing the progression of OA associated cartilage loss with OP-1. Two Phase II clinical trials are currently enrolling patients using single intraarticular dosing of OP-1. While the pro-anabolic strategy is a sensible one, and results of these trials remain to be seen, it is challenging to envision lasting structure modification effects from a single injection, given the very short half life of proteins (hours) in the joint. If the approach shows some potential in these trials, sustained release formulations may be indicated.
4.3.2 Fibroblast growth factor-18
FGF-18 is another predominantly pro-anabolic growth factor for cartilage but one that also has chondrocyte proliferative and in some cases pro-catabolic activity in chondrocytes in an OA setting.77,78,79 Recent microarray analysis of the effects of FGF-18 on IL-1 stimulated human articular chondrocytes in culture demonstrated increased gene expression of cartilage protective factors aggrecan, BMP-2, and COL2A1 with concomitant upregulation of catabolic factors such as the aggrecanases ADAMTS-4 and -5, IL-1β, IL-6, and MMP-13.79 The fact that FGF-18 can provide both proliferative and directional cues in airway cartilage in culture80 suggests that this balance may have the potential to not only ramp up cartilage formation in osteoarthritis but also to permit beneficial structural remodeling of newly formed repair tissue. Indeed this has been demonstrated in animal models,81 and intraarticularly delivered FGF-18 is currently in clinical development as an osteoarthritis structure modifying agent.
4.3.4 Calcitonin
Calcitonin is one of a number of natural regulators of calcium and phosphate balance in the body, produced primarily by parafollicular C cells in the thyroid. While its importance in maintaining normal calcium homeostasis is not well established, it has the ability to counteract the blood calcium increasing effects of parathyroid hormone. In states of calcium mobilization (e.g. lactation, menopause), calcitonin may attempt to protect against calcium mobilization from bones. The ability to inhibit osteoclast activity has also been ascribed to calcitonin.82
While historically most of the effects of calcitonin have been studied in bone, over the past decade evidence has accumulated that cartilage affects both bone and cartilage directly.83 There is in vitro evidence that calcitonin can directly inhibit MMPs and block collagen degradation in articular chondrocytes exposed to TNFα/oncostatin M to induce an OA phenotype.84 Recently, oral salmon calcitonin has been shown, in a rat ovariectomy model of the post-menopausal state common to OA patients, to significantly mitigate damage induced by a meniscectomy OA model.85 The dual activities on bone and cartilage may make this an ideal strategy for treating OA, which has pathology in both of these tissues. A clinical trial evaluating oral salmon calcitonin for the treatment of OA has just been completed, and trial results are pending.
4.3.5 Chondroitin sulfate
Chondroitin sulfate is a glycosaminoglycan that in articular cartilage attaches, along with keratan sulfate, in multiples to the core protein of the proteoglycan aggrecan, a major structural component that draws water into the cartilage as part of a process that, in concert with swelling resistance provided by Type II collagen, maintains the viscoelastic and force resistance properties of the tissue.86 Aggrecan loss, and thus chondroitin sulfate loss, are key features of progressing OA and as such, replacement of those lost components by oral supplementation could be considered a sound strategy for positively influencing disease progression. Various formulations of chondroitin sulfate are commercially available, by prescription in Europe, and over the counter sold as nutraceuticals in the United States. This latter fact may reflect on the product quality available in the EU versus US, with the potential for a higher level of quality control in the prescribed products which undergo a more rigorous level of regulatory scrutiny, as by contrast nutraceuticals tend to have very little regulation. There are 16 trials evaluating chondroitin sulfate for osteoarthritis registered with clinicaltrials.gov, seven recently completed, three recruiting patients, four registered but not yet recruiting patients, and two active but not recruiting. There are also numerous reports in the literature of structure modification success treating clinical OA with chondroitin sulfate, most frequently detected by radiographic assessment of joint space narrowing, or in some cases by magnetic resonance imaging of the cartilage volume and/or thickness.87–90 Recent meta-analyses of chondroitin sulfate data have concluded there is a small but significant effect of chondroitin sulfate on slowing the rate of cartilage loss from OA joints.91,92 The sheer number of investigations that have been undertaken makes it one of the most studied agents for structure modification in OA, and one of the few to show a measurable effect with some consistency.
