Abstract
Effective management of bone metastases in men with castration-resistant prostate cancer (CRPC) remains an important unmet medical need. MET and VEGFR are rational targets for intervention in CRPC. Clinical trials involving agents that inhibit one but not both pathways have reported modest activity and no improvement in overall survival. Cabozantinib is an oral multitargeted tyrosine kinase inhibitor (TKI) that inhibits both MET and VEGFR2. A phase II randomized discontinuation study involving subjects with CRPC demonstrated that cabozantinib therapy is associated with improvement in bone scans, bone turnover markers, and pain response, but with significant adverse events leading to dose reduction and treatment discontinuation. Lower doses of cabozantinib retain high levels of activity with less toxicity. Ongoing phase III clinical trials will define the role of cabozantinib in CRPC. We summarize the rationale for targeting MET and VEGFR pathways in CRPC and the clinical data available to date.
Keywords: MET, VEGFR, cabozantinib, prostate cancer, bone metastasis
Introduction
Prostate cancer is the second most common cause of cancer death in men in the United States, with an estimated 28,000 deaths in 2012[1]. Bone metastases are present in 90% of men with fatal prostate cancer and represent the major cause of prostate cancer morbidity and mortality. Pain is a common symptom. Skeletal related events including pathologic fractures and spinal cord compression are significantly morbid and contribute to mortality related to castration-resistant prostate cancer (CRPC)[2]. Despite recent therapeutic advances in the management of CRPC, effective management of bone metastases remains an important unmet medical need.
Pathophysiology of bone metastasis in prostate cancer
Bone metastasis in prostate cancer involves complex heterotypic interactions between prostate cancer cells, osteoblasts, osteoclasts, bone matrix, and endothelial cells. The bone microenvironment contains high concentrations of growth factors in the marrow and immobilized in the matrix[2]. Increased bone turnover due to bone metastasis is reflected in elevated bone-specific alkaline phosphatase and urinary N-telopeptide (NTx), markers of osteoblast and osteoclast activities, respectively.
Prostate cancer bone metastases are characterized by radiographically dense “osteoblastic” lesions on plain films. Despite this appearance, bone metastases due to prostate cancer are associated with high osteoclast activity and consequently have compromised structural integrity [3]. The balance of bone turnover activity is mediated by receptor activator of nuclear factor-kappa-B (RANK) and RANK ligand (RANKL). RANK is present on osteoclasts. RANKL is expressed by osteoblasts and bone marrow stromal cells. RANKL binding to RANK leads to differentiation of osteoclast precursors as well as to activation and survival of mature osteoclasts[2].
MET and VEGFR pathways and bone turnover
In addition to RANK/RANKL activity, the MET receptor tyrosine kinase and the vascular endothelial growth factor (VEGF) signaling pathways play important roles in normal and pathological bone metabolism. Osteoblasts and osteoclasts express MET, and MET signaling is important for growth and activity of these cells[4]. Hepatocyte growth factor (HGF), the ligand for MET, can substitute for macrophage-colony stimulating factor (M-CSF) and promote proliferation, survival, and osteoclastogenesis from CD14(+) monocytes[5]. HGF induces osteoblasts to enter the cell cycle and to express bone morphogenetic protein-2 (BMP-2), a protein required for bone formation and repair[4, 6]. Both osteoblasts and osteoclasts have been shown to secrete HGF[4, 7]. Together, these findings suggest that HGF/MET signaling plays important dual roles in autocrine and paracrine regulation of growth, survival, and activity of osteoclasts and osteoblasts.
VEGF signaling is similarly important for osteoblast and osteoclast activity and survival. Osteoblasts and osteoclasts also express VEGF receptors (VEGFRs). Murine osteoblasts have been shown to express VEGFR1 and VEGFR2 during differentiation, as well as the ligand VEGF-A, which is maximally expressed during mineralization[8]. Human osteoblasts similarly express functional VEGFRs and VEGF[9]. VEGF treatment inhibits apoptosis of human osteoblasts via increased expression of the anti-apoptotic protein Bcl-2 in vitro, whereas neutralizing antibodies to VEGF induced apoptosis[9]. Human osteoclasts express VEGFR-1, −2, and −3 throughout fetal and adult life[10]. Human osteoclasts express VEGF-A in vitro in a RANKL/NF-κB/HIF1α-dependent manner, especially in large osteoclasts[11]. Thus, similar to HGF/MET signaling, VEGF/VEGFR signaling mediates both autocrine and paracrine roles in activity and survival of osteoblasts and osteoclasts. Although the precise interactions between vascular cells and bone forming cells are unclear, VEGFR signaling may mediate the close association between angiogenesis and bone formation in fracture healing, and may play similar roles in pathogenesis of bone metastasis.
MET and VEGFR pathways and prostate cancer
MET and VEGFR signaling pathways play important roles in both prostate cancer progression and bone metastasis. MET is expressed in basal and atrophic luminal cells of normal prostate epithelium[12], and is present at low levels in prostate cancer cells[12]. MET expression is repressed by the androgen receptor (AR) in a ligand-dependent manner in vitro[13]. Androgen deprivation increases MET expression in prostate cancer cells, and also increases tumor and stromal expression of HGF[13, 14]. Increased expression of MET and/or HGF correlate with prostate cancer metastasis and disease recurrence[14, 15]. In a comparison of bone, lymph node, and soft tissue metastases from patients with prostate cancer, MET expression was highest in bone metastases[15]. Thus, increased expression of MET and/or HGF may contribute to prostate cancer metastasis to bone. Androgen deprivation therapy, the standard initial therapy for metastatic prostate cancer, may enhance MET signaling by increased expression of both MET and its ligand HGF.
VEGFR signaling is critical for angiogenesis, a key step in tumor growth. Compared with normal prostate and high grade prostatic intraepithelial neoplasia (PIN), prostate cancers have significantly higher microvessel density that correlates with higher tumor grade and pathologic stage[16]. Prostate cancers express VEGFR-2, with higher levels in high-grade disease[16]. Prostate cancer cells, but not benign prostatic epithelial cells, express VEGF[17]. Patients with metastatic prostate cancer have higher plasma VEGF levels[18]. Levels of plasma or urine VEGF are independent predictors of overall survival (OS) in men with metastatic CRPC[19, 20].
VEGF and MET interact in prostate cancer cells. Myeloid cell leukemia-1 (Mcl-1), a member of the Bcl-2 family of anti-apoptotic proteins, is significantly expressed in high grade prostate cancer and bone metastasis[21]. VEGF induces Mcl-1 expression in prostate cancer cells in a MET-dependent manner via the co-receptor neuropilin-1[21].
The activities of MET and VEGFR signaling in bone turnover and prostate cancer metastasis to bone provide a strong rationale for dual inhibition of VEGF and MET as a therapeutic strategy in men with CRPC and bone metastases.
