Abstract
Background
Homozygous or compound heterozygous mutations in KCNQ1 cause Jervell and Lange-Nielsen syndrome (JLNS), a rare, autosomal recessive form of long QT syndrome (LQTS) characterized by deafness, marked QT prolongation, and a high risk of sudden death. However, it is not understood why some individuals with mutations on both KCNQ1 alleles present without deafness. Here, we sought to determine the prevalence and genetic determinants of this phenomenon in a large referral population of LQTS patients.
Methods and Results
Retrospective analysis of all LQTS patients evaluated from July 1998 to April 2012 was used to identify those with ≥1 KCNQ1 mutation. Of the 249 KCNQ1-positive patients identified, 15 patients (6.0%) harbored a rare putative pathogenic mutation on both KCNQ1 alleles. Surprisingly, 11 (73%) of these patients presented without the sensorineural deafness associated with JLNS. The degree of QT interval prolongation and number of breakthrough cardiac events were similar between cases with and without deafness. Interestingly, truncating mutations were more prevalent in JLNS (79%) than non-deaf cases (36%, p<0.001) derived from this study and those in the literature.
Conclusions
Here, we provide evidence that the “recessive” inheritance of a severe LQT1 phenotype in the absence of an auditory phenotype may represent a more common pattern of LQTS inheritance than previously anticipated and that these cases should be treated as a higher-risk LQTS subset similar to their JLNS counterparts. Furthermore, mutation type may serve as a genetic determinant of deafness, but not cardiac expressivity, in individuals harboring ≥1 KCNQ1 mutation on each allele.
Keywords: long QT syndrome, genetics, ion channels, pediatrics, sudden cardiac death
Background
Congenital long QT syndrome (LQTS) is characterized by a prolonged heart rate-corrected QT interval (QTc) on electrocardiogram (ECG) and an increased risk of syncope and sudden death secondary to polymorphic ventricular arrhythmias.1 Loss-of-function mutations in the KCNQ1-encoded Kv7.1 potassium channel, which conducts the slowly activating delayed rectifier outward K+ current (IKs) in the heart2 and inner ear3, cause type 1 LQTS (LQT1)4 the most common genetic subtype of LQTS.5, 6 Classically, LQTS assumes two clinically recognized forms: autosomal dominant Romano-Ward syndrome (RWS) which affects between 1:2000 and 1:5000 individuals and presents with only a cardiac phenotype7 and the extremely rare (< 1 per 4 million), autosomal recessive Jervell and Lange-Nielsen syndrome (JLNS) that presents with a malignant cardiac phenotype and congenital bilateral sensorineural deafness.8, 9
Although individuals homozygous or compound heterozygous for KCNQ1 disease-causative mutations typically present with JLNS, over the past decade isolated reports of homozygous or compound heterozygous individuals presenting with only cardiac manifestations in the absence of deafness (so called “autosomal recessive” RWS/LQT1 or AR LQT1) have surfaced in the literature.10–14 It has been postulated that hearing preservation in these KCNQ1 homozygotes/compound heterozygotes stems from the presence of “milder” mutations that fail to completely abolish Kv7.1 function resulting in enough residual IKs current to maintain normal K+ cycling in the inner ear, but not the normal electrical activity of the heart.10, 11, 15 However, aside from a single individual with intact hearing who was found to harbor a homozygous splice-site mutation (c.387-5 T>A) in exon 2 of KCNQ1 that results in incomplete exon skipping14, few insights into the overall prevalence of KCNQ1 homozygosity/compound heterozygosity without sensorineural deafness and the genetic determinants underlying hearing preservation in these individuals exists.
As such, we performed a retrospective analysis of LQTS patients evaluated at the Mayo Clinic to determine: 1) the overall prevalence, degree of cardiac expressivity, and spectrum of auditory phenotypes associated with individuals harboring a mutation on both KCNQ1 alleles and 2) if mutation type or topological location of mutations in the Kv7.1 channel represents a genetic determinant of sensorineural deafness in KCNQ1 homozygotes/compound heterozygotes.
Methods
Study population
In this Mayo Clinic IRB-approved study, a retrospective review of all patients seen in the LQTS Clinic from July 1998 to April 2012 was used to identify all patients with ≥ 1 LQT1-associated mutation(s) in the KCNQ1-encoded Kv7.1 potassium channel. Of the 249 KCNQ1-positive patients (average age at evaluation 21 ± 16 years and average QTc 466 ± 42 msec), those patients harboring either i) a single KCNQ1 mutation, ii) mutation(s) observed in > 0.5% of ostensibly healthy individuals in either the National Heart Lung and Blood Institute (NHLBI) exome sequencing project (ESP) or 1000 genomes public datasets, iii) multiple KCNQ1 mutations on the same allele, or iv) additional mutations in other channelopathic genes were excluded from the analysis. Furthermore, individuals with acquired cardiac diseases, electrolyte abnormalities, or receiving QT prolonging medications were also excluded.
The following parameters were obtained from the electronic medical records of each family member: age, sex, proband status, family history of sudden death, incidence of TdP, documented VF, syncopal episodes, β-blocker status, ICD status, pertinent surgical interventions such as left cardiac sympathetic denervation (LCSD) and right cardiac sympathetic denervation (RCSD), and type of KCNQ1 mutation(s). The researcher (JG) who obtained the clinical parameters and analyzed ECGs was blinded initially with respect to the mutation type and auditory phenotype of each patient.
ECG analysis
Twelve-lead electrocardiograms were analyzed manually and the QT duration (lead II or V5) was corrected for heart rate using Bazett’s formula (QTc = QT/√RR). The QTc value for each individual represents the mean value of five consecutive beats and is reported in milliseconds.
Genetic analysis
The identification of LQT1-associated mutations in KCNQ1 was accomplished using either commercial genetic testing or through the use of laboratory-based genetic testing using mutation detection protocols described previously.16
Genotype-phenotype correlation analysis
Given the rare nature of bi-allelic inheritance of two KCNQ1 mutations, for the purposes of identifying potential genotype-phenotype correlations the results of the present study were combined with several large JLNS cases series17, 18 and AR LQT1 case reports10, 11, 13, 14 in the literature where full genotypic information was available. A list of the literature-derived unrelated JLNS and AR LQT1 cases used in this analysis are detailed in Table S1 of the supplemental appendix.
Statistical methods
Univariate analysis was employed to compare groups identified on the basis of clinical (e.g. sensorineural deafness) or molecular (e.g. truncating mutations) characteristics. All continuous variables are presented as mean ± standard deviation. Fisher’s exact test was used to compare categorical variables and Wilcoxon Rank Sum/Mann-Whitney U test was employed to compare continuous variables. For both tests a p value ≤ 0.05 was considered statistically significant.