4.3.6 iNOS inhibition
The enzyme responsible for the production by chondrocytes of high and sustained levels of nitric oxide (NO) in response to cytokines (e.g. IL-1β), endotoxin, and other pathologic stresses is inducible nitric oxide synthase (iNOS). NO itself as well as its primary metabolites (e.g. peroxynitrite, a potent pro-oxidant) are toxic to cells and create cartilage matrix and synovial tissue damage in OA.93 In studies using a canine model of experimental OA, iNOS inhibition decreased OA induced cartilage damage including a reduction in the size of cartilage lesions and the size and number of osteophytes. These reductions correlated with decreases in IL-1β, MMPs, and prostaglandins, all potential drivers of structural damage and/or pain sensitization. A reduction in chondrocyte apoptosis was noted as well.94–96 Although it remains unclear how significant the role of NO is in OA, the available body of evidence around this target justifies evaluation of the target in the clinic. As such, multiple phase I/II studies have been performed, and a phase III trial is currently ongoing with a two year primary endpoint of reduction in joint space narrowing.
4.3.7 Cells
Cell therapy for osteoarthritis has generated increasing interest in the last few years. Osiris demonstrated a trend toward efficacy in a completed phase II trial in OA using allogeneic mesenchymal stem cells, looking at pain relief and structure modification. Four cell therapy based clinical trials are currently registered evaluating efficacy for OA treatment. Most of these trials have both pain and structure based endpoints. The mechanism of action of stem cells in a disease such as OA may be linked to the pluripotent differentiation capability of these cells providing additional stem cells and chondrocytes that are geared toward restoration of damaged tissues, but these cells are also factories for balanced levels of growth factors, cytokines, chemokines, and other factors that influence tissue repair and remodeling, potentially making this a more biologically relevant solution than single growth factor or cytokine pathway inhibitor strategies alone. Mesenchymal stem cells have been shown to have anti-inflammatory and immune modulatory properties as well.
These same factors that make cell therapy potentially beneficial for OA may also cripple it as a commercializable product. Understanding mechanism of action and developing acceptable methods for establishing identity, purity, and potency represent key challenges in the development of cell therapy products for any indication. Sourcing also needs to be taken into consideration if allogeneic mesenchymal stem cells are used. Product developers and regulatory agencies will grapple with these challenges for some time to come if these cell based treatments demonstrate clinically relevant efficacy.
5 Competitive Environment
The majority of therapeutics currently in clinical development (generally Phase II+) for OA pain and/or structural modification are listed in Table 1. These do not include herbal supplements and other non-traditional treatments such as magnetic or radiofrequency therapy, acupuncture, leeches, cherry juice, or gold beads, all of which are currently being studied for OA treatment. In terms of numbers of pain trials, two categories of pain therapeutics dominate the development pipeline: current therapeutic reformulations/additives/dose changes (NSAIDS, opioids, hyaluronans), and nerve growth factor inhibitors, the latter of which have all been put on clinical hold by the Food and Drug Administration due to safety issues referred to above seen in a small number of patients treated with tanezumab, Pfizer’s anti-NGF monoclonal antibody. Structure modification trials are predominantly testing pro-anabolics (growth factors/hormones), with a modest selection of anti-catabolics still in trials (IL-1, IKK, and iNOS inhibitors). Cell therapy trials using primarily mesenchymal stem cells from either bone marrow or adipose tissue could fall into pro-anabolic, anti-catabolic, pain relief, and/or structure categories, as the mechanism of action of these cells may be multi-faceted and is incompletely understood.
6 Potential Development Issues
As alluded to previously, there are some potentially ominous development hurdles to overcome in producing effective OA therapeutics. Because the precise cause of OA is relatively unknown, target selection and validation has been challenging. The multi-factorial etiology and the variability in disease manifestation creates a situation that may be best suited to subset development, but there is not consensus on what those subsets should be.
The next piece in drug development progression involves selecting effective candidates to put into human clinical trials. There is no shortage of in vitro and in vivo models that can be used for eliminating candidates from consideration, but establishing the validity and utility of these models for this selection and facilitating ready translation into the human model in the face of a paucity of successful candidates is challenging.
Last but certainly not least, trial endpoints can be problematic. Pain trials generally use subjective patient reported outcomes, and these are susceptible to a high degree of variability, with resultant consequences on their responsiveness and ability to disentangle therapeutic effects from placebo. Placebo effects in OA trials can be high and persist over several months.97 Structure modification trials are hindered by a combination of slow and unpredictable disease progression and relatively insensitive detection tools (imaging, biochemical biomarkers). This combination often leads to trial failure simply because the control group did not progress during the study period, thus no treatment effect could be measured.98 One solution to this is to perform larger and longer clinical trials, which is not generally in the strategic best interests of a drug developer. Another solution is to select individuals likely to progress more quickly, which tend to be those with more severe disease and limb malalignment. The issue here is that this more severe disease is likely much harder to alter with an intervention.99 The pain/structure disconnect that tends to be a prominent feature of OA further complicates development of these therapeutics. Based on the regulatory guidelines for structure modification, unless there is complete cessation of disease progression (or reversal!), a clinically meaningful benefit of the observed structural change needs to be demonstrated. Thus pain, function, or similar data needs to be collected in structure modification trials along with the information on effect on parameters such as cartilage loss. In summary, safety issues for pain drugs and obtaining clinically relevant endpoints for structure modifying drugs may each contribute in the future to the requirement for long, large, expensive trials, which will serve to further hinder drug development in these areas.
