Abstract
Background
Segmental handling of sodium along the proximal and distal nephron might be heritable and different between black and white participants.
Methods
We randomly recruited 95 nuclear families of black South African ancestry and 103 nuclear families of white Belgian ancestry. We measured the (FENa) and estimated the fractional renal sodium reabsorption in the proximal (RNaprox) and distal (RNadist) tubules from the clearances of endogenous lithium and creatinine. In multivariable analyses, we studied the relation of RNaprox and RNadist with FENa and estimated the heritability (h2) of RNaprox and RNadist.
Results
Independent of urinary sodium excretion, South Africans (n =240) had higher RNaprox (unadjusted median, 93.9% vs. 81.0%; P < 0.001) than Belgians (n =737), but lower RNadist (91.2% vs. 95.1%; P < 0.001). The slope of RNaprox on FENa was steeper in Belgians than in South Africans (−5.40 ±0.58 vs. −0.78 ±0.58 units; P < 0.001), whereas the opposite was true for the slope of RNadist on FENa (−3.84 ± 0.19 vs. −13.71 ± 1.30 units; P < 0.001). h2 of RNaprox and RNadist was high and significant (P < 0.001) in both countries. h2 was higher in South Africans than in Belgians for RNaprox (0.82 vs. 0.56; P < 0.001), but was similar for RNadist (0.68 vs. 0.50; P = 0.17). Of the filtered sodium load, black participants reabsorb more than white participants in the proximal nephron and less postproximally.
Conclusion
Segmental sodium reabsorption along the nephron is highly heritable, but the capacity for regulation in the proximal and postproximal tubules differs between whites and blacks.
Keywords: clinical genetics, epidemiology, kidney, lithium clearance, salt sensitivity, segmental tubular sodium transport
Introduction
African–Americans, as compared with European Americans, have a higher prevalence [1] and incidence [2,3] of hypertension and experience higher rates of hypertensive complications – in particular, stroke [4] and end-stage renal disease [5]. Furthermore, several studies [6–8] have suggested that blood pressure might be more sensitive to sodium depletion or salt loading in African–Americans than in European Americans. However, researchers have not consistently observed this ethnic difference in normotensive and hypertensive participants or in women and men [8]. In the Dietary Approaches to Stop Hypertension (DASH) – Sodium Trial [9], African–Americans showed a greater blood pressure response to dietary sodium restriction than non-African–Americans, but only on the control diet, and not on the DASH diet. The existing literature therefore highlights the need for further studies addressing the ethnic diversity in renal sodium handling.
Of the sodium load filtered at the glomerulus, about 60–70% is reabsorbed at the proximal renal tubules, whereas about 90% of the sodium escaping the proximal nephron is distally reabsorbed in the postproximal nephron [10]. Measurement of the fractional excretion of endogenous lithium enables the differentiation of proximal from distal (postproximal) tubular sodium reabsorption [10–12]. We hypothesized that segmental handling of sodium along the proximal and distal nephron might be heritable and that it might be different between black Africans and white Europeans. To test our hypothesis, we measured the endogenous lithium clearance in families randomly recruited from a South African [13] and a Belgian [14] population.
Methods
Field work
We enrolled a random sample of nuclear families of African ancestry (Nguni and Sotho chiefdoms) from the metropolitan area of Johannesburg [13]. In the framework of the Flemish Study on Environment, Genes and Health Outcomes (FLEMENGHO), we also enrolled a random sample of families from a geographically defined area in northern Belgium [14]. We re-invited FLEMENGHO participants for measurement of their endogenous lithium clearance [15]. Lithium measurements, done at the Nephrology Division, University Hospital of Lausanne, Switzerland [12,16], were available for 296 South Africans (participation rate 85.3%) and 804 Belgians (re-examination rate 64.3%). We excluded 56 South Africans and 67 Belgians, because of high lithium concentrations in serum (>3 μmol/l) or urine (>40 μmol/l) suggestive of external contamination (n =1 and 1, respectively) or because of missing covariates (55 and 66, respectively). The number of participants analyzed therefore totaled 240 and 737.