Quantitative evaluation of bone lesions
End points in clinical trials of agents affecting bone metastases are an important consideration in prostate cancer and other malignancies with high prevalence of bone metastasis. Bone lesions change slowly over time and are considered nonmeasurable sites of disease by modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria[22]. 99mTc-methylene diphosphonate (MDP) bone scans measure osteoblastic activity and do not directly measure tumor activity. Post-treatment bone scan changes reflect altered osteoblast activity due to changes in tumor perfusion, death of tumor cells, direct downregulation of osteoblast function, or a combination of factors. There are no standard criteria for bone scan imaging results or post-treatment changes, which can lead to subjective interpretation. End points involving bone scan changes typically involve increase in bone scan lesion number but lack quantifiable metrics to evaluate for post-treatment response[22].
Recently, a computer-aided detection (CAD) assessment system was developed to provide objective, reproducible, and quantifiable measurements[23]. The CADrx system integrates image intensity normalization, anatomic region-specific intensity thresholding, and quantitation of disease burden, along with independent nuclear medicine physician review. Bone scan lesion area (BSLA), bone scan lesion intensity, and bone scan lesion count were tested as metrics to assess post-treatment effects in prostate cancer subjects receiving cabozantinib, a MET/VEGFR inhibitor (see below). The most significant metric to differentiate between untreated and treated subjects was BSLA[23]. Validation of BSLA as an objective measurement of post-treatment response compared with other clinically relevant outcome measures is underway.
VEGFR- and MET-targeted therapies in CRPC: Clinical evidence
Several agents targeting either VEGFR activity, MET activity, or both have been evaluated in men with CRPC. Notable contemporary trials are summarized in Table 1.
Table 1.
Notable contemporary trials of VEGFR, MET, or dual inhibition for CRPC.
| Trial name / agent |
Identifier / reference |
No. of subjects |
Population | Treatment arms | Primary endpoint |
Outcome/Comments |
|---|---|---|---|---|---|---|
| Notable VEGF/VEGFR-targeted phase III trials | ||||||
| Bevacizumab | CALGB 90401 NCT00110214 |
1,050 [25] |
Metastatic CRPC, chemo-naïve |
Docetaxel, prednisone, and bevacizumab (15mg/kg IV every 3 weeks) or placebo |
OS | No difference in OS (22.6 vs. 21.5 months, p=0.181). Median PFS and OR higher in bevacizumab group as were toxicities including treatment-related deaths |
| SUN1120, Sunitinib |
NCT00676650 [28] |
873 | Metastatic CRPC, docetaxel- pretreated |
Sunitinib (37.5mg PO daily) or placebo |
OS | Randomized 2:1. Prematurely discontinued in September 2010 after second interim analysis showed no improvement in OS (13.1 vs. 12.8 months, p=0.5813). Presented at ASCO Annual Meeting 2011. |
| MAINSAIL, Lenalidomide |
NCT00988208 [31] |
1,059 | Metastatic CRPC, chemo-naïve |
Docetaxel, prednisone, and lenalidomide (25mg PO daily, days 1-14 in 21-day cycle) or placebo |
OS | Prematurely discontinued in November 2011 after data monitoring committee stated it was unlikely to meet its endpoint. Median OS was 77 weeks vs. not reached Presented at ESMO 2012 Congress |
| VENICE, Aflibercept |
NCT00519285 | 1,224 | Metastatic CRPC, chemo-naïve |
Docetaxel, prednisone, and aflibercept (6mg/kg IV every 3 weeks) or placebo |
OS | Press release in April 2012 by the study sponsors indicated no difference in OS between treatment arms. Not yet presented or published. |
| Tasquinimod | NCT01234311 | 1,200 (target) |
Metastatic CRPC, chemo-naïve |
Tasquinimod (0.25, 0.5, or 1mg/day) or placebo |
PFS | Ongoing study, enrolling patients. Randomization 2:1 to treatment vs. placebo. |
| Notable MET-targeted phase II trials | ||||||
| Rilotumumab | NCT00770848 [34] |
142 | Metastatic CRPC, prior taxane-based treatment |
Mitoxantrone, prednisone, and rilotumumab (15mg/kg or 7.5mg/kg IV every 3 weeks) or placebo |
OS | The phase II portion of combined phase Ib/II study examined OS. Randomized 1:1:1. No difference in OS (13.4 vs. 11.6 vs. 11.1 months). No significant differences in PFS or PSA response. Presented at ASCO Genitourinary Cancers Symposium 2011. |
| Tivantinib | NCT01519414 | N/A | Metastatic CRPC, chemo-naïve |
Tivantinib (PO twice daily) or placebo |
PFS | Ongoing study, enrolling patients. |
| Notable trials inhibiting both MET and VEGFR pathways | ||||||
| XL184-203, | NCT00940225 | 177 | Metastatic CRPC, | Cabozantinib (100mg PO | PFS | Phase II randomized discontinuation |
| Cabozantinib | [45] | (CRPC subset) |
measurable disease. |
daily) for 12-week lead- in stage, then randomized according to response per RECIST |
study of 9 selected tumor types. Due to unexpected high rate of bone scan improvement during lead-in, CRPC cohort was expanded from 122 to 177 total subjects with nonrandomized extension. 4% response by RECIST; 75% improvement on bone scans. Dose reductions necessary for 51% by week 12; 16% discontinuation by week 12 due to AEs. |
|
| Cabozantinib | NCT01347788 [46] |
36 | Metastatic CRPC with bone metastases |
Cabozantinib at two doses: 40mg PO daily and 20mg PO daily and 20mg PO daily |
Bone scan response rate |
A dose-finding study to evaluate lowest active dose of cabozantinib. 40mg dose was associated with 64% bone scan response rate at 6 weeks at 76% at 12 weeks; no dose reductions by week 6 or 12; and 12% discontinuation rate by week 12 due to AEs. Presented at ASCO Annual Meeting 2012 and submitted, 2012. |
| COMET-1, Cabozantinib |
NCT01605227 | 960 (target) |
Metastatic CRPC with bone- dominant disease, prior docetaxel, prior abiraterone or MDV3100 |
Cabozantinib (60mg daily) or prednisone (5mg twice daily) |
OS | Phase III trial. Ongoing study, enrolling patients |
| COMET-2, Cabozantinib |
NCT-1522443 | 246 (target) |
Metastatic CRPC with bone metastases and pain requiring opioid narcotic intervention; prior docetaxel, prior abiraterone or MDV3100 |
Cabozantinib (60mg daily) or mitoxantrone (IV every 3 weeks) and prednisone |
Confirmed pain response |
Phase III trial. Ongoing study, enrolling patients. Maximum of 10 infusions for mitoxantrone (or placebo). |
VEGF/VEGFR inhibition in clinical trials in CRPC
Five agents targeting angiogenesis have reached phase III clinical trials in CRPC. To date, inhibition of VEGF/VEGFR signaling has not improved OS in men with CRPC.