7 Conclusion
Osteoarthritis is a widespread serious debilitating disease causing enormous medical expense burden on the public health system. This combined with the fact that OA also represents an enormous unmet medical need, as the majority of patients are not satisfied with their current therapy, provides for a very attractive market for newly developed OA therapeutics. Recent breakthroughs in pain management, while currently plagued by potential safety issues, represent the first bright spot in many years for sufferers of chronic pain. Focused effort on refinement of imaging endpoints for trials and revision of regulatory guidance on development of structure modifying drugs provides some hope for movement in this area as well, which to date has had no successful therapeutics brought to market. Development of drugs in both pain and structure modification realms is challenging, given high placebo effects and subjective patient reported outcomes in pain trials and slow disease progression and lack of adequately sensitive detection methods for loss of articular cartilage in structure modification trials. The latter are also saddled with the burden of demonstrating a clinically meaningful result (e.g. pain relief, functional improvement) in addition to preservation of joint structure.
8 Expert Opinion
Osteoarthritis treatment is an important area for a substantial segment of the world’s population that is in serious need of increased investment and research attention. In the past few years, many key corporate players have exited this arena due to the challenges of developing effective, approvable, and marketable therapeutics. As a result of this, as well as failure of many product candidates in clinical trials, the numbers of novel targets being tested has dwindled significantly. Rejuvenation of investment interest in this area will be critical to meeting this unmet medical need. Clinical and commercial success of a breakthrough product would drive this, and there is hope around the recent results of anti-NGF strategies for pain, although the safety issues are currently clouding the optimism around this approach. The ion channel inhibitors are likely to be a reasonable back-up, although validation of these targets for OA pain treatment is considerably less robust. It is unlikely, given the results of clinical trials and the continued elucidation of the presence and role of cytokines in OA, that inhibitors of factors such as IL-1, IL-6 and TNF, are likely to be successful, even if delivered for sustained periods of time. Success with structure modifying drugs will likely follow (or more optimistically move in parallel with) development of appropriate biomarkers (imaging, biochemical, and/or genetic) that will facilitate development, and there is clearly focused work going on in this area. A second critical piece for success of this type of program, however, rests in the ability to understand the disease well enough to make a currently missing connection between structural changes and clinically meaningful changes (e.g. pain, function, need for joint replacement, etc.). This is a bigger challenge, and may require thinking differently about the disease and joint as a whole. It is indeed fertile ground for future research.
Acknowledgments
Declaration of interest
G Matthews is an employee of Genzyme Corporation, a company which develops and markets osteoarthritis drugs. However, no products or product concepts associated with the company are presented in the manuscript and the content is completely without bias.
D Hunter receives grant funding from the NIH and ARC.
Contributor Information
David J Hunter, Professor of Medicine, Northern Clinical School - Rheumatology, University of Sydney, Sydney, New South Wales, Australia, David.Hunter@sydney.edu.au.
Gloria Matthews, Senior Scientific Director, Genzyme Corporation - Orthopaedics, 49 New York Avenue, Framingham, Massachusetts 01701, United States, gloria.matthews@genzyme.com.
Bibliography
- 1.Zebrowski M. The Pain Market Outlook to 2011. Business Insights Ltd. 2005 [Google Scholar]
- 2.Stakeholder Opinions: Osteoarthritis. Datamonitor. 2006:17. [Google Scholar]
- 3. http://www.cdc.gov/nccdphp/arthritis.
