Abstract
Microglia, the resident immune cells of the central nervous system, are normally quiescent but become activated after infection or injury. Their properties then change, and they promote both repair and damage processes. The extent of microglial activation is regulated, in part by activation-induced cell death (AICD). Although many apoptotic aspects of the microglial AICD mechanism have been elucidated, little is known about the connection between the activation step and the death process. Using mouse primary microglial cultures, we show that the ectoenzyme CD38, via its calcium-mobilizing metabolite cyclic-ADP-ribose (cADPR), helps promote microglial activation and AICD induced by LPS plus IFNγ (LPS/IFNγ), suggesting that CD38 links the two processes. Accordingly, CD38 expression and activity, as well as intracellular calcium concentration ([Ca2+]i) in the primary microglia were increased by LPS/IFNγ treatment. Moreover, CD38 deficiency or treatment with cADPR antagonists conferred partial resistance to LPS/IFNγ-induced AICD and also reduced [Ca2+]i. Microglial activation, indicated by induced expression of nitric-oxide-synthase-2 mRNA and production of NO, secretion and mRNA expression of TNFα and IL-12 p40, and expression of IL-6 mRNA, was attenuated by CD38 deficiency or cADPR-antagonist treatment. The observed effects of CD38 on microglial activation is probably mediated via a cADPR-dependent increase in [Ca2+]i, and the effect on AICD by regulation of NO production. Our results thus suggest that CD38 significantly affects regulation of the amount and function of activated microglia, with important consequences for injury and repair processes in the brain.
Keywords: Cell activation, CD38, microglia, cell surface molecule, apoptosis, nitric oxide, neuroimmunology
Introduction
Microglia, the resident immune cells of the CNS, are quiescent in the mature brain under physiological conditions. Following acute brain injury microglia migrate to the damaged sites, where they proliferate and become activated (1, 2). Activation is accompanied by morphological changes, up-regulated expression of cell-surface proteins (e.g. MHC class-II molecules, CD11b and scavenger receptors), cytokines (e.g. TNFα, IL-6 and IL-12), chemokines (e.g. CCL12), and NO production (3, 4).
Activated microglia can promote both protective and harmful effects. Beneficial effects include removal of cell debris and myelin fragments, buffering of toxic compounds, and secretion of neurotrophins and cytokines capable of supporting survival of injured neurons (5, 6). Microglia can also be beneficial in neurodegenerative diseases, for example by clearing β-amyloid (7). However, they can also produce and secrete toxic factors, including TNFα and IL-1β and free radicals such as NO (8–10); accordingly, some authors have postulated that microglial activation participates in the pathogenesis of several neurodegenerative diseases, including Alzheimer’s and Parkinson’s (11–13). Activated microglia might also be at least partially responsible for secondary damage after traumatic brain injury (14, 15); thus, their numbers and activity need to be strictly regulated. Indeed, following the initial rapid increase in activated microglia after acute brain injury, their numbers gradually decrease (16, 17). Among the mechanisms thought to help control the disappearance of activated microglia after brain damage is their elimination via cell death, termed microglial activation-induced cell death (AICD)1, and observed for example in animal models of brain damage and microglial activation (18–22). Consistently with those observations, treatment with agents known to induce microglial activation, such as LPS and IFNγ, induces apoptosis in microglial cell lines and primary cultures (23–26).
Our group and others have characterized the apoptotic pathways of AICD induced by LPS and IFNγ (LPS/IFNγ) and demonstrated their mediation via the NO-promoted (24) Bak/Bax-activated mitochondrial gateway (26) and caspase 11 (23). Although these studies have helped to elucidate this AICD mechanism, little is known about the relationship between the activation step and the death process. Here we show that CD38, an ectoenzyme that uses β-NAD to generate multiple Ca2+-mobilizing metabolites (27–29), participates in both the activation and the AICD processes of LPS/IFNγ-treated microglia. The CD38 effects were mediated, at least in part, via cyclic ADP-ribose (cADPR).
Materials and Methods
Animals, drugs and reagents
CD38-deficient mice (Cd38−/−) (30), backcrossed 12 generations to BALB/cBy mice (31), were obtained from the Trudeau Institute Breeding Facility (Saranac Lake, NY) and were maintained in accordance with all applicable rules and guidelines of the Animal Care and Use Committee of Tel Aviv University.
Reagents used were purchased from Sigma (St. Louis, MO) unless indicated otherwise. Mouse IFNγ was purchased from R&D Systems, (Minneapolis, MN); ryanodine was from Calbiochem (La Jolla, CA); actinomycin D (AMD) was from ICN Biomedicals (Aurora, OH); Hanks’s buffered salt solution (HBSS) was from Biological Industries (Kibbutz Beit Ha-Emek, Israel); DMEM, fura-2 AM, and Pluronic F-127 were from Invitrogen Life Technologies (Paisley, UK). Nicotinamide 8-bromoadenine dinucleotide N(8-Br-A)D+ was prepared as described (32).
Antibodies
Rabbit anti-inducible nitric oxide synthetase (iNOS) polyclonal Abs (#AB1631) were from Chemicon (Temecula, CA). Rat anti-caspase-11 (17D9, #C1354) and mouse anti-β-tubulin monoclonal Abs (#T4026) were purchased from Sigma (St. Louis, MO). Phycoerythrin (PE)-conjugated rat anti-CD38 (NIMR-5, #1635–09), low endotoxin/azide free rat anti-CD38 (90, #1640–14) monoclonal Abs and low endotoxin/azide free Rat IgG2a isotype control Ab (#0117–14) were from Southern Biotechnology Associates (Birmingham, AL), and PE-conjugated rat anti-CD11b monoclonal Ab (M1/70, 101207) was from Biolegend (San Diego, CA). Purified rat IgG2a anti-mouse monoclonal CD38 antibody (clone NIM-R5) was produced by the Trudeau monoclonal antibody facility. Secondary Ab F(ab′)2 fragment mouse anti-rat IgG (H+L) (#212-006-168) was purchased from Jackson ImmunoResearch, West Grove, PA.
Cell culture
N9 mouse microglial cell line (33) was maintained in DMEM supplemented with 10% FCS) (Invitrogen). CD38 expressing (CD38exp) and non-expressing [empty vector transfected Ba/F3 cells (CD38neg)] Ba/F3 cells (34) were cultured in RPMI 1640 supplemented with 10% WEHI-3 supernatant (containing IL-3), 10% FCS, non-essential amino acids, sodium pyruvate, L-glutamine, HEPES, β-mercaptoethanol and antibiotics. Mouse primary microglia were prepared as previously described (26), with minor modifications. Cerebral cortices from 1- to 3-day-old neonatal BALB/c [(wild-type (WT)] and Cd38−/− mice were dissected in ice-cold HBSS, carefully stripped of their meninges, and digested with 0.25% trypsin for 10 min at 37°C. Trypsinization was stopped by addition of an equal volume of culture medium [DMEM, 10% low endotoxin (≤10 EU/ml) FCS (Hyclone, Logan, UT), penicillin 100 U/ml and streptomycin 100 μg/ml] to which we added 0.02% deoxyribonuclease I. The cells were dispersed into a single cell level by repeated pipetting, then pelleted and re-suspended in culture medium. The cell suspension was then passed through a 100–3m pore mesh. Cells were seeded at a density of 300,000 cells/ml (equivalent to 62,500 cells/cm2) in 6-well, 12-well or 96-well plates and cultured at 37°C in humidified 5% CO2-95% air. Medium was replaced every 4–5 days. These cultures (‘mixed glia’) reached confluence after 7–10 days and were used between 15 and 20 days after preparation. Microglia were isolated from the mixed glia cultures by a mild trypsinization procedure as previously described (35). Briefly, treatment of the confluent mixed glial cultures with trypsin (0.06%) resulted in detachment of an intact layer of cells containing almost all the astrocytes and leaving behind a highly enriched population of microglia [>98%, as determined by staining with fluorescein-conjugated Griffonia simplicifolia isolectin B4 (Vector Laboratories, Peterborough, UK) and by flow cytometric analysis using the PE conjugated CD11b Ab (data not shown)]. The attached microglia were allowed to recover for 24 h and then were subjected to the different treatments.
Cell viability assays
Primary microglial cultures prepared in 96-well plates as described above were treated with LPS (100 ng/ml) and IFNγ (100 units/ml) (hereafter termed LPS/IFNγ treatment) for the indicated time periods. In some experiments the cells were preincubated with 8-Br-cADPR (100 μM), ryanodine (30 μM), N(8-Br-A)D+ (100 μM), cycloheximide [CHX (50–100 ng/ml)], or AMD (5–25 ng/ml) for 1–2 h prior, to the addition of LPS/IFNγ. In the cross-linking experiments primary microglia were cultured in 24-well plates in the absence or presence of anti-CD38 Abs (clone 90 or NIM-R5) or a rat IgG2a isotype control Ab with or without 5μg/ml secondary Ab F(ab′)2. On termination of the experiments, the medium from each well was carefully collected and replaced with an equal volume of fresh medium. Cell viability was then determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. MTT was added to the new medium at a final concentration of 0.5 mg/ml, followed by incubation at 37°C in a CO2 incubator. After 2 h the medium was aspirated and dimethyl sulfate (100 μl) was added to the wells. Insoluble crystals were dissolved by mixing and the plates were read on a SpectraMax micro-ELISA plate reader, using a test wavelength of 570 nm and a reference wavelength of 690 nm. Cell death was assessed by monitoring the release of lactate dehydrogenase (LDH) to the culture medium (which was collected at the end of the experiments, see above) using the CytoTox-One™ Homogeneous Membrane Integrity Assay kit (Promega, Madison, WI) according to the manufacturer’s instructions. The LDH signal was read using a test wavelength of 560 nm and a reference wavelength of 590 nm in a Synergy™ HT Multi-Detection Microplate Reader. The viability of the Ba/F3 cells was assessed as previously described (26). Briefly, cells were plated in 96-well plates (2 × 104 cells per well). After 18 h primary Abs were added to the culture medium and the cells were grown in the presence or absence of the Abs for additional 24 h. Then MTT was added to the culture medium at a final concentration of 0.5 mg/ml, followed by incubation at 37°C in a CO2 incubator for 2 h. Acid-isopropanol (100 μl of 0.04 M HCl in isopropanol) was then added to the wells and mixed. The plates were read on a micro-ELISA reader (SpectraMax microplate reader) as described above.
Nitrite quantification
Nitrite, measured as a reflection of NO production in culture supernatants, was assayed using modified Griess reagent. In brief, upon termination of the experiment the culture medium was replaced with an equal volume of new medium, and 50 μl of the removed medium was mixed with an equal volume of Griess reagent in 96-well plates and incubated at room temperature for 15 min. Absorbance at 540 nm was then measured using a micro-ELISA reader (SpectraMax). To normalize the amount of nitrite measured in each well to the amount of cells present in that well, MTT assay was performed after the new medium was added to the cultured cells, as described above.
cADPR measurements
WT and Cd38−/− primary microglia were washed twice with ice cold PBS and resuspended on ice with 25 mM sodium phosphate buffer at pH 7.4, homogenized by passing through a 23g needle (using the 500 μl Bee Stinger Gastight Syringe) and aliquot was removed from each sample to determine the protein content. Perchloric acid (HClO4) was then added to a final concentration of 0.6 M and homogenates were incubated on ice for 10 min followed by centrifugation (16,000 × g, 10 min, 4°C). Supernatants were transferred to new tubes and the pH adjusted to pH 7 by K2CO3 titration. Homogenates were left on ice for 30 min to enable gas evaporation after which the samples were then centrifuged (2,000 × g, 5 min, 4°C) to eliminate salts. Supernatants were collected into new tubes and kept frozen until used.
