Abstract
Postsynaptic molecules with PDZ domains (PDZ proteins) interact with various glutamate receptors and regulate their subcellular trafficking and stability. In rat neocortical development, the protein expression of AMPA-type glutamate receptor GluR1 lagged behind its mRNA expression and rather paralleled an increase in PDZ protein levels. One of the neurotrophins, brain-derived neurotrophic factor (BDNF), appeared to contribute to this process, regulating the PDZ protein expression. In neocortical cultures, BDNF treatment upregulated SAP97, GRIP1, and Pick1 PDZ proteins. Conversely, BDNF gene targeting downregulated these same PDZ molecules. The BDNF-triggered increases in PDZ proteins resulted in the elevation of their total association with the AMPA receptors GluR1 and GluR2/3, which led to the increase in AMPA receptor proteins. When Sindbis viruses carrying GluR1 or GluR2 C-terminal decoys disrupted their interactions, GluR2 C-terminal decoys inhibited both BDNF-triggered GluR1 and GluR2/3 increases, whereas GluR1 C-terminal decoys blocked only the BDNF-triggered GluR1 increase. In agreement, coexpression of SAP97 and GluR1 in nonneuronal HEK293 cells increased both proteins compared with their single transfection, implying mutual stabilization. This work reveals a novel function of BDNF in postsynaptic development by regulating the PDZ protein expression.
Keywords: BDNF, GluR1, GluR2, GRIP1, Neurotrophin, Postsynaptic density, SAP97
Introduction
Neurotrophic factors and cytokines regulate neuronal differentiation and synaptic maturation/plasticity during brain development (for review, see Thoenen, 2000; Poo, 2001). Such factors, including brain-derived neurotrophic factor (BDNF), have been classified into retrograde factors, and their functions in presynaptic neurons or terminals have been studied extensively. The latest studies, however, demonstrate that BDNF is also released from nerve terminals and acts on postsynaptic neurons (Griesbeck et al., 1999; Kohara et al., 2001). Accordingly, BDNF also contributes to the morphologic and neurochemical development of the postsynaptic compartment. For instance, it enhances the dendritic growth of hippocampal neurons (McAllister et al., 1997) and increases nitric oxide synthase (Xiong et al., 1999), calbindin-D (Mizuno et al., 1994; Marty et al., 1996), glutamic acid decarboxylase (Hyman et al., 1994; Mizuno et al., 1994), AMPA receptor levels (Narisawa-Saito et al., 1999), and Homer mRNA (Sato et al., 2001). This argument is further supported by the presence of neurotrophic factor receptors on the dendritic processes or in the postsynaptic compartment (Aoki et al., 2000; Yacoubian and Lo, 2000). These findings suggest the importance of neurotrophic factors in both structural and phenotypic development of postsynaptic neurons. In agreement, BDNF influences on synaptic AMPA receptor sensitivity have also been documented by using the chronic BDNF application into culture (Rutherford et al., 1998; Sherwood and Lo, 1999; McLean-Bolton et al., 2000). However, the molecular nature of the BDNF activity on developing postsynaptic sites has not been fully understood. For example, the BDNF-dependent AMPA receptor increase is not regulated at the mRNA level but presumably at posttranslational levels (Narisawa-Saito et al., 1999a, 1999b).
Postsynaptic density (PSD) proteins, such as PSD-95, SAP97, and GRIP1, are a group of scaffolding molecules that use their PDZ domains to interact with distinct glutamate receptors and voltage-gated ion-channels as well as with various signaling molecules in neurons (for review, see Fanning and Anderson, 1999). The PDZ domain is a stretch of 80–90 amino acids initially identified in three proteins (PSD-95, Dlg, and ZO-1). They participate in the clustering of glutamate receptors or in their movement into and out of the synaptic membrane. As such, the interaction of the AMPA receptor subunits with specific PDZ proteins is implicated in various forms of synaptic plasticity. The expression of several PDZ proteins is first detected in embryonic stages, however, and is upregulated when immature neurons undergo synaptogenesis (Bruckner et al., 1999; Dong et al., 1999). The regulation of PDZ proteins during development and whether such regulation leads to the induction/maturation of the synaptic compartments, including AMPA receptors, remain to be determined.
Here, we found that the developmental regulation of AMPA receptor protein is distinct from that of its mRNA in rat neocortex. Is BDNF involved in this developmental process by regulating the expression of their interacting scaffolding proteins? Using neocortical cultures and a heterologous transient expression system in HEK293 cells, we tested the two possibilities: (1) Exogenous or endogenous BDNF influences the expression of PDZ proteins and (2) the increase in PDZ proteins leads to the stabilization of AMPA receptors through their mutual interactions. The potential contribution of BDNF activity to synaptic development and plasticity is also discussed.
Materials and methods
BDNF knockout mice and neocortical neuronal culture
Total protein was obtained from neocortices of six newborn wild-type mice and from six BDNF homozygous mutant littermates with a C57BL/6 background (Jackson Laboratory, Bar Harbor, Maine) and was used for immunoblotting as indicated below. For neuronal cultures, pregnant Sprague-Dawley rats were purchased from SLC Ltd. (Japan) and cerebral neocortices of day (d) 18 embryos were dissociated with papain (1 mg/ml) and plated onto poly-D-lysine-coated dishes at a cell density (500–800 cells/mm2). Cortical neurons were grown with serum-free Dulbecco’s modified Eagle medium (DMEM) containing nutrient mixture N2 (Narisawa-Saito et al., 1999a). This procedure reduced glial contamination to less than 5% of total cells (Narisawa-Saito et al., 1999a). Untreated control cultures or cultures that were supplemented daily with purified human recombinant BDNF (50 ng/ml; gift from Sumitomo Chemical Ltd., Tokyo, Japan) for 5 d were used for all the experiments (see below). Alternatively, long-term cortical culture containing a lower density of cells (100–200 cells/mm2) was maintained in N2-DMEM medium plus 5% calf serum.
RNA analysis
Total RNA was extracted from Sprague-Dawley rats (SLC, Shizuoka, Japan). PolyA + RNA were purified and denatured in 50% formamide, 6% formaldehyde, 20 mM 3-(N-morpholino) propanesulfonic acid buffer (pH 7.0), and 1 mM EDTA, separated on a 1.5% formaldehyde-agarose gel, and transferred onto a nylon membrane. A 32P-labeled cDNA probe to rat GluR1 (a 2666-nt EcoRI–EcoRI fragment), mouse GluR2 mRNA (a 699-nt ApaI–HindIII fragment), rat SAP97 (a 1915-nt AccI–SpeI fragment) rat PSD95 (a 2730-nt PCR product for the entire cording region), or rat GRIP1 (a 2511-nt PCR product for the entire cording region) was generated by using the Random-primed DNA labeling kit (Roche Diagnostics, Tokyo, Japan). The probe (2 × 106 cpm/ml) was hybridized to filters for 20 h at 42°C in 50% formamide, 5× SSC, 5× Denhardt’s solution, and 1% SDS followed by washing with 0.1× SSC, 0.1% SDS at 60°C and film exposure.
Reverse transcriptase–polymerase chain reaction (RT-PCR)
Using an RT-PCR High kit (Toyobo), cDNA fragments for SAP97, GRIP1, Pick1, and β-Actin were synthesized from total RNA and amplified within the following linear range amplification. The oligonucleotide primers were designed as follows: 5′-CTCAGAAGTTCCATAGAGCG-3′ and 5′-GCTTCTGTGGGCTTTATTGG-3′ generating a 407-bp fragment for SAP97 (30 cycles); 5′-ACCATGT-GAAAATTCAGAGG-3′ and 5′-ATTCCAAAGCCAGT-GACAGG-3′ generating a 415-bp fragment for GRIP1 (28 cycles); 5′-TGAGAAGTTCGGCATTCGGC-3′ and 5′-GCACACACCACTCTAGCCTA-3′ generating a 461-bp fragment for Pick1 (28 cycles); and 5′-CACAGCTGAG-AGGGAAATCG-3′ and 5′-CACACAGAGTACTTGCG-CTC-3′ generating a 348-bp product for β-Actin (24 cycles). The PCR products were electrophoresed through a 2% agarose gel, stained with a sensitive DNA dye, SyberGreen I (Molecular Probes), and imaged with a CCD camera (Cosmicar; Pentax).
