Abstract
Scope
Green tea has been shown to ameliorate symptoms of metabolic syndrome in vivo. The effects could be due, in part, to modulation of postprandial blood glucose levels.
Methods and results
We examined the effect of coadministration of (−)-epigallocatechin-3-gallate (EGCG, 100 mg/kg, i.g.) on blood glucose levels following oral administration of common corn starch (CCS), maltose, sucrose, or glucose to fasted CF-1 mice. We found that cotreatment with EGCG significantly reduced postprandial blood glucose levels after administration of CCS compared to control mice (50 and 20% reduction in peak blood glucose levels and blood glucose area under the curve, respectively). EGCG had no effect on postprandial blood glucose following administration of maltose or glucose, suggesting that EGCG may modulate amylase-mediated starch digestion. In vitro, EGCG noncompetitively inhibited pancreatic amylase activity by 34% at 20 μM. No significant change was induced in the expression of two small intestinal glucose transporters (GLUT2 and SGLT1).
Conclusions
Our results suggest that EGCG acutely reduces postprandial blood glucose levels in mice when coadministered with CCS and this may be due in part to inhibition of α-amylase. The relatively low effective dose of EGCG makes a compelling case for studies in human subjects.
Keywords: Amylase, Blood glucose, EGCG, Metabolic syndrome, Starch
1 Introduction
Tea is second only to water in terms of global popularity as a beverage [1]. Green tea consumption has been suggested to have preventive activity against many chronic diseases, including Metabolic Syndrome [2, 3]. By contrast, diets containing foods with a high glycemic index/load are associated with increased incidence of chronic diseases such as type 2 diabetes [4]. It has been observed in rats that green tea catechins reduce blood glucose levels when included in the diet [5, 6].
(−)-Epigallocatechin-3-gallate (EGCG), is the most abundant catechin in green tea and may be the most biologically active. EGCG has been shown to reduce symptoms associated with Metabolic Syndrome in mice and rats: inhibition of lipid absorption, enhancement of fat oxidation, and improved insulin signaling, as well as inhibition of gluconeogenesis [7–14]. It has also been proposed that EGCG treatment can modulate blood glucose levels by increasing glucose uptake by skeletal muscles via glucose transporter (GLUT)-4 [15], or by increasing phosphorylation of forkhead box proteins [16].
In postmenopausal overweight women, 150 mg EGCG given twice/day for 12 weeks lowered blood glucose levels in those with poor glucose tolerance [17], other studies have been less promising [18, 19]. The variability in human observational studies may be due to varying composition of diet. For example, EGCG or green tea may inhibit postprandial increases in blood glucose levels following a meal containing high levels of starch, but not following a meal containing high levels of simple sugars. One study found that green tea (0.1 g solid in warm water) decreased carbohydrate absorption of a rice meal by 25% in healthy human subjects [20]. This suggests that green tea polyphenols may inhibit the breakdown of starch in vivo.
Green tea polyphenols may affect regulation of blood glucose levels at a number of different points including at the level of carbohydrate digesting enzymes, specifically by inhibiting enzymes α-amylase and α-glucosidase in the small intestine (SI) [21]. Two different types of α-amylase exist in the human body, salivary α-amylase and pancreatic α-amylase. Both types of α-amylase aid in the digestion of starch, through the hydrolysis of interior α-1,4-glucose linkages. The resulting oligosaccharides are broken down to glucose by other digestive enzymes such as maltase [22]. Both green and black teas (2 g in 200 mL boiling water) have been shown to inhibit potato starch breakdown by α-amylase in vitro using human saliva. In the same study, the effect of green and black teas on salivary amylase-mediated carbohydrate breakdown was examined in human subjects. In that experiment, human subjects were asked to chew crackers, followed by rinsing with the above tea solutions. From analyzing the breakdown products of cracker carbohydrates formed in the subject’s mouths, the investigators concluded that both teas inhibited salivary amylase in vivo [23]. Here, we investigate the effects of EGCG on changes in postprandial blood glucose levels induced by common corn starch (CCS), maltose, and glucose, as well as sucrose, in mice. We explored the underlying mechanisms of action both in vitro and in vivo.
2 Materials and methods
2.1 Chemicals
Melojel CCS was obtained from National Starch and Chemical Co. (Bridgewater, NJ, USA). EGCG (93% pure) was purchased from Taiyo Green Power (Jiangsu, China). All other chemicals were of the highest grade commercially available.