Bevacizumab
Bevacizumab is a humanized monoclonal antibody that neutralizes VEGF activity. A phase II study (CALGB 90006) of bevacizumab combined with docetaxel and estamustine suggested an improvement in OS[24]. CALGB 90401 enrolled 1,050 subjects with progressive, metastatic CRPC[25]. The primary endpoint was OS. Subjects were randomized to receive docetaxel (75mg/m2 every 21 days) with prednisone (5mg BID) and either bevacizumab (15mg/kg IV every 21 days) or placebo. There was no statistically significant difference in OS (22.6 vs. 21.5 months, respectively; HR 0.91; 95% CI, 0.78-1.05; p = 0.181). Median progression-free survival (PFS) and objective response (OR) were higher in the bevacizumab group, as were grade 3 or greater treatment-related toxicity and treatment-related deaths[25].
Sunitinib
Sunitinib, an oral tyrosine kinase inhibitor (TKI) of VEGFR and PDGFR, demonstrated activity in two phase II studies of subjects with progressive metastatic CRPC who had received docetaxel chemotherapy[26, 27]. A phase III trial randomized 873 subjects with progressive metastatic CRPC after docetaxel chemotherapy to sunitinib (37.5mg daily) or placebo, in a 2:1 ratio. The primary endpoint was OS. Results from a second interim analysis demonstrated no improvement in OS (13.1 vs. 12.8 months, respectively; HR 1.03; 95% CI 0.80-1.32; p = 0.5813). These results were presented at the 2011 Annual Meeting of the American Society for Clinical Oncology (ASCO) [28].
Unplanned post-hoc analyses of bone scans from a phase II study of sunitinib[27] evaluated baseline and 12-week scans in 25 subjects[29]. Two radiologists specialized in nuclear medicine and one nuclear medicine physician assessed all images and identified “partial responses” (PR) as subjective ≥ 50% overall improvement in total abnormal tracer uptake (intensity × volume) of previous metastatic bone lesions, or patients with extensively diffuse skeletal metastasis (superscan) that changed to recognizable individual metastatic lesions. Overall, six of 25 subjects (24%) demonstrated a response by bone scan using these criteria. One of the 6 subjects had complete response (CR, defined as no lesions indicating metastatic disease). None of the 6 demonstrated a response by PSA or RECIST criteria[29]. These data, in light of significant bone scan improvement in CRPC subjects taking cabozantinib (below), highlight the potential discordance between 99mTc-MDP bone scans and other assessments of post-treatment changes using TKIs or other targeted therapies.
Lenalidomide
Lenalidomide is an oral immunomodulatory agent with potential antineoplastic activity that also inhibits VEGF signaling and angiogenesis[30]. The MAINSAIL study randomized 1,059 subjects with CRPC to docetaxel (75mg/m2, once every 3 weeks) with prednisone (5mg twice daily) and either lenalidomide (25mg daily, days 1-14) or placebo. The primary endpoint was OS; secondary endpoints included PFS and overall response rate (ORR). In November, 2011, the data monitoring committee recommended that the study be stopped, as it was unlikely to meet its primary endpoint. This study was described at the European Society for Medical Oncology (ESMO) 2012 Congress[31]. The median OS of the lenalidomide group was 77 weeks; the median was not reached in the placebo arm. Treatment with lenalidomide was associated with more neutropenia, febrile neutropenia, and diarrhea.
Aflibercept
Aflibercept, also known as VEGF-Trap, is a protein composed of the extracellular domains of VEGFR-1 and −2 fused with the constant region (Fc) of the human IgG1 antibody. Aflibercept acts as a decoy receptor, preventing VEGF from binding to VEGFRs. The VENICE study randomized 1,224 subjects with CRPC to docetaxel (75mg/m2, once every 3 weeks) with prednisone (10mg daily) and either aflibercept (6mg/kg intravenous, once every 3 weeks) or placebo. The primary endpoint was OS; secondary endpoints included PSA response, pain response, skeletal-related events, and PFS. In April, 2012, the study sponsors, Sanofi and Regeneron, reported in a press release that no difference in OS was observed between the treatment arms. This study has not been presented or published for further details.
Tasquinimod
Among other therapies targeting angiogenesis in CRPC, tasquinimod has advanced to phase III testing. Tasquinimod is an oral agent with antiangiogenic and potential antineoplastic activities, and has been shown to decrease blood vessel density, although the precise mechanism of action is unknown[32]. In a randomized, double-blind phase II study, 201 subjects with metastatic CRPC were assigned to either tasquinimod (1mg daily) or placebo after a titration phase (0.25mg daily for 2 weeks followed by 0.5mg daily for 2 weeks)[32]. After a maximum of 6 months of double-blind treatment, asymptomatic subjects on the placebo arm were offered open-label tasquinimod. Subjects on the tasquinimod arm without disease progression at 6 months could continue open-label treatment, and subjects with progression were withdrawn from study. The primary endpoint was disease progression by RECIST, Prostate Cancer Working Group[33], or pain criteria. Six-month progression-free proportion favored the tasquinimod arm over placebo (69% vs. 37%, p < 0.001). Median PFS was longer in the tasquinimod arm over placebo (7.6 vs. 3.3 months, p = 0.0042). There was no significant difference in post-treatment PSA change between the two groups. Grade 3-4 adverse events were more common in the tasquinimod arm (40% vs. 10%)[32]. In the phase III study, a total of 1,200 subjects with chemotherapy-naïve CRPC will be randomized 2:1 to tasquinimod or placebo. The primary endpoint is PFS. The study is powered to detect an improvement in OS as a secondary endpoint. This study is ongoing.
MET inhibition in clinical trials in CRPC
MET-targeted therapies in development include monoclonal antibodies and small molecule TKIs. Two therapies that inhibit MET signaling have reached phase II clinical trials specifically enrolling CRPC patients.
Rilotumumab
Rilotumumab (AMG102) is a fully human monoclonal antibody to HGF. A phase II study randomized 142 men with progressive CRPC after taxane chemotherapy 1:1:1 to mitoxantrone (12mg/m2 every 3 weeks) with prednisone (5mg twice daily) and either rilotumumab (15mg/kg intravenous every 3 weeks), rilotumumab (7.5mg/kg intravenous every 3 weeks) or placebo. The primary endpoint was overall survival. Rilotumumab did not improve OS (13.4 vs. 11.6 vs. 11.1 months, respectively). Rilotumumab was not associated with any significant differences in PFS or PSA response. These data were presented at the 2012 ASCO Genitourinary Cancers Symposium[34].