The measurement of cADPR from samples was then performed essentially as already described for determining cADPR content of synaptosomes (36). The contaminating nucleotides were eliminated by incubation overnight in NaH2PO4 at pH 8.0 with a mix of three nucleotide hydrolytic enzymes (nucleotide pyrophosphatase, alkaline phosphatase and NAD+-glycohydrolase). Then, the conversion of cADPR into NAD+ in the presence of Aplysia ADP-ribosyl cyclase, and the cycling reaction were allowed to proceed. Results were compared to standard solutions of cADPR analyzed in the same experimental conditions.
Immunoblot analysis
Total extracts (30 μg protein) from each treatment were separated by 12.5% SDS-PAGE and electroblotted onto supported nitrocellulose. Each blot was blocked for 1 h in Tris-buffered saline-Tween-20 (10 mM Tris base, 150 mM NaCl and 0.05 % Tween-20) containing 5% fat-free milk, and then incubated with a primary antibody overnight at 4°C. Washing of membranes three times (10 min each) with Tris-buffered saline-Tween-20 was followed by incubation for 1 h at room temperature with the appropriate second antibody (goat anti-mouse, donkey anti-rat, or goat anti-rabbit IgG peroxidase conjugate; Jackson ImmunoResearch Laboratories, West Grove, PA). The blots were developed using the Supersignal West Pico chemiluminescence kit (Pierce Biotechnology, Rockford, IL). Each blot was reprobed with β-tubulin to verify that protein was uniformly loaded across the gel.
Assays of GDP-ribosyl cyclase activity
The ectocellular cyclase activity of CD38 was measured using nicotinamide–guanine dinucleotide (NGD+) instead of NAD+ as a surrogate substrate because the product, cyclic GDP-ribose (cGDPR), is fluorescent and is not susceptible to enzymatic hydrolysis (37). GDP-ribosyl cyclase activity was assayed by incubating NGD+ with intact microglial primary cultures or N9 microglial cells. Microglia grown in 96-well plates, untreated or treated with LPS/IFNγ for 24 h (primary cultures) or 15 h (N9 cells), were washed once with HBSS and then incubated with HBSS (100 μl/well) at 37°C. After 20 min, NGD+ (40 μM) was added to each well and the enzymatic conversion of NGD+ to cGDPR was measured fluorometrically by monitoring the increase in fluorescence at 420 nm upon excitation at 300 nm for 10 min, as described previously (32).
Flow cytometry
Microglia were cultured in 6-well plates and treated as indicated. After incubation the microglia were gently removed from the tissue culture plates by pipetting, then centrifuged (700 × g, 10 min, 4°C) and incubated (30 min, 4°C) in flow cytometry buffer (PBS containing 1% BSA and 0.05% sodium azide) containing PE-conjugated anti-CD38 (NIM-R5) monoclonal Ab, PE-conjugated anti CD11b monoclonal Ab, or isotype-matched control Abs. After washing, antigen expression on 5,000 live cells was determined by FACS analysis (FACSort; Becton Dickinson, Mountain View, CA) and CellQuest software (Becton Dickinson).
Gene Expression Profiling
RNA extraction, sample preparation, hybridization, and array processin
N9 cells (106 per plate) were washed once with 10 ml of ice cold PBS, then lysed with 1 ml/plate of TRIzol reagent (Invitrogen) and RNA was prepared according to the manufacturer’s instructions. Total RNA from each sample was used to prepare biotinylated target RNA, with minor modifications from the manufacturer’s recommendations. Briefly, 10 μg of total RNA was used to generate first-strand cDNA by using a T7-linked oligo (dT) primer. After second-strand synthesis, in vitro transcription was performed with biotinylated UTP and CTP (Enzo Diagnostics), resulting in approximately 100-fold amplification of RNA, which was then processed as per manufacturer’s recommendation using an Affymetrix GeneChip Instrument System. Briefly, spike controls were added to 10 μg fragmented cRNA before overnight hybridization. Arrays were then washed and stained with streptavidin-phycoerythrin, before being scanned on an Affymetrix GeneChip scanner. Quality and amount of starting RNA were confirmed using an agarose gel. Following scanning, array images were assessed by eye to confirm scanner alignment and the absence of significant bubbles or scratches. 3 ′/5 ′ ratios for GAPDH and β-actin were confirmed to be within acceptable limits range (0.80–0.85 and 1.14–1.2 respectively), and BioB spike controls were found to be present on 100%, with BioC, BioD and CreX also present in increasing intensity. When scaled to a target intensity of 150 (using Affymetrix MAS 5.0 array analysis software), scaling factors for all arrays were within acceptable limits (0.85–1.06) as were background, Q values and mean intensities. All experiments were performed using Affymetrix Mouse 430A 2.0 oligonucleotide arrays, as described at http://www.affymetrix.Com/products/arrays/specific/mgu74.affx. Genes were filtered using Mas 5 algorithm results. A list of 12,827 probe sets of “valid genes”, representing probe sets with signals higher than 20 and detected as “present” in at least one sample was obtained.
Analysis of gene expression data
DNA-microarray data were analyzed using the Expander 3.2 program (38). Data were normalized using Quantile normalization, filtered to include only genes that changed at least 2 fold form control (untreated sample), and standardized (mean 0, variation 1). Analysis yielded a list of 1,153 ‘active genes’.
Total RNA isolation, cDNA synthesis and semi-quantitative RT-PCR analysis
Primary microglia were untreated or treated with LPS/IFNγ for 10 h. In some experiments the cells were preincubated for 2 h with 8-Br-cADPR (100 μM). Total RNA was isolated using the RNeasy® Plus Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Total RNA (1 μg) was used as template for DNA synthesis, employing random decamer primers in a 20-μl reaction volume using the Reverse-iT™ 1st Strand Synthesis Kit (ABgene, Epsom, UK) according to the manufacturer’s instructions. The cDNA mixture was diluted 1:10 with PCR-grade water. mRNA expression of the indicated genes was measured using the semi-quantitative RT-PCR approach. The following primers were used according to the real-time PCR primer and probe database (39): TNF-α, sense 5′-GGC TTT CCG AAT TCA CTG GAG-3 ′, antisense 5 ′CCC CGG CCT TCC AAA TAA A-3 ′ (target size, 264 bp); IL 12 p40, sense 5 ′-GGA AGC ACG GCA GCA GAA TA-3 ′, antisense 5 ′-AAC TTG AGG GAG AAG TAG GAA TGG-3 ′ (target size, 180 bp); IL-6, sense 5 ′-GAG GAT ACC ACT CCC AAC AGA CC-3 ′, antisense 5 ′-AAG TGC ATC ATC GTT GTT CAT ACA-3 ′ (target size, 141 bp); nitric oxide synthase (NOS)2, sense 5 ′-CAG CTG GGC TGT ACA AAC CTT-3 ′, antisense 5 ′-CAT TGG AAG TGA AGC GTT TCG-3 ′ (target size, 95 bp); and GAPDH, sense 5 ′-CAT GGC CTT CCG TGT TCC TA-3 ′, antisense 5 ′-ATG CCT GCT TCA CCA CCT TCT-3 ′ (target size, 107 bp). PCR reactions were carried out using 5 μl of cDNA. The resulting PCR products, obtained after 24–26 cycles (in the logarithmic phase of the PCR reaction) were resolved on E-Gel® Pre-cast Agarose 2% (Invitrogen). The individual PCR products were scanned and their intensity determined (ImageMaster). The value of each of the IL-6, IL-12 p40, TNFα and NOS2 PCR products was then normalized to the intensity of its corresponding housekeeping gene, the GAPDH PCR product.
Measurement of TNFα and IL-12 p40 secretion
TNFα and IL-12 p40 secreted into the culture medium were assayed using a DuoSet® ELISA kit (R&D Systems) according to the manufacturer’s instructions. Primary microglia grown in 96-well plates (for TNFα) or 24-well plates (for IL-12 p40) were treated with LPS/IFNγ for 24 h. In some experiments, the cells were preincubated for 2 h with 8-Br-cADPR (100 μM) or N(8-Br-A)D+ (100 μM) prior to LPS/IFNγ treatment. The culture medium was carefully removed from each well, centrifuged at 1,000 × g (5 min, 4°C), and then kept frozen at −80°C until used. Cytokine quantity in each well was normalized to either the MTT value (TNFα) or the protein concentration (IL-12 p40) measured in the same well.
Calcium measurements
Intracellular calcium concentrations [Ca2+]i were monitored using the Ca2+-sensitive fluorescent indicator fura-2 AM, as described (40). Briefly, primary microglial cells were seeded on 18-mm poly-L-lysine-coated circular coverslips in a 12-well plate. Prior to treatment with LPS/IFNγ, cells were left untreated or were treated with LPS/IFNγ, in some experiments after preincubation for 2 h with 8-Br-cADPR (100 μM). After 24 h the cells were loaded with 2.5 μM fura-2 AM for 60 min at 37°C (in a 5% CO2 incubator). The fura-2 AM was loaded as a mixture (1:1 in culture medium) with the mild detergent pluronic F-127 (0.05%; used to facilitate loading of the AM ester indicator). Measurements were performed in an external solution (140 mM NaCl, 3 mM KCl, 2 mM CaCl2, 10 mM HEPES, and 2 mg/ml glucose, pH 7.2–7.4, 300–320 mOsm) at room temperature. For excitation of fura-2, cells were illuminated with two alternating wavelengths, 340 and 380 nm. Excitation was performed and [Ca2+]i values calculated from the ratio (R) of fluorescence recorded at excitation wavelengths of 340 and at 380 nm. Calibrations (by conversion of R340/380 values into molar calcium concentrations) were performed as described (41).
Statistical analysis
Data was expressed as mean values ± SEM. Statistical evaluation was determined by two-tailed Student’s t test. A value of p < 0.05 was considered to be statistically significant.
Results
Macromolecule synthesis as an essential requirement for microglial AICD
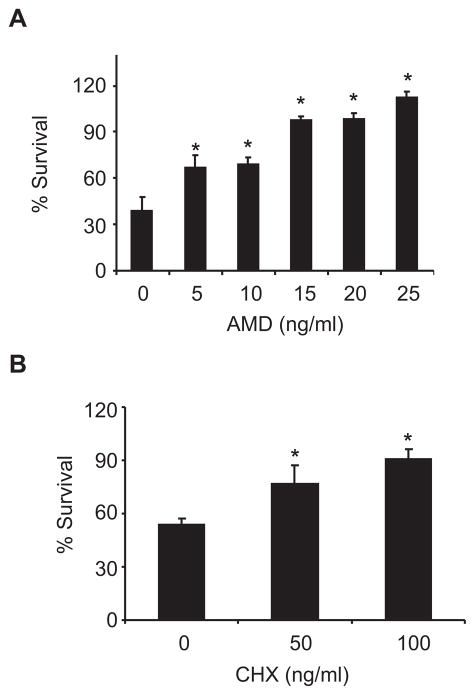
In some apoptotic systems the death process depends on synthesis of macromolecules. We were therefore interested in examining whether AICD in microglia is dependent on RNA or protein synthesis. AICD was induced by combined treatment with LPS (100 ng/ml) and IFNγ (100 units/ml) (LPS/IFNγ). N9 cells were treated with LPS/IFNγ in the absence or presence of the RNA-synthesis inhibitor AMD or the protein synthesis inhibitor CHX. Determination of cell viability by the MTT assay disclosed that death of the LPS/IFNγ-treated N9 cells was dose-dependently diminished by both inhibitors (Fig. 1A–B). CHX also inhibited the death of LPS/IFNγ-treated primary mouse microglia (data not shown). These results suggested that in order for microglial AICD to occur, some essential gene products need to be synthesized.
FIGURE 1.
