Abstract
IFN-γ is necessary in both humans and mice for control of Mycobacterium tuberculosis (M. tuberculosis). CD4 T cells are a significant source of IFN-γ during acute infection in mice and are required for control of bacterial growth and host survival. However, several other types of cells can and do produce IFN-γ during the course of the infection. We sought to determine whether IFN-γ from sources other than CD4 T cells was sufficient to control M. tuberculosis infection and whether CD4 T cells had a role in addition to IFN-γ production. To investigate the role of IFN-γ from CD4 T cells, a murine adoptive transfer model was developed in which all cells were capable of producing IFN-γ, with the exception of CD4 T cells. Our data in this system support that CD4 T cells are essential for control of infection but also that IFN-γ from CD4 T cells is necessary for host survival and optimal long-term control of bacterial burden. In addition, IFN-γ from CD4 T cells was required for a robust CD8 T cell response. IFN-γ from T cells inhibited intracellular replication of M. tuberculosis in macrophages, suggesting IFN-γ may be necessary for intracellular bactericidal activity. Thus, although CD4 T cells play additional roles in the control of M. tuberculosis infection, IFN-γ is a major function by which these cells participate in resistance to tuberculosis.
Introduction
Tuberculosis continues to be a global health crisis, causing ~1.7 million deaths per year. Lack of an effective vaccine and long treatment regimens with multiple chemotherapeutic agents make it imperative that research focuses on understanding the immune response to infection. The pro-inflammatory cytokine interferon γ (IFN-γ) promotes the development of a T-helper (Th) 1 T cell response [1]. In addition, it synergizes with TNF to activate macrophages (Mϕ), promoting the induction of nitric oxide synthase (NOS2) which participates in killing of M. tuberculosis [2]. Humans with loss-of-function genetic mutations in either IFN-γ or its receptor are very susceptible to mycobacterial infections [3]. Mice lacking IFN-γ or NOS2 are two of the most vulnerable strains, failing to control M. tuberculosis and succumbing within weeks of challenge [4, 5]. Thus, IFN-γ is required for containment of M. tuberculosis infection and host survival.
CD4 T cells are a primary source of IFN-γ during the adaptive immune response to M. tuberculosis infection and are required for host survival during both the acute and chronic stages of infection [6, 7]. CD4−/− and MHCII−/− mice are unable to control bacterial growth and succumb to infection significantly sooner than wild-type counterparts [6], yet these mice survive at least twice as long as those lacking IFN-γ or NOS2 [4-6, 8]. Lack of CD4 T cells during initial infection results in delayed IFN-γ and NOS2 production, but eventually levels comparable to wild type are reached, since other cells can produce this cytokine. However this did not rescue the CD4 T cell deficient mice from the infection [6]. When CD4 T cells are depleted during chronic infection, exponential bacterial growth and eventual host death occurs, despite maintenance of pre-depletion levels of IFN-γ and NOS2 [7].
As Th1 cells, CD4 T cells also produce IL-2 and TNF, interact with dendritic cells to help with T cell priming and provide T cell help to B cells [9]. CD8 T cells as well as other cells can and do produce IFN-γ during M. tuberculosis infection. Recent work by Gallegos et.al. supports that CD4 T cells have IFN-γ-independent mechanisms of controlling M. tuberculosis infection in vivo [10]. Taken together, these data suggest that CD4 T cells have roles in addition to IFN-γ production. These data lead to the hypotheses that IFN-γ from sources other than CD4 T cells is sufficient for bacterial containment and CD4 T cells have functions in addition to IFN-γ production.
For decades, knockout and transgenic animals as well as antibody depletion have been powerful tools for determining which immune factors are necessary for control of M. tuberculosis. However, these techniques are limited by studying the global effect of these immune mediators, and it can be difficult to determine which specific function of a cell type, for example, is causing the in vivo phenotype in a murine model. To determine whether IFN-γ from sources other than CD4 T cells were sufficient to contain bacterial growth, a new model system was needed. An adoptive transfer model was designed to permit manipulation of the CD4 T cell population. The data from this novel adoptive transfer system, indicate that CD4 T cells are necessary as a source of IFNγ, as well as influencing the function of CD8 T cells in the immune response against M. tuberculosis. The adoptive transfer systems developed provide an opportunity to manipulate the immune response to identify specific factors important in control of M. tuberculosis infection.
Materials and Methods
Animals
8-12 week old Helicobacter pylori free RAG1−/− (B6.129S7-Rag1tm1Mom/J), Thy1.1 (B6.PL-Thy1a/CyJ), IFN-γ −/− (GKO) (B6.129S7-Ifngtm1Mom/J), IFN-γ receptor−/− (IFN-γR−/−) (B6.129S7-Ifngrtm1agt/J) and wild-type (WT) C57BL/6 mice were obtained from Jackson laboratories (Bar Harbor, ME) or maintained in an in-house breeding facility. Mice were maintained in a biosafety level 3 facility in micro-isolator cages, free fed a diet of mouse chow and autoclaved water. All animals were maintained as per the University of Pittsburgh Institutional Animal Care and Use Committee.