Immunoprecipitation and immunoblotting
Cultured neurons or rat neocortical tissues were lysed with sample buffer [10% glycerol, 2% sodium dodecyl sulfate (SDS), 65 mM Tris–HCl, pH 7.5]. Alternatively, cultured neurons were lysed with immunoprecipitation buffer (10 mM Tris–HCl, 140 mM NaCl, 1 mM EDTA, and 1% deoxycholate, pH 7.5) supplemented with protease and phosphatase inhibitors (Wyszynski et al., 1999). Typically, 10–20 μg of anti-N-terminal GluR1 (Ibaraki et al., 1999), anti-N-terminal GluR2 (Chemicon), or anti-Pick1 (Iwakura et al., 2001) antibodies, which had been preadsorbed to Protein A/Protein G–Sepharose beads, were incubated with 200–400 μg of total cell lysate at 4°C overnight. Immuno-precipitated or total cellular proteins were denatured by boiling in 3 × sample buffer containing 0.1 M dithiothreitol for 5 min, separated by SDS-polyacrylamide gel electrophoresis (PAGE) with 7.5 or 10% polyacrylamide slab gels, and blotted to nitrocellulose membranes. The membranes were treated with primary antibodies (see below) at 4°C overnight. Immunoreactivity was detected by using goat anti-rabbit (DAKO, Kyoto, Japan) or goat anti-mouse (Jackson ImmunoResearch Laboratories) antibodies conjugated to peroxidase (diluted 1:10000) followed by chemiluminescence reaction (ECL kit; Amersham) combined with film exposure. Primary antibodies used in this study were as follows: anti-SAP97 monoclonal (1 μg/ml; StressGen; Muller et al., 1995), anti-GRIP1 (1 μg/ml; Upstate Biotechnology; Dong et al., 1999), anti-SAP102, anti-PSD-93 (both 1 μg/ml; Fukuya and Watanabe, 2000), anti-Pick1 (2 μg/ml; Iwakura et al., 2001), anti-PSD95/panPDZ antibodies (3 μg/ml; Transduction Laboratories; Sans et al., 2000), anti-C-terminal GluR1 (1 μg/ml; Chemicon), anti-GluR2/3 (1 μg/ml; Chemicon), and anti-β-Actin (1 μg/ml; Boehringer Mannheim) antibodies. The specificity of the immunoreactivity was determined by comparing their sizes with those of the reported molecular weights.
Culture immunostaining
Cultured neurons were washed with phosphate-buffered saline and fixed for 20 min with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.3). Neurons were immuno-stained with anti-SAP97 antibody (30 μg/ml; StressGen), anti-GRIP1 (10 μg/ml; Upstate Biotechnology), or anti-PSD95/panPDZ (5 μg/ml; Transduction Laboratories). Immunoreactivity was revealed by using the ABC-diamino-benzidine method (Vector) and visualized with the aid of a microscope (Axioskop; Carl Zeiss) fitted with an LCD color camera (DP50-CU; Olympus). All pictures were taken with a 20× objective, at 1/300 s shutter speed using Studio Lite software (Pixera Corp.).
Cultures maintained for 2 weeks were immunostained with anti-PSD95/panPDZ monoclonal antibody (5 μg/ml; Transduction Laboratories), the same rabbit anti-SAP97 antibody, anti-synaptophysin monoclonal (20 μg/ml; Sigma), and anti-synaptobrevin monoclonal (10 μg/ml; Wako Chemical) antibodies. Immunoreactivity was revealed by using anti-mouse immunoglobulin conjugated to Alexa 546 (red; Molecular Probes) and anti-rabbit immunoglobulin conjugated to Alexa 488 (green; Molecular Probes).
Lipofection into HEK293 cells
Full-length cDNAs for rat GluR1, rat GluR2 (Boulter et al., 1990), or rat GRIP1 (Dong et al., 1999) were subcloned into pCI mammalian expression vectors (Invitrogen) and introduced into HEK293 cells. A pCMV vector carrying the cDNA for rat SAP97 (Muller et al., 1995) was used for transfection as well (gift from Drs. Y. Hata and Y. Takai). HEK293 cells were plated onto plastic dishes (10 cm diameter) at a density of 500–1000 cell/mm2 24 h prior to their transfection. Transfection of these plasmid constructs was performed by lipofection with FuGENE 6 (Roche).
Infection of Sindbis virus vector carrying GluR1- and GluR2-carboxyl terminal decoys
The modified enhanced green fluorescent protein (EGFP) that carried either the carboxyl terminal sequence of GluR1 (SSGMPLGATGL; Leonard et al., 1998) or that of the short C-terminal isoform of GluR2 (GYNVYGIESVKI; Kohler et al., 1994) was constructed as described previously (Iwakura et al., 2001). Viral genome RNAs were transcribed in vitro by SP6 RNA polymerase using the Invitroscript CAP kit (Invitrogen) and cotransfected with mRNAs coding viral coat proteins into baby hamster kidney (BHK) cells. The infectivity of the Sindbis virus particles was titered with BHK cells by serial dilution. Typically, a viral titer of greater than 107 pfu/ml was obtained.
Cultured neocortical neurons were exposed to the Sindbis viral vectors at a titer of greater than 107 pfu/ml for 1 h. After washing, the neurons were incubated in regular growth medium at 37°C for 24 h in the presence or absence of BDNF. The expression of EGFP was monitored with the aid of a fluorescence microscope. At the viral titer used for infection, more than 90% of neurons expressed green fluorescence (Iwakura et al., 2001). The BDNF effects on PDZ proteins and AMPA receptors were determined by immunoprecipitation and immunoblotting as described above.
Statistical analysis
The Student’s t-test was used to analyze differences in immunoreactivity when the number of samples per set included more than three. Alternatively, the set of control and experimental group(s) covered multiple independent experiments done at distinct periods of time, comparisons between BDNF-treated or control groups were made with the Mann-Whitney U-test. A single developmental curve of mRNA expression was compared with the standard distribution of the data set of protein expression. Results were expressed as means ± standard deviation (SD), and the number of similar experiments is indicated in each figure legend.
Results
Developmental regulation of PDZ proteins and their associating AMPA receptor subunits
Among many PDZ proteins, SAP97 specifically interacts with the C-terminal tail of GluR1 (Leonard et al., 1998), whereas GRIP1, GRIP2/ABP, and Pick1 bind to the tails of GluR2/3 subunits (Kohler et al., 1994; Srivastava et al., 1998; Dev et al., 1999; Dong et al., 1999; Wyszynski et al., 1999; Xia et al., 1999). On the other hand, SAP102, PSD-95, and PSD-93/Chapsin-110 associate with the NMDA receptor subunits (Kornau et al., 1995; Lau et al., 1996; Muller et al., 1996). How do their interactions influence their expression, stability, and functions during brain development?
In developing rat neocortex, we determined the protein expression of AMPA receptor subunits, GluR1 and GluR2/3, and their partner PDZ proteins, SAP97, GRIP1, and PSD95/panPDZ to compare with their regulation at the mRNA level (Fig. 1). Immunoblotting revealed that most of these postsynaptic proteins were expressed from late embryonic stages, before synaptic formation or maturation. Protein levels for GluR1 and GluR2/3 were first detected at E16–E18, when neurons underwent the final mitosis and migrated to the cortical plate, and gradually increased when cortical neurons actively form many synapses until P20–P30. A similar prenatal and postnatal developmental increase was seen in these PDZ proteins. The antibody directed against PSD-95 recognized homologous PDZ proteins (also referred to as panPDZ antibody). GRIP1 protein and PSD95/panPDZ-like immunoreactivity were detectable as early as E14 in rat cortex. The developmental patterns of their mRNA expressions were determined by RNA blotting to compare with those of the proteins (Fig. 2A). GluR1 and GluR2 mRNAs exhibited a relatively sharp increase around birth (P0). The developmental increase in GluR1 mRNA significantly preceded that in GluR1 protein, whereas the developmental change in GluR2 mRNA partially disagreed but almost paralleled with that in GluR2/3 protein (Fig. 2B). Similar to GluR1 mRNA, mRNA levels for GRIP1 and PSD95 markedly increased between E14 and E19 or between E19 and P7, and gradually decreased in the later stages of development. The apparent discordance between mRNA and protein levels suggest that the developmental regulation of the postsynaptic proteins may also involve a posttranscriptional regulatory mechanisms such as synaptic stabilization.
Fig. 1.

Developmental regulation of AMPA receptor subunits and PDZ proteins. Relative protein levels for GluR1, GluR2/3, GRIP1, SAP97, and PSD95 (panPDZ) were determined in rat neocortex by immunoblotting. Rat cerebral cortex was isolated from rats at various ages and total protein was extracted. Protein (40 μg) was separated on a 7.5% SDS-PAGE and blotted onto a nitrocellulose membrane. Lanes correspond to embryonic days (E16 and E18) and postnatal days (P0, P5, P10, P20, P30, and P60).
Fig. 2.