The participants refrained from smoking, heavy exercise, and drinking alcohol or caffeine-containing beverages for at least 4 h prior to the examination [13,15]. Trained nurses measured the participants’ anthropometric characteristics and blood pressure. They also administered a questionnaire to collect information about each participant’s recent medical history, smoking and drinking habits, and intake of medications. Blood pressure was the average of five consecutive readings obtained at the participants’ homes. BMI was weight in kilograms divided by the square of height in meters. Hypertension was defined as a systolic blood pressure of at least 140 mmHg or a diastolic blood pressure of 90 mmHg or the use of antihypertensive drugs.
Biochemical measurements
On the examination days, participants collected an exactly timed urine sample and gave a venous blood sample for the measurement of serum sodium, lithium, and creatinine. We determined the sodium and creatinine concentrations by standard automated methods and the lithium concentration in serum and urine using an electrothermal atomic absorption spectrophotometer (model 1100B with HGA-700 graphite furnace; Perkin Elmer, Waltham, Massachusetts, USA). We computed the clearance of a substance x (Cx) as (Ux ×V)/Sx, where Ux and Sx represent the urinary and serum concentrations of x (lithium, sodium, or creatinine) and V the urine volume in milliliters per minute [12,15]. The fractional excretion of x (FEx) was Cx/Ccreatinine. We defined the fractional proximal sodium reabsorption (RNaprox) as (1 – FELi) and the fractional distal sodium reabsorption (RNadist) as 1 – (CLi/CNa) [12,15].
Statistical analyses
For data management and statistical analysis, we used SAS, version 9.1 (SAS Institute, Cary, North Carolina, USA), Stata, version 9.2 (StataCorp LP, College Station, Texas, USA), and the Statistical Analysis for Genetic Epidemiology, version 5.3 (SAGE; Case Western Reserve University, Cleveland, Ohio, USA). We compared means, medians, and proportions by a large-sample z-test, a nonparametric K-sample test, and Fisher’s exact test, respectively. We searched for possible covariates of the phenotypes under study, using stepwise multiple regressions with the P-values for independent variables to enter and stay in the model set at 0.15. To estimate heritability, we applied a maximum likelihood approach, as implemented in the ASSOC procedure of the SAGE package. We estimated heritability (h2) by assuming multivariate normality after a simultaneously estimated power transformation. ASSOC uses a linear regression model and partitions the residual variance into the sum of an additive polygenic component and a participant-specific random component. Heritability is the polygenic component divided by the total residual variance after adjusting for covariates. We also estimated h2 in the presence of an additional sibship component of variance, which captures both the common environment and a dominance genetic variance. For each phenotype of interest, severe outliers were values more than three interquartile ranges outside the percentiles 25 and 75.
Results
Study populations
The median number of relatives (interquartile range) among the 95 and 103 one-generation to three-generation families available, respectively, for analysis in South Africa and Belgium, amounted to three (three to four) and 21 (8–45), respectively. The families included 169 and 488 parent–offspring pairs and 57 and 497 sibling pairs, respectively.
South Africans differed from Belgians in most characteristics, with the exception of age and the urinary creatinine excretion rate (Table 1). The serum lithium concentration was also similar in both countries (0.31 μmol/l), whereas the urinary lithium excretion rate was lower in South Africans than in Belgians (0.13 vs. 0.35 μmol/h). In line with the sodium excretion rate (4.5 vs. 8.3 mmol/h), the serum sodium concentration (137.0 vs. 142.2 mmol/l), as well as the fractional excretion of sodium (0.55% vs. 0.88%), was lower in the South Africans. The mean [95% confidence interval (CI), range] duration of the timed urine collection was 24.0 h (23.9–24.0, 20.0–24.0) in South Africans and 4.6 h (4.5–4.7, 1.8–13.3) in Belgians. For volume, the corresponding numbers were 1278 ml (1194–1361, 200–3500) in South Africans and 347 ml (331–362, 25–1480) in Belgians.
Table 1.