Microglial AICD depends on synthesis of macromolecules. N9 cells were treated without or with LPS/IFNγ in the absence or presence of the indicated concentrations of the RNA synthesis inhibitor AMD (A) or the protein synthesis inhibitor CHX (B). Cell viability was determined by the MTT assay as described in Materials and Methods. Survival was assessed in terms of MTT values after treatment, expressed as a percentage of the MTT values without treatment. Values for each treatment are expressed as percentages of the corresponding control (cells untreated or treated with the inhibitor). Values for a given treatment relative to inhibitor untreated cells are defined as significant if *P < 0.05 (Student’s t-test; n = 3).
DNA-microarray analysis for identification of genes that are preferentially induced by LPS/IFNγ
In an attempt to identify the genes whose upregulated expression is needed for microglial AICD, we employed the DNA-microarray technology. In a previous study we showed that treatment of N9 cells with LPS (100 ng/ml) alone or IFNγ (100 units/ml) alone did not substantially affect their viability while the combination of LPS/IFNγ induced AICD (26). We therefore prepared total RNA from N9 cells that had been untreated (control) or treated with LPS/IFNγ, LPS, or IFNγ for 6 h, a time point by which the death gene mRNAs had already been synthesized. This was the earliest time after LPS/IFNγ treatment by which the AMD could not rescue the cells from LPS/IFNγ-induced death and the cell morphology still appeared healthy (data not shown).
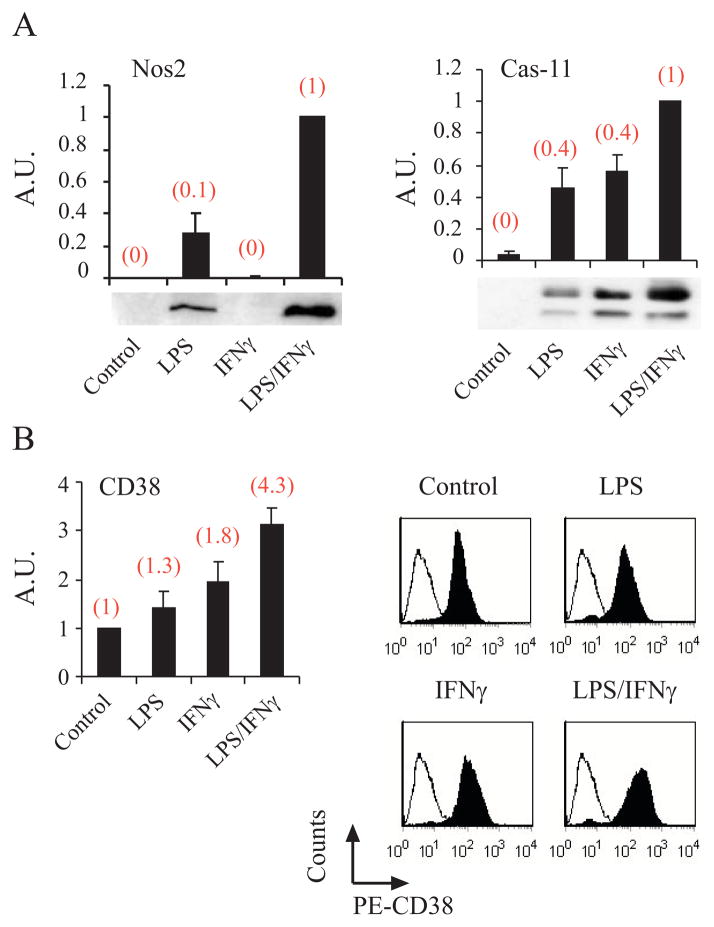
The global gene-expression profiles in the above-mentioned treatments were recorded by Affymetrix oligonucleotide microarrays. Comparative analysis of the DNA-microarray results from the four different samples identified 1,153 probe sets that were changed by at least twofold in one or more of the samples. Of all of these probe sets only 89 genes were preferentially induced by the LPS/IFN treatment and only 28 of these genes were induced synergistically by the combination of LPS/IFNγ. Among the genes identified by this analysis were Nos2 (which encodes the iNOS enzyme that catalyzes NO production) and caspase-11(Fig. 2A). As NO and caspase-11 are known to be upregulated and to promote microglial AICD by different pathways (23, 24, 26), it suggested to us that other genes identified in this analysis might also be AICD-promoting candidate genes.
FIGURE 2.
Nos2, caspase-11 or CD38 expression is preferentially induced by the LPS/IFNγ treatment; DNA-microarray and protein expression analysis. N9 cells were treated without (control) or with LPS (100 ng/ml) or IFNγ (100 units/ml) or LPS/IFNγ (LPS (100 ng/ml) and IFNγ (100 units/ml). RNA samples and protein extracts were prepared after 6 and 15 h respectively. The RNA was subjected to gene expression profiling and the results obtained were analyzed as described in Materials and Methods. The protein extracts were analyzed by immunoblotting (A) for the expression of Nos2, and caspase-11, as described in Materials and Methods. The immunoblotting results shown are from a representative experiment for each protein (one of 3–5 independent experiments). Immunoblots obtained in at least three independent experiments (including the one shown here) were scanned and the intensities of the different proteins were assessed by densitometric analysis. Data are presented as mean values of the intensities obtained for each protein after its normalization to β-tubulin. The data presented are expressed as the ratios of the mean intensities of caspase-11 and Nos2 in LPS-treated, IFNγ-treated, or control cells to those of LPS/IFNγ-treated cells (defined as 1 A.U). Values shown in the histograms are means ± SEM (bars) (n = 3–5). The gene-expression results (obtained from the DNA-microarray analysis) are shown (in parentheses) above each of the corresponding immunoblot results and are expressed for each gene in the same manner as described for the corresponding proteins. For determination of CD38 expression (B), N9 cells were treated as described above for 15 h and then were harvested, stained with PE-conjugated anti-CD38 Abs (PE-CD38), and analyzed by flow cytometry as described in Materials and Methods. The results of a representative experiment for each treatment (one of at least three independent experiments) are shown in the right panels. The empty histogram represents the background staining using an isotype-matched control Ab; the filled area represents CD38 staining. The mean fluorescence intensities (MFIs) for the different treatments of the (at least) three experiments were calculated and their mean values determined. The left panels present the quantification of the results of three independent experiments (including the one shown here), expressed as CD38 expression values (as determined by the MFIs) in the LPS-, IFNγ-, or LPS/IFNγ-treated cells relative to CD38 expression values in the control (defined as 1 A.U.). Values shown are means ± SEM (bars) (n = 3–4). The gene-expression results (obtained from the DNA-microarray analysis) are shown (in brackets) above each of the corresponding flow cytometry results.
CD38 plays an important role in LPS/IFNγ-induced AICD
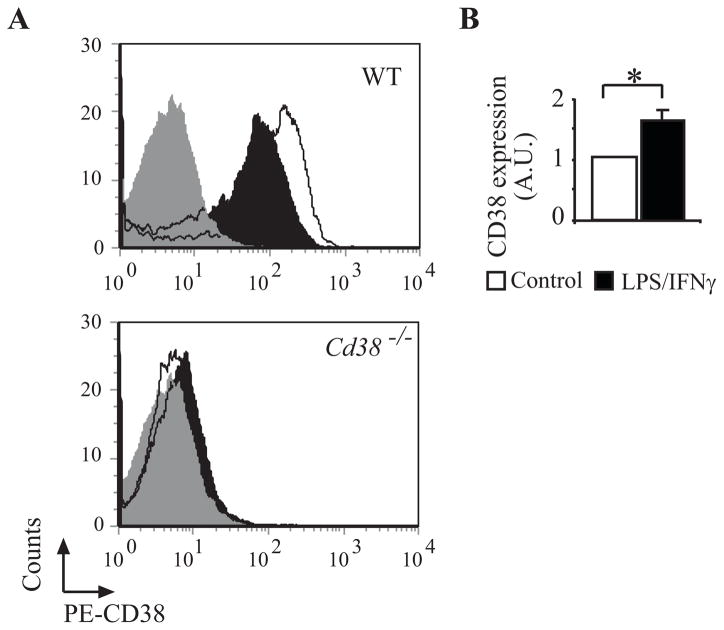
One of the 28 genes identified from the DNA microarray analysis as being synergistically induced by LPS/IFNγ treatment was Cd38 (Fig. 2B) that encodes the ecto-enzyme CD38. CD38 catabolizes its substrate, NAD+, to produce metabolites (e.g. cADPR) that increase cytosolic Ca2+ concentration. CD38 can also act as a cell-surface receptor capable of initiating a signal transduction cascade and cell death in immune cells (34, 42–47). Therefore, we suspected that it might play an important role also in microglial AICD. To examine this possibility, we first verified the DNA microarray results at the protein expression level. Membrane CD38 expression was determined by FACS analysis. As shown in Fig. 2B, and in agreement with the DNA-microarray results, CD38-protein expression in N9 cells was preferentially induced by the LPS/IFNγ treatment relative to treatment with LPS or IFNγ. Fig. 3 shows that the LPS/IFNγ treatment also induced an increase in membrane CD38 expression in the primary microglia (1.63-fold induction after 24 h) and that as expected, CD38-deficient primary microglia obtained from mice that lack CD38 did not express plasma membrane CD38.
FIGURE 3.
LPS/IFNγ induces CD38 expression in primary microglia. LPS/IFNγ induces CD38 expression in the plasma membrane. WT and Cd38−/− primary microglial cultures were grown in the absence or presence of LPS/IFNγ for 24 h and the cells were then harvested, stained with PE-conjugated anti-CD38 Abs (PE-CD38) and analyzed for CD38 expression by flow cytometry as described in Materials and Methods. The results of a representative experiment for each genotype (one of three independent experiments) are shown in A. The grey filled histogram represents the background staining obtained with an isotype-matched control Ab, the black filled histogram represents CD38 staining in untreated (control) cells, and the empty histogram represents CD38 staining in LPS/IFNγ-treated cells. MFIs of the different treatments in three independent experiments in WT microglia were calculated and their mean values determined. The results of this quantification are presented in B and are expressed as CD38 expression values (as determined by MFIs) in LPS/IFNγ-treated cells relative to the untreated control (defined as 1 A.U.). Values shown are means ± SEM (bars) (n = 3); *P < 0.05 compared to control (Student’s t-test).
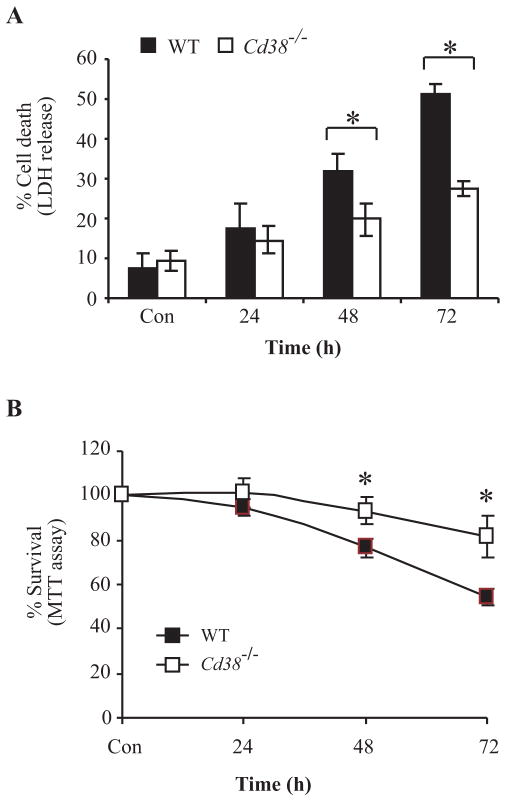
Having verified the DNA microarray results we next examined the need for CD38 in microglial AICD. WT and Cd38−/− primary microglia were treated with LPS/IFNγ for 24, 48, or 72 h, and the amount of cell death in each case was determined by measuring the activity of LDH released into the culture medium. Fig. 4A shows that LPS/IFNγ treatment induced a time-course-dependent release of LDH in both WT and Cd38−/− primary microglial cultures, but that the amount of LDH released from the CD38-deficient microglia was substantially and significantly lower (approximately 50% reduction 72 h after treatment) than observed in the WT cultures. The results were similar when cell death/viability was assessed by the MTT assay (Fig. 4B). Taken together, these results suggest that CD38−/− primary microglia are significantly more resistant than the WT microglia to LPS/IFNγ-induced AICD, and therefore that CD38 plays an important role in promoting this death process.