2.2 Animals and treatments
Male CF-1 mice (Charles River Laboratories, Wilmington, MA, USA) were selected for study because our laboratory has characterized the bioavailability of tea polyphenols in this strain [24, 25]. Mice were housed in gang cages (n = 10 per cage) on corn cob bedding and maintained on 12 h light/dark with standard chow and water provided ad libitum. All experiments were approved by the Institutional Animal Care and Use Committee at the Pennsylvania State University (IACUC #28962). Following a 2 week acclimatization period, mice were separated into groups based on body weight and fasted for 7 h (7 am–2 pm) prior to treatment. Mice were given CCS (5 g/kg b.w., i.g.), glucose (2 g/kg b.w., i.g.), maltose (2 g/kg b.w., i.g.), or sucrose (2 g/kg b.w., i.g.) alone or in combination with EGCG (100 mg/kg b.w., i.g.). All solutions were made in water. The dose of CCS was higher than that of the other carbohydrates, because we assumed that CCS would be less efficiently digested and therefore less bioavailable than the simpler mono- and disaccharides. The dose of each carbohydrate gave similar peak blood glucose levels. After dosing mice had ad libitum access to water but were kept without food for the duration of the experiment. Glucose concentration was determined in blood samples collected from the tail vein using the Ascensia Contour Blood Glucose Monitoring System (Bayer Healthcare, Tarrytown, NY, USA).
In experiments to determine expression of GLUTs, plasma insulin levels, and plasma and tissue EGCG levels, blood was collected via cardiac puncture from anaesthetized mice. Blood was centrifuged at 3200×g for 15 min at 4°C and plasma was aliquoted for plasma insulin analysis or EGCG analysis (combined with 0.1 vol. of 20% ascorbate/0.1% EDTA preservative) by LC-MS. The SI was removed, the contents expelled into a microfuge tube, and then rinsed with cold saline. Plasma, SI, and small intestinal contents (SIC) were snap frozen and kept at −80°C for later biochemical and chemical analysis.
2.3 Plasma insulin measurements
Plasma insulin levels were determined using the Rat/Mouse Insulin ELISA Kit from Millipore (Billerica, MA, USA), according to the manufacturer’s protocol. Plate readings were taken on a Thermo Scientific Multiskan Go Plate Reader (Waltham, MA, USA).
2.4 α-Amylase inhibition assay
Inhibition of α-amylase by EGCG and EGC was examined in vitro using a modified version of the chromogenic Red-starch method (Megazyme, Wicklow, Ireland). Inhibition studies were conducted by combining enzyme (0.3 U/mL) was suspended in 20 mM phosphate buffer (pH 6.9) containing 6.7 mM sodium chloride and Red-starch (7 mg/mL in 0.5 M potassium chloride). EGCG or EGC was added (0–200 μM). Following incubation at 37°C for 10 min, the reaction was terminated by adding 95% ethanol. The solution was brought to room temperature, and then centrifuged at 1000×g for 10 min. The absorbance of the supernatant was measured at 510 nm using a Beckman DU650 spectrophotometer. Kinetic studies were conducted similarly with the modification that EGCG concentration (50 μM) was held constant while Red-starch concentrations were varied (0–2.8 mg/mL).
2.5 Western blot analysis
Expression of GLUTs was measured according to a previously described method [26], with the following modifications. Tissue samples (50 mg) from SIs were extracted with 500 μL T-PER extraction reagent (Pierce Biotechnology, Rockford, IL, USA) containing phosphatase/protease inhibitors. The samples were homogenized using a Bullet Blender and Zirconium Silicate beads (Next Advance, Averill Park, NY, USA). The homogenate was centrifuged at high speed twice for 7 min, and the supernatant was collected for western blot. Protein samples were combined with loading buffer (30 μg per well) and resolved by polyacrylamide (10% w/v) gel electrophoresis, transferred to nitrocellulose membranes, and probed with primary antibodies GLUT-2, sodium-dependent glucose cotransporter (SGLT)-1, and β-actin (all 1:1000 dilution) in blocking solution (LI-COR Biosciences, Lincoln, NE) overnight at 4°C. GLUT antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and β-actin was from Cell Signaling Technology (Danvers, MA, USA). After incubation with a fluorescently labeled secondary antibody (LI-COR Biosciences), proteins were imaged with an Odyssey imaging system (LI-COR Biosciences).
2.6 Small intestinal and plasma concentrations of EGCG
Plasma, SIC, and small intestinal tissue were analyzed for EGCG concentration by LC-MS. Briefly, small intestinal tissue was homogenized in 5 volumes of buffer containing 1 part 0.3 M sodium hydrosulfite, 0.1% Na2EDTA in 0.4 M sodium monophosphate buffer (pH 6.8), and 1 part 50% methanol:50% ethyl acetate for 10 min in the Bullet Blender, and then centrifuged for 4 min at 14 000 rpm. The supernatant was removed and dried for 60 min. Each sample was resuspended in 0.4 mL water, hydrolyzed with glucuronidase (250 U/sample) and sulfatase (1 U/sample) enzymes at 37°C for 45 min, and extracted with dichloromethane and ethyl acetate as previously described [24]. Samples were resuspended in 10% acetonitrile, and analyzed by a previously described method [27]. EGCG levels in SIC samples were quantified using UV/Vis detection (280 nm). These samples represented total EGCG. Duplicate samples were prepared in an identical manner but without glucuronidase/sulfatase to quantify the free fraction of EGCG.