Characteristics of participants by ethnicity
| Variable | Black South Africans (n = 240) | White Belgians (n =737) |
|---|---|---|
| Women [n (%)] | 149 (62.0) | 374 (50.7) |
| Age (years) | 42.7 ± 18.6 | 40.4 ±15.8 |
| BMI (kg/m2) | 28.8 ± 7.1 | 25.0 ±4.4 |
| Blood pressure | ||
| Systolic blood pressure (mmHg) | 131.0 ± 24.0 | 125.3 ±14.2 |
| Diastolic blood pressure (mmHg) | 84.3 ± 11.5 | 77.7 ±10.3 |
| Hypertension [n (%)] | 101 (42.1) | 198 (26.9) |
| Antihypertensive therapy | ||
| β-Blocker [n (%)] | 1 (0.4) | 57 (7.7) |
| Diuretic [n (%)] | 39 (16.3) | 30 (4.1) |
| ACE inhibitor [n (%)] | 20 (8.3) | 15 (2.0) |
| Current smoking [n (%)] | 28 (11.7) | 200 (27.1) |
| Current alcohol intake [n (%)] | 53 (22.1) | 428 (58.1) |
| Serum concentration | ||
| Sodium (mmol/l) | 137.0 ± 2.4 | 142.2 ±2.6 |
| Lithium (μmol/l) | 0.31 ± 0.16 | 0.31 ±0.16 |
| Creatinine (μmol/l) | 80.6 ± 15.9 | 78.3 ±15.4 |
| Urinary excretion rate | ||
| Sodium (mmol/h) | 4.5 ± 2.3 | 8.3 ±5.2 |
| Lithium (μmol/h) | 0.13 ± 0.15 | 0.35 ±0.25 |
| Creatinine (mmol/h) | 0.44 ± 0.18 | 0.46 ±0.19 |
| Renal clearances (ml/min) | ||
| Sodium | 0.46 (0.42–0.50) | 0.81 (0.77–0.84) |
| Lithium | 5.2 (4.7–5.8) | 16.4 (15.8–17.1) |
| Creatinine | 84.0 (78.3–90.2) | 90.7 (88.2–93.2) |
| Renal sodium handling (%) | ||
| RNaprox | 91.9 (91.1–93.1) | 80.0 (79.4–80.6) |
| RNadist | 86.3 (84.2–88.4) | 93.9 (93.5–94.3) |
| FENa | 0.55 (0.51–0.59) | 0.88 (0.85–0.91) |
Plus–minus values are mean ± SD. The median (interquartile range) is presented for skewed distributions. All between-country differences were significant (P ≤ 0.05), with the exception of age (P = 0.06), serum lithium (P =0.74), and the urinary excretion rate of creatinine (P = 0.21). BMI is weight in kg/m2. Blood pressure is the average of five consecutive readings at a single home visit. ACE, angiotensin-converting enzyme. RNaprox, fractional renal sodium reabsorption in the proximal tubule; RNadist, fractional renal sodium reabsorption in the distal tubule; FENa, fractional excretion of sodium.