FIGURE 4.
CD38 plays an important role in LPS/IFNγ-induced microglial AICD. WT and Cd38−/− primary microglia were untreated (Con, for 24 h) or treated with LPS/IFNγ for the indicated time points. Cell viability was then assessed by monitoring LDH released to the culture medium (A) or by the MTT assay (B). Cell death was defined in terms of the LDH activity in the medium after treatment, expressed as a percentage of the total LDH activity of the cells. Values shown are means ± SEM (bars) (n = 3); *P< 0.05 compared to the corresponding treatment in Cd38−/− cells as indicated (Student’s t-test). The MTT assay and assessment of survival were performed as described in Fig. 1. Survival was expressed as a percentage of the MTT values without treatment. Values shown are means ± SEM (bars) (n = 3); *P < 0.05 compared to the corresponding treatment in WT cells as indicated (Student’s t-test).
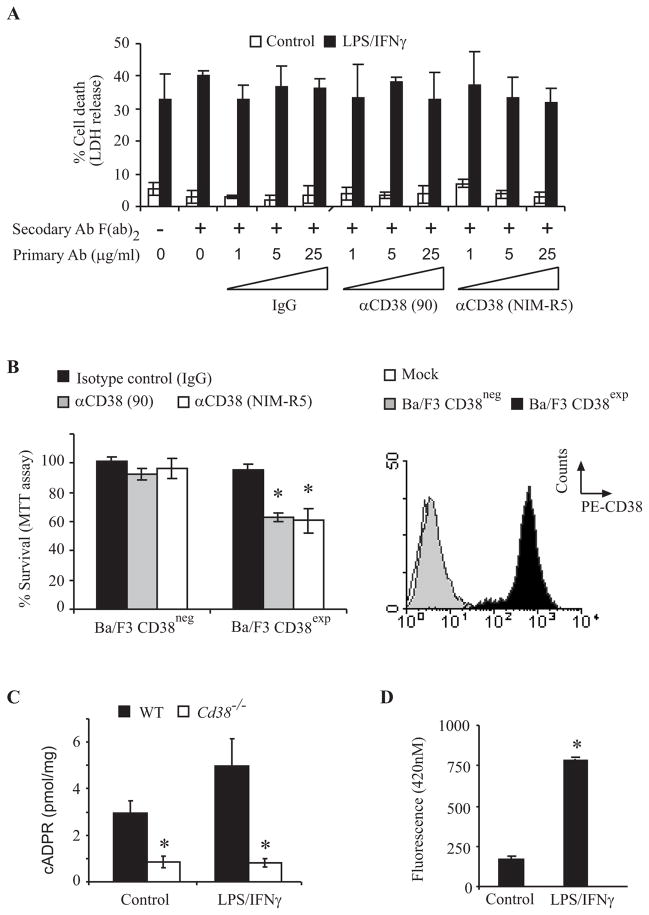
CD38 was reported to regulate apoptosis in hematopoietic cells independently of its enzymatic activity (34). We therefore examined the effect of cross-linking of CD38, using anti-CD38 agonist Abs (clone 90 and NIM-R5) (1, 5 and 25 μg/ml), on primary microglia treated for 72 h with or without LPS/IFNγ. None of these treatments, however, had any apparent effect on the viability of the cells (Fig. 5A). To assess the effectiveness of the cross-linking Abs, their ability to induce cell death of CD38neg and CD38exp Ba/F3 cells was examined. As shown in Fig. 5B both clone 90 and NIM-R5 anti-CD38 Abs induced cell death in Ba/F3 CD38exp but not in CD38neg Ba/F3 cells (Fig. 5B) as previously reported (34). Taken together these results suggest that the effect of CD38 on AICD is not mediated via its receptor signaling activity but by a different mechanism. In view of the important role of Ca2+ signaling in apoptosis (48), and the role of CD38/cADPR in intracellular Ca2+ levels regulation, we hypothesized that microglial AICD is mediated via CD38-dependent cADPR. However, it was previously reported that CD38 is not responsible for the production of most, if not all, of cADPR content in brain tissues (32, 36). Therefore, we first tested whether CD38 regulates cADPR content in primary microglia. WT and Cd38−/− primary microglial cultures were treated without or with LPS/IFNγ for 24 h and then the endogenous cADPR content in the WT and Cd38−/− primary microglial homogenates were determined by the sensitive cycling assay (36). As shown in Fig. 5C, the cADPR content of Cd38−/− primary microglial homogenates, either untreated or treated with LPS/IFNγ, were markedly lower than in WT primary microglial homogenates (3 and 7 fold reduction respectively). In addition, we observed that the cADPR content of the microglial cells was increased in the LPS/IFNγ-treated cells. These results thus suggest the CD38 is most likely responsible for the generation of most cADPR in microglia and thus that CD38-dependent cADPR might play a role in microglial responses. We therefore next examined whether LPS/IFNγ induces an increase in CD38 cyclase activity in intact primary microglia. Cyclase activity was monitored spectrofluorimetrically, using NGD+ as a substrate (NGD+-ribosyl cyclase activity). Fig. 5D shows that treatment of WT primary microglia with LPS/IFNγ for 24 h induced an increase of approximately 5 fold in ectocellular GDP-ribosyl cyclase activity relative to untreated cells. No GDP-ribosyl cyclase activity was detected in CD38-deficient primary microglia, indicating that the induced increase is dependent on CD38.
FIGURE 5.
Assessment of CD38 receptor and enzymatic activities in primary microglia. A, CD38 cross-linking does not affect the viability of primary microglia. WT primary microglia were untreated (Control) or treated with LPS/IFNγ for 72 h in absence or presence of the indicated concentrations of the primary monoclonal Abs: clone 90 (90) or NIM-R5 anti-CD38 Ab or with isotype control Ab [(rat IgG2a (IgG)], without (−) or with (+) the secondary anti-rat IgG Ab F(ab′)2 (3 μg/ml). Cell death was assessed by monitoring LDH released into the culture medium, and was defined and expressed as described in the legend of Fig. 4. Values shown are means ± SEM (bars) (n = 3). B, CD38 cross-linking induces cell death in CD38exp but not CD38neg Ba/F3 cells. CD38neg and CD38exp Ba/F3 cells were stimulated with the CD38 agonist Abs or isotype control Ab for 24 h. Cell viability was assessed by the MTT assay as described in Materials and Methods. Survival was defined and expressed (left panel) as described in the legend of Fig. 4. Values shown are means ± SEM (bars) (n = 3); *P < 0.05 compared to isotype control treated CD38exp Ba/F3 cells (Student’s t-test). Expression of CD38 in the CD38neg and CD38exp Ba/F3 cells (right panel). Ba/F3 cells were stained and analyzed for CD38 expression by flow cytometry as described in Fig. 3. The results of a representative experiment for each genotype (one of three independent experiments) are shown. The empty histogram represents the background staining (Mock) obtained with an isotype control Ab, the black filled histogram represents CD38 staining in the CD38exp cells, and the grey filled histogram represents CD38 staining in CD38neg Ba/F3 cells. C, cADPR content is lower in Cd38−/− primary microglia than in WT primary microglia. WT and Cd38−/− primary microglia were untreated (Control) or treated with LPS/IFNγ for 24 h and then extracts were prepared from the cells and their cADPR content was determined using the cycling assay (as described in the Material and Methods). Values shown are means ± SEM (bars) (n = 4 – 5, for untreated and LPS/IFNγ-treated respectively); *P< 0.05 compared to the corresponding treatment in Cd38−/− cells (Student’s t-test). D, LPS/IFNγ treatment induces GDP-ribosyl cyclase activity in WT primary microglia. WT and Cd38−/− primary microglia were grown in the absence (control) or presence of LPS/IFNγ for 24 h, and were then subjected to the GDP-ribosyl cyclase activity assay as described in Materials and Methods. Accumulation of the product, cGDPR, was measured fluorometrically. Data presented for WT primarymicroglia are the fluorescence values obtained after 10 min (no specific fluorescence was detected for Cd38−/− primarymicroglia). Values shown are means ± SEM (bars) (n = 3)
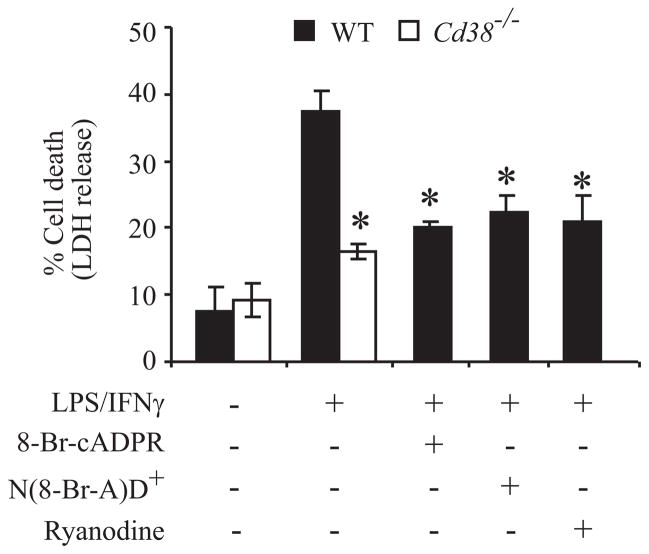
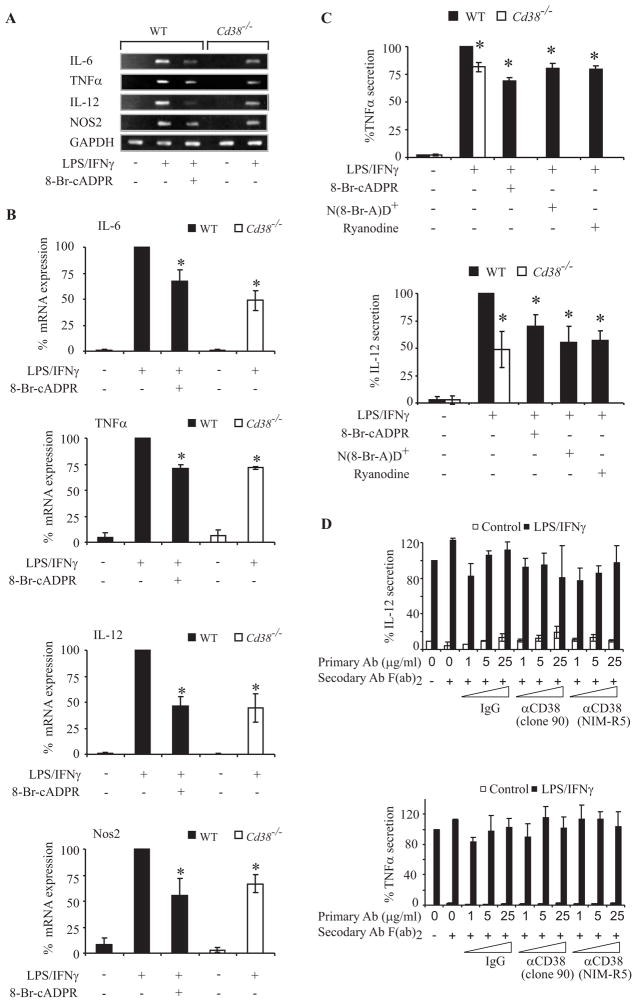
To further examine the role of CD38 enzymatic activity in microglial AICD, we examined the role of the CD38 metabolite cADPR in this process. WT primary microglia were treated with LPS/IFNγ for 48 h in the absence or presence of the cADPR inhibitors 8-Br-cADPR (a potent cADPR antagonist) and N(8-Br-A)D+ [an NAD+ analog that can be cyclized by CD38 into the cADPR antagonist 8-Br-cADPR (34)] as well as ryanodine, a cADPR antagonist (49, 50). Fig. 6 shows that all three compounds inhibited the LPS/IFNγ-induced AICD to the same extent as CD38 deficiency.