Probiotic treatment of RAG 1−/− mice
Initially we observed development of reconstitution induced inflammatory bowel disease [11-13] in our RAG 1−/− mice after T cell transfer but prior to M. tuberculosis challenge. After investigating several possible factors responsible for this syndrome, we treated RAG 1−/− mice prophylactically with probiotics, which eliminated the signs of inflammatory bowel disease. Prior to adoptive transfer, mice received one scoop (~0.25 gms) of Bene-Bac Powder (Pet Ag Inc. Hampshire, IL) (25 million Colony Forming Units (CFU) per gram, Lactobacillus fermentum, Enterococcus faecium, Lactobacillus plantarum, Lactobacillus acidophilus) in 350 ml of fresh, autoclaved water every day for 14 days. Water bottles were changed daily.
Isolation of cells and adoptive transfer
Thy1.1 mice were pretreated with 1mg/ mouse anti-CD4 antibody (clone GK1.5, NCCC, NIH Bethesda, MD) 7 days prior to splenocyte harvest. GKO and WT mice did not receive pretreatment with antibody. Naïve donor mice were anaesthetized by isoflurane and euthanized by cervical dislocation. Spleens were isolated under sterile conditions. A single cell suspension was obtained by crushing each spleen individually through a 40μm cell strainer in Dulbecco’s phosphate buffered saline (Sigma-Aldrich, St. Louis, MO) with the back of a sterile 5ml syringe. Erythrocytes were lysed with red blood cell lysis buffer (90ml 0.16M NH4, 10ml 0.17M Tris pH 7.65) for two minutes at room temperature. Following erythrocyte lysis and wash, remaining splenocytes from like animals were combined. Individual cell populations were isolated via Miltenyi MACS bead separation (Miltenyi Biotech, Auburn, CA) as per manufacture’s instructions. CD4+ cells were positively selected from either WT or GKO mice and naïve Thy1.1 mice were depleted of CD4 T cells by antibody neutralization and then CD4 T cells were positively selected T cells by magnetic bead separation as per manufacturer’s protocol prior to adoptive transfer. Recipient RAG1−/− mice received purified splenocytes (1.8×108 cells total 1:20 CD4 T cells to CD4 depleted splenocytes) via a tail vein injection in 300μl of PBS per mouse.
Aerosol infection
As previously described [5] M. tuberculosis strain Erdman was passed through mice, grown in culture once and frozen in aliquots in PBS 0.05% Tween-80 with 10% glycerol. Immediately prior to infection, an aliquot was thawed and diluted in PBS 0.05% Tween 80. Bacterial clumps were disaggregated by cup horn sonication. Mice were aerosol infected with low dose (~50 CFU, Erdman strain) using a nose-only exposure aerosolizer (InTox Products, Albuquerque, NM) [5]. Mice were exposed to aerosolized M. tuberculosis for 20 minutes then room air for 5 minutes. One day following infection, lungs of one mouse per aerosol group were crushed in 5 ml PBS-0.5% Tween-80 and plated neat on 7H10 or 7H11 plates (Difco Laboratories, Detroit, MI) to determine inoculum.
Sample harvest
At serial time points following infection, mice were anesthetized with isoflurane and euthanized by cervical dislocation. Under sterile conditions, organs were crushed in Dulbecco’s Modified Eagle Medium (Sigma-Aldrich) through a 40μm cell strainer with the plunger from a sterile 5ml syringe. Dilutions of cellular homogenate were plated on 7H10 agar plates for CFU determination. Cells were pelleted and washed, erythrocytes were lysed as described previously [14], and cells were resuspended for enumeration and immunologic asays.
Flow cytometry
For antigen specific responses and intracellular scytokine staining, cells were incubated with 1μl of either ESAT61-20aa MHC Class II multimer or GAP MHC Class I tetramer (Mtb32aa309-318, GAPINSATAM) NIAID tetramer facility, Bethesda, MD) [15] per 100μl at 37μC 5%CO2 for 60 minutes in media then washed. Once stained with tetramers, cells were stimulated with either ESAT61-20aa peptide or GAP peptide in the presence of monensin for 5 hours [16]. After staining with GAP tetramer, anti-CD107 (1D4B) antibody was added during stimulation to assess degranulation. Following stimulation, cells were surfaced stained for CD3, CD4 (clone L3T4), and CD8 (clone 53-6.7) at room temperature for 15 minutes in PBS 0.5% BSA 20% Mouse serum, washed and then fixed with 2% paraformaldehyde (Sigma-Aldrich) for one hour. Following fixation, cells were permeablized then washed with PBS 0.5% BSA 0.2% Saponin (Sigma-Aldrich). Cells were stained for intracellular cytokines IFN-γ (clone XMG-6.1) and TNF (clone MP6-XTT22) by incubating antibodies in PBS 0.5% BSA 0.2% Saponin and 20% mouse serum for 15 minutes at room temperature. All antibodies were from BD-Pharmingen unless otherwise noted. Cells were washed and resuspended in 1% paraformaldehyde. Data were collected on a FACS Aria and analyzed using FloJo software 8.6.3 (Treestar).
Histology sections
At necropsy, one quarter of lung was fixed in 10% normal buffered formalin, paraffin imbedded and sectioned. Slides were stained with hematoxylin and eosin. Granuloma formation was evaluated by two independent investigators in a blinded fashion.