Renal sodium handling
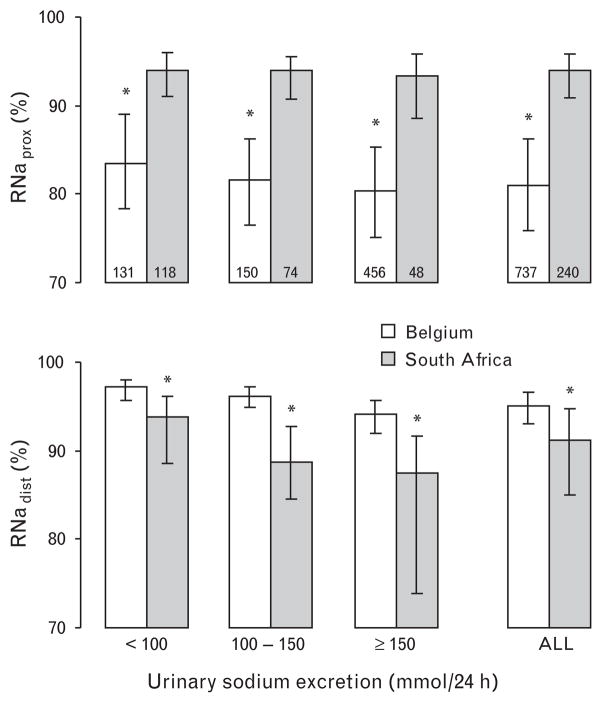
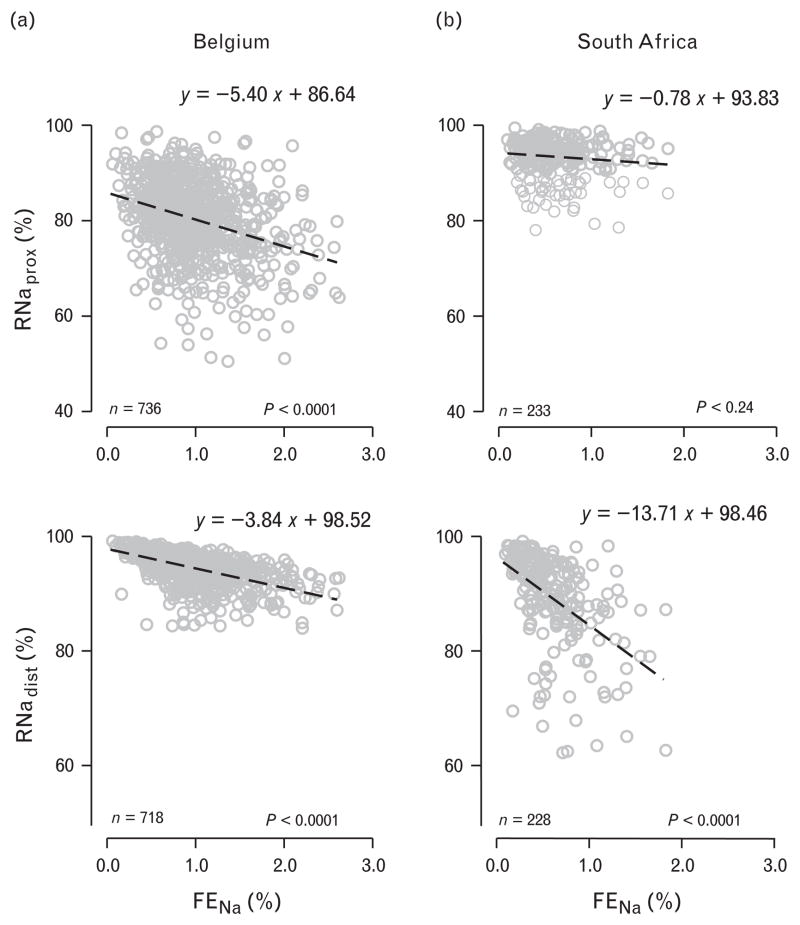
Compared with Belgians, South Africans had higher RNaprox, but lower RNadist. In unadjusted analyses, medians were 93.9% (interquartile range =90.8–95.8%) vs. 81.0% (75.9–86.3%; P <0.001) for RNaprox, and 91.2% (85.0–94.8%) vs. 95.1% (93.0–96.6%; P <0.001) for RNadist. These findings (P < 0.001) were consistent at various rates of sodium excretion (Fig. 1). The slope (±SE) of RNaprox on FENa was steeper in Belgians than in South Africans (−5.40 ±0.58 vs. −0.78 ±0.58 units; P <0.001; Fig. 2), whereas the opposite was true for the relation of RNadist on FENa (−3.84 ±0.19 vs. −13.71 ±1.30 units; P <0.001; Fig. 2). Analyses restricted to normotensive participants gave similar results.
Fig. 1.
Fractional sodium reabsorption at the proximal and distal nephron by country and category of urinary sodium excretion. Charted values are medians and bars represent interquartile ranges. Asterisk (*) denotes P-value less than 0.001 for differences in medians between South Africans and Belgians with Bonferroni’s adjustment for multiple testing. RNaprox, fractional renal sodium reabsorption in the proximal tubule; RNadist, fractional renal sodium reabsorption in the distal tubule.
Fig. 2.
Scatter plots of the fractional sodium reabsorption in the proximal and distal nephron vs. the fractional excretion of sodium, in Belgium (a) and in South Africa (b). Equations represent simple linear regression lines (y =reabsorption, x =fractional excretion of sodium), obtained from the ASSOC software in SAGE. P-values are for slopes, which were significantly different between countries (P <0.0001). Points more than three interquartile ranges away from the 25th and 75th percentiles were not plotted, but are included in the calculation of the regression lines. RNaprox, fractional renal sodium reabsorption in the proximal tubule; RNadist, fractional renal sodium reabsorption in the distal tubule; FENa, fractional excretion of sodium.