FIGURE 6.
The role of cADPR in LPS/IFNγ responses. cADPR plays an important role in LPS/IFNγ-induced microglial AICD. WT and Cd38−/− primary microglia were untreated or treated for 48 h in the absence (−) or presence (+) of the cADPR antagonists 8-Br-cADPR (100 μM) and ryanodine (30 μM) or the CD38 substrate analog N(8-Br-A)D+ (100 μM). Cell death was assessed by monitoring LDH released to the culture medium, and was defined and expressed as described in the legend of Fig. 4. Values shown are means ± SEM (bars) (n = 3); *P < 0.05 compared to WT microglia treated with LPS/IFNγ (Student’s t-test).
Taken together, these results suggest that CD38 regulates the LPS/IFNγ-induced AICD in primary microglia, and that this effect is due, at least in part, to the enzymatic activity of CD38 via its production of cADPR.
CD38 is an important player in LPS/IFNγ-induced NO production
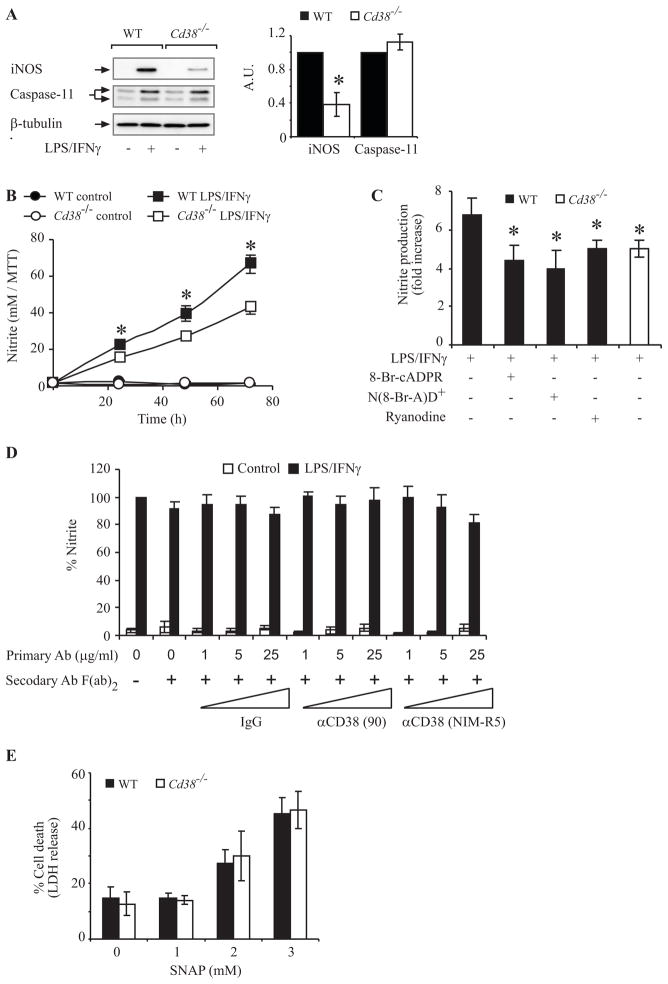
NO and caspase 11 are essential different mediators of LPS/IFNγ-induced microglial AICD (23, 24, 26). We were therefore interested in examining whether the effect of CD38 on LPS/IFNγ-induced AICD in primary microglia is mediated via CD38-dependent regulation of NO production, via caspase-11 expression, or both. Examination of the expression of iNOS and caspase-11 in LPS/IFNγ-treated microglia disclosed that iNOS expression induced by LPS/IFNγ in Cd38−/− primary microglia was markedly lower (by 2.5-fold) than in WT primary microglia. The induction of caspase-11, however, was not affected by CD38 expression (Fig. 7A). These results thus suggest that CD38 controls microglial AICD by promoting NO production (via its effect on iNOS) but not on caspase-11 activation. To further assess if and how CD38 regulates NO production we examined the effect of CD38 deficiency and cADPR antagonists as well as agonist anti-CD38 Abs on LPS/IFNγ-induced NO production. Fig. 7B shows that NO production was significantly lower in LPS/IFNγ-treated Cd38−/− primary microglia than in WT primary microglia. Fig. 7C shows that the cADPR antagonists 8-Br-cADPR and ryanodine and the CD38 substrate analog N(8-Br-A)D+ decreased LPS/IFNγ-induced NO production in WT primary microglia to similar extents (~40% inhibition). Importantly, the reduction in NO production caused by 8-Br-cADPR, ryanodine or by N(8-Br-A)D+ was similar to that caused by CD38 deficiency. In contrast to the effect of the cADPR antagonists on NO production, the crosslinking of CD38 did not have any effect on NO production in either LPS/IFNγ-untreated or treated WT primary microglia (Fig 7D). These results suggest that in primary microglia CD38 modulates LPS/IFNγ-induced NO production, and that this effect is mediated, at least in part, by the Ca2+-mobilizing cADPR metabolite. Taken together these results suggest that CD38 plays an important role in the regulation of LPS/IFNγ-induced NO production and that this effect is mediated via regulation of iNOS induction. In view of the essential role of NO in microglial AICD, these results further support the suggestion that CD38 regulates AICD by promoting NO production. To further assess the relationship between NO production and the effect of CD38 on AICD, we examined the susceptibility of WT and Cd38−/− primary microglia to external NO by treating them with the NO donor S-nitroso-N-acetylpenicillamine (SNAP). Fig. 7E shows that WT and Cd38−/− primary microglia exhibited similar susceptibilities to SNAP at all concentrations. This result suggested that the mechanism underlying the enhanced resistance of Cd38−/− cells to AICD is mediated not by an inhibition of downstream events in the NO-induced apoptotic pathway but rather by attenuation of NO production. Thus, once NO is generated, the sensitivity of Cd38−/− primary microglia to NO is similar to that of the WT cells.
FIGURE 7.
Participation of NO but not caspase 11 in the effect of CD38 on microglial AICD. A, iNOS and caspase-11 expression in WT and Cd38−/− primary microglia. Cells were treated without (−) or with LPS/IFNγ (+) for 24 h. Protein extracts were then prepared, and iNOS and caspase-11 expression were examined by immunoblot analysis (left panel). The results shown are from a representative experiment (one of three independent experiments). Immunoblots obtained from three independent experiments (including the one shown here) were scanned and the intensities of the different proteins were assessed by densitometric analysis. Data (right panel) are expressed as mean values of the intensities obtained for each protein after its normalization to β-tubulin, and are shown as the values obtained for each protein in LPS/IFNγ-treated Cd38−/− microglia relative to LPS/IFNγ-treated WT microglia (defined as 1 A.U.). B, C, the effect of CD38 and cADPR on NO production. WT and Cd38−/− primary microglia were untreated (control) or treated with LPS/IFNγ for the indicated times (B) or for 24 h in the absence (−) or presence (+) of 8-Br-cADPR (100 μM), ryanodine (30 μM) or N(8-Br-A)D+ (100 μM) (C). NO production (Griess reaction) and MTT values were then measured as described in Material and Methods. Nitrite levels in the medium of each treatment were normalized to the number of live cells in that treatment, as determined by MTT assay, and the results shown in B and C are expressed as the normalized nitrite values in each LPS/IFNγ treatment relative to the normalized nitrite values of its corresponding control [(24 h, untreated or treated with 8-Br-cADPR, ryanodine or N(8-Br-A)D+alone) (fold increase)]. Values shown are means ± SEM (bars) (n = 3). *P * 0.05 compared to the corresponding LPS/IFNγ-treated Cd38−/− microglia (B) or to LPS/IFNγ-treated WT microglia (C) (Student’s t-test). D, CD38 cross-linking does not affect NO production in LPS/IFNγ-untreated or treated primary microglia. WT primary microglia were untreated (Control) or treated with LPS/IFNγ for 24 h in absence or presence of primary or secondary Ab as described in the legend of Fig 5A. Nitrite levels in the medium of each treatment were normalized as described in B and C and expressed as the normalized nitrite values in each treatment relative to the normalized nitrite values in primary microglia treated only with LPS/IFNγ. Values shown are means ± SEM (bars) (n = 3). E, WT and Cd38−/− primary microglia exhibit similar susceptibilities to SNAP toxicity. WT and Cd38−/− primary microglia were treated with the indicated concentrations of the NO donor SNAP for 24 h, and cell death was then determined by the LDH assay, as described in the legend of Fig. 4A. Values shown are means ± SEM (bars) (n = 3).
CD38 is an important mediator of LPS/IFNγ-induced microglial activation
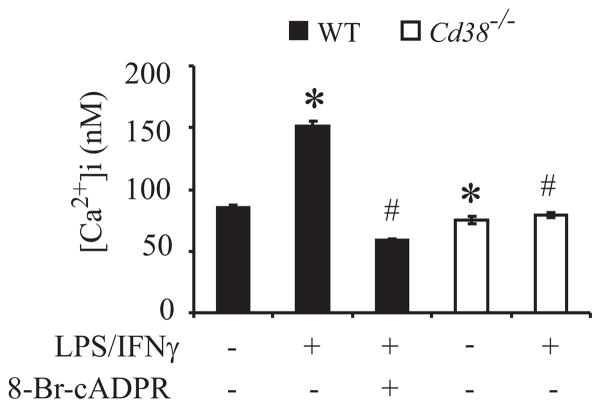
Apart from its role in microglial AICD, NO production is an important feature of microglia activation. In view of our results that CD38/cADPR regulates NO production and the notion that elevation of intracellular Ca2+ plays a central role in microglial activation (51–53) we hypothesize that CD38 might act also as a mediator of microglial activation via a cADPR-dependent increase in [Ca2+]i. To test this hypothesis we examined whether LPS/IFNγ increases [Ca2+]i and assessed the effects of CD38 deficiency and the presence of the cADPR antagonist 8-Br-cADPR on any such LPS/IFNγ-induced increase in [Ca2+]i. Fig. 8 shows that the basal level of [Ca2+]i in Cd38−/− primary microglia was lower than in WT microglia (75.12 ± 2.9 nM and 85.25 ± 2.19 nM, respectively). Treatment with LPS/IFNγ for 24 h increased [Ca2+]i in the WT but not in the Cd38−/− primary microglia. Furthermore, 8-Br-cADPR completely blocked the LPS/IFNγ-induced increase in [Ca2+]i.
FIGURE 8.
LPS/IFNγ induces enhancement in [Ca+2]i via the CD38/cADPR pathway. WT and Cd38−/− primary microglia were untreated (−) or treated with LPS/IFNγ (+) for 24 h in the absence (−) or presence (+) of the cADPR antagonist 8-Br-cADPR. The cells were loaded with fura-2 AM as described in Material and Methods. [Ca+2]i levels were determined on the basis of the fluorescence intensity ratio (F340/380). Values shown are means ± SEM (bars) from 2–3 independent experiments (n = 100–140 cells); *P < 0.05 relative to untreated WT cells; #P < 0.05 relative to LPS/IFNγ-treated WT cells.