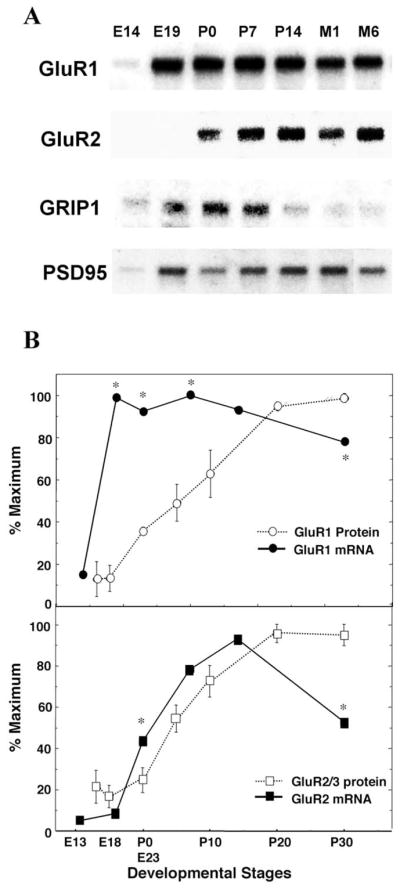
mRNA expression of AMPA receptors and PDZ proteins during rat neocortical development. (A) Brain mRNA levels for GluR1, GluR2, GRIP1, and PSD95 were examined during rat development by RNA blotting. Rat cerebral cortex was isolated from rats at various ages, and total RNA was extracted. PolyA + RNA (1.8 μg) was separated on a 1.5% formamide agarose gel and blotted onto a nylon membrane. Lanes correspond to embryonic days (E14 and E19), postnatal days (P0, P7, and P14), and postnatal months (M1 and M6) from left to right. (B) mRNA levels for GluR1 and GluR2 were determined by densitometric analysis of the blots and compared with their protein levels. The protein levels were similarly measured by three independent immunoblots as shown in Fig. 1. *P < 0.05, out of ±2 SD range.
BDNF increases the expression of AMPA receptor-associated PDZ proteins
Cultured neocortical neurons were prepared from embryonic day 18 rats and grown in a serum-free condition for 5 days in the presence or absence of BDNF to examine its influences on PDZ proteins and their mRNAs (Fig. 3). The presence of BDNF made no difference in cell densities in insulin-enriched serum-free culture as reported previously (see details in Fig. 5 legend) (Narisawa-Saito et al., 1999a). Immunoblotting of culture lysates revealed that BDNF significantly enhanced the expression of SAP97, GRIP1, and Pick1 in cortical neurons. In addition, the intensity of bands immunoreactive for the anti-PSD95/panPDZ antibody (83–110 kDa), which recognizes PSD-95 and crossreacts with common PDZ domains, was increased by chronic BDNF stimulation. BDNF effects on PDZ proteins were not blocked by glutamate receptor antagonists (CNQX, AP-5; data not shown). In contrast, BDNF had no effects on SAP102 and PSD-93. Protein levels for β-actin were not influenced, either. The ineffectiveness of BDNF on SAP102 and PSD-93 suggests that the above BDNF activity did not represent a general neurotrophic function on neurons and synaptic molecules. Quantification of mRNA had slightly different results, however: The effects of BDNF at the PDZ protein level were reflected by an increase in GRIP1 and Pick1 mRNAs but not by that of SAP97. BDNF had no effects on β-actin mRNA. Consistent results were obtained when the amounts of RT-PCR products were normalized with an internal control of β-actin mRNA (data not shown). These observations suggest that BDNF specifically enhances the protein expression of the AMPA receptor-associated PDZ molecules, SAP97, GRIP1, and Pick1, although the mechanism underlying the BDNF regulation of GRIP1 and Pick1 is apparently different from that affecting SAP97.
Fig. 3.
BDNF differentially regulates the expression of various PDZ proteins. Neocortical neurons were cultured in serum-free medium in the presence or absence of BDNF (50 ng/ml) for 5 days. Total cellular protein was collected and processed for immunoblotting. (A) Typical examples of immunoblotting are displayed. (B) The blotting results of similar culture experiments (four cultures and blots with duplicated lanes; total n = 8) were quantified by densitometry and plotted. (C) cDNA bands amplified by RT-PCR for the indicated molecules showed that BDNF chronic application resulted in increased signals for GRIP1 and Pick1. The same treatment did not modify SAP97 nor β-Actin mRNAs. “−” represents PCR reaction without mRNA as a negative control. mRNA was derived from two independent cultures. **P < 0.005 with the Mann-Whitney U-test.
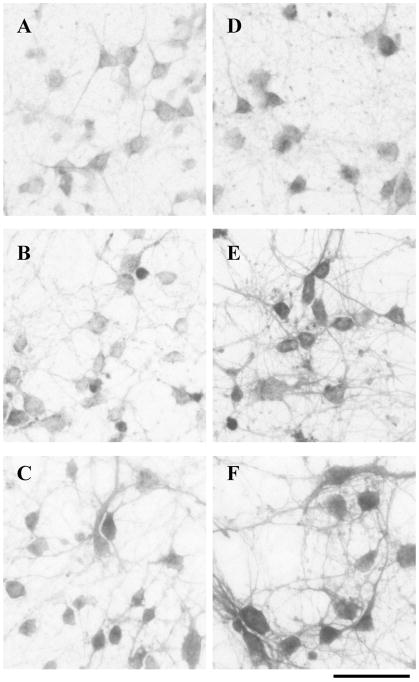
Fig. 5.
BDNF enhanced PDZ protein immunoreactivity in cultured neocortical neurons. Control (A–C) or BDNF-treated (D–F) neocortical neurons in culture were fixed and incubated with anti-SAP97 antibody (A, D), anti-GRIP1 antibody (B, E), or anti-PSD95/panPDZ antibody (C, F). Immunoreactivity was visualized with the DAB-peroxidase technique. Chronic incubation with BDNF (50 ng/ml daily for 5 days) significantly enhanced the staining with the three antibodies. Cell density was 3500 ± 400 cells/mm2 in control culture and 3100 ± 300 cells/mm2 in BDNF-treated culture. Scale bar, 30 μm.
The BDNF knockout mice were used to validate the in vitro effects of BDNF on PDZ proteins in vivo. Total protein was extracted from the neocortex of the homozygous mutants and control mice on postnatal day 1 and subjected to immunoblotting for SAP97, GRIP1, and Pick1 (Fig. 4). BDNF gene disruption reduced the levels of SAP97, GRIP1, and Pick1 proteins in the neocortex. In contrast, β-actin protein levels were not affected by the absence of BDNF. These observations suggest that the neurotrophin regulates the expression of this particular set of PDZ proteins during development.
Fig. 4.
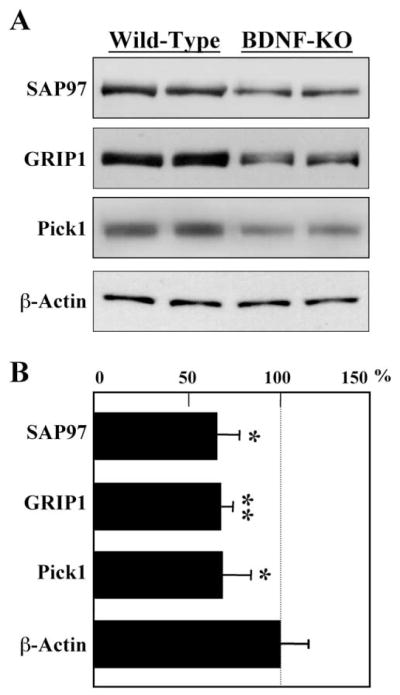
Impaired expression of PDZ proteins in the neocortex of BDNF-gene knockout mice. On postnatal day 1 the neocortex was dissected from homozygous BDNF knockout mice and from wild-type littermates. SAP97, GRIP1, and Pick1 protein levels were analyzed by immunoblotting. (A) The expression of β-actin was not affected by the lack of BDNF. (B) Total levels of PDZ proteins were quantified by densitometry and compared in the wild type and BDNF-homozygous mutant mice (n = 6 mice, each). *P < 0.05; **P < 0.005 with the Mann-Whitney U-test.
Enhanced immunoreactivity for the PDZ proteins in BDNF-treated neocortical culture
Immunostaining of cultured neurons confirmed the effects of BDNF on SAP97 and GRIP1 proteins, as well as on PDZ proteins reactive to the anti-PSD95/panPDZ antibody (Fig. 5). BDNF-treated cultures had enhanced SAP97-like and GRIP1-like immunoreactivities, compared with controls (Fig. 5A and D, and B and E, respectively). Immuno-reactivity to the anti-PSD95/panPDZ antibody had a similar BDNF-dependent intensification (Fig. 5C and F) and reflected the increase detected with immunoblotting. The neuronal populations immunoreactive to each individual antibody seem to have different cell morphologies, despite some overlap. For instance, the anti-SAP97 and anti-PSD95/panPDZ antibodies stained a heterogeneous cell population with a predominance of large cells (mean longer axis; 14 ± 2.3 μm). Conversely, the vast majority of cells with strong immunoreactivity to the anti-GRIP1 antibody were smaller (mean longer axis; 8.5 ± 2.1 μm).