- 4.Arthr Rheum. 1999;42(3):415–420. doi: 10.1002/1529-0131(199904)42:3<415::AID-ANR4>3.0.CO;2-Z. iOA, RA, fibromyalgia, gout. [DOI] [PubMed] [Google Scholar]
- 5.NINDS/NIH. 1999 migraine. [Google Scholar]
- 6.Wieland HA, Michaelis M, Kirschbaum BJ, et al. Osteoarthritis - an untreatable disease? Nat Rev Drug Discov. 2005;4(4):331–344. doi: 10.1038/nrd1693. [DOI] [PubMed] [Google Scholar]
- 7.Reijman M, Bierma-Zeinstra SM, Pols HA, et al. Is there an association between the use of different types of nonsteroidal antiinflammatory drugs and radiologic progression of osteoarthritis? The Rotterdam Study. Arthritis Rheum. 2005;52(10):3137–3142. doi: 10.1002/art.21357. [DOI] [PubMed] [Google Scholar]
- 8.Wood JN. Nerve Growth Factor and Pain N Engl J Med. 2010;363:1572–1573. doi: 10.1056/NEJMe1004416. Comment on Discussion section of reference 9 below. [DOI] [PubMed] [Google Scholar]
- 9.Lane NE, Schnitzer TJ, Birbara CA, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med. 2010;363(16):1521–1531. doi: 10.1056/NEJMoa0901510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nüesch E, Rutjes AW, Trelle S, et al. Doxycycline for osteoarthritis of the knee or hip. Cochrane Database Syst Rev. 2009;7(4) doi: 10.1002/14651858.CD007323.pub2. CD007323. [DOI] [PubMed] [Google Scholar]
- 11.Brandt KD, Mazzuca SA, Katz BP, et al. Effects of doxycycline on progression of osteoarthritis: results of a randomized, placebo-controlled, bouble-blind trial. Arthritis Rheum. 2005;52:2015–2025. doi: 10.1002/art.21122. [DOI] [PubMed] [Google Scholar]
- 12.Felson DT, Chaisson CE, Hill CL, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134(7):541–549. doi: 10.7326/0003-4819-134-7-200104030-00007. [DOI] [PubMed] [Google Scholar]
- 13.Hill CL, Gale DG, Chaisson CE, et al. Knee effusions, popliteal cysts, and synovial thickening: association with knee pain in osteoarthritis. J Rheumatol. 2001;28(6):1330–1337. [PubMed] [Google Scholar]
- 14.Hill CL, Hunter DJ, Niu J, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66(12):1599–1603. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chevalier X, Goupille P, Beaulieu AD, et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2009;61(3):344–352. doi: 10.1002/art.24096. [DOI] [PubMed] [Google Scholar]
- 16.Malemud CJ. Anticytokine therapy for osteoarthritis: evidence to date. Drugs Aging. 2010;27(2):95–115. doi: 10.2165/11319950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.Westacott CI, Whicher JT, Barnes IC, et al. Synovial fluid concentration of five different cytokines in rheumatic diseases. Ann Rheum Dis. 1990;49(9):676–681. doi: 10.1136/ard.49.9.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertazzolo N, Punzi L, Stefani MP, et al. Interrelationships between interleukin (IL)-1, IL-6 and IL-8 in synovial fluid of various arthropathies. Agents Actions. 1994;41(1–2):90–92. doi: 10.1007/BF01986402. [DOI] [PubMed] [Google Scholar]
- 19.Brenner SS, Klotz U, Alscher DM, et al. Osteoarthritis of the knee - clinical assessments and inflammatory markers. Osteoarthritis Cartilage. 2004;12(6):469–475. doi: 10.1016/j.joca.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008 Feb;16(2):137–162. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 21.American College of Rheumatology guidelines on the treatment of osteoarthritis. 1997 [Google Scholar]
- 22.Peoplemetrics Patient Segmentation Research. 2007 [Google Scholar]
- 23.National Council on Aging, Harris Interactive Survey. 2005 [Google Scholar]
- 24.Read SJ, Dray A. Osteoarthritic Pain: A review of current, theroretical, and emerging therapeutics. Expert Opin Investig Drugs. 2008;17(5):619–640. doi: 10.1517/13543784.17.5.619. [DOI] [PubMed] [Google Scholar]
- 25.Hunter DJ. Pharmacologic therapy for osteoarthritis - the era of disease modification. Nat Rev Rheumatol. 2011;7(1):13–22. doi: 10.1038/nrrheum.2010.178. [DOI] [PubMed] [Google Scholar]
- 26.Hunter DJ, Hellio Le Graverand-Gastineau MP. How close are we to having structure modifying drugs available? Rheum Dis Clin North Am. 2008;34(3):789–802. doi: 10.1016/j.rdc.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Baerwald C, Verdecchia P, Duquesroix B, Frayssinet H, Ferreira T. Efficacy, safety, and effects on blood pressure of naproxcinod 750 mg twice daily compared with placebo and naproxen 500 mg twice daily in patients with osteoarthritis of the hip: a randomized, double-blind, parallel-group, multicenter study. Arthritis Rheum. 2010;62(12):3635–3644. doi: 10.1002/art.27694. [DOI] [PubMed] [Google Scholar]