Taken together, our results show that treatment with LPS/IFNγ induced an increase in [Ca2+]i in primary microglia and that this increase was mediated by the CD38/cADPR pathway. They therefore suggest that, in addition to its effect on AICD, CD38 might also participate in the Ca2+-dependent microglial activation events induced by LPS/IFNγ. In the next experiments, therefore, we examined the role of CD38 in LPS/IFNγ-induced microglial activation by assessing the effects of CD38 expression and cADPR antagonists on such activation. Microglial activation was assessed in terms of the induced expression of TNFα, IL-12 p40, IL-6, and NOS2 mRNAs, as determined by semi-quantitative RT-PCR analysis. WT and Cd38−/− primary microglia were treated with or without LPS/IFNγ for 10 h, and total RNA was then prepared. Fig. 9A shows that the expression levels of TNFα, IL-12 p40, IL-6, and NOS2 mRNAs were lower in Cd38−/− primary microglia than in the WT, and that the cADPR inhibitor 8-Br-cADPR reduced the amounts of IL-6, NOS2, TNFα, and IL-12 p40 (IL-12) mRNAs in LPS/IFNγ-treated WT microglia to approximately the levels detected in LPS/IFNγ-treated Cd38−/− primary microglia. Quantitative analysis (Fig. 9B) of the intensities of each of the different RT-PCR products normalized to the intensity of GAPDH in the same treatment from 3–5 different independent experiments confirmed the results of the representative experiments shown in Fig. 9A. To further verify the results of the semi-quantitative RT-PCR, we also examined the effects of CD38 deficiency and the cADPR antagonist on the LPS/IFNγ-induced secretion of TNFα and IL-12 p40 protein. Fig. 9C shows that the amounts of TNFα and IL-12 p40 proteins (measured by ELISA) secreted after 24 h from LPS/IFNγ-treated Cd38−/− primary microglia were significantly lower (by 20% and 50%, respectively) than those secreted from WT LPS/IFNγ-treated primary microglia. Furthermore, treatment with 8-Br-cADPR, ryanodine or N(8-Br-A)D+ reduced the amounts of LPS/IFNγ-induced TNFα and IL-12 p40 secretion in WT primary microglia to about the same levels as those secreted by Cd38−/− primary microglia. In contrast, treatment with the cross-liking Abs did not affect TNFα or IL-12 p40 secretion in LPS/IFNγ-untreated or treated primary microglia (Fig. 9D). Taken together these results show that CD38 deficiency, and cADPR antagonists all attenuate the induction of key players in microglial activation, and thus suggest that CD38, via its metabolite cADPR, regulates the LPS/IFNγ signaling that triggers microglial activation.
FIGURE 9.
CD38 and cADPR are important players in microglial activation. WT and Cd38−/− primary microglia were grown without (−) or with (+) LPS/IFNγ in the absence (−) or presence (+) of 8-Br-cADPR (100 μM), ryanodine (30 μM) or N(8-Br-A)D+ (100 μM), as described in Materials and Methods. Semi-quantitative RT-PCR analysis was performed as described in Materials and Methods. PCR products were separated on agarose gel electrophoresis and the results of a representative experiment (one of 3–5 independent experiments) are shown in A. B, Quantification of the semi-quantitative RT-PCR results. Intensities of the PCR products in the different independent experiments (including the one shown) were measured and normalized to GAPDH, as described in Materials and Methods. Results are recorded as the normalized PCR product intensity value in each treatment expressed as a percentage of the value obtained for the LPS/IFNγ treatment. Values shown are means ± SEM (bars) (n = 3–5); *P < 0.05 relative to LPS/IFNγ treatment. For analysis of TNFα and IL-12 p40 secretion (C), media were collected and the concentrations of TNFα and IL-12 p40 in the media were determined by ELISA, as described in Materials and Methods. Results are expressed as the amounts of TNFα and IL-12 p40 released to the medium, normalized to the number of cells (MTT assay) or protein concentration, respectively, in the corresponding plates, as described in Materials and Methods. Data are recorded as the normalized values in the different treatments expressed as percentages of the value obtained for the LPS/IFNγ treatment in the WT. Values shown are means ± SEM (bars) (n = 4); *P < 0.05 relative to LPS/IFNγ treatment in the WT. D, CD38 cross-linking does not affect TNFα and IL-12 p40 secretion in LPS/IFNγ-untreated or treated primary microglia. WT primary microglia were untreated (Control) or treated with LPS/IFNγ for 72 h in absence or presence of primary or secondary Abs as described in the legend of Fig 5A. Analysis of TNFα and IL-12 p40 secretion and expression of the data are as described in C. Values shown are means ± SEM (bars) (n = 3);
Discussion
Microglial activation plays an important role both in the beneficial and in the harmful actions of microglia in the CNS. Microglial AICD is one of the processes suggested to regulate the numbers of microglia that become activated after brain derangement. In the present study we sought to identify the mechanism connecting microglial activation to AICD. Our experiments with the inhibitors of macromolecule synthesis disclosed that newly synthesized mRNA and proteins are needed for microglial AICD. Analysis of a DNA microarray enabled us to identify CD38 as a potential candidate for mediating microglial AICD. CD38 is an ectoenzyme that catabolizes NAD(P)+ to generate multiple Ca2+-mobilizing metabolites, including ADPR, cADPR, and nicotinic acid adenine dinucleotide phosphate (NAADP+) (27, 54, 55). CD38 can also act as a cell-surface receptor capable of initiating a signal transduction. Our results show that CD38, via its metabolite cADPR, but not its receptor activity, plays an important role in mediating LPS/IFNγ-induced microglial AICD and activation.
Evidence for the participation of CD38 and cADPR in microglial activation and AICD came from the following observations: (i) CD38 expression and enzymatic activity were induced in microglia by treatment with LPS/IFNγ. (ii) CD38 is the main contributor of cADPR content in both LPS/IFNγ treated and untreated primary microglia. (iii) CD38 expression in primary microglia was found to be needed for LPS/IFNγ-induced increase in [Ca2+]i and, at least in part, for induction of various features of microglial activation, including NOS2 induction and NO production, the mRNA induction and protein secretion of TNFα and IL-12 p40, and IL-6 mRNA induction. (iv) CD38-deficient primary microglia were more resistant than WT microglia to LPS/IFNγ-induced AICD. (v) cADPR antagonists inhibited LPS/IFNγ-induced microglial activation and AICD in WT primary microglia and the extent of this inhibition was similar to that obtained by CD38 deficiency.
The finding that CD38 plays an important role in microglial activation is in line with published studies in which CD38 was assigned as a key regulator of diverse biological processes, particularly in hematopoietic cells. Such processes include response to inflammatory challenges (32, 56), activation of B, T, and NK cells (57–60), and Ig isotype switching (61). In addition, CD38 was shown to mediate chemotaxis in response to formyl peptide receptor ligands such as fMLF in primary human monocytes and N9 cells (56, 62).
The results showing that CD38 is the main contributor of primary microglia cADPR content are in line with previous studies which showed that CD38 is the main ADP-ribosyl-cyclase in bone marrow myeloid cells (32). However, it was reported that the cADPR content in Cd38−/− in adult (32, 36) and newborn (36) brain tissues is nearly equivalent to that of the corresponding WT brain tissues and that CD38-deficient neural cells exhibit an ADP-ribosyl cyclase activity (36, 63). These results lead to the suggestion that CD38 may not be the major cADPR producing enzyme in brain (36). We believe that the likely reason for the discrepancy between our results and the published findings is that the published experiments examined the cADPR content in total brain tissue, whereas microglial cells only represent a small proportion (5–12% in different areas of the brain) (64) of the total cells in the brain. Thus, some of the cell types in the brain, such as the neural cells, express the still unknown cyclase, while other cell types, like microglial cells express CD38.
Mechanism whereby CD38 regulates LPS/IFNγ-induced microglial activation and AICD
CD38, via its metabolite cADPR, regulates Ca2+-mediated signal transduction by increasing the amounts of intracellular free Ca2+ in cells [by promoting Ca2+ release from ryanodine receptor-gated stores and by regulating the influx of extracellular Ca2+(32, 65)]. It thus seems feasible that CD38 mediates cADPR-associated LPS/IFNγ responses by increasing [Ca2+]i. Our results showed that LPS/IFNγ indeed induces an increase in [Ca2+]i and that this increase was mediated by CD38 and its metabolite cADPR. Ca2+ is also an important mediator of the activation of LPS signaling in primary microglia (51). There is an interesting similarity between the presently observed effects of CD38 deficiency and cADPR antagonists on LPS/IFNγ-induced primary microglial activation and the previously reported effect of the Ca2+ chelator BAPTA (51) on primary microglia activation induced by LPS. According to that report, BAPTA inhibited LPS-induced production of NO and secretion of TNFα and IL-12 in WT primary microglial cultures.
It should be noted that CD38 produces, in addition to cADPR, two other important Ca2+-mobilizing metabolites, NAADP+ and ADPR (27). NAADP+ induces Ca2+ release from intracellular lysosomal stores whereas ADPR, the major metabolite produced by CD38, induces Ca2+ influx via the transient receptor potential (TRPM2) cation channels (66–68). Our data does not exclude the possibility that these additional metabolites generated by CD38 may also play a role in regulating microglial cell activation or AICD. In addition, we cannot exclude the possibility that the loss of CD38 alters NAD+ homeostasis (69, 70), which may lead in turn to “disregulated” activity of other NAD+-utilizing enzymes such as ecto-ADP-ribosyltransferases (ART) (31) and poly(ADP-ribose) polymerases (PARP). While NAD dysregulation or production of the other metabolites produced by CD38 might also participate in LPS/IFNγ-mediated microglial activation and AICD, our data clearly indicate that cADPR is important in these processes. Furthermore, our experiments using the CD38 substrate analog N(8-Br-A)D+ also strongly suggested that CD38 mediates effects through an enzyme-dependent mechanism and not by functioning as a cell-surface signaling receptor as reported in hematopoietic cells (34, 43). This conclusion is further supported by our results which show that in primary microglia the cross-linking of CD38 using two different agonist anti-CD38 Abs did not affect either the viability of NO production or cytokine secretion by LPS/IFNγ-treated or untreated WT microglia.
Our results, point to a regulatory role for CD38 in LPS/IFNγ-induced microglial activation, and are in line with studies that suggested that cADPR has an important function in mediating the effects of LPS (71) and of fMLF (56) in the N9 cell line. In our study, however, the role of CD38 in LPS/IFNγ-induced microglial activation was demonstrated directly, and this CD38 effect was shown to occur in primary microglial cultures. The former finding is of great interest because it is becoming increasingly evident that microglia do not constitute a single, uniform cell population but rather comprise a family of cells (72). It is therefore of the utmost importance to study the characteristics of microglial activation in primary microglial cultures.
It should be noted that although microglial activation and AICD were attenuated by CD38 deficiency and cADPR antagonists, the inhibition was not complete. With regard to microglial AICD, it is known that this process is mediated by several pathways and molecules (23, 26). CD38 is thus one of several molecules whose induced expression and combined action culminates in microglial cell death. Similarly, additional signaling systems beside CD38/cADPR are likely to participate in the induction of microglial activation by LPS/IFNγ. Indeed, we found that CD38/cADPR does not regulate caspase 11 expression, an important mediator ofAICD pathway. Furthermore, data from others have shown that enhancement of [Ca 2+]i alone did not induce NO production or cytokine secretion in primary microglia (51). Thus, it is clear that the CD38/cADPR-dependent signaling pathway is only one of perhaps many pathways that contribute to microglial activation and AICD.