Ethical statement
Both authors had full access to and take full responsibility for the integrity of the data. Both authors have read and agree to the manuscript as written.
Results
Prevalence and clinical evaluation of KCNQ1 homozygotes/compound heterozygotes referred to the Mayo Clinic
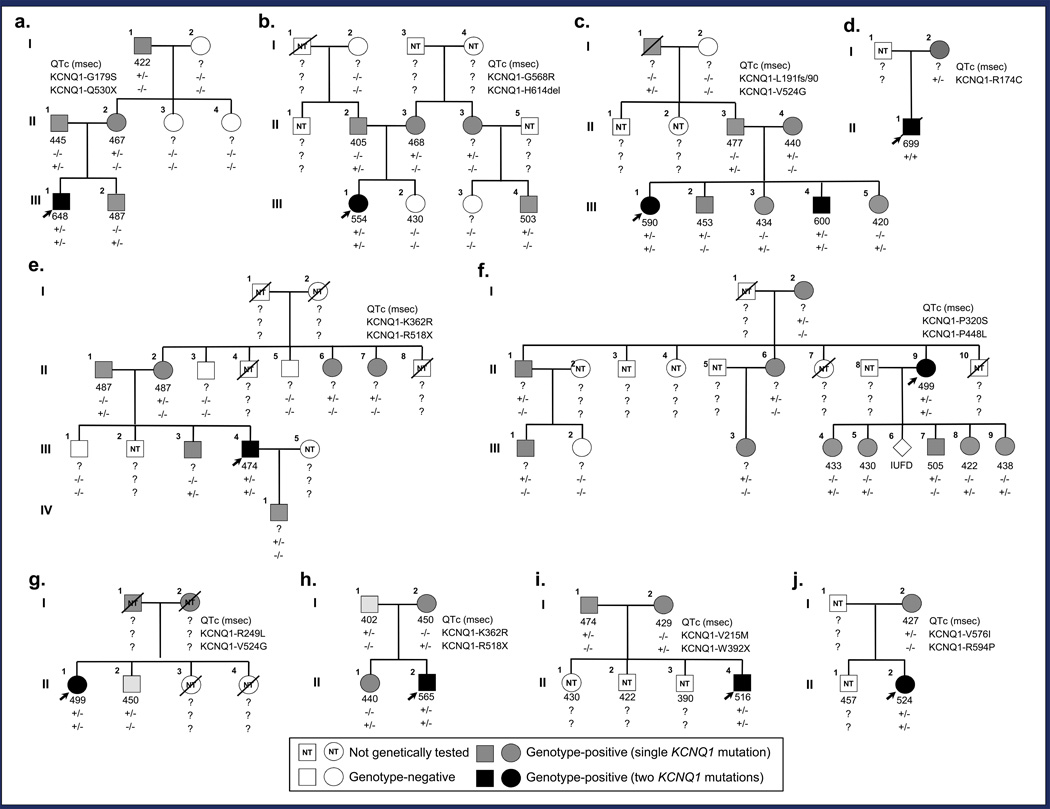
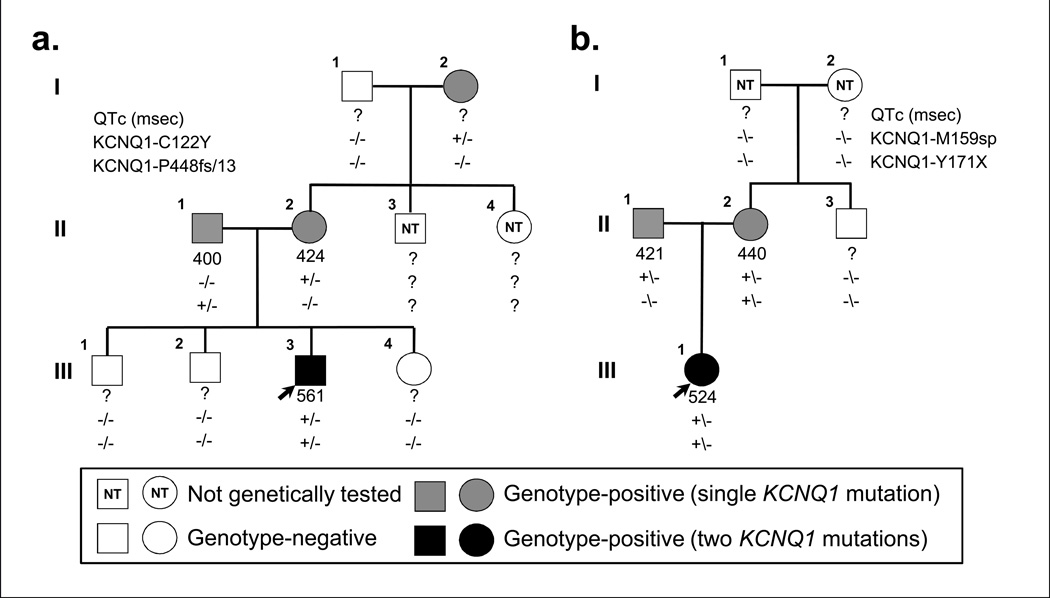
Out of the 249 KCNQ1-positive patients evaluated at Mayo Clinic’s Long QT Syndrome Clinic between July 1998 and April 2012, 15 patients (6.0%) harbored rare, potentially pathogenic mutations on both KCNQ1 alleles. Surprisingly, 11 (73%) of these double KCNQ1-positive individuals presented without the sensorineural deafness/hearing loss typically associated with autosomal recessive JLNS1 and thus are best defined as “recessive” LQT1 (AR LQT1) cases. Pertinent clinical information on cardiac expressivity and auditory phenotype of each KCNQ1 homozygote/compound heterozygote is summarized in Table 1. Pedigree structures and genotypic information of AR LQT1 and JLNS1 families are detailed in Figure 1 and Figure 2, respectively. Brief clinical/family histories for the 14 cases not previously described in the literature are provided in the supplement (see supplemental text).
Table 1.