The effects of BDNF on the expression of PDZ proteins in neocortical cultures having more developed synapses were evaluated as well (Fig. 6). The neocortical cultures were maintained for 2 weeks in the presence or absence of BDNF. The expression of PDZ proteins was assessed by using the anti-SAP97 antibody as well as the anti-PSD95/panPDZ antibody. BDNF treatment influenced the PSD95/panPDZ immunoreactivity along the dendrites. Punctate staining for the PSD95/panPDZ antibody was more frequent and stronger along the dendrites of BDNF-treated neurons. The number of strong PSD95/PDZ-positive spots was 730 ± 140 spots/mm dendrite in the BDNF-treated culture and 60 ± 8 spots/mm dendrite in control, respectively (P < 0.001). In contrast, there was no apparent difference in SAP97 immunoreactivity in this long-term cortical culture. The number of presynaptic sites that were marked with anti-synaptobrevin and anti-synaptophysin monoclonal antibodies was similar between the cultures (see Fig. 6 legend). This result indicates that the BDNF activity might have different time windows to regulate the individual AMPA receptor-associated PDZ proteins.
Fig. 6.
Effects of BDNF on the immunoreactivity for PDZ proteins in mature cortical culture. Lower density neocortical cultures were grown for 2 weeks with or without BDNF. After fixation, immunoreactivity for the anti-PSD95/panPDZ antibody was visualized with Alexa 546-conjugated secondary antibody (red) (A). (B) Immunostaining of presynaptic structures was marked with the mixture of anti-synaptobrevin and anti-synaptophysin monoclonal antibodies followed by the Alexa 546-conjugated secondary antibody (red). Immunostaining for SAP97 was simultaneously visualized with the Alexa 488-conjugated secondary antibody (green). Dendritic processes were randomly pictured (n = 15 each for A and B) and immunoreactive spots along dendritic processes were counted. Densities of SAP97-positive spots and synaptophysin/synaptobrevin-positive terminals were 243.4 ± 71.0 and 295.4 ± 102.8 spots/mm dendrite in the BDNF-treated culture and 289.2 ± 114.8 and 245.2 ± 118.6 spots/mm dendrite in control, respectively; P = 0.67 for SAP97-positive spots and 0.83 for synaptophysin/synaptobrevin-positive spots. Pictures of dendrites having higher immunoreactivities were chosen for display (A; n = 5 each, B; n = 2 each). Scale bar, 10 μm.
BDNF elevates the amounts of SAP97 and GRIP1 associating with AMPA receptor subunits
The BDNF-enhanced PDZ proteins, SAP97, GRIP1, and Pick1, were identical to those that are known to interact with the AMPA receptor subunits (Leonard et al., 1998; Dev et al., 1999; Dong et al., 1999; Iwakura et al., 2001). Does the BDNF-triggered increase in the PDZ proteins influence their association with the AMPA receptors and stabilize the AMPA receptor expression? To study the secondary effects of BDNF on AMPA receptors via PDZ proteins, culture lysates were subjected to immunoprecipitation to examine the interaction between GluR1 and SAP97, GluR2/3 and GRIP1, or Pick1 and GluR2/3 (Fig. 7). BDNF elevated the interaction between GluR1 and SAP97 as well as between GluR2 and GRIP1. In contrast, Pick1 association with GluR2/3 was not altered by chronic incubation of the neurons with BDNF, even though the incubation did indeed upregulate Pick 1 expression. Thus, chronic BDNF treatment also elevated total amounts of the interaction of SAP97 and GRIP1 with the AMPA receptors. The specificity of the combination having the enhanced interactions implies that the increase in total amounts of these two classes of molecules may not simply result in their mutual interaction, however. Accordingly, we cannot rule out the possibility that BDNF might enhance the affinity of their interaction as well.
Fig. 7.

BDNF enhanced the association of GluR1 and GluR2/3 with SAP97 and GRIP1 in neocortical neuronal cultures. Neurons were incubated, with or without BDNF (50 ng/ml), for 5 days. Protein samples were 1% DOC-extracted and used for coimmunoprecipitation (co-IP). Co-IP of SAP97 with anti-N-terminal GluR1 antibody (A), GRIP1 with anti-N-terminal GluR2 antibody (B), or GluR2/3 with anti-Pick1 antibody (C) revealed that only SAP97-GluR1 and GRIP1-GluR2/3 interactions were enhanced by BDNF in the treated cultures. To control the above co-IP reaction, immunoblots were reprobed with anti C-terminal GluR1 and anti C-terminal GluR2/3 antibodies (lower panels in A and B). Because of the similarity in the molecular sizes of Pick1 and the immunoglobulin heavy chain, the co-IP reaction was started with the anti-Pick1 antibody and immunoblotting was carried out with anti-GluR2/3. In addition, total cellular lysates were immunoblotted with anti-Pick1 antibody, which revealed that Pick1 protein was enhanced by BDNF (C). Each lane represents independent sister culture.
Disruption of GluR1 and GluR2 interactions with their partner PDZ proteins is detrimental to the BDNF-dependent increase in the AMPA receptors
Sindbis viruses carrying the cDNA of EGFP alone or that of EGFP attached to either the C-terminal decoy of GluR1 (11 amino acids; EGFP-R1) or that of the short isoform of GluR2 (12 amino acids; EGFP-R2) (Kohler et al., 1994) were used to perturb the endogenous interactions between the AMPA receptor subunits and the PDZ proteins (Iwakura et al., 2001). These C-terminal decoys code for the amino acid sequences that are involved in the interaction of GluR1 with SAP97 (Leonard et al., 1998) or GluR2 with GRIP1 (Dev et al., 1999; Dong et al., 1999) and Pick1 (Iwakura et al., 2001). The interaction with these PDZ proteins is implicated in trafficking and stability of AMPA receptors (Kohler et al., 1994; Srivastava et al., 1998; Dev et al., 1999; Dong et al., 1999; Wyszynski et al., 1999; Xia et al., 1999). Therefore, disruption of such interactions might clarify the role of BDNF on the increase in AMPA receptor proteins.
The viral transcripts carrying these decoys were synthesized in vitro by T4 RNA polymerase and transfected into cultured neocortical neurons (Fig. 8A and B). The individual expression of native GluR1 and GluR2/3 proteins was examined by immunoblotting (Fig. 8C and D, respectively). Infecting neocortical cultures with the control EGFP vector alone did not inhibit the BDNF-dependent increase in GluR1 and GluR2/3 proteins (Fig. 8E and F). Infection with the EGFP-R1 virus and the resultant overexpression of the GluR1 C-terminal decoy counteracted the BDNF-mediated increase in native GluR1 (Fig. 8E), while the BDNF-dependent increase was still detectable in GluR2/3 (Fig. 8F). Similarly, EGFP-R2 infection led to its overexpression but decreased the basal expression of GluR2/3 protein (Fig. 8F). It also blocked the BDNF-dependent increase in GluR2/3. In agreement with the results from both decoys, they indeed diminished the interactions between GluR1 and SAP97 and between GluR2/3 and GRIP1 in the presence of BDNF (Fig. 8G). Interestingly, EGFP-R2 decoy expression appeared to counteract the BDNF-triggered increase in native GluR1 as well (Fig. 8C and E). These results indicate that the competing decoys were sufficient to reverse the BDNF-mediated increase in GluR1 and GluR2, although the influence of each AMPA receptor decoy differed slightly on each subunit, presumably because it depends on the subunit composition of the AMPA receptor complexes in the infected neocortical neurons (Kondo et al., 1997). These findings support the hypothesis that the BDNF-triggered total protein increase in AMPA receptors involves their molecular interaction via their carboxyl termini with PDZ proteins.
Fig. 8.