2.7 Statistical analysis
All values are reported as means ± SEM. Area under the curve (AUC) values were calculated using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA). The kinetic parameters (Vmax and Km) of α-amylase inhibition by EGCG were calculated using GraphPad. Two-tailed Student’s t-tests were performed to determine statistical significance of western blot results, AUC values, and Vmax/Km parameters. Two-way ANOVA with a Bonferroni correction was used to determine the statistical significance of blood glucose and insulin measurements. A p value < 0.05 was considered statistically significant.
3 Results
3.1 Effect of EGCG on postprandial blood glucose in mice
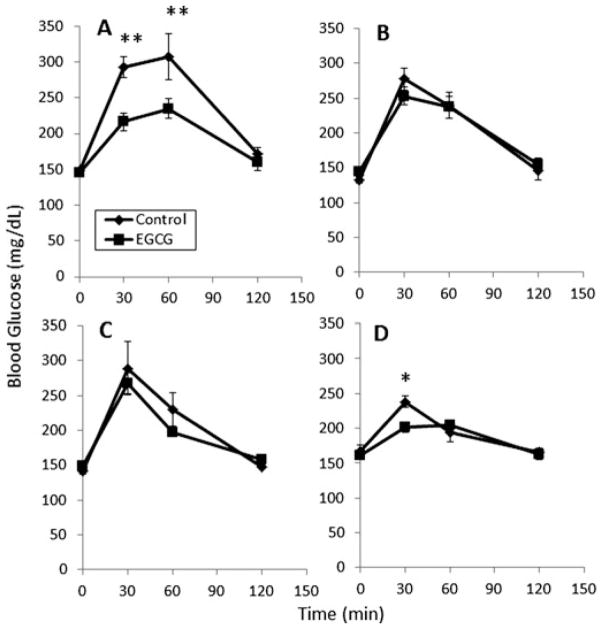
EGCG treatment significantly reduced the increase in blood glucose levels induced by oral administration of CCS to mice (Fig. 1A). Blood glucose levels were 77 and 73 mg/dL lower in the EGCG-treated mice after 30 and 60 min, respectively. EGCG caused a significant (p < 0.05) decrease in the blood glucose AUC in mice treated with CCS (Table 1). When mice were treated with either glucose or maltose, blood glucose levels increased: interestingly, EGCG had no significant effect on this rise (Fig. 1B and C). Coadministration of EGCG to mice treated with sucrose did reduce the peak blood glucose concentrations compared to sucrose alone (p < 0.05, Fig. 1D), although the effect was transient. Overall, EGCG did not significantly reduce the blood glucose AUC in mice treated with sucrose. Treatment with EGCG showed no clear effect on plasma insulin levels following oral administration of starch (Fig. 2).
Figure 1.
Impact of EGCG on the postprandial blood glucose in male CF-1 mice. Mice were treated with EGCG (100 mg/kg, i.g.) in combination with (A) common corn starch, (B) glucose, (C) maltose, and (D) sucrose. Results are shown as means ± SEM (n = 6). *p< 0.05 or **p< 0.01.
Table 1.
Effect of EGCG treatment on postprandial blood glucose in CF-1 micea)
| Blood glucose AUC (g/dL × min)
|
||||
|---|---|---|---|---|
| CCS | Glucose | Maltose | Sucrose | |
| Control | 30.0 ± 1.6 | 25.5 ± 1.8 | 25.4 ± 1.5 | 23.3 ± 0.9 |
| EGCG | 24.1 ± 1.1 | 23.8 ± 0.8 | 25.1 ± 1.2 | 22.5 ± 0.8 |
| p value | 0.01 | 0.39 | 0.84 | 0.51 |
Results are shown as means ± SEM (n = 6).
Figure 2.
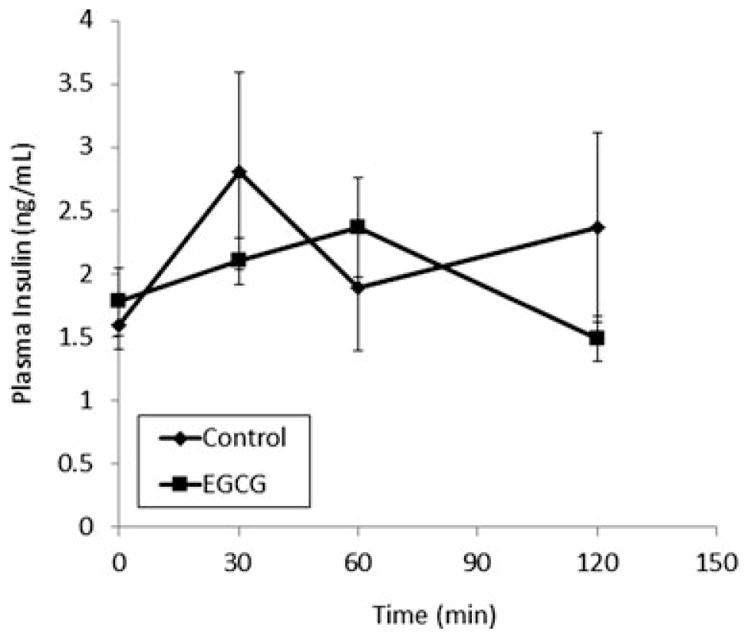
Impact of EGCG on insulin levels in male CF-1 mice. Mice were treated with EGCG (100 mg/kg, i.g.) in the absence or presence of common corn starch (control). Results are shown as means ± SEM (n = 6).