KCNQ1 homozygotes/compound heterozygotes referred to the Mayo Clinic for evaluation
| Family ID | Sex | Auditory Phenotype |
Syncopal episodes |
Documented VF/TdP |
OHC A |
QTc (ms) |
β- blocker |
ICD | Sympathetic Denervation |
KCNQ1 Allele #1 |
KCNQ1 Allele #2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AR LQT1a | M | Intact | Multiple | 0 | 0 | 648 | Yes | Yes | Left | G179S | Q530X |
| AR LQT1b | F | Intact | Multiple | 0 | 0 | 554 | Yes | No | Left | G568R | H614del |
| AR LQT1c | F | Intact | 1 | 1 | 1 | 590 | Yes | Yes | No | L191fs/90 | V524G |
| AR LQT1c | M | Intact | 1 | Multiple | 0 | 600 | Yes | Yes | Bilateral | L191fs/90 | V524G |
| AR LQT1d | M | Intact | Multiple | Multiple | 1 | 699 | Yes | Yes | Left | R174C | R174C |
| AR LQT1e | M | Intact | Multiple | 0 | 0 | 474 | Yes | No | No | K362R | R518X |
| AR LQT1f | F | Intact | 1 | 0 | 0 | 499 | Yes | No | No | P320S | P448L |
| AR LQT1g | F | Intact | 1 | 0 | 0 | 499 | Yes | Yes | No | R259L | V524G |
| AR LQT1h | M | Intact | Multiple | Multiple | 0 | 565 | Yes | Yes | Left | K362R | R518X |
| AR LQT1i | M | Intact | Multiple | 0 | 2 | 516 | Yes | No | Left | V215M | W392X |
| AR LQT1j | F | Intact | 1 | 0 | 0 | 524 | Yes | No | Left | V576I | R594P |
| JLNS1a | M | Deaf | Multiple | Multiple | 0 | 561 | Yes | Yes | Left | C122Y | P448fs/13 |
| JLNS1b | F | Deaf | 0 | 0 | 0 | 524 | Yes | No | Left | M159sp | Y171X |
| JLNS1c* | F | Deaf | 0 | 0 | 0 | 584 | Yes | No | Left | R195fs/40 | D202N |
| JLNS1d* | M | Deaf | Multiple | Multiple | 0 | 521 | Yes | Yes | Left | R518X | Q530X |
Figure 1.
Pedigree structure and genotypic information for families of KCNQ1 homozygotes/compound heterozygotes with intact hearing. Genotype-positive individuals indicated by gray (single KCNQ1 mutation) or black (two KCNQ1 mutations) squares (male) and circles (female). Genotype-negative individuals indicated by open symbols; index cases by black arrows; and deceased individuals by slashes. Lastly, squares or circles containing “NT” (not tested) indicate individuals who have yet to undergo or have refused genetic testing. QTc intervals and genotypes are displayed beneath each symbol. a. Family AR LQT1a. This three-generation pedigree is notable for the index case with severe QT prolongation that suffered from breakthrough syncopal episodes prior to undergoing a LCSD (III.1). b. Family AR LQT1b. This three-generation pedigree is notable for the index case with severe QT prolongation and a history of breakthrough cardiac events (III.1). c. Family AR LQT1c. This three-generation pedigree is notable for the index case with severe QT prolongation and a history of breakthrough cardiac events including an out-of-hospital cardiac arrest (III.1) and her brother who also has severe QT prolongation and has suffered multiple breakthrough cardiac events including an ICD storm (III.4). d. Family AR LQT1d. This two-generation pedigree is notable only for the index case that displays extreme QT prolongation and suffered multiple breakthrough cardiac events prior to succumbing to his malignant LQTS phenotype at the age of 3 (II.1). e. Family AR LQT1e. This four-generation pedigree is notable for the index case with QT prolongation that suffered numerous exertional syncopal episodes during childhood (III.4). f. Family AR LQT1f. This three-generation pedigree is notable for the index case who features a prolonged QT interval but has remained asymptomatic for the last 25 years (III.9) and the sudden unexplained deaths of her siblings at the age of 13 and 3, respectively (III.7 and III.10). g. Family AR LQT1g. A two-generation pedigree notable for the index case with QT prolongation (III.1) and the swimming-triggered sudden unexplained death of the index case’s 13-year old sister (III.3). h. Family AR LQT1h. A two-generation pedigree notable for the index case who has severe QT prolongation and suffered several breakthrough ICD shocks prior to undergoing a LCSD (III.2). i. Family AR LQT1i. This two-generation pedigree is notable for the index case who has QT prolongation and suffered several breakthrough cardiac events including an out-of-hospital cardiac arrest (III.5). j. Family AR LQT1j. This two-generation pedigree is notable for the symptomatic index case that has QT prolongation (III.2).
Figure 2.
Pedigree structure and genotypic information for families of KCNQ1 homozygotes/compound heterozygotes with bilateral sensorineural hearing loss. a. Family JLNS1a.This three-generation pedigree is only significant for the index case with severe QT prolongation and suffered from numerous breakthrough syncopal events during childhood including several appropriate VF-terminating ICD shocks (III.3). b. Family JLNS1b. This two-generation pedigree is only significant for the index case who features QT prolongation but is otherwise asymptomatic (III.1). Genotype-positive individuals indicated by gray (single KCNQ1 mutation) or black (two KCNQ1 mutations) squares (male) and circles (female). Genotype-negative individuals indicated by open symbols; index cases by black arrows; and deceased individuals by slashes. Lastly, squares or circles containing “NT” indicate individuals who have yet to undergo or have refused genetic testing. QTc intervals and genotypes are displayed beneath each symbol.
Clinical characteristics of patients homozygous or compound heterozygous for mutations in the KCNQ1-encoded Kv7.1 potassium channel
The clinical characteristics of the 15 individuals homozygous or compound heterozygous for mutations in KCNQ1 are summarized in Table 2. Interestingly, those cases defined as AR LQT1 (i.e. intact hearing) were significantly more likely to have a positive family of SUD or LQTS (91%) in comparison to JLNS cases (25%, p ≤ 0.05). Indicators of the severity of the cardiac phenotype such as previously suffering a cardiac event (defined as syncope, documented VF/VT, or cardiac arrest) and the degree of QT interval prolongation were statistically similar between JLNS and AR LQT1 cases (Table 2). Furthermore, AR LQT1 cases trended towards a higher likelihood of suffering from at least one syncopal episode (100%) in comparison to their JLNS counterparts (50%, p = 0.06; Table 2). However, this observation may reflect the small sample size.
Table 2.