Linking microglial activation to AICD
Studies by our group (26) and by other (23, 24) have shown that NO is an essential mediator of LPS/IFNγ-induced AICD. Our present finding that CD38 promotes the LPS/IFNγ-induced production of NO in primary microglia suggests that the effect of CD38 on microglial AICD is mediated via this CD38-mediated regulation of iNOS induction and NO production. This likelihood is further supported by the observation that CD38 does not affect caspase-11 expression, also an essential step in LPS/IFNγ-induced AICD (23). Moreover, we did not observe any substantial differences in the expression levels of key apoptotic proteins (Bcl-xL, MCl-1, Bid, Bim caspase-1, and caspase-7) between CD38-expressing and CD38-deficient primary microglia (data not shown). Furthermore, in contrast to the observed resistance of CD38-deficient primary microglia to LPS/IFNγ, these cells were as sensitive as CD38-expressing microglia to NO donor toxicity and to the well established apoptotic trigger staurosporine (data not shown). Taken together, these results suggest that the lack of CD38 does not endow the cells with a general resistance to apoptosis but rather confers a specific resistance to LPS/IFNγ-induced AICD, probably by reducing the amount of NO. Our results further suggest that CD38 links activation to AICD by controlling the amount of NO that the cells produce.
Activated microglia are present in almost all neurological diseases, where they may promote both beneficial and harmful effects (72). Our demonstration of the important role of CD38 in microglial activation and AICD suggests that CD38, via its regulatory effect on microglial functions and fate, might play an important role in acute and chronic brain derangements, and hence that manipulation of CD38 expression and/or activity might represent a useful future approach for treating neurological diseases. Towards that aim it will be necessary to extend the present in-vitro study to systems in vivo, and to study microglial activation and brain susceptibility to injury and inflammation in CD38-deficient mice after various brain insults. Such studies are currently in progress in our laboratory.
Acknowledgments
We are grateful to Ms. Shirley Smith for excellent editorial assistance. We thank Dr. Uri Ashery (Tel Aviv University, Israel) for help with calcium measurements and Dr. Norman Oppenheimer (Univ. California, San Francisco) for providing N(8-Br-A)D+.
Abbreviations used in this paper
- AICD
activation-induced cell death
- cADPR
cylic ADP-ribose
- AMD
actinomycin D
- N(8-Br-A)D+
nicotinamide 8-bromoadenine dinucleotide
- iNOS
inducible nitric oxide synthetase
- WT
wild-type
- CHX
cycloheximide
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- LDH
lactate dehydrogenase
- NGD
nicotinamide–guanine dinucleotide
- cGDPR
cyclic GDP-ribose
- SNAP
S-nitroso-N-acetylpenicillamine
- MFI
mean fluorescence intensity
Footnotes
Disclosure
The authors have no financial conflict of interest.
This work was supported by the Israel Science Foundation (643/02), the Adams Super-Center for Brain Research, and National Institutes of Health (R01-AI-057996 to F.E.L.).
References
- 1.Raivich G, Bohatschek M, Kloss CU, Werner A, Jones LL, Kreutzberg GW. Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain Research Reviews. 1999;30:77–105. doi: 10.1016/s0165-0173(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 2.Streit WJ, Conde JR, Fendrick SE, Flanary BE, Mariani CL. Role of microglia in the central nervous system’s immune response. Neurol Res. 2005;27:685–691. doi: 10.1179/016164105X49463a. [DOI] [PubMed] [Google Scholar]
- 3.Roy A, Fung YK, Liu X, Pahan K. Up-regulation of microglial CD11b expression by nitric oxide. J Biol Chem. 2006;281:14971–14980. doi: 10.1074/jbc.M600236200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiner HL, Frenkel D. Immunology and immunotherapy of Alzheimer’s disease. Nat Rev Immunol. 2006;6:404–416. doi: 10.1038/nri1843. [DOI] [PubMed] [Google Scholar]
- 5.Batchelor PE, Liberatore GT, Wong JY, Porritt MJ, Frerichs F, Donnan GA, Howells DW. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J Neurosci. 1999;19:1708–1716. doi: 10.1523/JNEUROSCI.19-05-01708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz M. Macrophages and microglia in central nervous system injury: are they helpful or harmful? J Cereb Blood Flow Metab. 2003;23:385–394. doi: 10.1097/01.WCB.0000061881.75234.5E. [DOI] [PubMed] [Google Scholar]
- 7.Paresce DM, Ghosh RN, Maxfield FR. Microglial cells internalize aggregates of the Alzheimer’s disease amyloid beta-protein via a scavenger receptor. Neuron. 1996;17:553–565. doi: 10.1016/s0896-6273(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 8.Boje KM, Arora PK. Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res. 1992;587:250–256. doi: 10.1016/0006-8993(92)91004-x. [DOI] [PubMed] [Google Scholar]
- 9.Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol. 1992;149:2736–2741. [PubMed] [Google Scholar]
- 10.Chao CC, Hu S, Peterson PK. Glia, cytokines, and neurotoxicity. Crit Rev Neurobiol. 1995;9:189–205. [PubMed] [Google Scholar]
- 11.McGeer EG, McGeer PL. The importance of inflammatory mechanisms in Alzheimer disease. Exp Gerontol. 1998;33:371–378. doi: 10.1016/s0531-5565(98)00013-8. [DOI] [PubMed] [Google Scholar]
- 12.Halliday G, Robinson SR, Shepherd C, Kril J. Alzheimer’s disease and inflammation: a review of cellular and therapeutic mechanisms. Clin Exp Pharmacol Physiol. 2000;27:1–8. doi: 10.1046/j.1440-1681.2000.03200.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim YS, Joh TH. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson’s disease. Exp Mol Med. 2006;38:333–347. doi: 10.1038/emm.2006.40. [DOI] [PubMed] [Google Scholar]
- 14.Beschorner R, Nguyen TD, Gozalan F, Pedal I, Mattern R, Schluesener HJ, Meyermann R, Schwab JM. CD14 expression by activated parenchymal microglia/macrophages and infiltrating monocytes following human traumatic brain injury. Acta Neuropathology. 2002;103:541–549. doi: 10.1007/s00401-001-0503-7. [DOI] [PubMed] [Google Scholar]
- 15.Koshinaga M, Katayama Y, Fukushima M, Oshima H, Suma T, Takahata T. Rapid and widespread microglial activation induced by traumatic brain injury in rat brain slices. Journal of Neurotrauma. 2000;17:185–192. doi: 10.1089/neu.2000.17.185. [DOI] [PubMed] [Google Scholar]
- 16.Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Prog Neurobiol. 1999;57:563–581. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 17.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 18.Dihne M, Block F, Korr H, Topper R. Time course of glial proliferation and glial apoptosis following excitotoxic CNS injury. Brain Res. 2001;902:178–189. doi: 10.1016/s0006-8993(01)02378-2. [DOI] [PubMed] [Google Scholar]
- 19.Vela JM, Yanez A, Gonzalez B, Castellano B. Time course of proliferation and elimination of microglia/macrophages in different neurodegenerative conditions. J Neurotrauma. 2002;19:1503–1520. doi: 10.1089/089771502320914723. [DOI] [PubMed] [Google Scholar]
- 20.Bonetti B, Pohl J, Gao YL, Raine CS. Cell death during autoimmune demyelination: effector but not target cells are eliminated by apoptosis. J Immunol. 1997;159:5733–5741. [PubMed] [Google Scholar]
- 21.Gehrmann J, Banati RB. Microglial turnover in the injured CNS: activated microglia undergo delayed DNA fragmentation following peripheral nerve injury. J Neuropathol Exp Neurol. 1995;54:680–688. doi: 10.1097/00005072-199509000-00010. [DOI] [PubMed] [Google Scholar]
- 22.White CA, McCombe PA, Pender MP. Microglia are more susceptible than macrophages to apoptosis in the central nervous system in experimental autoimmune encephalomyelitis through a mechanism not involving Fas (CD95) Int Immunol. 1998;10:935–941. doi: 10.1093/intimm/10.7.935. [DOI] [PubMed] [Google Scholar]
- 23.Lee J, Hur J, Lee P, Kim JY, Cho N, Kim SY, Kim H, Lee MS, Suk K. Dual role of inflammatory stimuli in activation-induced cell death of mouse microglial cells. Initiation of two separate apoptotic pathways via induction of interferon regulatory factor-1 and caspase-11. J Biol Chem. 2001;276:32956–32965. doi: 10.1074/jbc.M104700200. [DOI] [PubMed] [Google Scholar]
- 24.Lee P, Lee J, Kim S, Lee MS, Yagita H, Kim SY, Kim H, Suk K. NO as an autocrine mediator in the apoptosis of activated microglial cells: correlation between activation and apoptosis of microglial cells. Brain Res. 2001;892:380–385. doi: 10.1016/s0006-8993(00)03257-1. [DOI] [PubMed] [Google Scholar]
- 25.Liu B, Wang K, Gao HM, Mandavilli B, Wang JY, Hong JS. Molecular consequences of activated microglia in the brain: overactivation induces apoptosis. J Neurochem. 2001;77:182–189. doi: 10.1046/j.1471-4159.2001.t01-1-00216.x. [DOI] [PubMed] [Google Scholar]
- 26.Mayo L, Stein R. Characterization of LPS and interferon-gamma triggered activation-induced cell death in N9 and primary microglial cells: induction of the mitochondrial gateway by nitric oxide. Cell Death Differ. 2007;14:183–186. doi: 10.1038/sj.cdd.4401989. [DOI] [PubMed] [Google Scholar]
- 27.Schuber F, Lund FE. Structure and enzymology of ADP-ribosyl cyclases: conserved enzymes that produce multiple calcium mobilizing metabolites. Current Molecular Medicine. 2004;4:249–261. doi: 10.2174/1566524043360708. [DOI] [PubMed] [Google Scholar]
- 28.Funaro A, Ferrero E, Mehta K, Malavasi F. Schematic portrait of human CD38 and related molecules. Chemical Immunology. 2000;75:256–273. doi: 10.1159/000058773. [DOI] [PubMed] [Google Scholar]
- 29.Fliegert R, Gasser A, Guse AH. Regulation of calcium signalling by adenine-based second messengers. Biochem Soc Trans. 2007;35:109–114. doi: 10.1042/BST0350109. [DOI] [PubMed] [Google Scholar]
- 30.Cockayne DA, Muchamuel T, Grimaldi JC, Muller-Steffner H, Randall TD, Lund FE, Murray R, Schuber F, Howard MC. Mice deficient for the ecto-nicotinamide adenine dinucleotide glycohydrolase CD38 exhibit altered humoral immune responses. Blood. 1998;92:1324–1333. [PubMed] [Google Scholar]
- 31.Krebs C, Adriouch S, Braasch F, Koestner W, Leiter EH, Seman M, Lund FE, Oppenheimer N, Haag F, Koch-Nolte F. CD38 controls ADP-ribosyltransferase-2-catalyzed ADP-ribosylation of T cell surface proteins. J Immunol. 2005;174:3298–3305. doi: 10.4049/jimmunol.174.6.3298. [DOI] [PubMed] [Google Scholar]
