Abstract
Background
Mycobacterium tuberculosis (M. tuberculosis) infection in humans results in either latent infection or active tuberculosis (TB). We sought to determine whether a higher frequency of regulatory T cells predispose an individual toward active disease or whether the Tregs develop in response to active disease.
Methods
In cynomolgus macaques infected with a low dose M. tuberculosis approximately 50% develop primary TB and 50% present with latent infection. 41 animals were followed for 6-8 months to correlate the frequency of Foxp3+ cells in peripheral blood and airways with outcome of infection.
Results
In all animals, the frequency of Tregs (CD4+Foxp3+) in peripheral blood rapidly decreased and simultaneously increased in the airways. Latently infected monkeys had a significantly higher frequency of Tregs in peripheral blood prior to infection and during early infection than those that developed active disease. Monkeys with active disease had increased Tregs in PBMC as they developed disease.
Conclusions
Our data suggest that increased Tregs in active disease occur in response to more inflammation, rather than act as a causative factor in progression to active disease.
Keywords: Mycobacterium tuberculosis, Regulatory T cells, non-human primate
Introduction
Tuberculosis is the leading cause of death by a single infectious agent [1]. Although 5-10% of infected persons progress to primary TB, the majority control the infection, are asymptomatic and considered to be latently infected (LTBI)[2]. Factors contributing to these infection outcomes are not well understood. The extended chemotherapy, increase in drug resistant strains [3], and lack of an effective vaccine [4] make identification of factors that affect infection outcome imperative.
Once infected, the host mounts a robust Th1 response, and forms a granuloma, which can function to contain the bacilli. Presumably, the immune response must be controlled to limit damage of surrounding tissue. There is likely a fine balance in each granuloma of effector, inflammatory and regulatory mechanisms. Patients with active TB produce more anti-inflammatory cytokines such as IL-10 [5, 6] and TGFβ [6, 7], compared to LTBI, but whether aberrant immune modulation contributes to development of active disease is unknown. The CD4+ T regulatory cell (Treg) has been suggested as a possible factor in promotion of active TB.
CD4 T cells expressing CD25 are potent inhibitors of autoimmunity [8]; the transcription factor Foxp3 is a defining characteristic of these Treg cells [9]. Tregs inhibit inflammatory responses and proliferation by production of cytokines, cell-to-cell contact and inhibiting IL-2 [10]. Natural Tregs are thought to be self-reactive and prevent autoimmunity [11]. Tregs can also potentiate persistence of certain pathogens [12-16].
Recent data suggest a role for Tregs in M. tuberculosis persistence. Removal of Tregs in mice resulted in decreased bacterial burden in lungs [17, 18], indicating Tregs may down regulate M. tuberculosis specific immune responses. PBMCs from active TB patients had increased frequencies of Tregs and decreased IFNγ production in response to certain M. tuberculosis antigens when compared to LTBI [19-21]. In vitro depletion of CD25+ cells in active TB patients increased M. tuberculosis specific IFNγ production, suggesting Tregs are suppressing specific responses [19-21]. These studies are unable to differentiate between increased Tregs contributing to development of active TB or occurring in response to inflammation in active disease. Studies with human TB patients are complicated by difficulties in defining time of infection, extent of disease, mycobacterial strain and size of inoculum, which may contribute to the quality of immune responses and disease outcome.
To address whether an increased frequency of Tregs affects development of active disease or occurs in response to inflammation caused by active disease, we used a non-human primate (NHP) model of M. tuberculosis infection. This is the only established model to accurately mimic human latent infection [2]. When cynomolgus macaques are infected with a low dose via bronchoscope, ~50% of animals exhibit no signs of disease despite being tuberculin skin test positive and are considered latently infected by six months. The other 50% develop primary tuberculosis [2]. These clinical classifications were validated by pathology and bacterial numbers at necropsy [22]. Using this model, we addressed the correlation between Tregs and outcome of infection, and the dynamics of Treg in the periphery and airways.
Materials and Methods
Experimental animals
Cynomolgus macaques (Macaca fascicularis)(Alpha-Genesis,Yamassee, SC; Covance, Madison, WI; Valley Biosystems, Sacramento, CA), were ≥4 years of age, 3.5-10kg, housed in a biosafety level 3 facility [23], and free of TB or other infections. The University of Pittsburgh School of Medicine Institutional Animal Care and Use Committee approved all procedures and protocols.