Tivantinib
Tivantinib (ARQ 197) is an oral TKI that binds to MET protein, disrupting MET signal transduction pathways. A randomized phase II study is currently enrolling subjects with chemotherapy-naïve, minimally symptomatic or asymptomatic metastatic CRPC. Subjects will receive tivantinib orally twice daily or placebo. The primary endpoint is PFS.
Other therapies targeting MET signaling are in earlier phases of clinical development with respect to CRPC. Monoclonal antibodies include onartuzumab (MetMab), TAK-701, and ficlatuzumab (SCH900105). Small molecule TKIs include SGX523, PF-04217903, EMD 1214063, EMD 1204831, PF-02341066 (crizotinib/Xalkori), BMS-777607, SAR125844, and JNJ-38877605.
Dual inhibition of MET and VEGFR signaling in CRPC: Clinical Evidence
TKIs that target both MET and VEGFR signaling are in development. Cabozantinib has advanced to phase III trials in CRPC and is the subject of the remainder of this review. Other therapies that target both MET and VEGFR signaling are in earlier phases of clinical development with respect to CRPC, including foretinib (GSK1363089), golvatinib (E7050), GSK1363089 (XL880), and MGCD265.
Cabozantinib
Cabozantinib (XL184) is an oral small molecule TKI. The primary targets of cabozantinib are VEGFR2, MET, a mutationally activated form of RET, and KIT[35]. Dual inhibition of VEGFR and MET signaling by cabozantinib has shown promise in metastatic CRPC[36] and other malignancies. In medullary thyroid cancer, activating RET mutations are commonly associated with both inherited and sporadic forms of the disease. The phase I trial of cabozantinib (140mg daily freebase weight) yielded early evidence of clinical benefit in medullary thyroid cancer, with 68% of subjects exhibiting stable disease or confirmed partial response[37]. The EXAM study is the only completed phase III study of cabozantinib to date. The EXAM study randomized 330 subjects with progressive, locally advanced or metastatic medullary thyroid cancer to cabozantinib or placebo. The primary endpoint was PFS. Cabozantinib improved PFS (11.2 vs. 4.0 months; HR 0.28; 95% CI 0.19-0.40; p < 0.0001). An interim analysis of OS (a secondary endpoint) at 44% of 217 required events did not show a difference between cabozantinib or placebo[38]. Other malignancies that have demonstrated responsiveness to cabozantinib in phase II clinical trials include renal[39], ovarian[40], liver[41], melanoma[42], breast[43], and non-small cell lung cancer[44].
Phase II randomized discontinuation study
Phase I trial evidence of tumor regression in response to cabozantinib in multiple tumor types led to a phase II randomized discontinuation study in 9 selected tumor types (study XL184-203), including CRPC. In the CRPC group, enrolled subjects had progressive metastatic CRPC with measurable disease by mRECIST criteria, continued castration therapy, and no more than one prior standard chemotherapy regimen. Cabozantinib (100mg freebase weight) was administered daily during a 12-week lead-in stage. Subsequent treatment was based on response at 12 weeks: subjects with response by mRECIST criteria continued open-label cabozantinib, subjects with progressive disease discontinued therapy, and subjects with stable disease were randomized to either placebo or continued cabozantinib. The primary endpoint for the lead-in stage was response rate per mRECIST criteria. The primary endpoint for the randomized stage was PFS. Bone disease was present at baseline in 87% of patients, and 43% were pretreated with docetaxel[45].
Randomization in the CRPC cohort was suspended after the first 122 CRPC subjects were enrolled due to unexpected high rates of bone scan improvement during the lead-in stage. An additional 49 subjects were enrolled in a nonrandomized extension, for a total of 177 CRPC subjects. Bone scans were assessed visually by an independent single reader comparing baseline scan with week 6 or week 12 scans. The response rate by mRECIST criteria was 4% although most subjects had minor improvements in measurable disease. Unexpectedly, 75% of subjects had rapid improvement of 99mTc-MDP bone scans, a post-hoc study outcome, including 19% with complete resolution (CR)[45]. Improvements in bone scans correlated with clinical benefit, as 67% of subjects with pain at baseline reported a decrease in pain. PSA changes and bone scan improvements were discordant in 40% of subjects. Dose reductions occurred in 51% of subjects by week 12, with 16% discontinuing treatment due to adverse events (AEs) prior to week 12. The most common grade 3 toxicities during the lead-in stage were fatigue (16%), hypertension (6%), and hand-foot syndrome (6%). Serious AEs included venous thromboembolic events (VTEs, 13 events), gastrointestinal perforation (2 events), and one unexplained death on study at week 33. The high rates of bone scan improvements coupled with clinical benefit supported further development of cabozantinib in men with CRPC and bone metastases.
Dose-ranging study
Due to the high rate of dose reduction and treatment discontinuation at 100mg daily, a dose-ranging study was conducted to determine the efficacy and tolerability of cabozantinib at lower starting doses (NCT01347788)[46, 47]. Eligible patients had progressive CRPC with bone metastases, continued castration therapy, and up to 2 prior chemotherapy regimens. The primary outcome was bone scan response at 6 weeks, measured as the post-treatment change in BSLA assessed using the CADrx system[23]. A decrease in BSLA of ≥ 30% was defined as a response. Bone scans were categorized as CR, PR, stable disease (SD), or unequivocal progressive disease (PD) based on comparison of week 6 and baseline imaging. Secondary outcome measures included markers of bone turnover and effects on circulating tumor cells (CTCs).
The study completed enrollment of 36 subjects. Median age was 66 years. Sixteen subjects (44%) had previously received docetaxel chemotherapy. An adaptive response design was used to determine the lowest active daily cabozantinib dose among dose level +1 (60mg), dose level 0 (40mg), and dose level −1 (20mg), per Sargent one-stage design[48].
Of the tested doses, a higher bone scan response rate at 6 weeks was observed with cabozantinib 40mg daily (67%) compared with 20mg daily (10%). Of 25 subjects who started treatment at 40mg daily (cohort 1 and expansion cohort), 16 (64%) exhibited at least PR by week 6. The best response at 40mg daily by week 12 was 16 PR and 3 CR, or 76% of 25 subjects. Post-treatment PSA change and bone scan change were discordant in 54%. These findings are comparable with the 75% bone scan improvement rate seen in the randomized discontinuation study starting with a daily cabozantinib dose of 100mg[45].
Subjects receiving the 40mg starting dose were on study for median of 27 weeks (range: 2-57 weeks). No subjects required dose reduction or delay at 6 or 12 weeks, compared with 51% in the randomized discontinuation study[45]. Discontinuations due to drug-related AEs occurred in 12% of subjects at the 40mg starting dose before 12 weeks, compared with the 16% rate seen in the randomized discontinuation trial[45]. Reasons for discontinuing therapy by 12 weeks included fatigue and anorexia (1 subject) and VTE (2 subjects).