Clinical characteristics of KCNQ1 homozygotes/compound heterozygotes referred to Mayo Clinic for evaluation
| Variable | All cases | “Recessive” LQT1 | JLNS |
|---|---|---|---|
| Clinical demographics and family history | |||
| Patients, n (%) | 15 (100) | 11 (73) | 4 (27) |
| Male/female, n | 7/8 | 6/5 | 2/2 |
| Age at diagnosis, years | 7.0 ± 9.5 | 8.8 ± 11.0 | 2.0 ± 2.7 |
| Family history of LQTS/sudden death, n (%) | 11 (73) | 10 (91)# | 1 (25) |
| Bilateral sensorineural deafness, n (%) | 4 (27) | 0 (0) | 4 (100)* |
| Symptomatology | |||
| Any cardiac event (%) | 13 (87) | 11 (100) | 2 (50) |
| Syncope, n (%) | 13 (87) | 11 (100) | 2 (50) |
| Documented TdP/VF, n (%) | 5 (39) | 4 (36) | 2 (50) |
| Out of hospital cardiac arrest, n (%) | 2 (15) | 3 (27) | 0 (0) |
| QTc (ms) | 557 ± 60 | 560 ± 69 | 549 ± 29 |
| Clinical management | |||
| β-blockers, n (%) | 15 (100) | 11 (100) | 4 (100) |
| Implantable cardioverter-defibrillator, n (%) | 8 (53) | 6 (55) | 2 (50) |
| Left cardiac sympathetic denervation, n (%) | 11 (73) | 7 (64) | 4 (100) |
Clinical characteristic and management values are expressed as number of patients (percentage of total) or as mean ± standard deviation. All ECG parameters represent mean ± standard deviation.
P ≤ 0.05 compared to JLNS cases.
P ≤ 0.05 compared to AR LQT1 cases.
Abbreviations: JLNS, Jervell and Lange-Nielsen syndrome; LQT1, type 1 long QT syndrome; TdP, torsades de pointes; VF, ventricular fibrillation.
Clinical characteristics of the parents of KCNQ1 homozygotes/compound heterozygotes
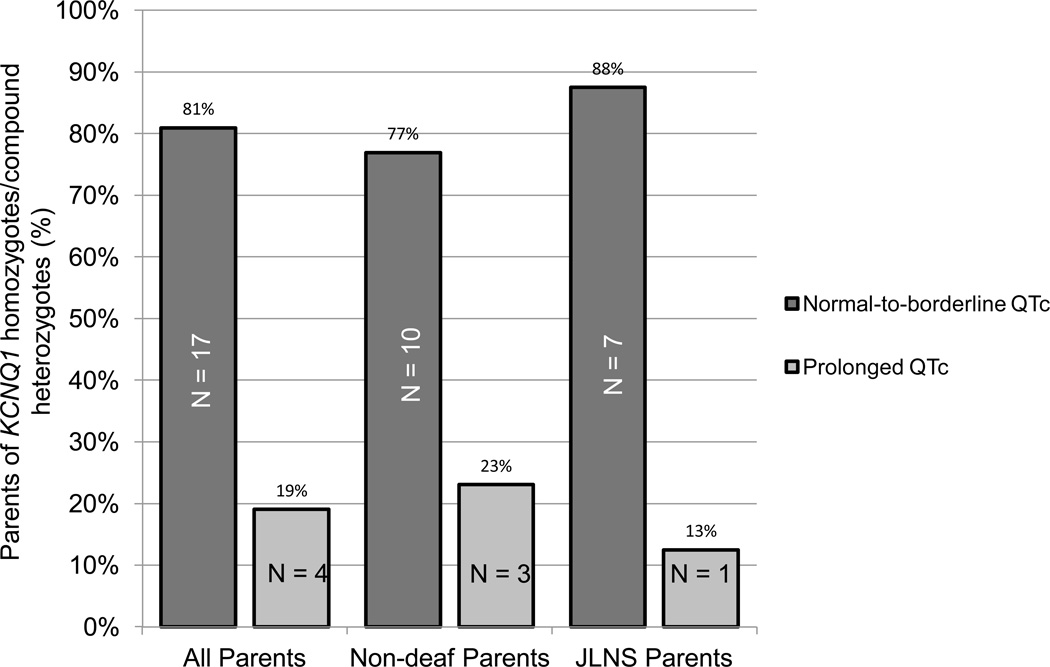
Complete clinical demographics were available on 21 out of 28 AR LQT1/JLNS parents (Table 3). Unfortunately, limited clinical information was not available on 7 AR LQT1/JLNS parents who either died of non-cardiac causes prior to their child’s LQTS diagnosis (3) or who declined further participation (4) in this study. Nevertheless, only 25% of the parents with available clinical information, who by definition are obligate positive for 1 KCNQ1 mutation, displayed phenotypic manifestations suggestive of LQTS (e.g. symptoms or a resting QTc ≥ 470 in males or ≥ 480 in females; Figure 3). There was no statistical difference in the percentage of parents of JLNS compound heterozygotes or parents of non-deaf compound heterozygotes that displayed objective phenotypic manifestations of LQTS (Figure 3). Furthermore, no statistically significant differences in the average QTc, percentage of symptomatic parents or percentage of parents requiring clinical management of their LQT1 genetic substrate was observed between parents of JLNS and non-deaf compound heterozygotes (Table 3).
Table 3.
Clinical characteristics of obligate LQT1-mutation carrying biological parents of KCNQ1 homozygotes/compound heterozygotes
| Variable | All parents# | “Recessive” LQT1 parents# |
JLNS parents |
|---|---|---|---|
| Clinical characteristics | |||
| Patients, n (%) | 21 (100) | 13 (63) | 8 (37) |
| Male/female, n | 10/11 | 6/7 | 4/4 |
| Family history of LQTS/sudden death, n (%) | 6 (29) | 5 (38) | 1 (13) |
| Bilateral sensorineural deafness, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Syncope, n (%) | 1 (5) | 1 (8) | 0 (0) |
| Documented TdP/VF, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Out of hospital cardiac arrest, n (%) | 0 (0) | 0 (0) | 0 (0) |
| QTc (ms) | 446 ± 29 | 450 ± 29 | 440 ± 31 |
| Clinical management | |||
| β-blockers, n (%) | 4 (19) | 4 (31) | 0 (0) |
| Implantable cardioverter-defibrillator, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Left cardiac sympathetic denervation, n (%) | 0 (0) | 0 (0) | 0 (0) |
Clinical characteristic and management values are expressed as number of patients (percentage of total) or as mean ± standard deviation.
Limited clinical information was available on seven parents of KCNQ1 homozygotes/compound heterozygotes with intact hearing who either died of non-cardiac causes prior to their child’s LQTS diagnosis (3) or declined further participation (4).
Abbreviations: JLNS, Jervell and Lange-Nielsen syndrome; LQT1, type 1 long QT syndrome; TdP, torsades de pointes; VF, ventricular fibrillation.
Figure 3.