Infection of NHP
Monkeys were infected with ~25 CFU M. tuberculosis strain Erdman as described [22, 23] by bronchoscopic instillation. CFU were determined in the inoculum by plating on 7H10 agar (Difco Laboratories, Detroit, MI). Infection of monkeys is confirmed by tuberculin skin test, and lymphocytic proliferation and IFN-γ production in response to mycobacterial antigens. Infection outcome was independent of age, gender and weight [22].
PBMC isolation
Blood was collected by percutaneous venipuncture [23]. Peripheral blood mononuclear cells (PBMCs) were isolated by Percoll gradient (Amersham Bioscience, Piscataway, NJ).
BAL Cells
Cells were sampled from airways by bronchoalveolar lavage (BAL)[23].
Necropsy of animals
Prior to necropsy, animals were sedated, then euthanized with sodium pentobarbital (Schering-Plough Animal Health, Union, NJ), as described [23]. A veterinary pathologist conducted all necropsies; tissues and samples were obtained in a sterile fashion [22].
Isolation of cells from necropsy tissue
At necropsy, granulomatous and non-granulomatous lung and lymph node were excised [22]. Cell suspensions were obtained by homogenizing tissues in PBS using a MediMixer (BD Biosciences, San Jose, CA). An aliquot of each suspension was plated for enumeration of M. tuberculosis colonies.
CD25 depletion from PBMCs
PBMCs were stained with PE anti-CD25 (Clone M-A251, BD Pharmingen, San Jose, CA); CD25+ cells were isolated using anti-PE beads (Miltenyi, Auburn, CA).
Lymphocyte proliferation assay
PBMCs prior to and at 6 weeks p.i. and for depletion studies were suspended in AIM V media (Invitrogen, Grand Island, NY) at 200,000 cells/well in 200 μl. Cells were stimulated with phytohemagglutinin (PHA 5μg/ml), culture filtrate protein (CFP, NIH-NIAID Contract HHSN266200400091C)(10 μg/ml), or media in triplicate wells for 60 hours at 37°C, 5% CO2; for the final 18 hours, [3H]-thymidine (1μCi/well, Amersham) was added. Cells were harvested onto filters and radioactive incorporation measured. Data were reported as a stimulation index (SI): fold increase in cpm over unstimulated control.
Flow cytometry
PBMCs, BAL cells, and cells from tissue were surface stained for CD3 (clone SP34, BD Pharmingen), CD4 (clone SK3, BD Biosciences), and CD25 (clone MA251, BD Biosciences) [23], and for CD39 (clone eBioA1, eBioxcience), GITR (clone eBioAITR, ebioscience) and intracellular CTLA-4 (clone BNI3, BD bioscience), then for Foxp3 (150D [BioLegend, San Diego, CA], PCH101, or 236A/E7 [eBioscience]) utilizing eBioscience Foxp3 staining kit. Data were collected on a FACSAria (BD Biosciences) and analyzed using FlowJo 8.6.3 (Tree Star, Inc., Ashland, OR).
Immunofluorescence tissue staining
Antigen retrieval on formalin fixed, deparaffinized tissue sections was performed with High pH Antigen Retrieval Buffer (Dako, Carpinteria, CA) at 95°C, 20 min. Slides were blocked with 2% goat serum and incubated with polyclonal rabbit anti-human CD3 (Dako) and biotinylated anti-human Foxp3 (clone 236A/E7;eBioscience) antibodies. Isotypes were stained using rabbit anti-human CD3 and mouse Universal Negative Control (Dako), 4°C overnight. Sections were stained with goat anti-rabbit AlexaFluor 488 and AlexaFluor 546 streptavidin conjugates (Invitrogen), and nuclei stained with Draq5 (Biostatus Limited). Sections were imaged with a Leica TCS-SL confocal microscope (Leica Microsystems), Z projections were made with ImageJ (http://rsb.info.nih.gov/ij/), and brightness and contrast adjusted with Adobe Photoshop (Adobe Systems).
Statistics
To compare two groups when data were determined to have normal distribution, a student t-test was used. If data did not have a Gaussian distribution, Mann-Whitney was used for unpaired data and Wilcox Signed Rank for paired data. Comparison of contiguous data over time was analyzed by repeated measures ANOVA and if significant, pair-wise comparison was by Tukey-Newman tests. p<0.05 was considered statistically significant.