CTC evaluation (CellSearch assay, Veridex LLC, Raritan, NJ) revealed that 12 subjects had “unfavorable” CTC levels of ≥ 5 CTCs per 7.5mL of peripheral blood at baseline. Eleven of the 12 subjects (92%) had a decrease in CTCs of >30% by 12 weeks, including 7 subjects who converted to the favorable level of <5 CTCs. These data are consistent with either a direct antitumor effect by cabozantinib and/or an alteration of the bone microenvironment by cabozantinib that affects cancer viability.
The high rate of bone scan response and improved tolerability of the 40mg daily starting dose have informed the design of other ongoing trials of cabozantinib in CRPC. The nonrandomized expansion cohort of trial XL184-203 was amended to include a 40mg daily dose of cabozantinib in CRPC subjects, in addition to the 100mg daily dose of the original protocol. The phase III trials of cabozantinib both use lower starting doses than the original phase II study.
Phase III trials
Two phase III, randomized, double-blind, controlled trials of cabozantinib (COMET-1 and COMET-2) at 60mg daily starting dose are currently enrolling patients with metastatic CRPC and disease progression despite prior treatment with docetaxel and either abiraterone or enzalutamide. COMET-1 compares cabozantinib with prednisone, with the primary outcome measure of OS. COMET-2 compares cabozantinib with mitoxantrone and prednisone in subjects with symptomatic disease, with the primary outcome measure of confirmed, durable pain response from week 6 to week 12.
Summary
Management of bone metastasis in CRPC remains an important unmet need. VEGFR and MET signaling pathways are thought to play important roles in prostate cancer progression and bone metastasis, and are thus rational targets for intervention in CRPC. Studies that evaluated agents that inhibit one but not both pathways have not yielded improvements in OS.
Cabozantinib, an inhibitor of both MET and VEGFR2, is associated with improvement in bone scans, pain response, bone turnover markers, and CTCs in men with CRPC and bone metastases. The phase II trial dose of 100mg daily was associated with frequent adverse events that led to dose reductions in most patients[45]. Lower doses of cabozantinib retain high levels of activity with less toxicity[47]. Ongoing phase III clinical trials will define the role of cabozantinib in CRPC.
The precise mechanism(s) of bone scan improvements with cabozantinib is undefined. Bone scans reflect altered osteoblast activity. Post-treatment bone scan changes may be caused by altered tumor perfusion, death of tumor cells, direct downregulation of osteoblast activity, or a combination of factors. Because cabozantinib and sunitinib share VEGFR as a common target and both TKIs demonstrated bone scan improvements in CRPC[29, 45], VEGFR inhibition may cause diminished perfusion, decreased osteoblast activity, and resultant improvement on bone scans. Cabozantinib may improve upon sunitinib’s effect by inhibition of another of its targets, MET. Animal models investigating sunitinib resistance mechanisms have demonstrated increased HGF production and induction of alternative angiogenic pathways via MET-expressing endothelial cells[49]. Combined inhibition of sunitinib with the MET inhibitor PF-04217903 effectively decreased tumor growth of sunitinib-resistant cancer cell lines in experimental animals[49]. Other studies examining a pancreatic neuroendocrine tumor model demonstrated similar inhibition of tumor growth, invasion, and metastasis using either cabozantinib or a combination of sunitinib and PF-04217903 in experimental animals[50].
The discordance between high rates of bone scan improvement and only mild effects of cabozantinib on visceral metastases[45] may reflect differential target inhibition depending on the tumor microenvironment. The coordinated MET and VEGFR inhibition may be more effective disrupting the bone microenvironment compared with visceral metastases due to the importance of these pathways in the interactions among prostate cancer cells, osteoblasts, osteoclasts, and vascular cells. Alteration of the bone microenvironment by cabozantinib may thus affect cancer viability, reflected in the decrease in CTCs. The decrease in CTCs could also reflect a direct antitumor effect of cabozantinib via inhibition of cell proliferation, invasion, and metastasis, similar to preclinical models[50].
Alternatively, cabozantinib may be tumoristatic in both bone and viscera via inhibition of both VEGFR- and MET-mediated angiogenesis[49]. The reduced perfusion of bone tumor deposits may cause bone scan improvement, clinical benefit, and overall static tumor burden, especially in patients with bone-dominant metastatic CRPC. Assuming that serum PSA reflects overall tumor burden, the discordance of bone scan improvements, clinical benefit, and PSA change may be consistent with this model. We have observed moderate fluctuation of PSA during prolonged (> 12 weeks) cabozantinib treatment, which could reflect a waxing and waning tumor burden that cannot escape effective angiogenesis inhibition (not shown). The decrease in CTCs seen in the dose-ranging studies may be consistent with this tumoristatic model due to diminished communication between vasculature and tumor deposits.
In summary, cabozantinib is a promising investigational therapy for metastatic CRPC. Cabozantinib is associated with a high rate of bone scan responses, decreases in measurable soft tissue disease, improvements in pain, and decreases in bone biomarkers and CTCs. The primary molecular targets of cabozantinib include VEGFR and MET although the precise mechanisms responsible for the activity of cabozantinib in metastatic prostate cancer are undefined. Two ongoing randomized controlled trials, COMET-1 and COMET-2, will evaluate the effects of cabozantinib on overall survival and pain in treatment-refractory metastatic CRPC.
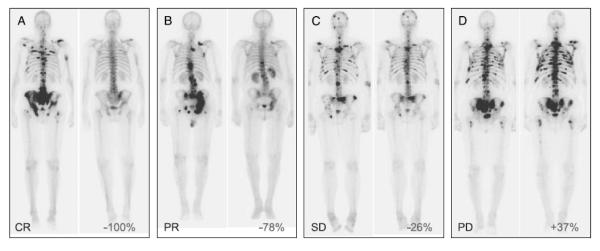
Fig. 1.
Representative bone scan responses from the dose-ranging cabozantinib trial. The left panel of each pair represents the baseline bone scan; the right panel is the scan from week 6 or 12. Percent change in BSLA is indicated. (A) Subject treated with 40mg daily, with complete response (CR) by BSLA at 12 weeks. (B) Subject treated with 40mg daily, with partial response (PR) at 6 weeks. (C) Subject treated with 40mg daily, with stable disease (SD) at 6 weeks. (D) Subject treated with 20mg daily, with progressive disease (PD) at 6 weeks.
Acknowledgments
Supported by: the Department of Defense Prostate Cancer Research Program under Award W81XWH-09-1-0471 and a Conquer Cancer Foundation Career Development Award (R.J.L.); by National Institutes of Health Midcareer Investigator Award No. 5K24CA121990 (M.R.S.); and competitive research awards from the Prostate Cancer Foundation.
Footnotes
Conflicts of Interest and Source of Funding: Dr. Lee has received sponsored research funding from Exelixis, Inc., South San Francisco, CA. Dr. Smith is a paid consultant of, and has received sponsored research funding from, Exelixis, Inc., South San Francisco, CA.