Electrocardiographic phenotype KCNQ1 compound heterozygote parents. Bar graph of the electrocardiographic phenotype of the parents of JLNS and non-deaf KCNQ1 compound heterozygotes. A normal-to-borderline QT interval was defined as a QTc < 470 in males and a QTc < 480 in females.
Potential genetic determinants of auditory/deafness phenotype in KCNQ1 homozygosity/compound heterozygosity
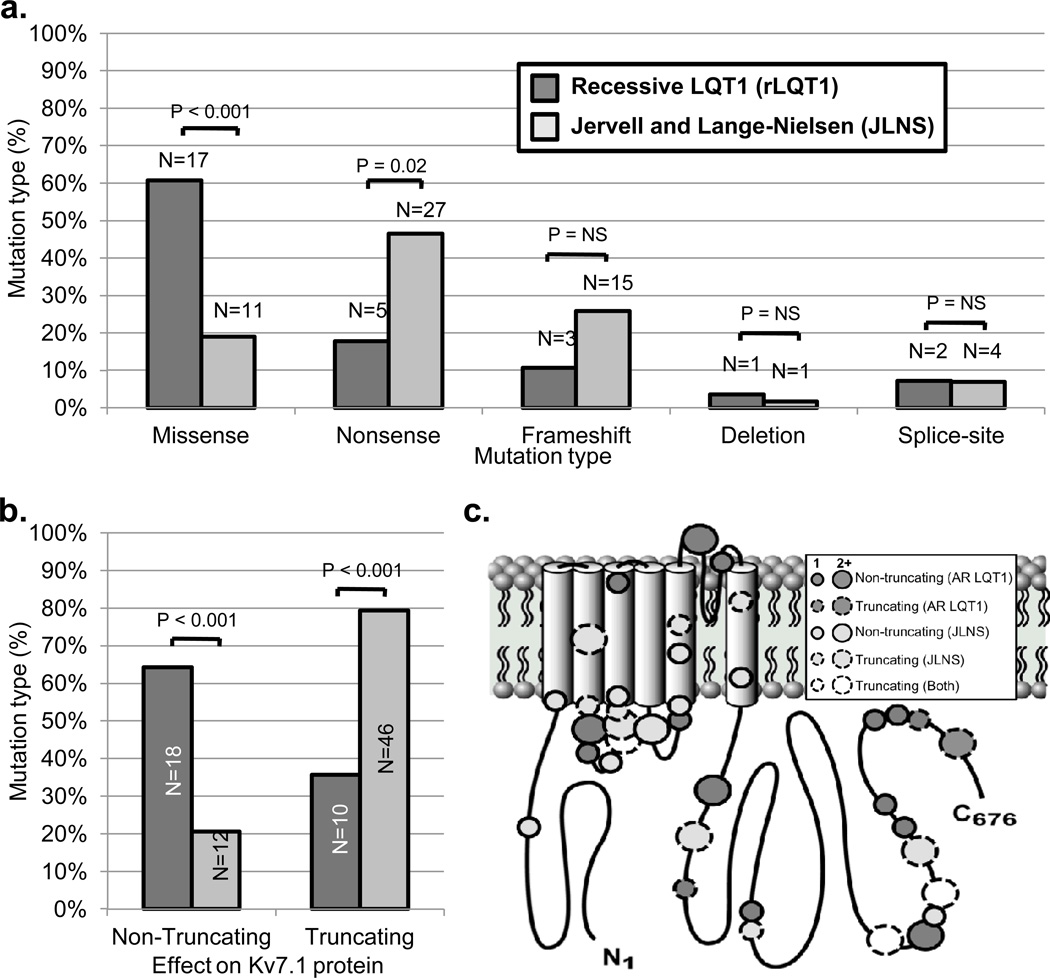
In order to strengthen the identification of potential AR LQT1/JLNS genotype-phenotype correlations, the cases described in the current study were combined and analyzed with JLNS and AR LQT1 cases derived from case series17, 18 or cases reports10, 11, 13, 14 in the literature. A breakdown of KCNQ1 mutations in this combined analysis by mutation type is summarized in Figure 4a. To allow for further statistical comparison, mutations were classified subsequently as either non-truncating (e.g. missense or in-frame deletion) or truncating (e.g. nonsense, frame-shift, or splice-site) mutations to reflect shared effects on protein structure. Interestingly, JLNS cases were significantly more likely to harbor truncating mutations (79%) than AR LQT1 cases (36%, p < 0.001; Figure 4b). No statistical difference in the localization of AR LQT1- or JLNS1-causative mutations to specific regions of the Kv7.1 channel topology was observed (Figure 4c).
Figure 4.
Potential genetic determinants of auditory phenotype in patients harboring mutations on both KCNQ1 alleles identified in the current study and previously in the literature. a. Comparison of mutation types identified in “recessive” type 1 long QT syndrome with intact hearing and Jervell and Lange-Nielsen syndrome cases. b. Percentage of truncating (e.g. nonsense, frameshift, etc.) and non-truncating (e.g. missense, in-frame deletion, etc.) mutations identified in “recessive” type 1 long QT syndrome with intact hearing and Jervell and Lange-Nielsen syndrome case, respectively. c. Location of putative “recessive” type 1 long QT syndrome with intact hearing- and Jervell and Lange-Nielsen syndrome-causative mutations in the Kv7.1 channel topology. Dark gray bars indicate “recessive” type 1 long QT syndrome cases, light gray bars indicate Jervell and Lange-Nielsen syndrome cases, and white circles indicate mutations seen in both “recessive” LQT1 and Jervell and Lange-Nielsen syndrome.
Discussion
Prevalence and inheritance patterns of KCNQ1 homozygosity/compound heterozygosity
The observation that marked variable penetrance and expressivity is a hallmark of LQTS has led some to hypothesize that widespread genetic testing in the general population may result in the identification of a surprisingly high number of low-frequency “reduced penetrance” mutations that manifest clinically only when other genetic (e.g. presence of a similar “reduced penetrance” mutation on the opposite allele) or environmental factors (e.g. QT prolonging drugs) are present.10, 21, 22
Indeed, the release of large-scale exome sequencing data from the NHLBI GO Exome Sequencing Project (ESP) revealed that 1:31 individuals (3%) harbor a rare genetic variant of in one of the 13 established LQTS-susceptibility genes.23 While the vast majority of established LQTS-associated mutations (94.8%), including all but the KCNQ1-R518X mutation described in the present study, are absent from the NHLBI ESP, nine rare missense or nonsense variants, previously associated with LQT1, were identified in KCNQ1 alone.23 This would place the prevalence of a heterozygous LQT1 genotype between 1:600 and 1:1080 in the general population, far greater than the estimated LQT1 disease prevalence of ~1:7500 (1:2500 estimate for LQTS of which approximately one-third is attributed to LQT1.24
While many of the rare LQTS-associated variants identified in ESP individuals are likely to be innocuous bystanders that slipped through the cracks, the discordance between the prevalence of functional/co-segregating KCNQ1 variants and overall LQTS disease prevalence may suggest that a subset of KCNQ1 variants, with defined pathogenic influence, are at best infrequent causes of monogenic disease in isolation. Furthermore, this observation suggests that the number of individuals who are compound heterozygous (or less likely homozygous) for rare potentially pathogenic KCNQ1 mutations exceeds the estimated prevalence of 1 in 4 million to 1 in 25 million derived from overall LQTS disease prevalence.