Overexpression of GluR1 and GluR2 C-terminal decoys disrupted the BDNF-mediated increase in AMPA receptors. Neocortical neurons were cultured for 4 days and then incubated with (+) or without (−) BDNF for the last 24 h before harvesting. The shorter BDNF treatment was still able to mimic a similar magnitude of the protein increase in AMPA receptors. During the incubation with BDNF, neurons were infected with the Sindbis viruses coding for EGFP alone, EGFP-R1 (A), or for EGFP-R2 (B) (Iwakura et al., 2001) to study the consequences of disrupting AMPA receptors’ interaction with their associated PDZ proteins. At the viral titre used (> 1 × 107), more than 90% of the cells were infected, as monitored by EGFP fluorescence (Iwakura et al., 2001). The control EGFP construct was essentially the same as the EGFP-R1 and EGFP-R2 constructs but lacked the decoy peptides. (A and B) nsp, nonstructural proteins; psg, subgenomic promoter; and poly A; poly-adenylated transcript tail. Hatched boxes fused to the EGFP represent the C-terminal decoys of GluR1 and GluR2 (short splice variant). (C) Probing with anti-C-terminal GluR1 revealed the native GluR1 band as well as a lower band corresponding to the expected size of EGFP-R1 fusion protein (28 kDa). (D) Similarly, the BDNF-induced increase in GluR2/3 protein was challenged by the overexpression of EGFP-R2 fusion protein. Statistical analysis of the GluR1 (E) and GluR2/3 (F) immunoreactivities between pairs of BDNF-plus and BDNF-minus cultures (n = 3). GluR1 or GluR2/3 immunoreactivity in virus-free control culture was set as 100%. Student’s t-test was applied to comparison between BDNF-plus and BDNF-minus cultures (*P < 0.05; **P < 0.01) or between the cultures transfected with EGFP-R2 and EGFP alone (+P < 0.05). (G) The overexpression of EGFP-R1 and EGFP-R2 decoys reduced the BDNF-stimulated interaction between GluR1 and SAP97 and between GluR2/3 and GRIP1, respectively, compared with that in the EGFP overexpression. Cell lysates were prepared from infected neocortical cultures and treated with anti-N-terminal GluR1 antibody or anti-N-terminal GluR2 antibody to immunoprecipiate the complexes of AMPA receptors and PDZ protein(s).
Coexpression of GluR1 and SAP97 elevates their protein levels in HEK293 cells
The previous lines of evidence suggest that BDNF increases the expression of AMPA receptor-associated PDZ proteins and then elevates the amount of their mutual interaction leading to receptor protein stabilization (Narisawa-Saito et al., 1999a,b). To confirm the significance of these interactions, we established a heterologous experimental system in the human kidney cell line, HEK293: cDNA for GluR1 or the short C-terminal isoform of GluR2 (Kohler et al., 1994) alone, or together with that of SAP97 or GRIP1 was transduced by lipofection and all were transiently expressed under the cytomegalovirus promoter (Fig. 9). Following the transient transfection, the magnitudes of their protein levels were examined by immunoblotting. Cotransfection of GluR1/SAP97 or GluR2/GRIP1 was compared with the single transfection of GluR1 or GluR2.
Fig. 9.
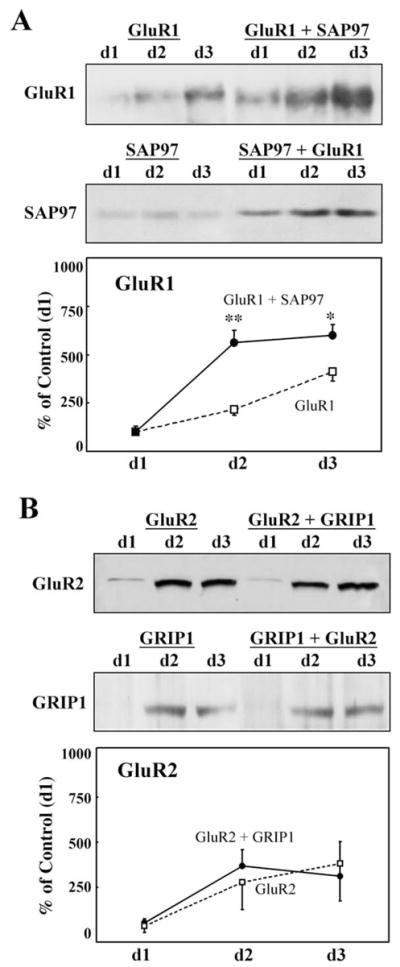
Effects of coexpression of SAP97 and GRIP1 proteins on AMPA receptor levels in HEK293 cells. cDNA for GluR1 or GluR2 in expression vector plasmids carrying the CMV promoter was transfected into human embryonic kidney cells, HEK293, together with that for SAP97 or GRIP1, respectively. Their transient expressions were monitored on days 1, 2, and 3 by immunoblotting. cDNAs used in each experiment are indicated on top of each blot. Three independent experiments were performed, and protein levels for GluR1 or GluR2 were quantified by densitometry for statistical analysis. Significant interaction between GluR1 and SAP97, or GluR2 and GRIP1, was indeed detected in the immunoprecipiates with the anti-N-terminal GluR1 antibody or the anti-N-terminal GluR2 antibody, respectively (data not shown). Results were obtained from three sets of cultures (n = 3). *P < 0.05; **P < 0.005 with paired Student’s t-test.
The cotransfection of GluR1 cDNA and SAP97 cDNA markedly enhanced GluR1 protein levels in comparison with those obtained with GluR1 cDNA transfection alone (Fig. 9A), and this increase was detected over the period of 3 days following transfection. Protein expression of SAP97 itself was also enhanced in the presence of GluR1. In contrast, the cotransfection of GluR2 cDNA and GRIP1 cDNA over the same period of time promoted neither the protein expression of GluR2 nor that of GRIP1 (Fig. 9B). The molecular behaviors of the AMPA receptors in the nonneuronal cells suggest that the presence and interaction of SAP97 facilitate the expression of the AMPA receptor subunit.
Discussion
Developmental regulation of PDZ protein expression
Postsynaptic density proteins with PDZ domains have been intensively studied in the context of synaptic plasticity in mature brain. However, some PDZ proteins are expressed in immature embryonic neurons (Dong et al., 1999; Osten et al., 2000) and some interact with cell adhesion molecules, such as neuroligins (Irie et al., 1997), vascular cell adhesion molecule, and neuron-specific synaptic scaffolding molecule (Ide et al., 1998). Moreover, recent studies indicate a role for PDZ proteins in the regulation of neuronal differentiation and synaptic development (El-Husseini et al., 2000; Penzes et al., 2001). In contrast to a large variety of molecular investigations on PDZ proteins, information on their developmental contribution is still limited. In the present study, the expression of several PDZ proteins begins during the embryonic development of the rat neocortex. Their binding partners, AMPA receptor proteins, display a similar developmental pattern of their expression. The present results reveal a novel role of BDNF in the developmental regulation of these PDZ proteins as well as in that of AMPA receptor proteins.
The developmental patterns of AMPA receptor protein expressions partially differ from those of their mRNA levels. For example, the developmental increase in GluR1 proteins markedly lagged behind that of its mRNA. The discordance between protein and mRNA levels of the postsynaptic proteins is also evident in previous literatures (Durand and Zukin, 1993; Arai et al. 1997). Both exogenous or endogenous BDNF not only upregulate the expression of PDZ proteins but also secondarily stabilize that of AMPA receptor subunits through their interaction with those PDZ proteins. Thus, the above developmental discordance between their proteins and mRNAs might also be ascribed to the secondary BDNF action through its regulation of PDZ proteins. The BDNF effects might not be limited to the above AMPA receptor-associating PDZ proteins but could also include potentially unidentified ones immunoreactive for the PSD95/panPDZ antibody (Kitano et al., 2002).
Postsynaptic effects of BDNF
BDNF has been implicated in dendritic growth as well as in synapse formation (Causing et al., 1997; McAllister et al., 1997; Kong et al., 2001), suggesting a potential influence on postsynaptic proteins. Chronic BDNF effects involve various types of intra- and intercellular molecules (Mizuno et al., 1994; Takei et al., 1997; Narisawa-Saito et al., 1999a; Xiong et al., 1999). The present results implicate a new series of molecules, namely the AMPA receptor-associated PDZ proteins, and suggest a molecular mechanism that might underlie our previous finding: BDNF enhances the protein expression of GluR1 and GluR2/3 without altering their mRNA levels (Narisawa-Saito et al., 1999b). Although BDNF is known to influence synaptic activity (Kang and Schuman, 1995; Figurov et al., 1996; Korte et al., 1996; Patterson et al., 1996) and neuronal translation rates (Takei et al., 2001), both processes appeared not to contribute to the AMPA receptor increase. First, BDNF failed to further elevate translation rates in the present culture condition containing high amounts of insulin (N.T., unpublished data). Secondly, the BDNF effects on PDZ proteins were resistant against glutamate receptor antagonists (CNQX, AP-5; data not shown), eliminating the possibility that the PDZ protein increase required endogenous synaptic activity. Furthermore, this explanation is also supported by two distinct approaches, the viral perturbation experiments and the reconstitution of the phenomenon in nonneuronal cells. Disruption of the interaction with GluR decoys inhibited not only the interactions between GluR1 and SAP97 and between GluR2/3 and GRIP1 but also the BDNF-triggered AMPA receptor increases. Transient coexpression of SAP97 together with GluR1 in HEK 293 cells resulted in their mutual interaction in parallel with the elevation in GluR1 protein. A similar scenario might be applicable to the BDNF-dependent upregulation of expression of other binding partner(s) for the above PDZ molecules. Thus, impaired learning ability of BDNF/TrkB knockout mice or BDNF/TrkB transgenic mice might also involve developmental deficits in these postsynaptic molecules or in such postsynaptic regulation (Linnarsson et al., 1997; Croll et al., 1999; Minichiello et al., 1999; Saarelainen et al., 2000; Xu et al., 2000).