3.2 Effect of EGCG on small intestinal expression of GLUTs
Green tea has been reported to modulate the expression and activity of GLUTs in vitro and in vivo [28–31]. The effect of EGCG on small intestinal expression of SGLT-1 and GLUT-2 was examined. Pretreatment with EGCG (100 mg/kg/day i.g.) for 4 days had no significant effect on postprandial blood glucose levels compared to naïve mice (Fig. 3A). Western blot analysis showed that EGCG did not significantly affect the expression of either SGLT-1 or GLUT-2 in the SI (Fig. 3B) compared to mice not pretreated with EGCG. Expression of SGLT-1 and GLUT-2 was also determined specifically in the jejunum, however again no significant differences were found (data not shown).
Figure 3.
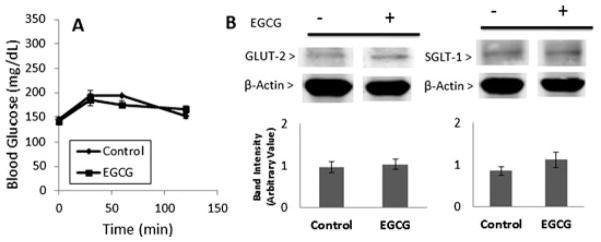
Effect of pretreatment with EGCG on postprandial blood glucose (A) and small intestinal expression of GLUT-2 and SGLT-1 (B) in mice. Mice were pretreated with saline or EGCG (100 mg/kg/day, i.g.) for 4 days. On day 5, all mice were treated with a single dose of glucose. Blood glucose measurements were taken and then mice were sacrificed and small intestinal tissue was harvested. GLUT-2 and SGLT-1 levels were determined by Western Blot. Results are shown as means ± SEM (n = 6).
3.3 Inhibition of α-amylase activity
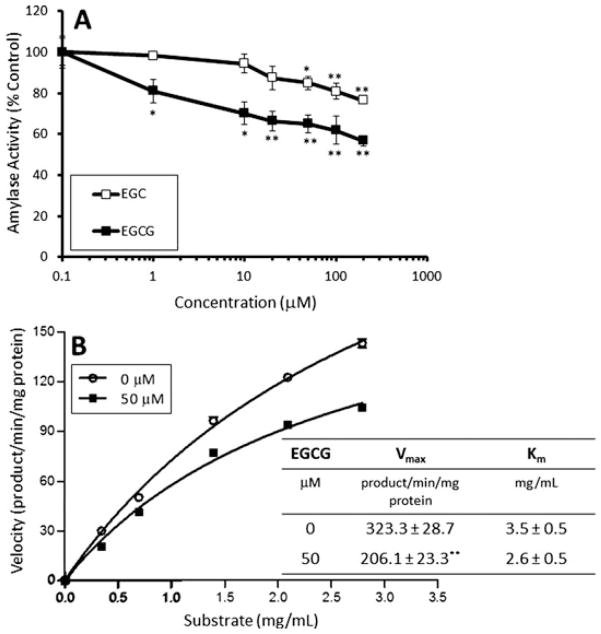
Starch is primarily metabolized by α-amylase resulting in the formation of glucose and maltose. We examined the inhibitory effect of EGCG and (−)-epigallocatechin (EGC) on α-amylase activity in a cell-free system. Both EGCG and EGC inhibited α-amylase activity with EGCG being the more potent inhibitor (Fig. 4A). At a concentration of 20 μM, EGCG inhibited α-amylase by 34%, whereas EGC caused only 13% inhibition at the same concentration. Kinetic analysis showed that 50 μM EGCG reduced the Vmax of α-amylase from 323.3 to 206.1 product/min/mg protein (p < 0.01), but did not significantly affect the Km (p = 0.1) in the presence of increasing concentrations of Red-starch substrate (Fig. 4B). These results suggest that EGCG inhibits α-amylase in a noncompetitive fashion with regard to substrate concentration.
Figure 4.