- 28.Schnitzer TJ, Kivitz A, Frayssinet H, et al. Efficacy and safety of naproxcinod in the treatment of patients with osteoarthritis of the knee: a 13-week prospective, randomized, multicenter study. Osteoarthritis Cartilage. 2010;18(5):629–639. doi: 10.1016/j.joca.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 29.Wild JE, Grond S, Kuperwasser B, et al. Long-term safety and tolerability of tapentadol extended release for the management of chronic low back pain or osteoarthritis pain. Pain Pract. 2010 Sep-Oct;10(5):416–427. doi: 10.1111/j.1533-2500.2010.00397.x. [DOI] [PubMed] [Google Scholar]
- 30.Iyengar S, Webster AA, Henrick-Lucke SK, et al. Efficacy of duloxetine, a potent and balanced serotonin-norepinephrine inhibitor in persistent pain models in rats. J Pharmacol Exp Ther. 2004;311:576–584. doi: 10.1124/jpet.104.070656. [DOI] [PubMed] [Google Scholar]
- 31.Wong DT, Bymaster FP. Dual serotonin and noradrenaline uptake inhibitor class of antidepressants—potential for greater efficacy or just hype? Prog Drug Res. 2002;58:169–222. doi: 10.1007/978-3-0348-8183-8_5. [DOI] [PubMed] [Google Scholar]
- 32.Chappell AS, Desaiah D, Liu-Seifert H, et al. A double-blind, randomized, placebo-controlled study of the efficacy and safety of duloxetine for the treatment of chronic pain due to osteoarthritis of the knee. Pain Pract. 2011;11(1):33–41. doi: 10.1111/j.1533-2500.2010.00401.x. [DOI] [PubMed] [Google Scholar]
- 33.Spector TD, Conaghan PG, Buckland-Wright JC, et al. Effect of risedronate on joint structure and symptoms of knee osteoarthritis: results of the BRISK randomized controlled trial. Arthritis Res Ther. 2005;7:R625–R633. doi: 10.1186/ar1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenbaum T, Simon SA. TRPV1 Receptors and Signal Transduction. Chapter 5. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press; 2007. Frontiers in Neuroscience. [Google Scholar]
- 35.Cesare P, Dekker LV, Sardini A, et al. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron. 1999;23:617–624. doi: 10.1016/s0896-6273(00)80813-2. [DOI] [PubMed] [Google Scholar]
- 36.Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- 37.Vellani V, Mapplebeck S, Moriondo A, et al. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat, and anandamide. J Physiol. 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhave G, Zhu W, Wang H, et al. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- 39.Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300:1284–1288. doi: 10.1126/science.1083646. [DOI] [PubMed] [Google Scholar]
- 40.Chuang HH, Prescott ED, Kong H, et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 41.Shin HJ, Gye MH, Chung KH, et al. Activity of protein kinase C modulates the apoptosis induced by polychlorinated biphenyls in human leukemic HL-60 cells. Toxicol Lett. 2002;135:25–31. doi: 10.1016/s0378-4274(02)00231-x. [DOI] [PubMed] [Google Scholar]
- 42.Gavva NR, Treanor JJS, Garami A, et al. Pharmacological blockade of the vanilloid receptor TRPv1 elicits marked hyperthermia in humans. Pain. 2008;136(1–2):202–210. doi: 10.1016/j.pain.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 43.Rowbotham MC, Nothaft W, Duan WR, et al. Oral and cutaneous thermosensory profile of selective TRPv1 inhibition by ABT-102 in a randomized healthy volunteer trial. Pain. 2011 Mar 3; doi: 10.1016/j.pain.2011.01.051. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 44.Wong GY, Gavva NR. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: Recent advances and setbacks. Brain Res Rev. 2009;60(1):267–277. doi: 10.1016/j.brainresrev.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 45.Remadevi R, Szallisi A. Adlea (ALGRX-4975), an injectable capsaicin (TRPV1 receptor agonist) formulation for longlasting pain relief. IDrugs. 2008;11(2):120–132. [PubMed] [Google Scholar]
- 46.Irving GA, Backonja MM, Dunteman E, et al. A multicenter, randomized, double-blind, controlled study of NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia. Pain Med. 2011 Jan;12(1):99–109. doi: 10.1111/j.1526-4637.2010.01004.x. [DOI] [PubMed] [Google Scholar]