Intracellular bactericidal assays
Bone marrow derived macrophages were obtained by culturing bone marrow from the long bones of mice with 25% L929-cell supernatant containing media for 5 days in 10cm culture dishes (2.5×106 cells/ plate in 10 ml media). Cells by this time had formed a monolayer of macrophages. Macrophages were removed by incubation with PBS on ice for 20 minutes followed by vigorous pipetting to dislodge any remaining adherent cells. Cells were pelleted, resuspended, counted and diluted to a concentration of 2.5×107 cells/ml with macrophage infection media (DMEM, 1% FBS, 1% sodium pyruvate, 1% L-glutamine, 1% non-essential amino acids). Macrophages were plated at 100μl/well in a 96 well plate and allowed to adhere for 45 minutes. Subsequently, macrophages were infected with M. tuberculosis at an MOI≤1 for 4 hours at 37°C 5% CO2. After infection, supernatant from three wells from both WT and IFN-γR−/− macrophages was saved and the macrophages in all wells washed. Three wells per each macrophage type were lysed with 1% Saponin and the saved supernatant and macrophage lysis were diluted and plated on 7H10 plates to determine the “input” bacterial numbers.
T cells were obtained from the lungs of wild type mice infected with M. tuberculosis for 4 weeks for culture with infected macrophages. Infected mice were anesthetized with isoflurane and euthanized by cervical dislocation. Lungs were retrieved under sterile conditions and crushed through a 40μm cell strainer with the back of a sterile 5 ml syringe. Once a single cell suspension was obtained and erythrocytes lysed, CD4 and CD8 T cells were purified by magnetic bead separation (Miltenyi) as per manufacturer’s instructions. T cells were incubated in wells with infected macrophages at a 1:1 ratio of T cell to macrophage (n=3 wells per condition) Media without T cells in wells with infected macrophages was used as a control for growth of M. tuberculosis. As a positive control, wells were treated with IFN-γ (250U/ml, Invitrogen) and LPS (3μg/ml, Sigma-Aldrich). Cells were incubated for 72 hours at 37°C 5% CO2. Following incubation, supernatant was removed and saved and cells were lysed with 1% Saponin. Supernatants and cell lysates were serially diluted and plated on 7H10 agar plates for determination of bacterial burden per well. Data are reported as a % killed intracellular bacteria ((output CFU/Input CFU)×100)).
Results
Adoptive transfer model
An adoptive transfer model was developed so that the presence or absence of CD4 T cells from IFN-γ−/− or wild type (WT) mice could be evaluated for ability to control M. tuberculosis infection. Three experimental groups were compared: 1) a control group lacking CD4 T cells (CTLAT), 2) a group in which CD4 T cells were from IFN-γ−/− mice (GammaAT) and 3) a group in which CD4 T cells were from WT mice (WTAT). In addition, the remainder of the immune system components was capable of producing IFNγ.
RAG1−/− mice were reconstituted with and without CD4 T cells from WT or IFN-γ deficient mice in conjunction with CD4-depleted whole spleen equivalents. The whole spleen equivalents were derived from CD4-depleted naïve Thy1.1 mice and adoptively transferred at a ration of 20:1 (CD4-splenocytes to CD4 T cells) (Figure 1A). Reconstitution of lymphopenic mice often results in homeostatic proliferation of the transferred cells and occasionally mice develop inflammatory bowel disease (IBD). Thus distinguishing between M. tuberculosis specific responses and the afore mentioned conditions can be difficult [13]. Prophylactic probiotic treatment prevented the development of IBD [11, 12]. Mice received cells 2.5 weeks prior to aerosol infection enabling differentiation between homeostatic proliferation and the immune response to M. tuberculosis (Figure 1B) [13]. To determine whether reconstitution of lymphopenic RAG1−/− mice was effective, we examined the lungs of mice in our experimental groups at 4 weeks post infection (6.5 weeks post transfer). Evaluation of the cellular composition in infected lung showed both CD4+ and CD8+ T cells were detectable in the lungs of GammaAT and WTAT, whereas only CD8+ T cells were found in the lungs of CTLAT mice (Figure 1C). When stimulated with anti-CD3 and anti-CD28 in the presence of monensin, CD8 T cells were able to make IFN-γ in all groups, whereas only CD4 T cells in the WTAT group made IFN-γ (figure 1 C). In mice that received CD4 T cells, the frequency of both CD4 and CD8 T cells (Figure 1D) were comparable. CTLAT mice (those lacking CD4 T cells) had a significantly higher frequency of CD8 T cells in the lungs compared to WTAT or GammaAT (Figure 2D). Finally, analysis of the monocyte populations (Figure 1E) (macrophages, dendritic cells and neutrophils) showed no significant differences in the frequency between reconstituted groups. These data indicate that experimental animals were successfully reconstituted and recruit a similar assortment of cell types to the lung during infection.
Figure 1. Experimental design.