Dose-dependent inhibition (A) and kinetic analysis of inhibition (B) of purified pancreatic α-amylase by EGCG or EGC. Activity was determined using the Red Starch Method. Reactions were incubated for 10 min at 37°C and absorbance was determined spectrophotometrically. Results are shown as the mean ± SD (n = 3). *p< 0.05; **p < 0.01 compared to control.
3.4 Small intestinal and plasma concentrations of EGCG
We determined the levels of EGCG in the plasma, SI, and SIC after treating CF-1 mice with EGCG (100 mg/kg, i.g.) and CCS (5 g/kg, i.g.) (Table 2). In the SIC and SI, EGCG was present largely in the unconjugated form, whereas in the plasma, EGCG was mostly conjugated. As expected, the SIC had the highest concentrations whereas plasma had the lowest levels of EGCG.
Table 2.
Plasma, small intestine, and small intestinal contents levels of EGCG in CF-1 mice after oral administration of 100 mg/kg, i.g. EGCG
| EGCG
|
||||
|---|---|---|---|---|
| 30 min | 60 min | 120 min | ||
| Plasma (nM) | Total | 49.9 ± 12.1 | 37.7 ± 5.1 | 16.3 ± 2.8 |
| Unconjugated | 2.5 ± 0.8 | 9.6 ± 2.0 | 1.4 ± 0.5 | |
| SIC (nmol/g) | Total | 2185.9 ± 700.1 | 1613.4 ± 325.7 | 2156.7 ± 588.8 |
| Unconjugated | 972.5 ± 116.8 | 1038.8 ± 186.5 | 2065.2 ± 642.6 | |
| SI tissue (nmol/g) | Total | 58.6 ± 23.8 | 82.7 ± 8.1 | 56.5 ± 15.8 |
| Unconjugated | 64.4 ± 28.0 | 96.8 ± 8.3 | 65.8 ± 20.0 | |
Unconjugated and total levels of EGCG in the small intestinal contents (SIC), small intestinal (SI) tissue, and plasma of CF-1 mice (n = 5–6) treated in combination with common corn starch.
4 Discussion
In the present study, we examined the acute effect of EGCG on the digestion and postprandial effects of oral carbohydrates in mice. We report for the first time that cotreatment with EGCG at a dose equivalent to 1.5 cups of green tea reduced the postprandial elevation in blood glucose levels induced by bolus dosing with CCS [25]. By contrast, no effect on the increase in postprandial blood glucose induced by maltose or glucose was observed following cotreatment with EGCG. In total, these results suggest that EGCG modulates an aspect of carbohydrate metabolism that is unique to starch. We hypothesized that this might be the result of inhibition of α-amylase by EGCG.
Supporting this hypothesis, we found that EGCG dose-dependently inhibited α-amylase in a cell-free system. This inhibition was noncompetitive with respect to substrate concentration. Treatment with 20 μM EGCG caused 34% inhibition. Analysis of the concentration of EGCG in the SIC of CF-1 mice after dosing with EGCG and CCS showed concentrations of 972–2065 nmol/g. Assuming that 1 g of contents is equivalent to one mL of volume, the concentration of EGCG in the SIC ranges from 972 to 2065 μM, which is sufficient to induce greater than 35% inhibition of α-amylase. These in vitro results are similar to previous studies by Hara and Honda (1990) who reported that EGCG inhibited salivary α-amylase noncompetitively in vitro with an IC50 = 260 μM [32]. Additionally, the results are supported by a second study that reported that EGCG can inhibit both salivary α-amylase and α-glucosidase in vitro with IC50 = 1.4 mM and 220 μM, respectively [33].
In contrast to the potent postprandial effects of EGCG following CCS administration, we found that EGCG less potently and only transiently affected postprandial blood glucose following oral administration of sucrose. These results suggest that EGCG has a more modest effect of intestinal sucrase activity. Honda and Hara (1992) have previously reported that EGCG inhibited sucrase in vitro with an IC50 of 100 μM [34]. It is possible that the more modest effects of EGCG against sucrose-induced blood glucose are due to the relatively lower expression of sucrase in the SI compared to α-amylase [35]. Further studies are needed to more fully understand this effect.
Although our in vivo findings are new, they support previous work by others using EGCG-containing extracts. For example, oligomeric procyanidins from persimmon leaf (including EGCG subunits) lower blood glucose levels in starch-fed rats [36]. Kucha tea extract, which contains EGCG, was able to inhibit blood glucose levels in mice after oral administration of starch and sucrose, but not glucose [37]. A previous study in humans reported that treatment with a beverage containing green, black, and mulberry extracts reduced the digestibility of a rice-based (carbohydrate) meal, but not the digestibility of a lipid-based meal [20]. Although suggestive for a role for green tea (and EGCG) at inhibiting starch digestion, the results are confounded by the complicated mixture of green, black, and mulberry teas, and the use of a rice-based meal rather than pure starch.