- 47.Szallasi A, Blumberg PM. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience. 1989;30(2):515–520. doi: 10.1016/0306-4522(89)90269-8. [DOI] [PubMed] [Google Scholar]
- 48.Peng CH, Kuo HC. Multiple intravesical instillations of low-dose resiniferatoxin in the treatment of refractory interstitial cystitis. Urol Int. 2007;78(1):78–81. doi: 10.1159/000096940. [DOI] [PubMed] [Google Scholar]
- 49.Priest BT. Future potential and status of selective sodium channel blockers for the treatment of pain. Current Opin in Drug Discovery and Development. 2009;12(5):682–692. [PubMed] [Google Scholar]
- 50.Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 51.Delcroix JD, Valletta JS, Wu C, et al. NGF signaling in sensory neurons: Evidence that early endosomes carry NGF retrograde signals. Neuron Jul. 2003;3(39):69–84. doi: 10.1016/s0896-6273(03)00397-0. [DOI] [PubMed] [Google Scholar]
- 52.Shu X-Q, Mendell LM. Acute sensitization by NGF of the response of small-diameter sensory neurons to capsaicin. J.Neurophysiol. 2001;86:2931–2938. doi: 10.1152/jn.2001.86.6.2931. [DOI] [PubMed] [Google Scholar]
- 53.Bonnington JK, McNaughton PA. Signalling pathways involved in the sensitization of mouse nociceptive neurons by nerve growth factor. J.Physiol. 2003;551:433–446. doi: 10.1113/jphysiol.2003.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ji R-R, Samad TA, Jin S-X, Schmoll R, Woolf CJ. p38 MAPK Activation by NGF in Primary Sensory Neurons after Inflammation Increases TRPV1 Levels and Maintains Heat Hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 56.Nicol GD, Vasko MR. Unraveling the story of NGF-mediated sensitization of nociceptive sensory neurons: on or off the Trks? Mol Interv. 2007 Feb;7(1):26–41. doi: 10.1124/mi.7.1.6. [DOI] [PubMed] [Google Scholar]
- 57.Chao MV. Neurotrophins and their receptors: A convergence point for many signaling pathways. Nature Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 58.Crowley C, Spencer SD, Nishimura MC, et al. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994 Mar 25;76(6):1001–1011. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- 59.Smeyne RJ, Klein R, Schnapp A, et al. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 1994 Mar 17;368(6468):246–249. doi: 10.1038/368246a0. [DOI] [PubMed] [Google Scholar]
- 60.Davis BM, Lewin GR, Mendell LM, et al. Altered expression of nerve growth factor in the skin of transgenic mice leads to changes in response to mechanical stimuli. Neuroscience. 1993 Oct;56(4):789–792. doi: 10.1016/0306-4522(93)90127-2. [DOI] [PubMed] [Google Scholar]
- 61.Stucky CL, Koltzenburg M, Schneider M, et al. Overexpression of nerve growth factor in skin selectively affects the survival and functional properties of nociceptors. J Neurosci. 1999 Oct 1;19(19):8509–8516. doi: 10.1523/JNEUROSCI.19-19-08509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lewin GR, Ritter AM, Mendell LM. Nerve growth factor-induced hyperalgesia in the neonatal and adult rat. J Neurosci. 1993 May;13(5):2136–2148. doi: 10.1523/JNEUROSCI.13-05-02136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Andreev NYu, Dimitrieva N, Koltzenburg M, et al. Peripheral administration of nerve growth factor in the adult rat produces a thermal hyperalgesia that requires the presence of sympathetic post-ganglionic neurones. Pain. 1995 Oct;63(1):109–115. doi: 10.1016/0304-3959(95)00024-M. [DOI] [PubMed] [Google Scholar]
- 64.Wild KD, Bian D, Zhu D, et al. Antibodies to nerve growth factor reverse established tactile allodynia in rodent models of neuropathic pain without tolerance. J Pharma Exp Ther. 2007;222(1):282–287. doi: 10.1124/jpet.106.116236. [DOI] [PubMed] [Google Scholar]
- 65.Tonoki H, Awaya Y, Matsuda I. Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nature Genet. 1996;13:485–488. doi: 10.1038/ng0896-485. [DOI] [PubMed] [Google Scholar]
- 66.Friess H, Zhu ZW, di Mola FF, et al. Nerve growth factor and its high-affinity receptor in chronic pancreatitis. Ann Surg. 1999;230(5):615–624. doi: 10.1097/00000658-199911000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sarchielli P, Alberti A, Floridi A, et al. Levels of nerve growth factor in cerebrospinal fluid of chronic daily headache patients. Neurology. 2001 Jul 10;57(1):132–134. doi: 10.1212/wnl.57.1.132. [DOI] [PubMed] [Google Scholar]