Given the severe clinical course experienced by 12 out of 14 (86%) unrelated individuals who have a rare variant on each KCNQ1 allele (e.g. QTc >500 msec with documented cardiac events) and the concealed phenotype observed in the majority of their heterozygous parents/family members assessed, it is not surprising that the pedigree structures of at least 11 out of the 14 (79%) families in this study display a probable autosomal recessive (AR) pattern of inheritance. While two of the remaining pedigrees (AR LQT1g and AR LQT1j) had insufficient information to clearly determine the pattern of inheritance, one pedigree (AR LQT1f) clearly represents classic autosomal dominant LQT1 as P320S-positive individuals display significant QT prolongation regardless of the P448L status on their opposite allele. Thus, given the relatively high prevalence of “private” innocuous KCNQ1 variants in the general population, it should not be assumed that the identification of a rare KCNQ1 mutation on both alleles directly correlates to an AR pattern of inheritance.
Spectrum of auditory/deafness phenotype and degree of cardiac expressivity in KCNQ1 compound heterozygotes
Over a decade ago, Priori et al provided the first clinical and molecular evidence that homozygous or compound heterozygous mutations in KCNQ1 do not invariably lead to JLNS.10 While several additional case reports confirmed that AR LQT1 is not an isolated phenomenon11–14 and previous studies have assessed the prevalence and phenotypic severity of a compound/digenic hetorozygosity involving the three major LQTS-susceptibility genes5, 25, 26, the prevalence of sensorineural hearing loss and degree of cardiac expressivity in individuals with mutations on both KCNQ1 alleles remains undefined.
Surprisingly, the present study suggests that the majority of unrelated KCNQ1 compound heterozygotes with a probable AR pattern of inheritance (7/11; 64%) present without sensorineural hearing loss. To our knowledge, even when cases with an uncertain pattern of inheritance are excluded, this study still more than doubles (from 4 to 11) the number of individuals with intact hearing despite homozygous or compound heterozygous mutations in KCNQ1 that have been described in the literature. As such, this report provides further evidence that the two traditional patterns of LQTS inheritance, autosomal dominant RWS with cardiac phenotype only and autosomal recessive JLNS with cardiac phenotype and sensorineural deafness, inadequately describe the full spectrum of inheritance patterns observed in LQTS cases.
In addition to providing evidence that the existing patterns of LQTS inheritance should be expanded to include instances where the LQTS cardiac phenotype is inherited in a “recessive” manner without a discernible auditory phenotype culminating in what is best described as recessive RWS/LQT1, this study also provides additional evidence that JLNS and recessive RWS/LQT1 compound heterozygotes represent equally high-risk LQTS subsets that are both prone to suffering breakthrough cardiac events. Given that JLNS1 and AR LQT1 individuals share similarly severe clinical courses, the early initiation of surgical options, such as prophylactic LCSD or ICD implantation for secondary prevention, in addition to β-blockers merits serious consideration as a means of further reducing the risk of sudden death in these higher-risk LQTS populations.
Genetic determinants of sensorineural hearing loss in KCNQ1 compound heterozygosity
While most LQT1-causative mutations are non-truncating, missense mutations capable of exerting a dominant-negative effect on channel function5, most JLNS1-causative mutations are truncating nonsense or frameshift mutations that likely abolish the ability of mutant α-subunits to post-translationally co-assemble thereby resulting in a non-functioning channel when expressed in isolation and haploinsufficiency when co-expressed with a wild type subunit.21, 27 Not surprisingly, 79% of JLNS mutations identified in the cases analyzed for this study were truncating, consistent with previous observations.17, 21, 28 However, this observation did not extend to the 14 newly and previously described unrelated AR LQT1 cases were only 36% of individuals harbored truncating mutations expected to result in KCNQ1 haploinsuffiency.
As such, this study provides preliminary evidence that KCNQ1 mutation type represents a major differentiating factor between JLNS and AR LQT1 and thus may play a role in determining the presence or absence of an auditory phenotype. Furthermore, this study suggests that the residual KCNQ1 function needed to maintain the normal secretion of K+ into the endolymphatic compartment partially responsible for generating the endocochlear potential in the inner ear must be fairly low. Interestingly, through the study of a patient homozygous for the incomplete exon skipping c.387 -5 T>A splice-site mutation, Bhuiyan and colleagues were able to demonstrate that the degree of residual KCNQ1 function required for hearing preservation is roughly 10%.14 While the current study cannot improve upon the numerical KCNQ1 “threshold” required for hearing preservation, it is clear from this study and others that the complete cessation of KCNQ1-mediated K+ secretion in the inner ear linked to the bi-allelic inheritance of two truncating/haploinsufficient mutations is the primary mechanism that disrupts cochlear fluid homeostasis resulting in the collapse of endolymphatic compartment and sensorineural deafness in JLNS patients and murine JLNS models.29, 30 As such, current evidence places the threshold for hearing preservation in KCNQ1 homozygosity/compound heterozygosity at > 0%, but ≤ 10% residual KCNQ1 function. Functional investigation of unique or unexpected AR LQT1 and JLNS mutation combinations, perhaps by employing patient-specific induced pluripotent stem cell (iPS) models that more accurately recapitulate a endocochlear/cardiac environment, may shed more light on this interesting phenomena and help precisely define the degree of residual KCNQ1 function required for hearing preservation in KCNQ1 homozygosity/compound heterozygosity.