Interestingly, a study by Liu et al., suggested that green tea polyphenols can enhance blood glucose levels in rats following administration of starch that was pregelatinized in the presence of the tea polyphenols. The authors suggest that these effects are due to effects of the tea polyphenols on starch structure that results in a more open structure that is more readily attacked by α-amylase [38]. The inconsistency of this finding with our results is likely due to the fact that we combined CCS and EGCG immediately prior to oral administration to mice, rather than combining the two, cooking them together, and then administering them. There is little chance of EGCG modifying the structure of starch in our experiments since the starch structure is not disrupted by heating.
In terms of human exposure, the study by Liu et al., is analogous to the development of a functional food that contains CCS and is supplemented with green tea prior to some cooking process. By contrast, our system models and exposure system where CCS is consumed as part of a food and that food is ingested with tea as a beverage. Both systems represent equally plausible mechanisms of human exposure, but demonstrate that the effects of green tea polyphenol supplementation on starch digestion may be significantly different depending on when and how green tea polyphenols are ingested.
Previously, it has been suggested that EGCG (and green tea) may modulate carbohydrate digestion by influencing the expression or activity of GLUTs, specifically SGLT-1 and GLUT-2/4 in the SI, adipose, and muscle, respectively [28–30]. Although effects on GLUT-2/4 have been explored in animal models, the effects of EGCG on SGLT-1 are based only on in vitro studies. Inhibition of glucose uptake from the intestine (via SGLT-1) on uptake into tissues (via GLUT-2/4) would be expected to impact blood glucose following oral administration of CCS, maltose, or glucose. In our study, we found that acute administration of EGCG did not impact the postprandial increase in blood glucose following administration of maltose or glucose, nor did we observe changes in glucose response or the expression of SGLT-1 or GLUT-2 following treatment with EGCG for 4 days. These results suggest that acute administration of EGCG does not inhibit glucose transport across the intestinal wall. Interestingly, it is possible that long-term treatment with EGCG (or green tea) may modulate other aspects of carbohydrate metabolism, for example, EGCG has been shown to inhibit gluconeogenesis in the intestine [39]. Green tea extract has also been shown to modulate GLUT-2/4 gene expression, and increase insulin signaling in the liver and skeletal muscle [31]. Given the acute nature of the present study, it is unlikely, that the effects of EGCG are due to inhibition of gluconeogenic enzymes. One can speculate that treatment with EGCG may acutely affect starch digestibility and, after chronic dosing, affect expression of genes related to glucose handling distal to the SI. Such a hypothesis should be further tested.
In conclusion, the green tea polyphenol EGCG caused decreased blood glucose levels in starch-fed mice, and to a lesser extent in sucrose-fed mice. Due to the relatively high concentrations of EGCG available in the SI following oral administration of EGCG, and the in vitro antiamylase activity of EGCG, it is likely that EGCG inhibition of pancreatic α-amylase plays a role in the observed decrease in postprandial blood glucose. These results suggest that coadministration of green tea or EGCG may represent an effective means to reduce the glycemic effect of high starch foods, and indicates that further studies in human subjects are warranted.
Acknowledgments
This study was supported by NIH grant AT004678 (to J. D. L.).
J. D. L. has served as a paid consultant for Kao Corporation, Tokyo.
Abbreviations
- CCS
common corn starch
- EGC
(−)-epigallocatechin
- EGCG
(−)-epigallocatechin-3-gallate
- GLUT
glucose transporter
- GTE
green tea extract
- SGLT1
sodium-dependent glucose cotransporter 1
- SI
small intestine
- SIC
small intestinal contents
References