- 68.Rukwied R, Mayer A, Kluschina O, et al. NGF induces non-inflammatory localized and lasting mechanical and thermal hypersensitivity in human skin. Pain. 2010;148(3):407–413. doi: 10.1016/j.pain.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 69.Svensson P, Cairns BE, Wang K, et al. Injection of nerve growth factor into human masseter muscle evokes long-lasting mechanical allodynia and hyperalgesia. Pain. 2003 Jul;104(1–2):241–247. doi: 10.1016/s0304-3959(03)00012-5. [DOI] [PubMed] [Google Scholar]
- 70.Wozney JM. Bone morphogenetic proteins. Prog Growth Factor Res. 1989;1(4):267–280. doi: 10.1016/0955-2235(89)90015-x. [DOI] [PubMed] [Google Scholar]
- 71.Chubinskaya S, Merrihew C, Cs-Szabo G, et al. Human articular chondrocytes express osteogenic protein-I. J Histochem Cytochem. 2000;48(2):239–250. doi: 10.1177/002215540004800209. [DOI] [PubMed] [Google Scholar]
- 72.Huch K, Wilbrink B, Flechtenmacher J, et al. Effects of recombinant human osteogenic protein1 on the production of proteoglycan, prostaglandin E2, and interleukin-I receptor antagonist by human articular chondrocytes cultured in the presence of interleukin-1. Arthritis Rheum. 1997;40:2157–2161. doi: 10.1002/art.1780401209. [DOI] [PubMed] [Google Scholar]
- 73.Koepp HE, Sampath KT, Kuettner KE, et al. Osteogenic protein-1 (OP-I) blocks cartilage damage caused by fibronectin fragments and promotes repair by enhancing proteoglycan synthesis. Inflamm Res. 1999;47:1–6. doi: 10.1007/s000110050446. [DOI] [PubMed] [Google Scholar]
- 74.Elshaier AM, Hakimiyan AA, Rappoport L, et al. Effect of interleukin-1beta on osteogenic protein 1-induced signaling in adult human articular chondrocytes. Arthritis Rheum. 2009 Jan;60(1):143–154. doi: 10.1002/art.24151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chubinskaya S, Kumar B, Merrihew C, et al. Age-related changes in cartilage endogenous osteogenic protein-1 (OP-1) Biochim Biophys Acta. 2002;1588:126–134. doi: 10.1016/s0925-4439(02)00158-8. [DOI] [PubMed] [Google Scholar]
- 76.Methylation of the OP-1 promoter: potential role in the age-related decline in OP-1 expression in cartilage Osteoarthritis Cartilage. 2009;17(4):513–517. doi: 10.1016/j.joca.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ellsworth JL, Berry J, Bukowski T, et al. Fibroblast growth factor-18 is a trophic factor for mature chondrocytes and their progenitors. Osteoarthritis Cartilage. 2002;10:308–320. doi: 10.1053/joca.2002.0514. [DOI] [PubMed] [Google Scholar]
- 78.Davidson D, Blanc A, Filion D, et al. Fibroblast growth factor (FGF) 18 signals through FGF receptor 3 to promote chondrogenesis. J Biol Chem. 2005;280:20509–20515. doi: 10.1074/jbc.M410148200. [DOI] [PubMed] [Google Scholar]
- 79.Sandell LJ, Xing X, Franz C, et al. Exuberant expression of chemokine genes by adult human articular chondrocytes in response to IL-1beta. Osteoarthritis Cartilage. 2008;16(12):1560–1571. doi: 10.1016/j.joca.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Elluru RG, Thompson F, Reece A. Fibroblast growth factor 18 gives growth and directional cues to airway cartilage. Laryngoscope. 2009;119(6):1153–1165. doi: 10.1002/lary.20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moore EE, Bendele AM, Thompson DL, et al. Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthritis Cartilage. 2005;13:623. doi: 10.1016/j.joca.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 82.Naot D, Cornish J. The role of peptides and receptors of the calcitonin family in the regulation of bone metabolism. Bone. 2008;43(5):813–818. doi: 10.1016/j.bone.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 83.Karsdal MA, Sondergaard BC, Arnold M, et al. Calcitonin affects both bone and cartilage: a dual action treatment for osteoarthritis? Ann N Y Acad Sci. 2007;1117:181–195. doi: 10.1196/annals.1402.041. [DOI] [PubMed] [Google Scholar]