Results
Regulatory T cells in lung granulomas and draining lymph nodes
The NHP is the only animal model that mimics the spectrum of M. tuberculosis infection (latent and active disease) seen in humans [22]. Infection outcomes are determined by clinical criteria and severity of disease quantified at necropsy [22].
Cells from involved lung and thoracic LN at necropsy were stained for CD3, CD4, CD25 and Foxp3 (Fig 1A, B). In tissues, not all Foxp3+ cells are CD25+ and not all CD25+ cells are Foxp3+. We further characterized CD4+Foxp3+ cells in PBMC and showed that they expressed Treg-associated cell surface markers CD25, CD39, CTLA-4, and GITR to varying degrees (Supplemental figure 1), and did not produce cytokine in response to M. tuberculosis antigens (data not shown). Thus we used CD3+CD4+Foxp3+ to define the Treg population.
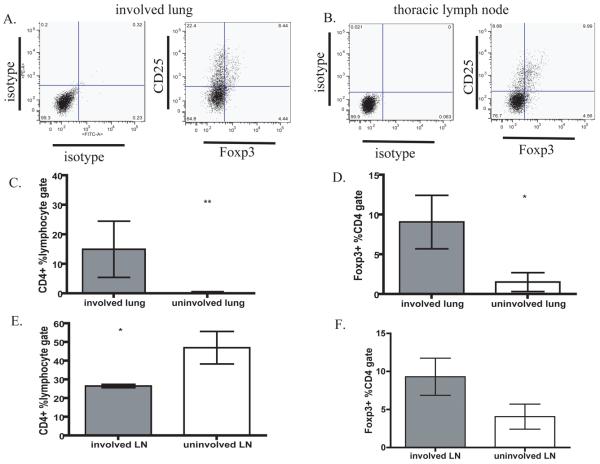
Figure 1. Regulatory T cells are enriched in involved lung tissue and lymph nodes.
The frequency of Tregs (CD3+CD4+Foxp3+) was determined by gating on live cells, lymphocytes and CD3+CD4+ cells in involved (M. tuberculosis+) and uninvolved (M. tuberculosis−) lung and thoracic lymph node at necropsy. Representative dot plots of A) involved lung and B) lymph node from an infected monkey. The frequency of CD4+ cells (C, E) and Tregs within CD4 gate (D, F) in lung or lymph node were compared within involved and uninvolved tissue.
To address whether Tregs were preferentially localized to areas of lung containing bacilli, tissue homogenates were divided into M. tuberculosis+ (involved) or M. tuberculosis− (uninvolved), based on whether M. tuberculosis was cultured from the sample. Involved lung tissue (granulomas) contained a significantly higher frequency of both total CD4+ T cells (Fig. 1C) and Tregs (Fig. 1D) than uninvolved lung. Similar to murine studies [18], lymphocytes localized to pulmonary sites that contained M. tuberculosis. Interestingly, when thoracic LN from infected NHP were divided into M. tuberculosis+ and M. tuberculosis− samples, involved LN had significantly fewer CD4 T cells than uninvolved LN (Fig 1E), but there was a trend toward more Tregs within the CD4 subset (Fig 1F). It may be that fewer T cells are present in involved LN because of effacement by granulomas with caseous necrosis.
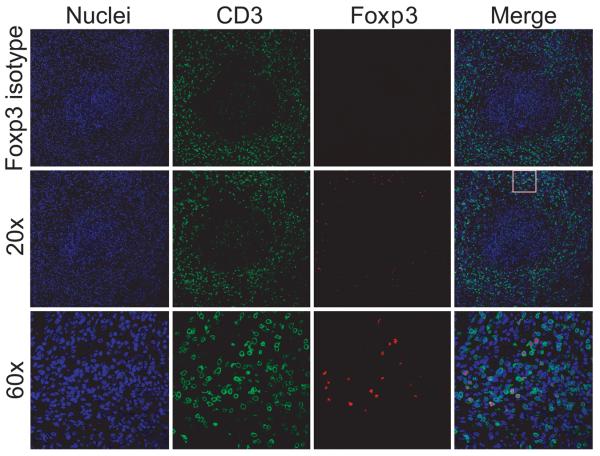
Immunofluorescent staining demonstrated that CD3+Foxp3+ Tregs are abundant within the lymphocyte cuff of granulomatous lung (Fig 2), but rare in uninvolved lung. These data support that Tregs preferentially localize to involved tissue (granulomas) and are proportionally increased in thoracic LN.