A.) RAG1−/− mice were reconstituted with CD4-depleted splenocytes (Thy1.1) in the presence or absence (CTLAT) of CD4 T cells from either C57BL/6 (WTAT) or IFN-γ deficient (GammaAT) mice. B.) Prior to adoptive transfer, RAG1−/− mice were treated with probiotics to prevent development of inflammatory bowel disease (see Materials and Methods). Cells were adoptively transferred and mice allowed to reconstitute for 2.5 weeks. Mice were infected via aerosol wth low dose M.tuberculosis and harvested at serial time points. The experiments were repeated at least 3 times. C.) Representative flow cytometry plots of CD4 and CD8 T cells are shown for each experimental group (SSC vs CD4 or CD8) Representative plots for CD4 v IFN-γ and CD8 v IFN-γ following stimulation with anti-CD3/CD28 in the presence of monensin. D.) The frequency of CD4 and CD8 T cells within the live cell gate. E.) The frequency of macrophages (CD11b+CD11cdim), dendritic cell (CD11c+CD11b-) and neutrophils (GR-1+) within the live cell gate.
Figure 2. Granuloma in lungs of reconstituted mice.

4 weeks p.i. one quarter of the lung was fixed, paraffin embedded and sectioned for H&E staining. Representative sections for each group are shown at 4X and 20X magnification.
Reconstituted mice form granulomas by 4 weeks post infection
Granuloma formation is the hallmark of M. tuberculosis infection as well as an integral part by which the host controls infection. Lung sections from infected mice were evaluated at 4 weeks post infection for granuloma formation by 2 independent investigators in a blinded fashion. Representative H&E sections found in Figure 2 show granulomas were present in all groups regardless of the presence of CD4 T cells. While wild type mice had more circumscribed granulomas, no differences were detected between the reconstituted groups when compared to each other. Thus, neither CD4 T cells nor IFN-γ from CD4 T cells was required for initial granuloma formation.
IFN-γ from CD4 T cells is necessary for control of bacterial burden and host survival
CD4 T cells are the primary source of IFN-γ during early M. tuberculosis infection[14]. However, other cells, including CD8 T cells and NK cells, can and do produce IFN-γ. For this study, because T cells are the main source of IFN-γ during M. tuberculosis infection, we chose to focus on production of IFN-γ by T cells on host survival and bacterial control. To determine whether IFN-γ from sources other than CD4 T cells was sufficient to control M. tuberculosis infection, the reconstituted mice were followed for bacterial burden in the lungs and monitored for survival (i.e. mice were euthanized when moribund). Mice reconstituted with WT CD4 T cells (WTAT) survived longer than either the CTLAT or GammaAT groups (~210 days vs. 45 days and 135 days post-infection, respectively, Figure 3A). In the absence of CD4 T cells, CTLAT mice failed to control bacterial burden (Figure 3B) and did not survive longer than mock reconstituted RAG 1−/− mice (data no shown). WTAT mice maintained the lowest lung bacterial burden for the duration of the experiment (Figure 3B). Interestingly, when CD4 T cells were present but unable to produce IFN-γ (GammaAT), infected animals were able to control bacterial burden and survive for a longer period of time, i.e. more than twice as long as animals that did not have CD4 T cells. Taken together, these data suggest that while IFN-γ from CD4 T cells is necessary for long term control of infection and host survival, CD4 T cells may have roles in addition to IFN-γ production that contribute to the protective immune response to M. tuberculosis.
Figure 3. CD4 T cells producing IFN-γ are needed for long-term survival of M. tuberculosis infection.
A.) Adoptively transferred mice were followed for survival post-infection (N=5 per group), p=0.001, Log rank test. B.) Bacterial burden (CFU) in the lungs of adoptively transferred mice following M. tuberculosis infection; week 6: ANOVA p=0.002, Tukey’s multiple comparisons: **p<0.01 CTLAT v GammaAT; p<0.01 CTLAT v WTAT; Week 10: Student’s T test: GammaAT v WTAT **p=0.01. N=4/group/time point C.) Total number of cells in lungs at 4 weeks post infection; each symbol is one mouse from each group. ANOVA p=0.002; Tukey’s multiple comparisons **p<0.01 CTLAT v Gamma AT; **p<0.01 CTLAT v WTAT. Experiments were repeated at least three times.
Lack of CD4 T cells results in fewer cells recovered from the lungs post-infection
In general, in M. tuberculosis infected mice, higher bacterial burdens result in higher numbers of cells in the lungs [17, 18], as an indication that bacterial burden drives recruitment of cells to the lung and detrimental pathology. To further characterize the immune response we focused on the peak of the immune response, 4 weeks post infection (p.i.). At 4 weeks p.i., mice lacking CD4 T cells had fewer total cells recovered from the lungs than those with CD4 T cells regardless of whether the CD4 T cells could produce IFN-γ (Figure 3C), suggesting that CD4 T cells are needed to sustain the local immune response.
Antigen specific CD4 T cells are functional in reconstituted mice
In addition to IFN-γ production, Th1 CD4 T cells also produce interleukin-2 (IL-2) and tumor necrosis factor (TNF). To determine whether CD4 T cells were functional and produced cytokine in response to antigen-specific stimulation, CD4 T cells from lungs were assessed for ESAT-6 specific responses by tetramer and intracellular cytokine staining (representative plots shown in Figure 4A). ESAT-6tet+ CD4 T cells were detected in both GammaAT and WTAT groups and, as expected, not in the group that did not receive CD4 T cells (CTLAT) (Figure 4B). Following in vitro stimulation, similar frequencies of CD4 T cells from both GammaAT and WTAT groups produced TNF (Figure 4D) and IL-2 (Figure 4E). In addition, CD4+ESAT6tet+ cells produced similar amounts of both IL-2 and TNF when mean fluorescence intensity (MFI) was measured (data not shown). Only CD4 T cells from WTAT-reconstituted mice produced IFN-γ (Figure 4C). Thus, CD4 T cells were functional in the adoptive transfer model and produced cytokines in an antigen-specific manner.