Limitations
As a result of the rarity of KCNQ1 homozygosity/compound heterozygosity and the single center nature of this study, only 15 patients from 14 unrelated families were enrolled in this study. However, to our knowledge, this study represents the only attempt in the literature to assess the overall prevalence and phenotypic spectrum of all KCNQ1 compound heterozygotes, not just JLNS, evaluated at a single referral center. While every attempt was made to gather clinical information and genetic material on as many family members as possible, due to death, or declining further participation, clinical information and DNA were not available on all parents and extended family members. As a result, we were unable to fully assess the inheritance patterns, clinical characteristics, and potential genetic determinants of cardiac/auditory phenotype in a few of the pedigrees included in this study.
Additionally, emerging evidence in the literature suggests that KCNQ1 homozygosity/compound heterozygosity resulting in JLNS may also be accompanied by additional hematologic and gastrointestinal manifestations.31, 32 Unfortunately, clinical data such as serum gastrin levels pertinent to these additional phenotypic manifestations were not available for the patients enrolled in this study. Thus, it remains to be seen if AR LQT1 patients are susceptible to similar hematologic and gastrointestinal pathologies as those described previously for JLNS.
Given the referral nature of our LQTS Clinic, it is also possible that the observed 6% frequency of bi-allelic KCNQ1 mutations is an overestimate of the actual frequency/burden of multiple hit KCNQ1-mediated disease. Patients with LQT1 and recurrent symptoms despite beta-blocker therapy have been referred increasingly over the years for LCSD therapy and thus may be over-represented in this cohort, which could inflate the apparent frequency of compound mutation LQT1.
Lastly, the vast majority of KCNQ1 mutations identified in this study have not been characterized functionally. Without an electrophysiological phenotype, it is difficult to assess the mechanism (e.g. dominant-negative vs. haploinsufficiency) and the relative strength of each mutation’s electrophysiological phenotype. It is certainly possible that both mutation mechanism and electrophysiological phenotype could shed even more light on the potential genetic mechanisms and determinants underlying the phenotypic presentation of KCNQ1 compound heterozygotes identified in this study. However, even without an electrophysiological phenotype on each of the 22 mutations identified, we were still able to glean new observations in regards to the genetic determinants that may partially define the severity of the cardiac/auditory phenotype in KCNQ1 compound heterozygosity.
Conclusions
By increasing the number of recessive LQT1 cases reported in the literature, this study provides compelling evidence that the “recessive” inheritance of a severe LQTS cardiac phenotype in the absence of an auditory phenotype may represent a more common pattern of LQTS inheritance than previously anticipated. Furthermore, given the severe clinical course and high rate of breakthrough cardiac events observed in recessive LQTS cases, this study suggests that these individuals should be treated as a higher-risk LQTS subgroup similar to their JLNS counterparts. In other words, normal hearing in a patient with multiple mutations in LQT1 should not result in a better cardiac prognosis than for a deaf patient with LQT1 (JLNS1). Lastly, this study provides preliminary evidence that KCNQ1 mutation type functions as a genetic determinant of sensorineural deafness, but not cardiac expressivity, in KCNQ1 homozygosity/compound heterozygosity.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by the Windland Smith Rice Sudden Comprehensive Sudden Cardiac Death Program (to M.J.A.). J.R.G is supported by a NIH/NHLBI NRSA Ruth L. Kirschstein individual pre-doctoral MD/PhD fellowship (F30-HL106993).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: MJA is a consultant for Transgenomic. Intellectual property derived from MJA’s research program resulted in license agreements in 2004 between Mayo Clinic Health Solutions (formerly Mayo Medical Ventures) and PGxHealth (formerly Genaissance Pharmaceuticals and now Transgenomic).
References
- 1.Moss AJ. Long QT Syndrome. JAMA. 2003;289:2041–2044. doi: 10.1001/jama.289.16.2041. [DOI] [PubMed] [Google Scholar]
- 2.Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, et al. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 3.Wangemann P. Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J Physiol. 2006;576:11–21. doi: 10.1113/jphysiol.2006.112888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, VanRaay TJ, et al. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- 5.Tester DJ, Will ML, Haglund CM, Ackerman MJ. Compendium of cardiac channel mutations in 541 consecutive unrelated patients referred for long QT syndrome genetic testing. Heart Rhythm. 2005;2:507–517. doi: 10.1016/j.hrthm.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Giudicessi JR, Ackerman MJ. Potassium-channel mutations and cardiac arrhythmias-diagnosis and therapy. Nat Rev Cardiol. 2012;9:319–332. doi: 10.1038/nrcardio.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz PJ, Stramba-Badiale M, Crotti L, Pedrazzini M, Besana A, Bosi G, et al. Prevalence of the congenital long-QT syndrome. Circulation. 2009;120:1761–1767. doi: 10.1161/CIRCULATIONAHA.109.863209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jervell A, Lange-Nielsen F. Congenital deaf-mutism, functional heart disease with prolongation of the Q-T interval and sudden death. Am Heart J. 1957;54:59–68. doi: 10.1016/0002-8703(57)90079-0. [DOI] [PubMed] [Google Scholar]
- 9.Fraser GR, Froggatt P, Murphy T. Genetical Aspects of the Cardio-Auditory Syndrome of Jervell and Lange-Nielsen (Congenital Deafness and Electrocardiographic Abnormalities) Ann Hum Genet. 1964;28:133–157. doi: 10.1111/j.1469-1809.1964.tb00469.x. [DOI] [PubMed] [Google Scholar]
- 10.Priori SG, Schwartz PJ, Napolitano C, Bianchi L, Dennis A, De Fusco M, et al. A recessive variant of the Romano-Ward long-QT syndrome? Circulation. 1998;97:2420–2425. doi: 10.1161/01.cir.97.24.2420. [DOI] [PubMed] [Google Scholar]