- 32.Partida-Sanchez S, Cockayne DA, Monard S, Jacobson EL, Oppenheimer N, Garvy B, Kusser K, Goodrich S, Howard M, Harmsen A, Randall TD, Lund FE. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat Med. 2001;7:1209–1216. doi: 10.1038/nm1101-1209. [DOI] [PubMed] [Google Scholar]
- 33.Righi M, Mori L, De Libero G, Sironi M, Biondi A, Mantovani A, Donini SD, Ricciardi-Castagnoli P. Monokine production by microglial cell clones. Eur J Immunol. 1989;19:1443–1448. doi: 10.1002/eji.1830190815. [DOI] [PubMed] [Google Scholar]
- 34.Lund FE, Muller-Steffner H, Romero-Ramirez H, Moreno-Garcia ME, Partida-Sanchez S, Makris M, Oppenheimer NJ, Santos-Argumedo L, Schuber F. CD38 induces apoptosis of a murine pro-B leukemic cell line by a tyrosine kinase-dependent but ADP-ribosyl cyclase- and NAD glycohydrolase-independent mechanism. Int Immunol. 2006;18:1029–1042. doi: 10.1093/intimm/dxl037. [DOI] [PubMed] [Google Scholar]
- 35.Saura J, Tusell JM, Serratosa J. High-yield isolation of murine microglia by mild trypsinization. Glia. 2003;44:183–189. doi: 10.1002/glia.10274. [DOI] [PubMed] [Google Scholar]
- 36.Ceni C, Pochon N, Villaz M, Muller-Steffner H, Schuber F, Baratier J, De Waard M, Ronjat M, Moutin MJ. The CD38-independent ADP-ribosyl cyclase from mouse brain synaptosomes: a comparative study of neonate and adult brain. Biochem J. 2006;395:417–426. doi: 10.1042/BJ20051321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graeff RM, Walseth TF, Fryxell K, Branton WD, Lee HC. Enzymatic synthesis and characterizations of cyclic GDP-ribose. A procedure for distinguishing enzymes with ADP-ribosyl cyclase activity. J Biol Chem. 1994;269:30260–30267. [PubMed] [Google Scholar]
- 38.Shamir R, Maron-Katz A, Tanay A, Linhart C, Steinfeld I, Sharan R, Shiloh Y, Elkon R. EXPANDER--an integrative program suite for microarray data analysis. BMC Bioinformatics. 2005;6:232. doi: 10.1186/1471-2105-6-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pattyn F, Robbrecht P, De Paepe A, Speleman F, Vandesompele J. RTPrimerDB: the real-time PCR primer and probe database, major update 2006. Nucleic Acids Res. 2006;34:D684–688. doi: 10.1093/nar/gkj155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yizhar O, Matti U, Melamed R, Hagalili Y, Bruns D, Rettig J, Ashery U. Tomosyn inhibits priming of large dense-core vesicles in a calcium-dependent manner. Proc Natl Acad Sci U S A. 2004;101:2578–2583. doi: 10.1073/pnas.0308700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 42.Lee HC. Enzymatic functions and structures of CD38 and homologs. Chemical Immunology. 2000;75:39–59. doi: 10.1159/000058774. [DOI] [PubMed] [Google Scholar]
- 43.Lund FE, Cockayne DA, Randall TD, Solvason N, Schuber F, Howard MC. CD38: a new paradigm in lymphocyte activation and signal transduction. Immunological Reviews. 1998;161:79–93. doi: 10.1111/j.1600-065x.1998.tb01573.x. [DOI] [PubMed] [Google Scholar]
- 44.Lund FE, Muller-Steffner HM, Yu N, Stout CD, Schuber F, Howard MC. CD38 signaling in B lymphocytes is controlled by its ectodomain but occurs independently of enzymatically generated ADP-ribose or cyclic ADP-ribose. J Immunol. 1999;162:2693–2702. [PubMed] [Google Scholar]
- 45.Gregorini A, Tomasetti M, Cinti C, Colomba D, Colomba S. CD38 expression enhances sensitivity of lymphoma T and B cell lines to biochemical and receptor-mediated apoptosis. Cell Biology International. 2006;30:727–732. doi: 10.1016/j.cellbi.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Kumagai M, Coustan-Smith E, Murray DJ, Silvennoinen O, Murti KG, Evans WE, Malavasi F, Campana D. Ligation of CD38 suppresses human B lymphopoiesis. J Exp Med. 1995;181:1101–1110. doi: 10.1084/jem.181.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silvennoinen O, Nishigaki H, Kitanaka A, Kumagai M, Ito C, Malavasi F, Lin Q, Conley ME, Campana D. CD38 signal transduction in human B cell precursors. Rapid induction of tyrosine phosphorylation, activation of syk tyrosine kinase, and phosphorylation of phospholipase C-gamma and phosphatidylinositol 3-kinase. J Immunol. 1996;156:100–107. [PubMed] [Google Scholar]
- 48.Rizzuto R, Pinton P, Ferrari D, Chami M, Szabadkai G, Magalhaes PJ, Di Virgilio F, Pozzan T. Calcium and apoptosis: facts and hypotheses. Oncogene. 2003;22:8619–8627. doi: 10.1038/sj.onc.1207105. [DOI] [PubMed] [Google Scholar]
- 49.Guse AH. Second messenger function and the structure-activity relationship of cyclic adenosine diphosphoribose (cADPR) Febs J. 2005;272:4590–4597. doi: 10.1111/j.1742-4658.2005.04863.x. [DOI] [PubMed] [Google Scholar]
- 50.White TA, Kannan MS, Walseth TF. Intracellular calcium signaling through the cADPR pathway is agonist specific in porcine airway smooth muscle. Faseb J. 2003;17:482–484. doi: 10.1096/fj.02-0622fje. [DOI] [PubMed] [Google Scholar]
- 51.Hoffmann A, Kann O, Ohlemeyer C, Hanisch UK, Kettenmann H. Elevation of basal intracellular calcium as a central element in the activation of brain macrophages (microglia): suppression of receptor-evoked calcium signaling and control of release function. J Neurosci. 2003;23:4410–4419. doi: 10.1523/JNEUROSCI.23-11-04410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moller T. Calcium signaling in microglial cells. Glia. 2002;40:184–194. doi: 10.1002/glia.10152. [DOI] [PubMed] [Google Scholar]
- 53.Farber K, Kettenmann H. Functional role of calcium signals for microglial function. Glia. 2006;54:656–665. doi: 10.1002/glia.20412. [DOI] [PubMed] [Google Scholar]
- 54.Howard M, Grimaldi JC, Bazan JF, Lund FE, Santos-Argumedo L, Parkhouse RM, Walseth TF, Lee HC. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science. 1993;262:1056–1059. doi: 10.1126/science.8235624. [DOI] [PubMed] [Google Scholar]
- 55.Lee HC. Nicotinic acid adenine dinucleotide phosphate (NAADP)-mediated calcium signaling. J Biol Chem. 2005;280:33693–33696. doi: 10.1074/jbc.R500012200. [DOI] [PubMed] [Google Scholar]
- 56.Partida-Sanchez S, Iribarren P, Moreno-Garcia ME, Gao JL, Murphy PM, Oppenheimer N, Wang JM, Lund FE. Chemotaxis and calcium responses of phagocytes to formyl peptide receptor ligands is differentially regulated by cyclic ADP ribose. J Immunol. 2004;172:1896–1906. doi: 10.4049/jimmunol.172.3.1896. [DOI] [PubMed] [Google Scholar]
- 57.Funaro A, Spagnoli GC, Ausiello CM, Alessio M, Roggero S, Delia D, Zaccolo M, Malavasi F. Involvement of the multilineage CD38 molecule in a unique pathway of cell activation and proliferation. J Immunol. 1990;145:2390–2396. [PubMed] [Google Scholar]
- 58.Sconocchia G, Titus JA, Mazzoni A, Visintin A, Pericle F, Hicks SW, Malavasi F, Segal DM. CD38 triggers cytotoxic responses in activated human natural killer cells. Blood. 1999;94:3864–3871. [PubMed] [Google Scholar]
- 59.Mallone R, Funaro A, Zubiaur M, Baj G, Ausiello CM, Tacchetti C, Sancho J, Grossi C, Malavasi F. Signaling through CD38 induces NK cell activation. Int Immunol. 2001;13:397–409. doi: 10.1093/intimm/13.4.397. [DOI] [PubMed] [Google Scholar]
- 60.Lund FE, Solvason NW, Cooke MP, Health AW, Grimaldi JC, Parkhouse RM, Goodnow CC, Howard MC. Signaling through murine CD38 is impaired in antigen receptor-unresponsive B cells. Eur J Immunol. 1995;25:1338–1345. doi: 10.1002/eji.1830250531. [DOI] [PubMed] [Google Scholar]
- 61.Yasue T, Baba M, Mori S, Mizoguchi C, Uehara S, Takatsu K. IgG1 production by sIgD+ splenic B cells and peritoneal B-1 cells in response to IL-5 and CD38 ligation. Int Immunol. 1999;11:915–923. doi: 10.1093/intimm/11.6.915. [DOI] [PubMed] [Google Scholar]
- 62.Pfister M, Ogilvie A, da Silva CP, Grahnert A, Guse AH, Hauschildt S. NAD degradation and regulation of CD38 expression by human monocytes/macrophages. Eur J Biochem. 2001;268:5601–5608. doi: 10.1046/j.1432-1033.2001.02495.x. [DOI] [PubMed] [Google Scholar]
- 63.Ceni C, Muller-Steffner H, Lund F, Pochon N, Schweitzer A, De Waard M, Schuber F, Villaz M, Moutin MJ. Evidence for an intracellular ADP-ribosyl cyclase/NAD+-glycohydrolase in brain from CD38-deficient mice. J Biol Chem. 2003;278:40670–40678. doi: 10.1074/jbc.M301196200. [DOI] [PubMed] [Google Scholar]
- 64.Lawson LJ, V, Perry H, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- 65.Guse AH, da Silva CP, Berg I, Skapenko AL, Weber K, Heyer P, Hohenegger M, Ashamu GA, Schulze-Koops H, Potter BV, Mayr GW. Regulation of calcium signalling in T lymphocytes by the second messenger cyclic ADP-ribose. Nature. 1999;398:70–73. doi: 10.1038/18024. [DOI] [PubMed] [Google Scholar]
- 66.Sano Y, Inamura K, Miyake A, Mochizuki S, Yokoi H, Matsushime H, Furuichi K. Immunocyte Ca2+ influx system mediated by LTRPC2. Science. 2001;293:1327–1330. doi: 10.1126/science.1062473. [DOI] [PubMed] [Google Scholar]
- 67.Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R, Kinet JP, Scharenberg AM. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature. 2001;411:595–599. doi: 10.1038/35079100. [DOI] [PubMed] [Google Scholar]
- 68.Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, Shimizu N, Kurose H, Okada Y, Imoto K, Mori Y. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell. 2002;9:163–173. doi: 10.1016/s1097-2765(01)00438-5. [DOI] [PubMed] [Google Scholar]
- 69.Young GS, Choleris E, Lund FE, Kirkland JB. Decreased cADPR and increased NAD+ in the Cd38−/− mouse. Biochem Biophys Res Commun. 2006;346:188–192. doi: 10.1016/j.bbrc.2006.05.100. [DOI] [PubMed] [Google Scholar]
- 70.Aksoy P, White TA, Thompson M, Chini EN. Regulation of intracellular levels of NAD: a novel role for CD38. Biochem Biophys Res Commun. 2006;345:1386–1392. doi: 10.1016/j.bbrc.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 71.Franco L, Bodrato N, Moreschi I, Usai C, Bruzzone S, Scarfi S, Zocchi E, De Flora A. Cyclic ADP-ribose is a second messenger in the lipopolysaccharide-stimulated activation of murine N9 microglial cell line. J Neurochem. 2006;99:165–176. doi: 10.1111/j.1471-4159.2006.04031.x. [DOI] [PubMed] [Google Scholar]
- 72.Schwartz M, Butovsky O, Bruck W, Hanisch UK. Microglial phenotype: is the commitment reversible? Trends Neurosci. 2006;29:68–74. doi: 10.1016/j.tins.2005.12.005. [DOI] [PubMed] [Google Scholar]