Figure 4. Adoptively transferred CD4 T cells produce cytokines in the lungs in response to M. tuberculosis antigens.
Cells from lung homogenates were stained with ESAT-6 tetramer and stimulated with ESAT-6 peptide (aa1-20) for 5 hours in monenesin, then for CD4 and Thy1.2.,and intracellular cytokines IL-2, IFN-γ, and TNF. A.) Representative plot: cells are gated on lymphocytes by size (forward and side scatter), then on Thy1.2 , then on CD4 and expressed as CD4 v ESAT-6 tetramer. B.) GammaAT and WTAT have similar frequencies of ESAT-6 tetramer+ cells in lungs at 4 weeks post-infection. C.) Only WTAT mice produce IFN-γ from CD4 T cells. GammaAT and WTAT CD4 T cells produce TNF (D) and IL-2 (E). As expected there are no CD4 T cells in the CTLAT lungs. N=4 mice/group. CD4+ESAT6tet+ T cells in both GammaAT and WTAT produced similar amounts of IL-2 (F) and TNF (G) per cell as measured by mean fluorescence intensity (MFI). Experiment was repeated at least 3 times.
The quality of the CD8 T cell response is truncated in the absence of IFN-γ from CD4 T cells
Studies of M. tuberculosis infection in CD4−/− and MHCII−/− mice have shown that CD8 T cells can be a significant source of IFN-γ [6, 14]. Previously work published by our lab has shown that the cytotoxic function of CD8 T cells is not as robust in the absence of CD4 T cells [14]. In the adoptive transfer model, we sought to determine the quality of the CD8 T cell response generated was directly influenced by the presence of CD4 T cells. Antigen-specific CD8 T cells were identified by staining samples with GAP tetramer (GAPtet) [19] stimulated with GAP peptide in the presence of monensin. GAPtet is a fluoroflor linked MHC I molecule that holds the peptide GAPINSATAM, from Rv0125 (Mtb32A309-319) which has been demonstrated previously to stimulate 3-10% of lung CD8 T cells following M. tuberculosis-infection of C57BL/6 mice [15, 16] (representative plots shown in Figure 5A). Animals lacking CD4 T cells had a reduced frequency of overall CD8 T cells in the lung (Figure 5B). Fewer CD8 T cells from CD4 deficient animals produce either IFN-γ (Figure 5C) or TNF (Figure 5E) as well as a reduced frequency of CD107+ cells (Figure 5D). These data are consistent with recently published literature reporting the presence of CD4 T cells directly affected the production of IFN-γ by CD8 T cells [20]. When CD4 T cells could not make IFN-γ (GammaAT), the total frequency of CD8 T cells was decreased by 20% compared to those with wild type CD4 T cells. In addition, the lack of IFN-γ from CD4 T cells resulted in fewer CD8 T cells capable of producing IFN-γ (Figure 5C), TNF (Figure 5E), or CD107 (Figure 5D). Despite fewer antigen specific CD8+GAPtet+ cells present in the absence of either CD4 T cells or IFN-γ from CD4 T cells, CD8+GAPtet+ T cells in all groups produced similar amounts of both IFN-γ and TNF when measured by MFI (Data not shown). Of note, the observed truncated CD8 T cell response was restored with as little as 3% contamination with CD4 T cells from a wild type donor (data not shown). These data indicate that CD4 T cells are required for a quality CD8 T cell response which is consistent with recent literature [20]. In contrast our data suggest IFN-γ from CD4 T cells may act directly on CD8 T cells to boost the frequency of cells capable of producing cytokine.
Figure 5. CD8 T cell function is impaired in the absence of IFN-γ from CD4 T cells.
Lung homogenates at 4 weeks post-infection were stimulated with GAPtetramer and GAP peptide and CD107 in the presence of monensin. Following stimulation cells were stained for CD8,Thy1.1., and intracellular cytokines TNF and IFN-γ. A.) Representative plots: live cell gate, lymphcyte gate, Thy1.1, CD8+ gate and GAPtet+ within the CD8+ gate. B.) WTAT lungs have more CD8+GAPtet+ cells compared to CTLAT lungs; ANOVA p=0.01, Tukey’s multiple comparisons: **p<0.01 CTLAT v WTAT. C. Number of CD8+ GAPtet+IFN-g+ T cells in lungs of WTAT, GammaAT and CTLAT mice. ANOVA p=0.015, Tukey’s *p<0.05 CTLAT v WTAT. D. Number of CD8+GAPtet+CD107+ cells in lungs; ANOVA p=0.008; Tukey’s *p<0.05 WTAT v CTLAT and GammaAT v WTAT. E. CD8+GAPtet+TNF+ cells in lungs; ANOVA p=0.015; Tukey’s *p<0.05 CTLAT v WTAT.