Figure 2. Regulatory T cells in granulomas.
Paraffin-embedded granulomatous lung tissue was immunofluorescently stained for nuclei (blue), CD3 (green) and Foxp3 (red). The 60x section is an enlargement of box indicated on merged image from 20x.
Tregs migrate from peripheral blood to airways during early M. tuberculosis infection
We analyzed PBMCs from 41 NHPs (infected for other studies) for six months after inoculation. The frequency of Tregs decreased dramatically within the first two weeks and was significantly lower than pre-infection levels 8-12 weeks p.i. (Fig 3A), while CD4 T cell frequencies were unchanged (Fig 3C). By 16 weeks the Treg levels in all monkeys return to baseline levels (0 wk vs 16wk p > 0.05 for both active and latent), but continue to increase in monkeys developing active TB.
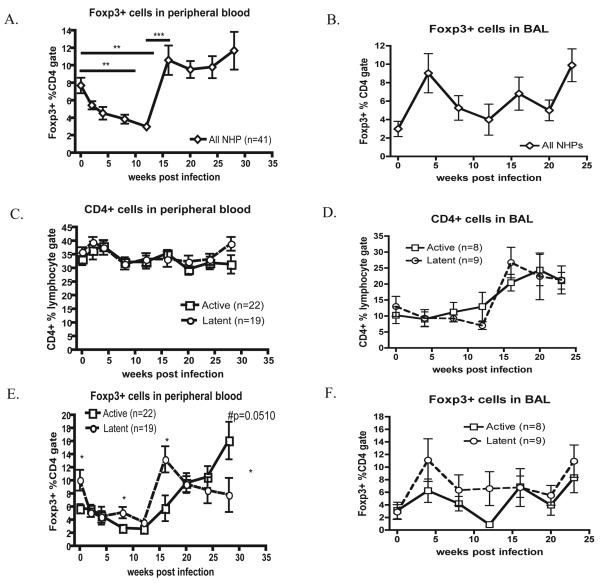
Figure 3. Tregs in PBMC and airways during M. tuberculosis infection.
A) The frequency of Tregs (CD3+CD4+Foxp3+) in PBMC during M. tuberculosis infection (n=41 monkeys) was determined by gating on live cells, lymphocytes and CD3+CD4+ cells. B) BAL cells from infected NHPs (N=17) were stained and gated as above. p <0.05 was considered significant. PBMCs and BAL cells were stained and compared between active disease (open squares) and latently infected (open circles) groups. The frequency of CD4 T cells within the lymphocyte gate (C,D) or Foxp3+ cells within CD4 gate (E, F) for blood (C, E) and airways (B, D) are represented. Statistical significance over time (A) was determined by repeated measure ANOVA (p<0.0001) with a Tukey-Newman post-test. (C-F) Mann-Whitney determined significance between each group at each time point.
The reduction in peripheral Treg frequencies during early infection suggested that Tregs were migrating to the lungs. BAL is the only relatively non-invasive way to serially sample the pulmonary environment. For 6 months, BAL cells were obtained monthly from 17 macaques and stained for Tregs (Fig 3B). By 4 weeks p.i., the frequency of Tregs increased in airways, corresponding to the reduction in the periphery. After an initial influx of Tregs into airways, the frequency of Tregs fluctuated until 16 weeks p.i., when both Tregs and CD4 T cells (Fig 3B,D) increased.
Pre-infection levels of Tregs in PBMC correlate with clinical outcome
Of 41 macaques followed, 22 developed active disease and 19 had latent infections. Surprisingly, the animals that became latent had significantly higher frequencies of Tregs prior to infection in PBMCs compared to monkeys that developed active disease (p=0.0116)(Fig 3E). Although all NHP exhibited an initial decrease of Tregs, latently infected animals maintained higher levels of Foxp3+ cells in PBMCs compared to those with active disease at 8 weeks p.i. (Fig 3E). By 16 weeks, all animals returned to pre-infection levels of Tregs. Those that would develop latent infection maintained pre-infection Treg frequencies, while Foxp3+ cells continued to increase up to 28 weeks p.i. in those developing active disease. Latent NHP demonstrate a trend of more Tregs in BAL by 4 wks p.i. compared with those that would develop active disease (Fig 3F), although this was not statistically significant.