In further analyses, we adjusted the relations of RNaprox and RNadist with FENa in South Africans and Belgians for all covariates listed in Table 2, while accounting for familial correlations. The partial regression coefficients (±SE) of RNaprox on FENa were −0.96 ±0.62 units (P =0.12) in South Africans and −5.27 ±0.61 units (P <0.0001) in Belgians. The corresponding partial regression coefficients for RNadist in relation to FENa were −13.41 ±1.39 units (P <0.0001) and −3.90 ±0.20 units (P <0.0001), respectively. The between-country differences in these partial regression coefficients were significantly different from 0 (P <0.0001). Analyses restricted to normotensive participants gave similar results.
Table 2.
Covariates of renal phenotypes
| Black South Africans (n =240)
|
White Belgians (n =737)
|
|||||
|---|---|---|---|---|---|---|
| RNaprox | RNadist | FENa | RNaprox | RNadist | FENa | |
| R2 | 0.030 | 0.238 | 0.161 | 0.031 | 0.375 | 0.111 |
| Intercept | 92.20 | 90.84 | 1.36 | 80.49 | 95.05 | 1.22 |
| Partial regression coefficients | ||||||
| Women (1) vs. men (0) | NS | NS | −0.077 ± 0.044* | NS | NS | −0.11 ± 0.03*** |
| Age years | NS | NS | NS | −0.069 ±0.021‡ | −0.0014 ± 0.0055 | 0.0031 ± 0.0012† |
| Age2 years2 × 103 | NS | −2.10 ± 1.7 | 0.30 ± 0.06 | 3.30 ± 1.0 | −1.00 ± 0.3 | −0.13 ± 0.06 |
| Mean arterial pressure (mmHg) | NS | −0.074 ± 0.033* | 0.0023 ±0.0016* | NS | NS | 0.0046 ± 0.0019† |
| Current smoking (0, 1) | NS | NS | NS | NS | NS | −0.12 ± 0.04 |
| Use of diuretics (0, 1) | NS | NS | NS | NS | −1.16 ±0.56** | 0.43 ± 0.12*** |
| Use of renin inhibitors (0, 1) | NS | NS | NS | NS | −0.68 ±0.31** | NS |
| Sodium excretion (mmol/h) | NS | −1.29 ± 0.20*** | NS | −0.39 ±0.02** | ||
Models were run in ASSOC (SAGE, version 5.3). The power transformations applied to South African and Belgian data were 9.6 and 2.8 for RNaprox, 6.6 and 17.3 for RNadist, and −1.0 (fixed to lower bound) and −0.32 for FENa, respectively. ‘NS’ indicates that covariates did not attain a significance of 0.15. BMI and current alcohol intake (0, 1) were not significant at the 0.15 level in any model. Significance of the partial regression coefficients. RNaprox, fractional renal sodium reabsorption in the proximal tubule; RNadist, fractional renal sodium reabsorption in the distal tubule; FENa, fractional excretion of sodium.
0.15 <P ≤0.05.
0.05 <P ≤ 0.01.
P <0.01.
Heritability
With adjustments applied as before, RNaprox and RNadist were highly heritable in both South Africans and Belgians (Table 3). Estimates of h2 for RNaprox and RNadist were significantly higher in South Africans than in Belgians. Adding a sibship component of variance did not substantially modify the h2 estimates. Among South Africans, there were five outliers for RNaprox and 10 for RNadist, whereas among Belgians, these numbers were 1 and 18, respectively. Sensitivity analyses, with outliers excluded, restricted to normotensive participants, restricted to untreated participants, or without adjustment for mean arterial pressure, produced similar h2 estimates as those reported in Table 3 and did not alter our conclusions.
Table 3.