Carboxyl terminal domains of AMPA receptors are important for stabilization
The perturbation experiments using Sindbis viruses carrying the GluR1 and GluR2 C-terminal decoys revealed the molecular significance of these C-terminal domains in the expression and stabilization of the AMPA receptors in neurons. Disrupting their binding with Sindbis viruses carrying the GluR1 and GluR2 C-terminal decoys inhibited the BDNF-mediated enhancement of their individual expression.
The overexpression of GluR2 C-terminal decoys resulted in marked reduction in basal GluR2 expression. In addition, the coexpression of GRIP1 and the AMPA receptors failed to stabilize the receptors in HEK293 cells, although GRIP1 binds to GluR2/3 subunits and is suggested to stabilize the AMPA receptor subunits in a phosphorylation-independent manner (Dong et al., 1999; Wyszynski et al., 1999; Matsuda et al., 2000; Osten et al., 2000). The difference between the results from cortical neurons and HEK293 cells could be ascribed to the lack of essential component(s) for the PDZ protein(s) or for its posttranslational modification in the nonneuronal cells (Craven et al., 1999; Chung et al., 2000; Daw et al., 2000). In fact, it has already been suggested that the cellular and molecular behaviors of individual AMPA receptor complexes are governed by a variety of PDZ proteins, by their specific cellular environment, as well as by other partner molecules (Shi et al., 2001; McLean-Bolton et al., 2000). Apparently, BDNF could contribute to this phenotypic difference in the relationship between these PDZ proteins and their interacting individual AMPA receptor subunits. This implies the existence of distinct regulatory mechanisms leading to unique responses to BDNF depending on the subunit composition of the complexes and the expression of various PDZ proteins in different neuronal populations.
The molecular interaction between the GluR1 C-terminal motif with partner PDZ proteins, potentially with SAP97, can lead to the translocation of the GluR1-carrying AMPA receptor complexes to postsynaptic compartments (Hayashi et al., 2000; Shi et al., 2001). In fact, SAP97 protein associates with GluR1 in the intracellular biosynthetic membranes during development but is distributed at the postsynaptic sites in mature synapses (Valtschanoff et al., 2000; Sans et al., 2001). Consistent with these observations, immunoreactivity for SAP97 was more distributed in somatic regions rather than in dendrites in young neocortical cultures. In mature culture, however, strong immunoreactivity for SAP97 appeared as spots along the dendrites, presumably marking postsynaptic sites. The ultrastructural distribution of the BDNF-affected PDZ proteins needs to be characterized at the electron microscopic level, however.
Our latest study indicates that the acute stimulation of BDNF rapidly induces surface translocation of GluR2 subunit through its interaction with N-ethylmaleimide-sensitive factor but not with PDZ protein (Narisawa-Saito et al., 2002). Accordingly, the chronic BDNF stimulation might trigger both processes involving GluR1 and GluR2 to aid the synaptic translocation and stabilization of the PDZ molecule–AMPA receptor complexes. Recently, Chetkovich et al. (2002) found that another PDZ molecule, PSD-95, indirectly interacts with GluR1 via Stargazin to translocate it to the postsynaptic sites. In this context, we cannot deny the possibility that PSD-95 might also contribute to the above process, although we failed to obtain consistent effects of BDNF on this PDZ molecule. BDNF is released from nerve terminals and acts on postsynaptic sites (Griesbeck et al., 1999; Kohara et al., 2001) to translocate AMPA receptors (Narisawa-Saito et al., 2002) and enhance the expression of their associating PDZ proteins. Accordingly, the local action of BDNF presumably contributes to selective postsynaptic development.
Differential regulation of postsynaptic differentiation and/or maturation by neurotrophic molecules
Many neurotrophic factors and cytokines regulate neuronal differentiation and development (Thoenen, 2000; Poo, 2001). The members that regulate postsynaptic structure and/or phenotype, however, have been poorly characterized. Neuregulins are important effectors in synaptic development in the brain. These factors regulate synaptic expression of alpha acetylcholine receptor subunits in the periphery and influence the alpha 7 subunit and NMDA receptors in the brain (Ozaki et al., 1997; Rieff et al., 1999; Liu et al., 2001).
In neuronal cultures, BDNF had a much greater effect, compared with nerve growth factor and neurotrophin-3, on the expression of both AMPA-type glutamate receptor subunits (Narisawa-Saito et al., 1999a) and PDZ proteins (unpublished data). Conversely, basic fibroblast growth factor downregulates the expression of a different subset of PDZ proteins (Jourdi and Nawa, 2002). All the above observations led us to presume that many neurotrophic factors and cytokines can influence the expression of various PDZ proteins during brain development and could contribute to the activity-dependent glutamate receptor expression as well (Gold et al., 1996). There is a distinct distribution of neurotrophic factor and cytokine receptors in the brain. Therefore, it will be interesting to elucidate whether and how these neurotrophic molecules differentially affect PDZ proteins, contributing to the divergence in individual synaptic functions and/or their development.
Acknowledgments
We thank Dr. S. Heinemann for GluR cDNAs, Drs. Y. Hata and Y. Takai for SAP97 plasmid, Dr. O. Hanyu for advice, Mr. K. Araki, Mrs. E. Higuchi, and Ms. K. Ishii for technical assistance, and Sumitomo Pharmaceuticals for rec BDNF. The preliminary form of the present study was shown in Soc. Neurosci. Abstr. (2001). Supported by: The Japanese Society for the Promotion of Science (RFTF-96L00203) and Grant-in-Aid for Creative Scientific Research.