Foxp3+ cells affect proliferative responses in peripheral blood
To determine whether increased Tregs correlated with a reduced proliferative response, PBMCs were stimulated with the mitogen PHA or mycobacterial CFP. Prior to infection, PBMCs from animals that would become latent demonstrated significantly less proliferation in response to PHA than PBMCs from animals destined to develop active TB (Fig 4A), correlating with more peripheral Tregs in latent monkeys (Fig 3E). By 6 weeks p.i., the latent group exhibited a trend toward decreased proliferation in response to both PHA (Fig 4A) and CFP (Fig 4B). Tregs were not measured at 6 weeks, but the frequency of Tregs is significantly increased at 8 weeks p.i. in latently infected monkeys compared to those that would develop active disease. All NHPs showed increased proliferation to both stimuli by 6 weeks p.i. and had significantly lower frequencies of Treg by 8 weeks p.i. compared to pre-infection. These data support an association between decreased proliferation and more Tregs, suggesting that Tregs may limit T cell proliferation.
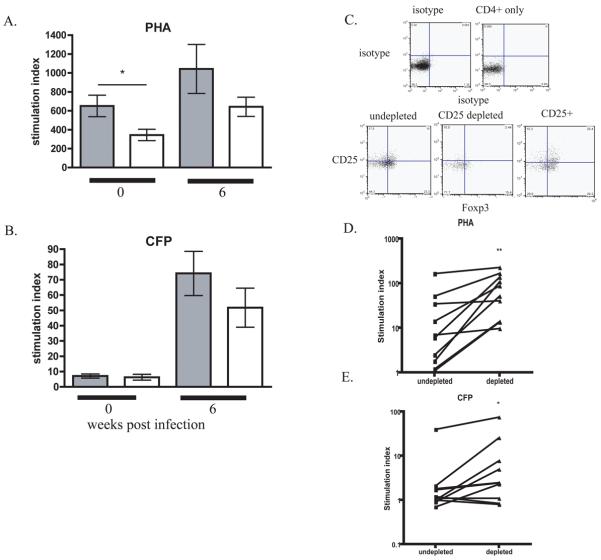
Figure 4. Treg inhibit PBMC proliferation.
PBMCs from animals that would develop active disease (shaded bars, N=22) or latent infection (open bars, N=19) at 0 and 6 weeks p.i. were stimulated with A) PHA and B) CFP, and proliferation measured. Data reported as stimulation index. Statistical significance was determined by Mann-Whitney. CD25+ cells were depleted from PBMCs (N=10) (C), and stimulated as above. Comparison of PBMC proliferative response to PHA (D) and CFP (E) before and after CD25 depletion is reported as a stimulation index statistical significance was determined by Wilcox Signed Rank.
We tested whether depletion of Tregs would reverse this reduced response. No convenient method for depleting Foxp3+ cells from primates exists, so CD25+ cells were depleted from PBMC (Fig 4C). Depletion of CD25+ cells resulted in increased proliferation in seven of ten animals for both stimuli (Fig 4D,E).
Discussion
The immune response against M. tuberculosis is a tightly controlled balance between sufficient inflammation to limit M. tuberculosis growth and regulatory factors that prevent damage of surrounding tissue. We sought to determine whether the frequency of regulatory T cells in blood and airways was associated with outcome of infection in a macaque model of TB. We hypothesized that if Tregs were involved in modulating the immune response to M. tuberculosis and limiting damage to tissue, Tregs would be found at the site of infection. If regulatory T cells contributed to development of active disease, we would expect the following: First, animals that develop primary TB would have a higher frequency of Tregs in blood and airways prior to or during early infection when compared to those that develop latent infection. Second, an increased frequency of Tregs would correspond with decreased proliferation when PBMCs were stimulated with antigens. Conversely, if increased Tregs were not a causative factor for active TB, but a response to inflammation, a disparity in Tregs would become apparent between the two groups when those with active disease failed to control bacterial growth.
In healthy individuals, M. tuberculosis infection is predominantly localized to the lung and thoracic LN. Bacilli and granulomas are most commonly found in lung parenchyma, rather than airways, making sampling involved tissues difficult. In humans, timing of infection, as well as dose and strain of inoculum are unknown, and samples prior to infection are not generally available for comparison. Since these factors can contribute to the quality of the immune response and infection outcome, we used the macaque model of TB. This model resembles human TB in disease outcome and pathology, and inoculum size, strain and timing of infection can be controlled [23]. PBMC and BAL cells can be obtained frequently, and tissue samples are available at necropsy.