Heritability estimates by ethnicity
| Black South Africans (n = 240)
|
White Belgians (n = 737)
|
||||
|---|---|---|---|---|---|
| h2 ± SE | P | h2 ±SE | P | PEthnicity | |
| Serum concentrations | |||||
| Sodium (mmol/l) | 0.62 ± 0.14 | <0.0001 | 0.38 ± 0.09 | <0.0001 | 0.14 |
| Lithium (μmol/l) | 0.78 ± 0.09 | <0.0001 | 0.46 ± 0.08 | <0.0001 | 0.009 |
| Creatinine (μmol/l) | 0.73 ± 0.12 | <0.0001 | 0.38 ± 0.07 | <0.0001 | 0.007 |
| Renal clearances (ml/min) | |||||
| Sodium | 0.48 ± 0.12 | <0.0001 | 0.16 ± 0.09 | 0.03 | 0.03 |
| Lithium | 0.76 ± 0.10 | <0.0001 | 0.45 ± 0.07 | <0.0001 | 0.01 |
| Creatinine | 0.33 ± 0.14 | 0.01 | 0.09 ± 0.07 | 0.12 | 0.13 |
| Renal sodium handling (%) | |||||
| RNaprox | 0.82 ± 0.08 | <0.0001 | 0.56 ± 0.07 | <0.0001 | 0.02 |
| RNadist | 0.68 ± 0.11 | <0.0001 | 0.50 ± 0.07 | <0.0001 | 0.17 |
| FENa | 0.55 ± 0.13 | <0.0001 | 0.09 ± 0.07 | 0.10 | 0.002 |
Plus–minus values are heritability (h2) estimates ± SE. Models were adjusted for sex, the linear and squared terms of age, BMI, smoking and alcohol intake, mean arterial blood pressure, treatment with antihypertensive drugs (diuretics and inhibitors of the renin system), and the urinary sodium excretion rate. FENa and the sodium clearance were not adjusted for urinary sodium excretion. P values refer to the significance of h2. PEthnicity indicates the significance of the difference between blacks and whites. RNaprox, fractional renal sodium reabsorption in the proximal tubule; RNadist, fractional renal sodium reabsorption in the distal tubule; FENa, fractional excretion of sodium.
Discussion
The main finding of our study is that, independent of sodium excretion and other covariates, blacks reabsorb more of the filtered sodium load in the proximal nephron, but less in the distal (postproximal) renal tubules, than do whites. Furthermore, segmental sodium reabsorption along the nephron is highly heritable, with significantly greater heritability of proximal sodium reabsorption in blacks than in whites. Heritability was also high in both ethnic groups for the clearances of lithium and sodium, which proves that the high heritability of proximal and distal tubular sodium handling, as estimated by the clearance of endogenous lithium, cannot be due only to the heritability of the creatinine clearance.
The lower fractional sodium excretion in black Africans than in white participants suggests that the former tend to reabsorb more sodium than the latter. We showed that differences in dietary salt intake, as reflected by the urinary sodium excretion rate, could not explain this ethnic difference. In keeping with one previous study [17], our study showed that the proximal sodium reabsorption under usual dietary conditions was significantly higher in participants of African descent than in whites. Indeed, Barbato et al. [17] noticed that the fractional excretion of lithium, which reflects the amount of sodium escaping proximal tubular reabsorption, was lower in participants of African descent (15.6%; 95% CI =15.0–16.3) than in whites (18.4%; CI 17.6–19.1) or in South Asians (18.4%; CI 17.4–18.9). Our current observations, taken together with those of Barbato et al. [17], suggest that, compared with other ethnic groups, blacks reabsorb more of the filtered sodium load in the proximal renal tubules. In contrast, black South Africans had lower fractional distal sodium reabsorption than white Belgians, which may be a compensatory mechanism in response to the enhanced proximal sodium reabsorption. We are not aware of any other previous study with similar observations.
Our current findings are in line with previous studies by Pratt et al. [18,19]. These investigators noticed that black compared with white adolescents had lower plasma levels of renin activity and aldosterone, and a lower urinary excretion rate of aldosterone [18]. In basal conditions and after stimulation with adrenocorticotrophic hormone, blacks also had lower plasma deoxycorticosterone levels [18]. In a further study, Pratt et al. [19] found that amiloride, an inhibitor of the aldosterone-regulated epithelial sodium channel (ENaC) in the distal nephron, reduced blood pressure in white, but not in black, adolescents. Pratt et al. [19] hypothesized that, in blacks, sodium reabsorption is enhanced at other sites of the nephron and this enhanced sodium retention reduces plasma renin activity, the aldosterone excretion rate, and ENaC activity.