- 84.Sondergaard BC, Wulf H, Henriksen K, et al. Calcitonin directly attenuates collagen type II degradation by inhibition of matrix metalloproteinase expression and activity in articular chondrocytes. Osteoarthritis Cartilage. 2006 Aug;14(8):759–68. doi: 10.1016/j.joca.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 85.Nielsen RH, Bay-Jensen AC, Byrjalsen I, et al. Oral salmon calcitonin reduces cartilage and bone pathology in an osteoarthritis rat model with increased subchondral bone turnover. Osteoarthritis Cartilage. 2011 Jan 18; doi: 10.1016/j.joca.2011.01.008. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 86.Hardingham TE, Fosang AF, Dudiah J. Aggrecan, the chondroitin sulfate/keratan sulfate proteoglycan from cartilage. In: Kuettner KE, Schleyerback R, Peyron JG, et al., editors. Articular Cartilage and Osteoarthritis. New York: Raven Press; 1992. p. 6. [Google Scholar]
- 87.Wildi LM, Raynauld JP, Martel-Pelletier J, et al. Chondroitin sulphate reduces both cartilage volume loss and bone marrow lesions in knee osteoarthritis patients starting as early as 6 months after initiation of therapy: a randomised, double-blind, placebo-controlled pilot study using MRI. Ann Rheum Dis. 2011 Mar 1; doi: 10.1136/ard.2010.140848. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kahan A, Uebelhart D, De Vathaire F, et al. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60(2):524–533. doi: 10.1002/art.24255. [DOI] [PubMed] [Google Scholar]
- 89.Michel BA, Stucki G, Frey D, et al. Chondroitins 4 and 6 sulfate in osteoarthritis of the knee: a randomized, controlled trial. Arthritis Rheum. 2005;52(3):779–786. doi: 10.1002/art.20867. [DOI] [PubMed] [Google Scholar]
- 90.Möller I, Pérez M, Monfort J, et al. Effectiveness of chondroitin sulphate in patients with concomitant knee osteoarthritis and psoriasis: a randomized, double-blind, placebo-controlled study. Osteoarthritis Cartilage. 2010;18(Suppl 1):S32–S40. doi: 10.1016/j.joca.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 91.Hochberg MC. Structure-modifying effects of chondroitin sulfate in knee osteoarthritis: an updated meta-analysis of randomized placebo-controlled trials of 2-year duration. Osteoarthritis Cartilage. 2010;18(Suppl 1):S28–S31. doi: 10.1016/j.joca.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 92.Lee YH, Woo JH, Choi SJ, et al. Effect of glucosamine or chondroitin sulfate on the osteoarthritis progression: a meta-analysis. Rheumatol Int. 2010;30(3):357–363. doi: 10.1007/s00296-009-0969-5. [DOI] [PubMed] [Google Scholar]
- 93.Abramson SB, Amin AR, Clancy RM, et al. The role of nitric oxide in tissue destruction. Best Pract Res Clin Rheumatol. 2001;15:831–845. doi: 10.1053/berh.2001.0196. [DOI] [PubMed] [Google Scholar]
- 94.Pelletier JP, Jovanovic D, Fernandes JC, et al. Reduced progression of experimental osteoarthritis in vivo by selective inhibition of inducible nitric oxide synthase. Arthritis Rheum. 1998;41:1275–1286. doi: 10.1002/1529-0131(199807)41:7<1275::AID-ART19>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 95.Pelletier JP, Jovanovic D, Fernandes JC, et al. Selective inhibition of iNOS in experimental OA is associated with reduction in tissue levels of catabolic factors. J Rheumatol. 1999 [PubMed] [Google Scholar]
- 96.Pelletier J-P, Jovanovic DV, Lascau-Coman V, et al. Selective inhibition of inducible nitric oxide synthase reduces progression of experimental osteoarthritis in vivo: possible link with the reduction in chondrocyte apoptosis and caspase 3 level. Arthritis Rheum. 2000;43:1290–1299. doi: 10.1002/1529-0131(200006)43:6<1290::AID-ANR11>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 97.Bijlsma JWJ, Welsing PMJ. The art of medicine in treating osteoarthritis: I will please. Ann Rheum Dis. 2008;67:1653–1655. doi: 10.1136/ard.2008.097006. [DOI] [PubMed] [Google Scholar]
- 98.Bingham CO, 3rd, Buckland-Wright JC, Garnero P, et al. Risedronate decreases biochemical markers of cartilage degradation but does not decrease symptoms or slow radiographic progression in patients with medial compartment osteoarthritis of the knee: results of the two-year multinational knee osteoarthritis structural arthritis study. Arthritis Rheum. 2006;54(11):3494–3507. doi: 10.1002/art.22160. [DOI] [PubMed] [Google Scholar]
- 99.Mazzuca SA, Brandt KD, Chakr R, et al. Varus malalignment negates the structure-modifying benefits of doxycycline in obese women with knee osteoarthritis. Osteoarthritis Cartilage. 2010;18(8):1008–1011. doi: 10.1016/j.joca.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]