References
- [1].Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: A Cancer Journal for Clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- [2].Saylor PJ, Lee RJ, Smith MR. Emerging Therapies to Prevent Skeletal Morbidity in Men With Prostate Cancer. J. Clin. Oncol. 2011;29:3705–3714. doi: 10.1200/JCO.2010.34.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Clarke NW, McClure J, George NJ. Osteoblast function and osteomalacia in metastatic prostate cancer. Eur. Urol. 1993;24:286–290. doi: 10.1159/000474311. [DOI] [PubMed] [Google Scholar]
- [4].Grano M, Galimi F, Zambonin G, Colucci S, Cottone E, Zallone AZ, Comoglio PM. Hepatocyte growth factor is a coupling factor for osteoclasts and osteoblasts in vitro. Proc. Natl. Acad. Sci. USA. 1996;93:7644–7648. doi: 10.1073/pnas.93.15.7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Adamopoulos IE, Xia Z, Lau YS, Athanasou NA. Hepatocyte growth factor can substitute for M-CSF to support osteoclastogenesis. Biochemical and Biophysical Research Communications. 2006;350:478–483. doi: 10.1016/j.bbrc.2006.09.076. [DOI] [PubMed] [Google Scholar]
- [6].Tsai S-Y, Huang Y-L, Yang W-H, Tang C-H. Hepatocyte growth factor-induced BMP-2 expression is mediated by c-Met receptor, FAK, JNK, Runx2, and p300 pathways in human osteoblasts. Int. Immunopharmacol. 2012;13:156–162. doi: 10.1016/j.intimp.2012.03.026. [DOI] [PubMed] [Google Scholar]
- [7].Taichman RS, Reilly MJ, Verma RS, Ehrenman K, Emerson SG. Hepatocyte growth factor is secreted by osteoblasts and cooperatively permits the survival of haematopoietic progenitors. Br J Haematol. 2001;112:438–448. doi: 10.1046/j.1365-2141.2001.02568.x. [DOI] [PubMed] [Google Scholar]
- [8].Deckers MML, Karperien M, van der Bent C, Yamashita T, Papapoulos SE, Löwik CWGM Expression of Vascular Endothelial Growth Factors and Their Receptors during Osteoblast Differentiation. Endocrinology. 2000;141:1667–1674. doi: 10.1210/endo.141.5.7458. [DOI] [PubMed] [Google Scholar]
- [9].Street J, Lenehan B. Vascular endothelial growth factor regulates osteoblast survival -evidence for an autocrine feedback mechanism. J. Orthop. Surg. Res. 2009;4:19. doi: 10.1186/1749-799X-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Marini M, Sarchielli E, Toce M, Acocella A, Bertolai R, Ciulli C, Orlando C, Sgambati E, Vannelli GB. Expression and localization of VEGF receptors in human fetal skeletal tissues. Histol. Histopathol. 2012;27:1579–1587. doi: 10.14670/HH-27.1579. [DOI] [PubMed] [Google Scholar]
- [11].Trebec-Reynolds DP, Voronov I, Heersche JNM, Manolson MF. VEGF-A expression in osteoclasts is regulated by NF-κB induction of HIF-1α. J. Cell. Biochem. 2010;110:343–351. doi: 10.1002/jcb.22542. [DOI] [PubMed] [Google Scholar]
- [12].van Leenders G, van Balken B, Aalders T, Hulsbergen-van de Kaa C, Ruiter D, Schalken J. Intermediate cells in normal and malignant prostate epithelium express c-MET: Implications for prostate cancer invasion. Prostate. 2002;51:98–107. doi: 10.1002/pros.10073. [DOI] [PubMed] [Google Scholar]
- [13].Verras M, Lee J, Xue H, Li T-H, Wang Y, Sun Z. The Androgen Receptor Negatively Regulates the Expression of c-Met: Implications for a Novel Mechanism of Prostate Cancer Progression. Cancer Res. 2007;67:967–975. doi: 10.1158/0008-5472.CAN-06-3552. [DOI] [PubMed] [Google Scholar]
- [14].Humphrey PA, Zhu X, Swanson PE, Ratliff TL, Vollmer RT, Day ML. Hepatocyte growth factor and its receptor (c-MET) in prostatic carcinoma. Am. J. Pathol. 1995;147:386–396. [PMC free article] [PubMed] [Google Scholar]
- [15].Knudsen BS, Gmyrek GA, Inra J, Scherr DS, Vaughan ED, Nanus DM, Kattan MW, Gerald WL, Vande Woude GF. High expression of the Met receptor in prostate cancer metastasis to bone. Urology. 2002;60:1113–1117. doi: 10.1016/s0090-4295(02)01954-4. [DOI] [PubMed] [Google Scholar]
- [16].Pallares J, Rojo F, Iriarte J, Morote J, Armadans LI, de Torres I. Study of microvessel density and the expression of the angiogenic factors VEGF, bFGF and the receptors Flt-1 and FLK-1 in benign, premalignant and malignant prostate tissues. Histol. Histopathol. 2006;21:857–865. doi: 10.14670/HH-21.857. [DOI] [PubMed] [Google Scholar]
- [17].Ferrer FA, Miller LJ, Andrawis RI, Kurtzman SH, Albertsen PC, Laudone VP, Kreutzer DL. Vascular Endothelial Growth Factor (VEGF) Expression in Human Prostate Cancer: In Situ and in Vitro Expression of VEGF by Human Prostate Cancer Cells. J. Urol. 1997;157:2329–2333. [PubMed] [Google Scholar]
- [18].Duque JLF, Loughlin KR, Adam RM, Kantoff PW, Zurakowski D, Freeman MR. Plasma levels of vascular endothelial growth factor are increased in patients with metastatic prostate cancer. Urology. 1999;54:523–527. doi: 10.1016/s0090-4295(99)00167-3. [DOI] [PubMed] [Google Scholar]
- [19].Bok RA, Halabi S, Fei DT, Rodriquez CR, Hayes DF, Vogelzang NJ, Kantoff P, Shuman MA, Small EJ. Vascular Endothelial Growth Factor and Basic Fibroblast Growth Factor Urine Levels as Predictors of Outcome in Hormone-refractory Prostate Cancer Patients: A Cancer and Leukemia Group B Study. Cancer Res. 2001;61:2533–2536. [PubMed] [Google Scholar]
- [20].George DJ, Halabi S, Shepard TF, Sanford B, Vogelzang NJ, Small EJ, Kantoff PW. The Prognostic Significance of Plasma Interleukin-6 Levels in Patients with Metastatic Hormone-Refractory Prostate Cancer: Results from Cancer and Leukemia Group B 9480. Clin. Cancer Res. 2005;11:1815–1820. doi: 10.1158/1078-0432.CCR-04-1560. [DOI] [PubMed] [Google Scholar]