IFN-γ from CD4 enhances the quality of the CD8 T cell responses
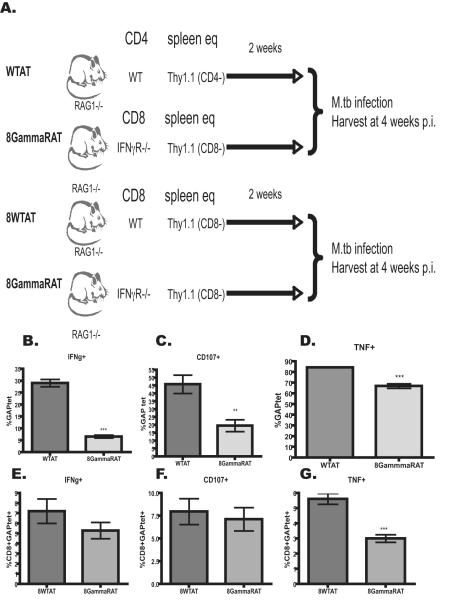
In the absence of IFN-γ from CD4 T cells, fewer CD8 T cells were present and those present were less capable of producing cytokine or exhibiting makers of cytotoxicity. Recent work has shown that CD4 T cells are necessary for sustained IFN-γ production during M. tuberculosis infection [20]. We hypothesized that IFN-γ directly affected development of CD8 T cell responses as has been shown in a viral infection model [21]. To test this hypothesis an additional experimental group was added in tandem with the previously described adoptive transfer groups. In the additional group, RAG1−/− mice were reconstituted with CD8-depleted Thy1.1 splenocytes and CD8 T cells from IFN-γ R −/− mice (8GammaRAT) and compared to the antigen specific CD8 T cell responses observed in the previously described WTAT group (Figure 6A). The CD8 T cell response was analyzed at 4 weeks p.i. as previously described in Figure 5. In the absence of IFN-γ receptor, fewer antigen specific CD8 T cells produced IFN-γ (Figure 6B), TNF (Figure 6D) and CD107+ (Figure 6C), suggesting that IFN-γ directly affects the quality of the CD8 T cell response during M. tuberculosis infection. To confirm these results the experiment and control for any affect CD8 T cell manipulation may present, RAG1−/− mice were reconstituted with CD8-depleted Thy1.1 splenocytes and CD8 T cells from either WT (8WTAT) or IFN-γR−/− mice (8GammaRAT) (Figure 6B). Interestingly, the trend remained the same. When CD8+GAPtet+ T cells were unable to respond to IFN-γ signaling, fewer cells produced IFN-γ (Figure 6E), TNF (Figure 6G), or were CD107+ (Figure 6F), thus suggesting that IFN-γ may act directly on CD8 T cells to enhance the quality of the response.
Figure 6. In the absence of IFN-γ the M. tuberculosis-specific CD8 T cell response is truncated.
A.) Experimental design: RAG1−/− mice were reconstituted with CD8 depleted Thy1.1 splenocytes in the presence of CD8 T cells from or IFN-γR −/− mice and compared against WTAT (previously described in figure 1) at 4 weeks post infection, B) RAG1 −/− mice were received naïve CD8 T cells from either WT or IFN-γR−/− mice in combination with Thy 1.1 CD8 Depleted naïve splenocytes. CD8 T cells from experimental design shown in A were evaluated for C.) IFN-γ (students t-test ***p<0.0001), D.) CD107+ (student t-test **p<0.001) and E.) TNF (student t-test ***p<0.0001)production. CD8 T cells from experimental design shown in B were analyzed for F) IFNγ, G) CD107 and H) TNF. Experiments were completed at least 2 times.
T cell:Mϕ cell to cell contact mediated killing of intracellular bacteria is enhanced by IFN-γ signaling
T cells that produce IFN-γ are capable of interacting with macrophages, via cytokine production (i.e. IFN-γ) and cell-to-cell contact. IFN-γ can synergize with TNF to activate the macrophage and mobilize mechanisms of intracellular killing. A recent report by Gallegos et.al. indicates that CD4 T cells have IFN-γ independent mechanisms of controlling bacterial growth [10]. However, that report was based on bacterial burden in vivo and was unable to distinguish between intracellular bacterial killing and inhibition of bacterial growth. We sought to determine whether T cell-macrophage interactions were sufficient in the absence of IFN-γ signal to activate the macrophage to kill intracellular M. tuberculosis. We hypothesized that one of three outcomes were possible in the absence of IFN-γ signal from T cell to macrophage: 1) Cell-to-cell contact would be sufficient to activate bactericidal activity, 2) in the absence of IFN-γ signal, bacterial growth would be inhibited (bacteriostatic), or 3) IFN-γ-receptor deficient Mϕ would fail to slow intracellular bacterial growth. To test our hypotheses, we utilized an in vitro intracellular bacterial killing assay. Bone marrow derived macrophages from either WT or IFN-γR−/− mice were infected with M. tuberculosis and incubated with media only, LPS/IFN-γ, CD4 T cells or CD8 T cells isolated from the lungs of M. tuberculosis-infected wild type mice for 72 hours. Following incubation, bacterial numbers per well were determined via plating for CFU, and compared initial input CFU to calculate a percent of bateria killed. In the presence or absence of IFN-γ signaling, wild type T cells (either CD4 or CD8 T cells) were able to induce killing of the intracellular bacteria (Figure 7). When IFN-γ signaling was intact and CD4 T cells present, there was a significant increase in intracellular bacteria killed (p<0.05) (Figure 7). Taken together these data suggest that IFN-γ signal enhances T cell:Mϕ cell to cell contact to induce bactericidal Mϕ activation.