Classically, Tregs were defined as CD4+CD25hi cells. However, CD25 is also found on activated effector T cells and where immune stimulation is continuous, (e.g. chronic infection), one is unable to distinguish Tregs from effector populations with this single marker. The transcription factor Foxp3 denotes a Treg phenotype [9], and transfection of constitutively active Foxp3 in human T cells conferred the Treg phenotype [24]. We defined our Treg cell population as CD3+CD4+Foxp3+, but used additional markers to confirm this. In blood we are most likely measuring Foxp3+ natural Tregs [10]; cells found within airways and tissues may be primed before migrating to the site of infection. Here we show that Tregs are present in involved lung and LN and localize to the lymphocyte cuff of granulomas, suggesting they may modulate immune responses within the granuloma.
We examined the association between Tregs in blood and airways of infected monkeys and development of active disease and found surprising results. Whereas the literature [20, 21, 25-27] supports an association between active disease and increased Tregs in blood of humans, we found that monkeys who developed latent infection had a significantly higher frequency of Tregs in PBMC prior to and during early infection than those that developed primary TB. The increased frequency of Tregs in monkeys that became latently infected correlated with decreased proliferation to mitogen prior to M. tuberculosis infection. When CD25+ cells were depleted from PBMCs, several NHPs exhibited a modest increase in proliferation when stimulated with CFP and PHA. Our findings do not support the hypothesis that more Tregs and a less vigorous lymphoproliferative response during the initial phase of M. tuberculosis infection would predispose an individual to development of primary TB. On the contrary, our data indicate that more Tregs prior to infection correlates with a better infection outcome.
These data led us to ask whether the increased frequency of Tregs associated with active disease is a response to increased inflammation. Regardless of infection outcome the frequency of Foxp3+ cells dramatically decreased in blood within the first 8 weeks. This early reduction in Foxp3+ cells suggests that these cells are poised to sense inflammation and migrate quickly to the site of infection, and mirrors a drop reported in peripheral blood of healthy human contacts in the Gambia [26]. At the same time, the frequency of Tregs increased in airways, supporting the hypothesis that these cells migrate to the lungs. In vitro experiments on lymphocytes from both humans and mice indicate that natural Tregs change their chemokine receptor phenotype more quickly than naïve/resting T cells in the periphery [28]. This may be a mechanism for rapidly increasing Tregs at the infection site for expansion during the initial burst of inflammation to protect surrounding tissue from damage. While all monkeys returned to baseline levels of Tregs in PBMC by 16 weeks p.i., those with latent infection remained steady while Tregs in NHPs with active disease began to increase.
In summary, these data indicate that following infection, Tregs rapidly decrease in peripheral blood, and are maintained at low levels in the periphery for ~4 months. When bacterial growth is under control, i.e. during latent infection, peripheral levels of Tregs return to pre-infection levels. However, when bacterial growth is not contained and active disease develops, Tregs continue to increase, perhaps acting to dampen peripheral inflammation as a protective mechanism. Based on this concept, we propose a model where Tregs in peripheral blood act as important regulators poised to protect uninvolved tissues from potentially damaging antimycobacterial immune responses. Upon infection Treg migrate to the lung and draining LN to protect “healthy” tissue and limit inflammation. Tregs are retained within granulomas and involved LN along with effector T cells. Provided the T cell response is sufficient to contain M. tuberculosis infection, Tregs control damage to surrounding tissue by limiting proliferation of effector T cells in granulomas. In latent infection, peripheral responses are limited due to containment of antigen within granulomas and control of bacterial growth. However if the immune response is insufficient to control bacterial replication, antigenic burden increases, resulting in increased inflammation. In this instance, Tregs in the periphery may limit the inflammation associated with active disease. The increased Treg frequency observed in blood of people with active TB is likely a response to inflammation and bacterial burden, and not a predetermining or contributing factor to active disease, at least during the initial stages of infection.
Control of M. tuberculosis is a dynamic balance between inflammation and immune regulation maintained by the host. Here we explored the role of Treg cells in the dynamics of this response during initial stages of infection. Further understanding of how these cells are functioning at the site of infection as well as the signals responsible for early exodus from the periphery will provide valuable information about the microenvironments needed to maintain control of M. tuberculosis infection.