- [21].Zhang S, Zhau H, Osunkoya A, Iqbal S, Yang X, Fan S, Chen Z, Wang R, Marshall F, Chung L, Wu D. Vascular endothelial growth factor regulates myeloid cell leukemia-1 expression through neuropilin-1-dependent activation of c-MET signaling in human prostate cancer cells. Mol. Cancer. 2010;9:9. doi: 10.1186/1476-4598-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Scher HI, Morris MJ, Kelly WK, Schwartz LH, Heller G. Prostate Cancer Clinical Trial End Points: “RECIST”ing a Step Backwards. Clin. Cancer Res. 2005;11:5223–5232. doi: 10.1158/1078-0432.CCR-05-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Brown MS, Chu GH, Kim HJ, Allen-Auerbach M, Poon C, Bridges J, Vidovic A, Ramakrishna B, Ho J, Morris MJ, Larson SM, Scher HI, Goldin JG. Computer-aided quantitative bone scan assessment of prostate cancer treatment response. Nucl. Med. Commun. 2012;33:384–394. doi: 10.1097/MNM.0b013e3283503ebf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Picus J, Halabi S, Kelly WK, Vogelzang NJ, Whang YE, Kaplan EB, Stadler WM, Small EJ, for Cancer and Leukemia Group B A phase 2 study of estramustine, docetaxel, and bevacizumab in men with castrate-resistant prostate cancer. Cancer. 2011;117:526–533. doi: 10.1002/cncr.25421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kelly WK, Halabi S, Carducci M, George D, Mahoney JF, Stadler WM, Morris M, Kantoff P, Monk JP, Kaplan E, Vogelzang NJ, Small EJ. Randomized, Double-Blind, Placebo-Controlled Phase III Trial Comparing Docetaxel and Prednisone With or Without Bevacizumab in Men With Metastatic Castration-Resistant Prostate Cancer: CALGB 90401. J. Clin. Oncol. 2012;30:1534–1540. doi: 10.1200/JCO.2011.39.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sonpavde G, Periman PO, Bernold D, Weckstein D, Fleming MT, Galsky MD, Berry WR, Zhan F, Boehm KA, Asmar L, Hutson TE. Sunitinib malate for metastatic castration-resistant prostate cancer following docetaxel-based chemotherapy. Ann. Oncol. 2010;21:319–324. doi: 10.1093/annonc/mdp323. [DOI] [PubMed] [Google Scholar]
- [27].Michaelson MD, Regan MM, Oh WK, Kaufman DS, Olivier K, Michaelson SZ, Spicer B, Gurski C, Kantoff PW, Smith MR. Phase II study of sunitinib in men with advanced prostate cancer. Ann. Oncol. 2009;20:913–920. doi: 10.1093/annonc/mdp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Michaelson MD, Oudard S, Ou Y, Sengelov F, Saad F, Houede N, Ostler PJ, Stenzl A, Daugaard G, Jones RJ, Laestadius F, Bahl A, Castellano DE, Gschwend J, Maurina T, Ye D, Chen I, Wang S, Maneval EC. Randomized, placebo-controlled, phase III trial of sunitinib in combination with prednisone (SU+P) versus prednisone (P) alone in men with progressive metastatic castration-resistant prostate cancer (mCRPC) J. Clin. Oncol. 2011;29(suppl) doi: 10.1200/JCO.2012.48.5268. abstr 4515. [DOI] [PubMed] [Google Scholar]
- [29].Saylor PJ, Mahmood U, Kunawudhi A, Smith MR, Palmer EL, Michaelson MD. Multitargeted Tyrosine Kinase Inhibition Produces Discordant Changes Between 99mTc-MDP Bone Scans and Other Disease Biomarkers: Analysis of a Phase II Study of Sunitinib for Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2012;53:1670–1675. doi: 10.2967/jnumed.112.105007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lu L, Payvandi F, Wu L, Zhang L-H, Hariri RJ, Man H-W, Chen RS, Muller GW, Hughes CCW, Stirling DI, Schafer PH, Bartlett JB. The anti-cancer drug lenalidomide inhibits angiogenesis and metastasis via multiple inhibitory effects on endothelial cell function in normoxic and hypoxic conditions. Microvasc. Res. 2009;77:78–86. doi: 10.1016/j.mvr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- [31].Petrylak DP, Fizazi K, Sternberg CN, Budnik N, Wit Rd, Wiechno PJ, Bellmunt J, Barton D, Fandi A, Jungnelius U, Li S, Vogelzang NJ, Investigators M. A Phase 3 Study to Evaluate the Efficacy and Safety of Docetaxel and Prednisone (DP) With or Without Lenalidomide in Patients With Castrate-resistant Prostate Cancer (CRPC): The MAINSAIL Trial. ESMO 2012 Congress. 2012 abstract LBA24. [Google Scholar]
- [32].Pili R, Haggman M, Stadler WM, Gingrich JR, Assikis VJ, Bjork A, Nordle O, Forsberg G, Carducci MA, Armstrong AJ. Phase II Randomized, Double-Blind, Placebo-Controlled Study of Tasquinimod in Men With Minimally Symptomatic Metastatic Castrate-Resistant Prostate Cancer. J. Clin. Oncol. 2011;29:4022–4028. doi: 10.1200/JCO.2011.35.6295. [DOI] [PubMed] [Google Scholar]
- [33].Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ, Dreicer R, Petrylak D, Kantoff P, Basch E, Kelly WK, Figg WD, Small EJ, Beer TM, Wilding G, Martin A, Hussain M. Design and End Points of Clinical Trials for Patients With Progressive Prostate Cancer and Castrate Levels of Testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J. Clin. Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ryan CJ, Rosenthal M, Ng S, Alumkal JJ, Picus J, Gravis G, Fizazi K, Forget F, Machiels J-PH, Zhu M, Jiang J, Dubey S, Loh E, Gerritsen WR. A multicenter, randomized phase II study of rilotumumab (R) (AMG 102) or placebo (Pbo) plus mitoxantrone (M) and prednisone (P) in patients (pts) with previously treated castrate-resistant prostate cancer (CRPC) J. Clin. Oncol. 2012;30(suppl 5) abstr 115. [Google Scholar]
- [35].Yakes FM, Chen J, Tan J, Yamaguchi K, Shi Y, Yu P, Qian F, Chu F, Bentzien F, Cancilla B, Orf J, You A, Laird AD, Engst S, Lee L, Lesch J, Chou Y-C, Joly AH. Cabozantinib (XL184), a Novel MET and VEGFR2 Inhibitor, Simultaneously Suppresses Metastasis, Angiogenesis, and Tumor Growth. Mol. Cancer Ther. 2011;10:2298–2308. doi: 10.1158/1535-7163.MCT-11-0264. [DOI] [PubMed] [Google Scholar]