References
- Aoki C, Wu K, Elste A, Len Gw, Lin Sy, McAuliffe G, Black IB. Localization of brain-derived neurotrophic factor and TrkB receptors to postsynaptic densities of adult rat cerebral cortex. J Neurosci Res. 2000;59:454–463. doi: 10.1002/(SICI)1097-4547(20000201)59:3<454::AID-JNR21>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Arai Y, Mizuguchi M, Takashima S. Developmental changes of glutamate receptors in the rat cerebral cortex and hippocampus. Anat Embryol (Berl) 1997;195:65–70. doi: 10.1007/s004290050025. [DOI] [PubMed] [Google Scholar]
- Boulter J, Hollmann M, O’Shea-Greenfield A, Hartley M, Deneris E, Maron C, Heinemann S. Molecular cloning and functional expression of glutamate receptor subunit genes. Science. 1990;249:1033–1037. doi: 10.1126/science.2168579. [DOI] [PubMed] [Google Scholar]
- Bruckner K, Pablo Labrador J, Scheiffele P, Herb A, Seeburg PH, Klein R. EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron. 1999;22:511–524. doi: 10.1016/s0896-6273(00)80706-0. [DOI] [PubMed] [Google Scholar]
- Causing CG, Gloster A, Aloyz R, Banji SX, Chang E, Fawcett J, Kuchel G, Miller FD. Synaptic innervation density is regulated by neuron-derived BDNF. Neuron. 1997;18:257–267. doi: 10.1016/s0896-6273(00)80266-4. [DOI] [PubMed] [Google Scholar]
- Chetkovich DM, Chen L, Stocker TJ, Nicoll RA, Bredt DS. Phosphorylation of the Postsynaptic Density-95 (PSD-95)/Discs Large/Zona Occludens-1 binding site of Stargazin regulates binding to PSD-95 and synaptic targeting of AMPA receptors. J Neurosci. 2002;22:5791–5796. doi: 10.1523/JNEUROSCI.22-14-05791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci. 2000;20:7258–7267. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven SE, El-Husseini AE, Bredt DS. Synaptic targeting of the postsynaptic density protein PSD-95 mediated by lipid and protein motifs. Neuron. 1999;22:497–509. doi: 10.1016/s0896-6273(00)80705-9. [DOI] [PubMed] [Google Scholar]
- Croll SD, Suri C, Compton DL, Simmons MV, Yancopoulos GD, Lindsay RM, Wiegand SJ, Rudge JS, Scharfman HE. Brain-derived neurotrophic factor transgenic mice exhibit passive avoidance deficits, increased seizure severity and in vitro hyperexcitability in the hippocampus and entorhinal cortex. Neuroscience. 1999;93:1491–1506. doi: 10.1016/s0306-4522(99)00296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw MI, Chittajallu R, Bortolotto ZA, Dev KK, Duprat F, Henley JM, Collingridge GL, Isaac JT. PDZ proteins interacting with C-terminal GluR2/3 are involved in a PKC-dependent regulation of AMPA receptors at hippocampal synapses. Neuron. 2000;28:873–886. doi: 10.1016/s0896-6273(00)00160-4. [DOI] [PubMed] [Google Scholar]
- Dev KK, Nishimune A, Henley JM, Nakanishi S. The protein kinase C alpha binding protein PICK1 interacts with short but not long form alternative splice variants of AMPA receptor subunits. Neuropharmacology. 1999;38:635–644. doi: 10.1016/s0028-3908(98)00230-5. [DOI] [PubMed] [Google Scholar]
- Dong H, Zhang P, Song I, Petralia RS, Liao D, Huganir RL. Characterization of the glutamate receptor-interacting proteins GRIP1 and GRIP2. J Neurosci. 1999;19:6930–6941. doi: 10.1523/JNEUROSCI.19-16-06930.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand GM, Zukin RS. Developmental regulation of mRNAs encoding rat brain kainate/AMPA receptors: a northern analysis study. J Neurochem. 1993;61:2239–2246. doi: 10.1111/j.1471-4159.1993.tb07465.x. [DOI] [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- Fanning AS, Anderson JM. Protein modules as organizers of membrane structure. Curr Opin Cell Biol. 1999;11:432–439. doi: 10.1016/S0955-0674(99)80062-3. [DOI] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Fukuya M, Watanabe M. Improved immunohistochemical detection of postsynaptically located PSD-95/SAP90 protein family by protease section pretreatment: a study in the adult mouse brain. J Comp Neurol. 2000;426:572–586. [PubMed] [Google Scholar]
- Gold SJ, Hennegriff M, Lynch G, Gall CM. Relative concentrations and seizure-induced changes in mRNAs encoding three AMPA receptor subunits in hippocampus and cortex. J Comp Neurol. 1996;365:541–555. doi: 10.1002/(SICI)1096-9861(19960219)365:4<541::AID-CNE3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Griesbeck O, Canossa M, Campana G, Gartner A, Hoener MC, Nawa H, Kolbeck R, Thoenen H. Are there differences between the secretion characteristics of NGF and BDNF? Implications for the modulatory role of neurotrophins in activity-dependent neuronal plasticity. Microsc Res Technol. 1999;45:262–275. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<262::AID-JEMT10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Hyman C, Juhasz M, Jackson C, Wright P, Ip NY, Lindsay RM. Overlapping and distinct actions of the neurotrophins BDNF, NT-3, and NT-4/5 on cultured dopaminergic and GABAergic neurons of the ventral mesencephalon. J Neurosci. 1994;14:335–347. doi: 10.1523/JNEUROSCI.14-01-00335.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibaraki K, Otsu Y, Nawa H. A novel two-site enzyme immunoassay reveals the regional distributions of and developmental changes in GluR1 and NMDAR1 protein contents in the rat brain. J Neurochem. 1999;73:408–417. doi: 10.1046/j.1471-4159.1999.0730408.x. [DOI] [PubMed] [Google Scholar]
- Ide N, Hirao K, Hata Y, Takeuchi M, Irie M, Yao I, Deguchi M, Toyoda A, Nishioka H, Mizoguchi A, Takai Y. Molecular cloning and characterization of rat lin-10. Biochem Biophys Res Commun. 1998;243:634–638. doi: 10.1006/bbrc.1998.8142. [DOI] [PubMed] [Google Scholar]
- Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, Takai Y, Rosahl TW, Sudhof TC. Binding of neuroligins to PSD-95. Science. 1997;277:1511–1515. doi: 10.1126/science.277.5331.1511. [DOI] [PubMed] [Google Scholar]
- Jourdi H, Nawa H. Basic fibroblast growth factor modulates the expression of PDZ-domain containing proteins. Acta Med Biol. 2002;50:107–115. [PMC free article] [PubMed] [Google Scholar]
- Iwakura Y, Nagano T, Kawamura M, Horikawa H, Ibaraki K, Takei N, Nawa H. NMDA-induced AMPA receptor down-regulation involves interaction of carboxyl terminus of GluR2/3 with Pick1. Ligand-binding studies using Sindbis vectors carrying AMPA receptor decoys. J Biol Chem. 2001;276:40025–40032. doi: 10.1074/jbc.M103125200. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Kitano J, Kimura K, Yamazaki Y, Soda T, Shigemoto R, Nakajima Y, Nakanishi S. Tamalin, a PDZ domain-containing protein, links a protein complex formation of group 1 metabotropic glutamate receptors and the guanine nucleotide exchange factor cytohesins. J Neurosci. 2002;22:1290–1302. doi: 10.1523/JNEUROSCI.22-04-01280.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara K, Kitamura A, Morishima M, Tsumoto T. Activity-dependent transfer of brain-derived neurotrophic factor to postsynaptic neurons. Science. 2001;291:2419–2423. doi: 10.1126/science.1057415. [DOI] [PubMed] [Google Scholar]
- Kohler M, Kornau HC, Seeburg PH. The organization of the gene for the functionally dominant alpha-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid receptor subunit GluR-B. J Biol Chem. 1994;269:17367–17370. [PubMed] [Google Scholar]
- Kondo M, Sumino R, Okado H. Combinations of AMPA receptor subunit expression in individual cortical neurons correlate with expression of specific calcium-binding proteins. J Neurosci. 1997;17:1570–1581. doi: 10.1523/JNEUROSCI.17-05-01570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong H, Boulter J, Weber JL, Lai C, Chao MV. An evolutionarily conserved transmembrane protein that is a novel downstream target of neurotrophin and ephrin receptors. J Neurosci. 2001;21:176–185. doi: 10.1523/JNEUROSCI.21-01-00176.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Korte M, Griesbeck O, Gravel C, Carroll P, Staiger V, Thoenen H, Bonhoeffer T. Virus-mediated gene transfer into hippocampal CA1 region restores long-term potentiation in brain-derived neurotrophic factor mutant mice. Proc Natl Acad Sci USA. 1996;93:12547–12552. doi: 10.1073/pnas.93.22.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau LF, Mammen A, Ehlers MD, Kindler S, Chung WJ, Garner CC, Huganir RL. Interaction of the N-methyl-D-aspartate receptor complex with a novel synapse-associated protein, SAP102. J Biol Chem. 1996;271:21622–21628. doi: 10.1074/jbc.271.35.21622. [DOI] [PubMed] [Google Scholar]
- Leonard AS, Davare MA, Horne MC, Garner CC, Hell JW. SAP97 is associated with the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit. J Biol Chem. 1998;273:19518–19524. doi: 10.1074/jbc.273.31.19518. [DOI] [PubMed] [Google Scholar]