- 1.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sae-Tan S, Grove KA, Lambert JD. Weight control and prevention of metabolic syndrome by green tea. Pharmacol Res. 2011;64:146–154. doi: 10.1016/j.phrs.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grove KA, Lambert JD. Laboratory, epidemiological, and human intervention studies show that tea (Camellia sinensis) may be useful in the prevention of obesity. J Nutr. 2010;140:446–453. doi: 10.3945/jn.109.115972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barclay AW, Petocz P, McMillan-Price J, Flood VM, et al. Glycemic index, glycemic load, and chronic disease risk—a metaanalysis of observational studies. Am J Clin Nutr. 2008;87:627–637. doi: 10.1093/ajcn/87.3.627. [DOI] [PubMed] [Google Scholar]
- 5.Igarashi K, Honma K, Yoshinari O, Nanjo F, et al. Effects of dietary catechins on glucose tolerance, blood pressure and oxidative status in Goto-Kakizaki rats. J Nutr Sci Vitaminol. 2007;53:496–500. doi: 10.3177/jnsv.53.496. [DOI] [PubMed] [Google Scholar]
- 6.Khan SA, Priyamvada S, Arivarasu NA, Khan S, et al. Influence of green tea on enzymes of carbohydrate metabolism, antioxidant defense, and plasma membrane in rat tissues. Nutrition. 2007;23:687–695. doi: 10.1016/j.nut.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Bose M, Lambert JD, Ju J, Reuhl KR, et al. The major green tea polyphenol, (−)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high fat-fed mice. J Nutr. 2008;138:1677–1683. doi: 10.1093/jn/138.9.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klaus S, Pultz S, Thone-Reineke C, Wolfram S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int J Obes. 2005;29:615–623. doi: 10.1038/sj.ijo.0802926. [DOI] [PubMed] [Google Scholar]
- 9.Sae-tan S, Grove KA, Kennett MJ, Lambert JD. (−)-Epigallocatechin-3-gallate increases the expression of genes related to fat oxidation in the skeletal muscle of high fat-fed mice. Food Funct. 2011;2:111–116. doi: 10.1039/C0FO00155D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin CL, Lin JK. Epigallocatechin gallate (EGCG) attenuates high glucose-induced insulin signaling blockade in human hepG2 hepatoma cells. Mol Nutr Food Res. 2008;52:930–939. doi: 10.1002/mnfr.200700437. [DOI] [PubMed] [Google Scholar]
- 11.Wolfram S, Raederstorff D, Wang Y, Teixeira SR, et al. TEAVIGO (TM) (epigallocatechin gallate) supplementation prevents obesity in rodents by reducing adipose tissue mass. Ann Nutr Metab. 2005;49:54–63. doi: 10.1159/000084178. [DOI] [PubMed] [Google Scholar]
- 12.Song EK, Hur H, Han MK. Epigallocatechin gallate prevents autoimmune diabetes induced by multiple low doses of streptozotocin in mice. Arch Pharm Res. 2003;26:559–563. doi: 10.1007/BF02976881. [DOI] [PubMed] [Google Scholar]
- 13.Abe K, Okada N, Tanabe H, Fukutomi R, et al. Effects of chronic ingestion of catechin-rich green tea on hepatic gene expression of gluconeogenic enzymes in rats. Biomed Res. 2009;30:25–29. doi: 10.2220/biomedres.30.25. [DOI] [PubMed] [Google Scholar]
- 14.Wolfram S, Raederstorff D, Preller M, Wang Y, et al. Epigallocatechin gallate supplementation alleviates diabetes in rodents. J Nutr. 2006;136:2512–2518. doi: 10.1093/jn/136.10.2512. [DOI] [PubMed] [Google Scholar]
- 15.Ueda M, Nishiumi S, Nagayasu H, Fukuda I, et al. Epigallocatechin gallate promotes GLUT4 translocation in skeletal muscle. Biochem Biophys Res Commun. 2008;377:286–290. doi: 10.1016/j.bbrc.2008.09.128. [DOI] [PubMed] [Google Scholar]
- 16.Anton S, Melville L, Rena G. Epigallocatechin gallate (EGCG) mimics insulin action on the transcription factor FOXO1 a and elicits cellular responses in the presence and absence of insulin. Cell Signal. 2007;19:378–383. doi: 10.1016/j.cellsig.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Hill AM, Coates AM, Buckley JD, Ross R, et al. Can EGCG reduce abdominal fat in obese subjects? J Am Coll Nutr. 2007;26:396S–402S. doi: 10.1080/07315724.2007.10719628. [DOI] [PubMed] [Google Scholar]
- 18.Brown AL, Lane J, Coverly J, Stocks J, et al. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: randomized controlled trial. Br J Nutr. 2009;101:886–894. doi: 10.1017/S0007114508047727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venables MC, Hulston CJ, Cox HR, Jeukendrup AE. Green tea extract ingestion, fat oxidation, and glucose tolerance in healthy humans. Am J Clin Nutr. 2008;87:778–784. doi: 10.1093/ajcn/87.3.778. [DOI] [PubMed] [Google Scholar]
- 20.Zhong LT, Furne JK, Levitt MD. An extract of black, green, and mulberry teas causes malabsorption of carbohydrate but not of triacylglycerol in healthy volunteers. Am J Clin Nutr. 2006;84:551–555. doi: 10.1093/ajcn/84.3.551. [DOI] [PubMed] [Google Scholar]