Figure 7. IFN-γ signal is required to induce intracellular bacterial killing in macrophages.
Infected bone marrow derived macrophages from C57BL/6 or IFNγR deficient mice were cultured for 72 hours with either media, IFN-Γ and LPS, CD4 T cells or CD8 T cells isolated from the lungs of M. tuberculosis infected mice. Data are reported as a percent killing ((CFU output/CFU input)×100)). (* p<0.05 Mann-whitney U test)
Discussion
We sought to investigate the role of IFN-γ from CD4 T cells during M. tuberculosis on host survival and overall immune response. We hypothesized that IFN-γ from cells other than CD4 T cells would be sufficient to control M. tuberculosis infection, however our data instead supported that IFN-γ must be produced by CD4 T cells for optimal protection. Using a novel adoptive transfer model in which lymphopenic RAG1 deficient mice were reconstituted with CD4 depleted naïve splenocytes with naïve CD4 T cells that either could or could not produce IFN-γ, we demonstrated that CD4 T cells are essential for the control of M. tuberculosis infection and host survival. While the presence of IFN-γ-deficient CD4 T cells significantly increased host survival, long-term control of infection was not achieved when compared to mice reconstituted with cells including wild type CD4 T cells. These data agree with published literature from both knockout and antibody depletion studies [6, 7] as well as with Gallegos et.al. [10] indicating that CD4 T cells contribute to initial control of M. tuberculosis infection. In addition, mice reconstituted without CD4 T cells had a truncated or abortive immune response in which higher bacterial burden failed to elicit an expected dramatic increase in cellular infiltrate. These data indicate that cell types other than CD4 T cells are capable of producing enough IFN-γ to initially impair growth of the bacteria, but in the long term, IFN-γ from CD4 T cells is necessary for survival.
In contrast to our findings, a recent publication by Gallegos et.al. showed that CD4 T cells can inhibit M. tuberculosis growth in vivo at 3 weeks post infection independent of the Th1 transcription factor T-bet and Th1 cytokines IFN-γ or TNF production [10]. While these data are very intriguing, the study did not address the long term role of IFN-γ from CD4 T cells on host survival as well as whether CD4 T cells in the absence of IFN-γ are able to induce macrophages to kill intracellular bacilli. The traditional approaches of knockout and transgenic mice, as well as antibody mediated depletion, have only provide information about the global effects of loss or gain of the cellular subset and fail to evaluate the individual contribution of each cell type. Other immune cells can and do produce IFN-γ over the course of infection and as previously reported, CD8 T cells are the major source of IFN-γ in CD4 deficient mice [22].
Mice lacking CD4 T cells were deficient in cytotoxic CD8 T cells when measured by CD107 expression after stimulation. These findings are consistent with previously published data in knock out mice that measured cytotoxicity by limiting dilution assay [14, 22]. In addition, unexpectedly, when CD4 T cells were present but unable to make IFN-γ, CD8 T cells also exhibited a decreased frequency of IFN-γ, TNF and CD107 expression, indicating that IFN-γ from CD4 T cells may act directly on the CD8 T cell. We confirmed this by modifying the adoptive transfer method and reconstituting animals with CD8 depleted splenocytes +/− CD8 T cells from either wild type or IFN-γ receptor-deficient mice and saw a similar trend. When CD8 T cells were unable to respond to IFN-γ, antigen-specific responses were less robust. Thus, IFN-γ from CD4 T cells may be necessary to enhance CD8 T cell responses in our adoptive transfer model. Similar effects of direct IFN-γ signaling have been reported in murine viral models [21], but these data are the first to show that IFN-γ directly improves CD8 T cell functions during M. tuberculosis infection. We were surprised by the data suggesting that IFN-γ from CD4 T cells may act directly on the CD8 T cells to enhance the response to M. tuberculosis infection in our model. These data are in contrast to two previous studies using knockout animals in which there were no observed differences in CD8 T cell cytokine production during M. tuberculosis infection [20, 22]. One possibility is that the previous studies assessed the global CD8 T cell response whereas we were able to follow antigen specific responses. In addition, studies reported by Bold et al. assessed the responses of CD8 T cells primed in the presence of CD4 T cells. Based on the data in our model, IFN-γ on CD8 T cells may be to enhance responses and therefore a key role of CD4 T cells during the initial response is to help CD8 T cell induction and function.
Finally, we evaluated the ability of CD4 T cells and CD8 T cells from the lungs of infected mice to activate macrophages and kill intracellular pathogens in the presence and absence of an IFN-γ signal. Here the data suggest that T cell:Mϕ cell to cell contact is sufficient, regardless of IFN-γ signaling, to kill intracellular bacteria in vitro, which leads naturally to questions of mechanism that are the focus of future studies. However, IFN-γ signaling was required for maximal intracellular bacterial killing.