- 11.Larsen LA, Fosdal I, Andersen PS, Kanters JK, Vuust J, Wettrell G, et al. Recessive Romano-Ward syndrome associated with compound heterozygosity for two mutations in the KVLQT1 gene. Eur J Hum Genet. 1999;7:724–728. doi: 10.1038/sj.ejhg.5200323. [DOI] [PubMed] [Google Scholar]
- 12.Wei J, Fish FA, Myerburg RJ, Roden DM, George AL., Jr Novel KCNQ1 mutations associated with recessive and dominant congenital long QT syndromes: evidence for variable hearing phenotype associated with R518X. Hum Mutat. 2000;15:387–388. doi: 10.1002/(SICI)1098-1004(200004)15:4<387::AID-HUMU26>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 13.Novotny T, Kadlecova J, Janousek J, Gaillyova R, Bittnerova A, Florianova A, et al. The homozygous KCNQ1 gene mutation associated with recessive Romano-Ward syndrome. Pacing Clin Electrophysiol. 2006;29:1013–1015. doi: 10.1111/j.1540-8159.2006.00478.x. [DOI] [PubMed] [Google Scholar]
- 14.Bhuiyan ZA, Momenah TS, Amin AS, Al-Khadra AS, Alders M, Wilde AA, et al. An intronic mutation leading to incomplete skipping of exon-2 in KCNQ1 rescues hearing in Jervell and Lange-Nielsen syndrome. Prog Biophys Mol Biol. 2008;98:319–327. doi: 10.1016/j.pbiomolbio.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Wollnik B, Schroeder BC, Kubisch C, Esperer HD, Wieacker P, Jentsch TJ. Pathophysiological mechanisms of dominant and recessive KVLQT1 K+ channel mutations found in inherited cardiac arrhythmias. Hum Mol Genet. 1997;6:1943–1949. doi: 10.1093/hmg/6.11.1943. [DOI] [PubMed] [Google Scholar]
- 16.Tester DJ, Will ML, Ackerman MJ. Mutation detection in congenital long QT syndrome: cardiac channel gene screen using PCR, dHPLC, and direct DNA sequencing. Methods Mol Med. 2006;128:181–207. doi: 10.1385/1-59745-159-2:181. [DOI] [PubMed] [Google Scholar]
- 17.Huang L, Bitner-Glindzicz M, Tranebjaerg L, Tinker A. A spectrum of functional effects for disease causing mutations in the Jervell and Lange-Nielsen syndrome. Cardiovasc Res. 2001;51:670–680. doi: 10.1016/s0008-6363(01)00350-9. [DOI] [PubMed] [Google Scholar]
- 18.Winbo A, Stattin EL, Diamant UB, Persson J, Jensen SM, Rydberg A. Prevalence, mutation spectrum, and cardiac phenotype of the Jervell and Lange-Nielsen syndrome in Sweden. Europace. 2012 doi: 10.1093/europace/eus111. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Li H, Moss AJ, Robinson J, Zareba W, Knilans T, et al. Compound heterozygous mutations in KvLQT1 cause Jervell and Lange-Nielsen syndrome. Mol Genet Metab. 2002;75:308–316. doi: 10.1016/S1096-7192(02)00007-0. [DOI] [PubMed] [Google Scholar]
- 20.Ning L, Moss AJ, Zareba W, Robinson J, Rosero S, Ryan D, et al. Novel compound heterozygous mutations in the KCNQ1 gene associated with autosomal recessive long QT syndrome (Jervell and Lange-Nielsen syndrome) Ann Noninvasive Electrocardiol. 2003;8:246–250. doi: 10.1046/j.1542-474X.2003.08313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz PJ, Spazzolini C, Crotti L, Bathen J, Amlie JP, Timothy K, et al. The Jervell and Lange-Nielsen syndrome: natural history, molecular basis, and clinical outcome. Circulation. 2006;113:783–790. doi: 10.1161/CIRCULATIONAHA.105.592899. [DOI] [PubMed] [Google Scholar]
- 22.Amin AS, Giudicessi JR, Tijsen AJ, Spanjaart AM, Reckman YJ, Klemens CA, et al. Variants in the 3' untranslated region of the KCNQ1-encoded Kv7.1 potassium channel modify disease severity in patients with type 1 long QT syndrome in an allele-specific manner. Eur Heart J. 2012;33:714–723. doi: 10.1093/eurheartj/ehr473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Refsgaard L, Holst AG, Sadjadieh G, Haunso S, Nielsen JB, Olesen MS. High prevalence of genetic variants previously associated with LQT syndrome in new exome data. Eur J Hum Genet. 2012;20:905–908. doi: 10.1038/ejhg.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hedley PL, Jorgensen P, Schlamowitz S, Wangari R, Moolman-Smook J, Brink PA, et al. The genetic basis of long QT and short QT syndromes: a mutation update. Hum Mutat. 2009;30:1486–1511. doi: 10.1002/humu.21106. [DOI] [PubMed] [Google Scholar]
- 25.Westenskow P, Splawski I, Timothy KW, Keating MT, Sanguinetti MC. Compound mutations: a common cause of severe long-QT syndrome. Circulation. 2004;109:1834–1841. doi: 10.1161/01.CIR.0000125524.34234.13. [DOI] [PubMed] [Google Scholar]
- 26.Itoh H, Shimizu W, Hayashi K, Yamagata K, Sakaguchi T, Ohno S, et al. Long QT syndrome with compound mutations is associated with a more severe phenotype: a Japanese multicenter study. Heart Rhythm. 2010;7:1411–1418. doi: 10.1016/j.hrthm.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 27.Goldenberg I, Moss AJ, Zareba W, McNitt S, Robinson JL, Qi M, et al. Clinical course and risk stratification of patients affected with the Jervell and Lange-Nielsen syndrome. J Cardiovasc Electrophysiol. 2006;17:1161–1168. doi: 10.1111/j.1540-8167.2006.00587.x. [DOI] [PubMed] [Google Scholar]
- 28.Tyson J, Tranebjaerg L, McEntagart M, Larsen LA, Christiansen M, Whiteford ML, et al. Mutational spectrum in the cardioauditory syndrome of Jervell and Lange-Nielsen. Hum Genet. 2000;107:499–503. doi: 10.1007/s004390000402. [DOI] [PubMed] [Google Scholar]
- 29.Friedmann I, Fraser GR, Froggatt P. Pathology of the ear in the cardioauditory syndrome of Jervell and Lange-Nielsen (recessive deafness with electrocardiographic abnormalities) J Laryngol Otol. 1966;80:451–470. doi: 10.1017/s002221510006552x. [DOI] [PubMed] [Google Scholar]
- 30.Casimiro MC, Knollmann BC, Ebert SN, Vary JC, Jr, Greene AE, Franz MR, et al. Targeted disruption of the Kcnq1 gene produces a mouse model of Jervell and Lange-Nielsen Syndrome. Proc Natl Acad Sci U S A. 2001;98:2526–2531. doi: 10.1073/pnas.041398998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rice KS, Dickson G, Lane M, Crawford J, Chung SK, Rees MI, et al. Elevated serum gastrin levels in Jervell and Lange-Nielsen syndrome: a marker of severe KCNQ1 dysfunction? Heart Rhythm. 2011;8:551–554. doi: 10.1016/j.hrthm.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 32.Winbo A, Sandstrom O, Palmqvist R, Rydberg A. Iron-deficiency anaemia, gastric hyperplasia, and elevated gastrin levels due to potassium channel dysfunction in the Jervell and Lange-Nielsen Syndrome. Cardiol Young. 2012:1–10. doi: 10.1017/S1047951112001060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.