- 21.Kwon YI, Apostolidis E, Shetty K. Inhibitory potential of wine and tea against alpha-amylase and alpha-glucosidase for management of hyperglycemia linked to type 2 diabetes. J Food Biochem. 2008;32:15–31. [Google Scholar]
- 22.Whitcomb DC, Lowe ME. Human pancreatic digestive enzymes. Dig Dis Sci. 2007;52:1–17. doi: 10.1007/s10620-006-9589-z. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Kashket S. Inhibition of salivary amylase by black and green teas and their effects on the intraoral hydrolysis of starch. Caries Res. 1998;32:233–238. doi: 10.1159/000016458. [DOI] [PubMed] [Google Scholar]
- 24.Lambert JD, Lee MJ, Lu H, Meng XF, et al. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J Nutr. 2003;133:4172–4177. doi: 10.1093/jn/133.12.4172. [DOI] [PubMed] [Google Scholar]
- 25.Lambert JD, Lee MJ, Diamond L, Ju JY, et al. Dose-dependent levels of epigallocatechin-3-gallate in human colon cancer cells and mouse plasma and tissues. Drug Metab Dispos. 2006;34:8–11. doi: 10.1124/dmd.104.003434. [DOI] [PubMed] [Google Scholar]
- 26.Lambert JD, Kennett MJ, Sang SM, Reuhl KR, et al. Hepatotoxicity of high oral dose (−)-epigallocatechin-3-gallate in mice. Food Chem Toxicol. 2010;48:409–416. doi: 10.1016/j.fct.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu H, Meng XF, Yang CS. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (−)-epigallocatechin gallate. Drug Metab Dispos. 2003;31:572–579. doi: 10.1124/dmd.31.5.572. [DOI] [PubMed] [Google Scholar]
- 28.Ashida H, Furuyashiki T, Nagayasu H, Bessho H, et al. Anti-obesity actions of green tea: possible involvements in modulation of the glucose uptake system and suppression of the adipogenesis-related transcription factors. BioFactors. 2004;22:135–140. doi: 10.1002/biof.5520220126. [DOI] [PubMed] [Google Scholar]
- 29.Hossain SJ, Kato H, Aoshima H, Yokoyama T, et al. Polyphenol-induced inhibition of the response of Na+/glucose cotransporter expressed in Xenopus oocytes. J Agric Food Chem. 2002;50:5215–5219. doi: 10.1021/jf020252e. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi Y, Suzuki M, Satsu H, Arai S, et al. Green tea polyphenols inhibit the sodium-dependent glucose transporter of intestinal epithelial cells by a competitive mechanism. J Agric Food Chem. 2000;48:5618–5623. doi: 10.1021/jf0006832. [DOI] [PubMed] [Google Scholar]
- 31.Cao H, Hininger-Favier I, Kelly MA, Benaraba R, et al. Green tea polyphenol extract regulates the expression of genes involved in glucose uptake and insulin signaling in rats fed a high fructose diet. J Agric Food Chem. 2007;55:6372–6378. doi: 10.1021/jf070695o. [DOI] [PubMed] [Google Scholar]
- 32.Hara Y, Honda M. The inhibition of alpha-amylase by tea polyphenols. Agric Biol Chem. 1990;54:1939–1945. [Google Scholar]
- 33.Koh LW, Wong LL, Loo YY, Kasapis S, et al. Evaluation of different teas against starch digestibility by mammalian glycosidases. J Agric Food Chem. 2010;58:148–154. doi: 10.1021/jf903011g. [DOI] [PubMed] [Google Scholar]
- 34.Honda M, Hara Y. Inhibition of rat small intestinal sucrase and alpha-glucosidase activities by tea polyphenols. Biosci Biotechnol Biochem. 1993;57:123–124. doi: 10.1271/bbb.57.123. [DOI] [PubMed] [Google Scholar]
- 35.Stevens JA, Kidder DE. Distribution of trehalase, sucrase, alpha amylase, glucoamylase, and lactase (beta-galactosidase) along the small intestine of five pigs. Br J Nutr. 1972;28:129–137. doi: 10.1079/bjn19720015. [DOI] [PubMed] [Google Scholar]
- 36.Kawakami K, Aketa S, Nakanami M, Iizuka S, et al. Major water-soluble polyphenols, proanthocyanidins, in leaves of persimmon (Diospyros kaki) and their alpha amylase inhibitory activity. Biosci Biotechnol Biochem. 2010;74:1380–1385. doi: 10.1271/bbb.100056. [DOI] [PubMed] [Google Scholar]
- 37.Xie G, He RR, Feng X, Yan T, et al. The hypoglycemic effects of Camellia assamica var. kucha extract. Biosci Biotechnol Biochem. 2010;74:405–407. doi: 10.1271/bbb.90618. [DOI] [PubMed] [Google Scholar]
- 38.Liu J, Wang MZ, Peng SL, Zhang GY. Effect of green tea catechins on the postprandial glycemic response to starches differing in amylose content. J Agric Food Chem. 2011;59:4582–4588. doi: 10.1021/jf200355q. [DOI] [PubMed] [Google Scholar]
- 39.Yasui K, Tanabe H, Miyoshi N, Suzuki T, et al. Effects of (−)-epigallocatechin-3-O-gallate on expression of gluconeogenesis-related genes in the mouse duodenum. Biomed Res. 2011;32:313–320. doi: 10.2220/biomedres.32.313. [DOI] [PubMed] [Google Scholar]