The data presented here provide novel data about the role of IFN-γ from CD4 T cells and its effects on survival, bacterial burden, and quality of the CD8 T cell response. Development of this adoptive transfer model opens opportunities for asking questions about the specific origin and function of cytokines from cellular sources. It provides a powerful tool to move beyond global systemic neutralization or loss of function and begin to discover when and from where the factors required for an effective immune response against M. tuberculosis originate.
Acknowledgments
We thank P. Ling Lin, MD for helpful discussions, Amy Frasier for her help during early development of the model and Judy Dininno for wonderful animal care.
Footnotes
This work was supported by NIH 2RO1AI50732-06A2 (JLF); AG was supported by T32 AI060525-02/03.
References
- 1.Lighvani AA, et al. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci U S A. 2001;98(26):15137–42. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saito S, Nakano M. Nitric oxide production by peritoneal macrophages of Mycobacterium bovis BCG-infected or non-infected mice: regulatory role of T lymphocytes and cytokines. J Leukoc Biol. 1996;59(6):908–15. doi: 10.1002/jlb.59.6.908. [DOI] [PubMed] [Google Scholar]
- 3.Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851. [DOI] [PubMed] [Google Scholar]
- 4.Flynn JL, et al. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178(6):2249–54. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scanga CA, et al. The inducible nitric oxide synthase locus confers protection against aerogenic challenge of both clinical and laboratory strains of Mycobacterium tuberculosis in mice. Infect Immun. 2001;69(12):7711–7. doi: 10.1128/IAI.69.12.7711-7717.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caruso AM, et al. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J Immunol. 1999;162(9):5407–16. [PubMed] [Google Scholar]
- 7.Scanga CA, et al. Depletion of CD4(+) T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon gamma and nitric oxide synthase 2. J Exp Med. 2000;192(3):347–58. doi: 10.1084/jem.192.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flynn JL, et al. Effects of aminoguanidine on latent murine tuberculosis. J Immunol. 1998;160(4):1796–803. [PubMed] [Google Scholar]
- 9.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 28:445–89. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallegos AM, et al. A Gamma Interferon Independent Mechanism of CD4 T Cell Mediated Control of M. tuberculosis Infection in vivo. PLoS Pathog. 7(5):e1002052. doi: 10.1371/journal.ppat.1002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Mahony L, et al. Probiotic impact on microbial flora, inflammation and tumour development in IL-10 knockout mice. Aliment Pharmacol Ther. 2001;15(8):1219–25. doi: 10.1046/j.1365-2036.2001.01027.x. [DOI] [PubMed] [Google Scholar]
- 12.Sheil B, Shanahan F, O’Mahony L. Probiotic effects on inflammatory bowel disease. J Nutr. 2007;137(3 Suppl 2):819S–24S. doi: 10.1093/jn/137.3.819S. [DOI] [PubMed] [Google Scholar]
- 13.Ancelet L, et al. Dissecting memory T cell responses to TB: Concerns using adoptive transfer into immunodeficient mice. Tuberculosis (Edinb) 2012;92(5):422–33. doi: 10.1016/j.tube.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Serbina NV, Lazarevic V, Flynn JL. CD4(+) T cells are required for the development of cytotoxic CD8(+) T cells during Mycobacterium tuberculosis infection. J Immunol. 2001;167(12):6991–7000. doi: 10.4049/jimmunol.167.12.6991. [DOI] [PubMed] [Google Scholar]
- 15.Einarsdottir T, Lockhart E, Flynn JL. Cytotoxicity and secretion of gamma interferon are carried out by distinct CD8 T cells during Mycobacterium tuberculosis infection. Infect Immun. 2009;77(10):4621–30. doi: 10.1128/IAI.00415-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irwin SM, et al. Tracking antigen-specific CD8 T lymphocytes in the lungs of mice vaccinated with the Mtb72F polyprotein. Infect Immun. 2005;73(9):5809–16. doi: 10.1128/IAI.73.9.5809-5816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott HM, Flynn JL. Mycobacterium tuberculosis in chemokine receptor 2-deficient mice: influence of dose on disease progression. Infect Immun. 2002;70(11):5946–54. doi: 10.1128/IAI.70.11.5946-5954.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolf AJ, et al. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J Exp Med. 2008;205(1):105–15. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chackerian AA, et al. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect Immun. 2002;70(8):4501–9. doi: 10.1128/IAI.70.8.4501-4509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bold TD, Ernst JD. CD4+ T Cell-Dependent IFN-gamma Production by CD8+ Effector T Cells in Mycobacterium tuberculosis Infection. Journal of immunology. 2012;189(5):2530–6. doi: 10.4049/jimmunol.1200994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sercan O, et al. IFN-gamma receptor signaling regulates memory CD8+ T cell differentiation. J Immunol. 184(6):2855–62. doi: 10.4049/jimmunol.0902708. [DOI] [PubMed] [Google Scholar]
- 22.Serbina NV, Lazarevic V, Flynn JL. CD4(+) T cells are required for the development of cytotoxic CD8(+) T cells during Mycobacterium tuberculosis infection. Journal of immunology. 2001;167(12):6991–7000. doi: 10.4049/jimmunol.167.12.6991. [DOI] [PubMed] [Google Scholar]