Supplementary Material
Cellular surface markers on Tregs In addition to CD25, CD39 [29], intracellular CTLA-4 [30] and GITR[30] have been reported to be associated with a Treg phenotype in individuals infected with M. tuberculosis. We sought to further characterize PBMC Tregs, CD4+Foxp3+ were analyzed for expression of Treg associated cell surface markers at 0, 8 and 24 weeks post infection. A) Shows representative flow plots of CD25, CD39, intracellular CTLA-4 and GITR within the CD4+Foxp3+ gate. The frequency of Tregs expressing CD25 (B), CD39 (C), CTLA-4 (D) and GITR (E) from n=7 NHP are expressed over time. CD4+Foxp3+ cells express Treg associated cell surface markers to varying degrees over the course of infection indicating this population of cells is Tregs.
Acknowledgements
This work was supported by NIH R01-075845 (JLF), R01-AI50732 (JLF), T32-AI060525 (JLF, AG), Bill and Melinda Gates Foundation, and Ellison Foundation. We are grateful to Lekneitah Smith and Mark Rodgers for technical assistance, and Melanie O'Malley, Jaime Tomko, Paul Johnston, Andre Samuel, and Jennifer Kerr for veterinary technician services. We thank Dr. Edwin Klein for clinical consultation and all necropsy specimens. Finally, we'd like to thank members of the Flynn lab for helpful discussions.
Footnotes
The authors have no conflict of interest to report.
This work was supported by NIH R01-075845 (JLF), R01-A150732 (JLF), T32-AI060525 (JLF, AG), Bill and Melinda Gates Foundation, and Ellison Foundation.
References
- 1.(WHO) WHO WHO annual report on global TB control- summary. 2008:10. [PubMed] [Google Scholar]
- 2.Capuano SV, 3rd, Croix DA, Pawar S, et al. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect Immun. 2003;71:5831–44. doi: 10.1128/IAI.71.10.5831-5844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs--worldwide, 2000-2004. MMWR Morb Mortal Wkly Rep. 2006;55:301–5. [PubMed] [Google Scholar]
- 4.Andersen P, Doherty TM. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nat Rev Microbiol. 2005;3:656–62. doi: 10.1038/nrmicro1211. [DOI] [PubMed] [Google Scholar]
- 5.Gong JH, Zhang M, Modlin RL, et al. Interleukin-10 downregulates Mycobacterium tuberculosis-induced Th1 responses and CTLA-4 expression. Infect Immun. 1996;64:913–8. doi: 10.1128/iai.64.3.913-918.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Othieno C, Hirsch CS, Hamilton BD, Wilkinson K, Ellner JJ, Toossi Z. Interaction of Mycobacterium tuberculosis-induced transforming growth factor beta1 and interleukin-10. Infect Immun. 1999;67:5730–5. doi: 10.1128/iai.67.11.5730-5735.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirsch CS, Hussain R, Toossi Z, Dawood G, Shahid F, Ellner JJ. Cross-modulation by transforming growth factor beta in human tuberculosis: suppression of antigen-driven blastogenesis and interferon gamma production. Proc Natl Acad Sci U S A. 1996;93:3193–8. doi: 10.1073/pnas.93.8.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 9.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 10.Wohlfert E, Belkaid Y. Role of endogenous and induced regulatory T cells during infections. J Clin Immunol. 2008;28:707–15. doi: 10.1007/s10875-008-9248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–7. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 13.Hisaeda H, Maekawa Y, Iwakawa D, et al. Escape of malaria parasites from host immunity requires CD4+ CD25+ regulatory T cells. Nat Med. 2004;10:29–30. doi: 10.1038/nm975. [DOI] [PubMed] [Google Scholar]
- 14.Boettler T, Spangenberg HC, Neumann-Haefelin C, et al. T cells with a CD4+CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection. J Virol. 2005;79:7860–7. doi: 10.1128/JVI.79.12.7860-7867.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rushbrook SM, Hoare M, Alexander GJ. T-regulatory lymphocytes and chronic viral hepatitis. Expert Opin Biol Ther. 2007;7:1689–703. doi: 10.1517/14712598.7.11.1689. [DOI] [PubMed] [Google Scholar]
- 16.Rushbrook SM, Ward SM, Unitt E, et al. Regulatory T cells suppress in vitro proliferation of virus-specific CD8+ T cells during persistent hepatitis C virus infection. J Virol. 2005;79:7852–9. doi: 10.1128/JVI.79.12.7852-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kursar M, Koch M, Mittrucker HW, et al. Cutting Edge: Regulatory T cells prevent efficient clearance of Mycobacterium tuberculosis. J Immunol. 2007;178:2661–5. doi: 10.4049/jimmunol.178.5.2661. [DOI] [PubMed] [Google Scholar]