- [36].Smith MR, Sweeney C, Rathkopf DE, Scher HI, Logothetis C, George DJ, Higano CS, Yu EY, Harzstark AL, Small EJ, Sartor AO, Gordon MS, Vogelzang NJ, Smith DC, Hussain M, De Bono JS, Haas NB, Scheffold C, Lee Y, Corn PG. Cabozantinib (XL184) in chemotherapy-pretreated metastatic castration resistant prostate cancer (mCRPC): Results from a phase II nonrandomized expansion cohort (NRE) J. Clin. Oncol. 2012;30(suppl) doi: 10.1200/JCO.2013.54.5954. abstr 4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kurzrock R, Sherman SI, Ball DW, Forastiere AA, Cohen RB, Mehra R, Pfister DG, Cohen EEW, Janisch L, Nauling F, Hong DS, Ng CS, Ye L, Gagel RF, Frye J, Muller T, Ratain MJ, Salgia R. Activity of XL184 (Cabozantinib), an Oral Tyrosine Kinase Inhibitor, in Patients With Medullary Thyroid Cancer. J. Clin. Oncol. 2011;29:2660–2666. doi: 10.1200/JCO.2010.32.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Schoffski P, Elisei R, Muller S, Brose MS, Shah MH, Licitra LF, Jarzab B, Medvedev V, Kreissl M, Niederle B, Cohen EEW, Wirth LJ, Ali HY, Hessel C, Yaron Y, Ball DW, Nelkin B, Sherman SI, Schlumberger M, Group ES. An international, double-blind, randomized, placebo-controlled phase III trial (EXAM) of cabozantinib (XL184) in medullary thyroid carcinoma (MTC) patients (pts) with documented RECIST progression at baseline. J. Clin. Oncol. 2012;(suppl) abstr 5508. [Google Scholar]
- [39].Choueiri TK, Pal SK, McDermott DF, Ramies DA, Morrissey S, Lee Y, Miles D, Holland J, Dutcher JP. Efficacy of cabozantinib (XL184) in patients (pts) with metastatic, refractory renal cell carcinoma (RCC) J. Clin. Oncol. 2012;30(suppl) abstr 4504. [Google Scholar]
- [40].Buckanovich RJ, Berger R, Sella A, Sikic BI, Shen X, Ramies DA, Smith DC, Vergote IB. Activity of cabozantinib (XL184) in advanced ovarian cancer patients (pts): Results from a phase II randomized discontinuation trial (RDT) J. Clin. Oncol. 2012;29(suppl) abstr 5008. [Google Scholar]
- [41].Cohn AL, Kelley RK, Yang T-S, Su W-C, Verslype C, Ramies DA, Lee Y, Shen X, Cutsem EV. Activity of cabozantinib (XL184) in hepatocellular carcinoma patients (pts): Results from a phase II randomized discontinuation trial (RDT) J. Clin. Oncol. 2012;30(suppl 4) abstr 261. [Google Scholar]
- [42].Gordon MS, Kluger HM, Shapiro G, Kurzrock R, Edelman G, Samuel TA, Moussa AH, Ramies DA, Laird AD, Schimmoller F, Shen X, Daud A. Activity of cabozantinib (XL184) in metastatic melanoma: Results from a phase II randomized discontinuation trial (RDT) J. Clin. Oncol. 2012;30(suppl) abstr 8531. [Google Scholar]
- [43].Winer EP, Tolaney S, Nechushtan H, Berger R, Kurzrock R, Ron I-G, Schoffski P, Awada A, Yasenchak CA, Burris HA, Ramies DA, Rafferty T, Shen X. Activity of cabozantinib (XL184) in metastatic breast cancer (MBC): Results from a phase II randomized discontinuation trial (RDT) J. Clin. Oncol. 2012;30(suppl) abstr 535. [Google Scholar]
- [44].Hellerstedt BA, Edelman G, Vogelzang NJ, Kluger HM, Yasenchak CA, Shen X, Ramies DA, Gordon MS, Lara P. Activity of cabozantinib (XL184) in metastatic NSCLC: Results from a phase II randomized discontinuation trial (RDT) J. Clin. Oncol. 2012;30(suppl) abstr 7514. [Google Scholar]
- [45].Smith DC, Smith MR, Sweeney C, Elfiky AA, Logothetis C, Corn PG, Vogelzang NJ, Small EJ, Harzstark AL, Gordon MS, Vaishampayan UN, Haas NB, Spira AI, Lara PN, Jr., Lin CC, Srinivas S, Sella A, Schoffski P, Scheffold C, Weitzman A, Hussain M. Cabozantinib for metastatic castration-resistant prostate cancer: results of a Phase II placebo-controlled randomized discontinuation study. J. Clin. Oncol. 2012;2012 in press. [Google Scholar]
- [46].Lee RJ, Michaelson MD, Saylor PJ, Gurski CA, Rothenberg SM, Miyamoto DT, Maheswaran S, Haber DA, Goldin JG, Smith MR. Investigator-sponsored trial of efficacy and tolerability of cabozantinib (cabo) at lower dose: A dose-finding study in men with castration-resistant prostate cancer (CRPC) and bone metastases. J. Clin. Oncol. 2012;30(suppl) abstr 4566. [Google Scholar]
- [47].Lee RJ, Saylor PJ, Michaelson MD, Rothenberg SM, Smas ME, Miyamoto DT, Gurski CA, Xie W, Maheswaran S, Haber DA, Goldin JG, Smith MR. A dose-ranging study of cabozantinib in men with castration-resistant prostate cancer and bone metastases. 2012 doi: 10.1158/1078-0432.CCR-13-0319. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sargent DJ, Chan V, Goldberg RM. A Three-Outcome Design for Phase II Clinical Trials. Controlled Clinical Trials. 2001;22:117–125. doi: 10.1016/s0197-2456(00)00115-x. [DOI] [PubMed] [Google Scholar]
- [49].Shojaei F, Lee JH, Simmons BH, Wong A, Esparza CO, Plumlee PA, Feng J, Stewart AE, Hu-Lowe DD, Christensen JG. HGF/c-Met Acts as an Alternative Angiogenic Pathway in Sunitinib-Resistant Tumors. Cancer Res. 2010;70:10090–10100. doi: 10.1158/0008-5472.CAN-10-0489. [DOI] [PubMed] [Google Scholar]
- [50].Sennino B, Ishiguro-Oonuma T, Wei Y, Naylor RM, Williamson CW, Bhagwandin V, Tabruyn SP, You W-K, Chapman HA, Christensen JG, Aftab DT, McDonald DM. Suppression of Tumor Invasion and Metastasis by Concurrent Inhibition of c-Met and VEGF Signaling in Pancreatic Neuroendocrine Tumors. Cancer Discov. 2012;2:270–287. doi: 10.1158/2159-8290.CD-11-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]