- Linnarsson S, Bjorklund A, Ernfors P. Learning deficit in BDNF mutant mice. Eur J Neurosci. 1997;9:2581–2587. doi: 10.1111/j.1460-9568.1997.tb01687.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ford B, Mann MA, Fischbach GD. Neuregulins increase alpha7 nicotinic acetylcholine receptors and enhance excitatory synaptic transmission in GABAergic interneurons of the hippocampus. J Neurosci. 2001;21:5660–5669. doi: 10.1523/JNEUROSCI.21-15-05660.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty S, Carroll P, Cellerino A, Castren E, Staiger V, Thoenen H, Lindholm D. Brain-derived neurotrophic factor promotes the differentiation of various hippocampal nonpyramidal neurons, including Cajal-Retzius cells, in organotypic slice cultures. J Neurosci. 1996;16:675–687. doi: 10.1523/JNEUROSCI.16-02-00675.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Launey T, Mikawa S, Hirai H. Disruption of AMPA receptor GluR2 clusters following long-term depression induction in cerebellar Purkinje neurons. EMBO J. 2000;19:2765–2774. doi: 10.1093/emboj/19.12.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron. 1997;18:767–778. doi: 10.1016/s0896-6273(00)80316-5. [DOI] [PubMed] [Google Scholar]
- McLean-Bolton M, Pittman AJ, Lo DC. Brain-derived neurotrophic factor differentially regulates excitatory and inhibitory synaptic transmission in hippocampal cultures. J Neurosci. 2000;20:3221–3232. doi: 10.1523/JNEUROSCI.20-09-03221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minichiello L, Korte M, Wolfer D, Kuhn R, Unsicker K, Cestari V, Rossi-Arnaud C, Lipp HP, Bonhoeffer T, Klein R. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron. 1999;24:401–414. doi: 10.1016/s0896-6273(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Carnahan J, Nawa H. Brain-derived neurotrophic factor promotes differentiation of striatal GABAergic neurons. Dev Biol. 1994;165:243–256. doi: 10.1006/dbio.1994.1250. [DOI] [PubMed] [Google Scholar]
- Muller BM, Kistner U, Kindler S, Chung WJ, Kuhlendahl S, Fenster SD, Lau LF, Veh RW, Huganir RL, Gundelfinger ED, Garner CC. SAP102, a novel postsynaptic protein that interacts with NMDA receptor complexes in vivo. Neuron. 1996;17:255–265. doi: 10.1016/s0896-6273(00)80157-9. [DOI] [PubMed] [Google Scholar]
- Muller BM, Kistner U, Veh RW, Cases-Langhoff C, Becker B, Gundelfinger ED, Garner CC. Molecular characterization and spatial distribution of SAP97, a novel presynaptic protein homologous to SAP90 and the Drosophila discs-large tumor suppressor protein. J Neurosci. 1995;15:2354–2366. doi: 10.1523/JNEUROSCI.15-03-02354.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narisawa-Saito M, Carnahan J, Araki K, Yamaguchi T, Nawa H. Brain-derived neurotrophic factor regulates the expression of AMPA receptor proteins in neocortical neurons. Neuroscience. 1999a;88:1009–1014. doi: 10.1016/s0306-4522(98)00496-5. [DOI] [PubMed] [Google Scholar]
- Narisawa-Saito M, Silva AJ, Yamaguchi T, Hayashi T, Yamamoto T, Nawa H. Growth factor-mediated Fyn signaling regulates alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor expression in rodent neocortical neurons. Proc Natl Acad Sci USA. 1999b;96:2461–2466. doi: 10.1073/pnas.96.5.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narisawa-Saito M, Iwakura Y, Kawamura M, Araki K, Kozaki S, Takei N, Nawa H. Brain-derived neurotrophic factor regulates surface expression of alpha-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid receptors by enhancing the N-ethyl-maleimide-sensitive factor/GluR2 interaction in developing neocortical neurons. J Biol Chem. 2002;277:40901–40910. doi: 10.1074/jbc.M202158200. [DOI] [PubMed] [Google Scholar]
- Ozaki M, Sasner M, Yano R, Lu HS, Buonano A. Neuregulin-beta induces expression of an NMDA-receptor subunit. Nature. 1997;390:691–694. doi: 10.1038/37795. [DOI] [PubMed] [Google Scholar]
- Osten P, Khatri L, Perez JL, Kohr G, Giese G, Daly C, Schulz TW, Wensky A, Lee LM, Ziff EB. Mutagenesis reveals a role for ABP/GRIP binding to GluR2 in synaptic surface accumulation of the AMPA receptor. Neuron. 2000;27:313–325. doi: 10.1016/s0896-6273(00)00039-8. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Penzes P, Johnson RC, Sattler R, Zhang X, Huganir RL, Kambampati V, Mains RE, Eipper BA. The neuronal Rho-GEF Kalirin-7 interacts with PDZ domain-containing proteins and regulates dendritic morphogenesis. Neuron. 2001;29:229–242. doi: 10.1016/s0896-6273(01)00193-3. [DOI] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Rieff HI, Raetzman LT, Sapp DW, Yeh HH, Siegel RE, Corfas G. Neuregulin induces GABA(A) receptor subunit expression and neurite outgrowth in cerebellar granule cells. J Neurosci. 1999;19:10757–10766. doi: 10.1523/JNEUROSCI.19-24-10757.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford LC, Nelson SB, Turrigiano GG. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and inter-neuron excitatory synapses. Neuron. 1998;21:521–530. doi: 10.1016/s0896-6273(00)80563-2. [DOI] [PubMed] [Google Scholar]
- Saarelainen T, Pussinen R, Koponen E, Alhonen L, Wong G, Sirvio J, Castren E. Transgenic mice overexpressing truncated trkB neurotrophin receptors in neurons have impaired long-term spatial memory but normal hippocampal LTP. Synapse. 2000;38:102–104. doi: 10.1002/1098-2396(200010)38:1<102::AID-SYN11>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Sans N, Petralia RS, Wang YX, Blahos J, 2nd, Hell JW, Wenthold RJ. A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J Neurosci. 2000;20:1260–71. doi: 10.1523/JNEUROSCI.20-03-01260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans N, Racca C, Petralia RS, Wang YX, McCallum J, Wenthold RJ. Synapse-associated protein 97 selectively associates with a subset of AMPA receptors early in their biosynthetic pathway. J Neurosci. 2001;21:7506–7516. doi: 10.1523/JNEUROSCI.21-19-07506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Suzuki K, Nakanishi S. NMDA receptor stimulation and brain-derived neurotrophic factor upregulate homer 1a mRNA via the mitogen-activated protein kinase cascade in cultured cerebellar granule cells. J Neurosci. 2001;21:3797–3805. doi: 10.1523/JNEUROSCI.21-11-03797.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood NT, Lo DC. Long-term enhancement of central synaptic transmission by chronic brain-derived neurotrophic factor treatment. J Neurosci. 1999;19:7025–7036. doi: 10.1523/JNEUROSCI.19-16-07025.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Osten P, Vilim FS, Khatri L, Inman G, States B, Daly C, DeSouza S, Abagyan R, Valtschanoff JG, Weinberg RJ, Ziff EB. Novel anchorage of GluR2/3 to the postsynaptic density by the AMPA receptor-binding protein ABP. Neuron. 1998;21:581–591. doi: 10.1016/s0896-6273(00)80568-1. [DOI] [PubMed] [Google Scholar]
- Takei N, Sasaoka K, Inoue K, Takahashi M, Endo Y, Hatanaka H. Brain-derived neurotrophic factor increases the stimulation-evoked release of glutamate and the levels of exocytosis-associated proteins in cultured cortical neurons from embryonic rats. J Neurochem. 1997;68:370–375. doi: 10.1046/j.1471-4159.1997.68010370.x. [DOI] [PubMed] [Google Scholar]
- Takei N, Kawamura M, Hara K, Yonezawa K, Nawa H. Brain-derived neurotrophic factor enhances neuronal translation by activating multiple initiation processes: comparison with the effects of insulin. J Biol Chem. 2001;276:42818–42825. doi: 10.1074/jbc.M103237200. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Neurotrophins and activity-dependent plasticity. Prog Brain Res. 2000;128:183–191. doi: 10.1016/S0079-6123(00)28016-3. [DOI] [PubMed] [Google Scholar]
- Valtschanoff JG, Burette A, Davare MA, Leonard AS, Hell JW, Weinberg RJ. SAP97 concentrates at the postsynaptic density in cerebral cortex. Eur J Neurosci. 2000;12:3605–3614. doi: 10.1046/j.1460-9568.2000.00256.x. [DOI] [PubMed] [Google Scholar]
- Wyszynski M, Valtschanoff JG, Naisbitt S, Dunah AW, Kim E, Standaert DG, Weinberg R, Sheng M. Association of AMPA receptors with a subset of glutamate receptor-interacting protein in vivo. J Neurosci. 1999;19:6528–6537. doi: 10.1523/JNEUROSCI.19-15-06528.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Zhang X, Staudinger J, Huganir RL. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein Pick1. Neuron. 1999;22:179–187. doi: 10.1016/s0896-6273(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Xiong H, Yamada K, Han D, Nabeshima T, Enikolopov G, Carnahan J, Nawa H. Mutual regulation between the intercellular messengers nitric oxide and brain-derived neurotrophic factor in rodent neocortical neurons. Eur J Neurosci. 1999;11:1567–1576. doi: 10.1046/j.1460-9568.1999.00567.x. [DOI] [PubMed] [Google Scholar]
- Xu B, Gottschalk W, Chow A, Wilson RI, Schnell E, Zang K, Wang D, Nicoll RA, Lu B, Reichardt LF. The role of brain-derived neurotrophic factor receptors in the mature hippocampus: modulation of long-term potentiation through a presynaptic mechanism involving TrkB. J Neurosci. 2000;20:6888–6897. doi: 10.1523/JNEUROSCI.20-18-06888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoubian TA, Lo DC. Truncated and full-length TrkB receptors regulate distinct modes of dendritic growth. Nat Neurosci. 2000;3:342–329. doi: 10.1038/73911. [DOI] [PubMed] [Google Scholar]