- 18.Scott-Browne JP, Shafiani S, Tucker-Heard G, et al. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J Exp Med. 2007;204:2159–69. doi: 10.1084/jem.20062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribeiro-Rodrigues R, Resende Co T, Rojas R, et al. A role for CD4+CD25+ T cells in regulation of the immune response during human tuberculosis. Clin Exp Immunol. 2006;144:25–34. doi: 10.1111/j.1365-2249.2006.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hougardy JM, Place S, Hildebrand M, et al. Regulatory T cells depress immune responses to protective antigens in active tuberculosis. Am J Respir Crit Care Med. 2007;176:409–16. doi: 10.1164/rccm.200701-084OC. [DOI] [PubMed] [Google Scholar]
- 21.Guyot-Revol V, Innes JA, Hackforth S, Hinks T, Lalvani A. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am J Respir Crit Care Med. 2006;173:803–10. doi: 10.1164/rccm.200508-1294OC. [DOI] [PubMed] [Google Scholar]
- 22.Lin PL, Rodgers M, Smith L, et al. Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect Immun. 2009;77:4631–42. doi: 10.1128/IAI.00592-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin PL, Pawar S, Myers A, et al. Early events in Mycobacterium tuberculosis infection in cynomolgus macaques. Infect Immun. 2006;74:3790–803. doi: 10.1128/IAI.00064-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allan SE, Song-Zhao GX, Abraham T, McMurchy AN, Levings MK. Inducible reprogramming of human T cells into Treg cells by a conditionally active form of FOXP3. Eur J Immunol. 2008;38:3282–9. doi: 10.1002/eji.200838373. [DOI] [PubMed] [Google Scholar]
- 25.Antas PR, Sampaio EP. Another round for the CD4+CD25+ regulatory T cells in patients with tuberculosis. Am J Respir Crit Care Med. 2007;176:214–5. doi: 10.1164/ajrccm.176.2.214. [DOI] [PubMed] [Google Scholar]
- 26.Burl S, Hill PC, Jeffries DJ, et al. FOXP3 gene expression in a tuberculosis case contact study. Clin Exp Immunol. 2007;149:117–22. doi: 10.1111/j.1365-2249.2007.03399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X, Zhou B, Li M, et al. CD4(+)CD25(+)FoxP3(+) regulatory T cells suppress Mycobacterium tuberculosis immunity in patients with active disease. Clin Immunol. 2007;123:50–9. doi: 10.1016/j.clim.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Lee JH, Kang SG, Kim CH. FoxP3+ T cells undergo conventional first switch to lymphoid tissue homing receptors in thymus but accelerated second switch to nonlymphoid tissue homing receptors in secondary lymphoid tissues. J Immunol. 2007;178:301–11. doi: 10.4049/jimmunol.178.1.301. [DOI] [PubMed] [Google Scholar]
- 29.Chiacchio T, Casetti R, Butera O, et al. Characterization of regulatory T cells identified as CD4(+)CD25(high)CD39(+) in patients with active tuberculosis. Clin Exp Immunol. 2009;156:463–70. doi: 10.1111/j.1365-2249.2009.03908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hougardy JM, Verscheure V, Locht C, Mascart F. In vitro expansion of CD4+CD25highFOXP3+CD127low/− regulatory T cells from peripheral blood lymphocytes of healthy Mycobacterium tuberculosis-infected humans. Microbes Infect. 2007;9:1325–32. doi: 10.1016/j.micinf.2007.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cellular surface markers on Tregs In addition to CD25, CD39 [29], intracellular CTLA-4 [30] and GITR[30] have been reported to be associated with a Treg phenotype in individuals infected with M. tuberculosis. We sought to further characterize PBMC Tregs, CD4+Foxp3+ were analyzed for expression of Treg associated cell surface markers at 0, 8 and 24 weeks post infection. A) Shows representative flow plots of CD25, CD39, intracellular CTLA-4 and GITR within the CD4+Foxp3+ gate. The frequency of Tregs expressing CD25 (B), CD39 (C), CTLA-4 (D) and GITR (E) from n=7 NHP are expressed over time. CD4+Foxp3+ cells express Treg associated cell surface markers to varying degrees over the course of infection indicating this population of cells is Tregs.