Sodium reabsorption in the proximal and postproximal tubules differed between black South Africans and white Belgians not only in quantitative terms, but also in their relation to the fractional sodium excretion. The proximal sodium reabsorption decreased more steeply with fractional sodium excretion in whites than in Black South Africans. This observation suggests that blacks of African descent are less capable of reducing their proximal sodium reabsorption in response to a higher salt intake. This feature is a marker of salt-sensitive hypertension [12]. Our current results, in keeping with several other studies [6–9], therefore imply that blacks might have a blood pressure that is more responsive to an excessive salt intake than whites.
To our knowledge, our study is the first to demonstrate heritability of renal sodium reabsorption separately for the proximal and postproximal renal tubules. The latter observation confirms that genetic determinants must play a major role in regulating sodium reabsorption in various segments of the nephron and through this mechanism might influence blood pressure. Monogenic forms of hypertension or hypotension are associated with alterations in renal tubular sodium handling [20]. The vast majority of allelic variants that have been linked and/or associated with blood pressure encode proteins that are either directly involved in sodium transport in the kidney or in the hormonal regulation of renal tubular sodium handling [20]. In line with our present observations, several candidate genes involved in renal sodium handling show important allelic differences across ethnic groups. For instance, the –344 C>T variant of the aldosterone synthase gene (CYP11B2) is more frequent in blacks than in whites (79% vs. 50%) [21]. Its association with the plasma aldosterone concentration and hypertension remains uncertain in whites and Asians [22]. However, in the African–American Heart Failure Trial, the –344 C>T variant was associated with higher aldosterone levels and poorer event-free survival in blacks with heart failure [23]. Another example of ethnic diversity is in the G protein-coupled receptor kinase 4 (GRK4). This enzyme influences sodium reabsorption by inhibiting the dopamine receptor D1 and is encoded by a gene that shows ethnic differences in allele frequencies and haplotype structure [24].
In our analyses, BMI was not a significant determinant of proximal tubular sodium reabsorption in either center, including in analyses restricted to normotensive participants. This is in contrast with previous findings showing a positive association of BMI with proximal tubular sodium reabsorption in whites [17,25]. In the study by Barbato et al. [17], this latter association was not, however, observed in Africans, a finding that is in line with our findings. It is not easy to explain why findings in whites are inconsistent. A potential explanation could be a difference in insulin resistance, but this phenotype is not available in our study. Further studies are needed to clarify this issue.
The present study has potential limitations and strengths. The two ethnic groups under study differed markedly in lifestyle, dietary habits as reflected by urinary sodium excretion, and their environment. However, our results remained consistent in multivariable-adjusted analyses. Our study is cross-sectional. We therefore cannot infer causality from the observed associations. Renal sodium handling varies substantially with differences in dietary sodium intake. We did not measure plasma renin activity or the plasma aldosterone concentration on the day of the urine collections. Participants collected their urine only once. We do not know how reproducible our results would be within each population, considering the large day-to-day variation in dietary salt intake [26]. We expressed urinary excretion of solutes as a rate per hour to address the difference in the durations of the urine collections in Belgium vs. South Africa. We cannot exclude that the shorter duration of urine collection in the Belgian sample resulted in slight differences for clearances. However, it should not have affected the results obtained for fractional excretions, which do not depend on urine volume and duration. RNaprox was always lower in Belgian participants than in black participants, irrespective of urine flow rate. Lithium is not a perfect marker of proximal sodium reabsorption. Micro-puncture studies showed lithium reabsorption beyond the proximal tubule in rats [27], in particular during sodium or potassium depletion. However, several other studies have demonstrated that, in humans, the distal tubular handling of lithium is minimal [28,29]. The endogenous lithium clearance currently represents the best noninvasive marker of proximal tubular sodium reabsorption in humans.
Perspectives
Our findings might have important clinical implications. A series of clinical observations, summarized elsewhere [30], suggests that blacks are more salt-sensitive than whites. Blacks have lower plasma renin activity [18] and experience a greater reduction in blood pressure in response to dietary sodium restriction [9] or thiazide diuretics [31,32]. Not surprisingly, hypertension is more frequent among African than European Americans [1–3], and leads to a greater incidence of complications, such as stroke [4] or end-stage renal disease [5]. Furthermore, several studies have showed that the consumption of high-salt foods is more widespread among African–American than non-African–Americans [33,34]. In Africa itself, migration from rural areas to cities and urbanization are also leading to an increase in sodium intake and blood pressure [35]. Our present findings, viewed against the background of the salt sensitivity of blacks [30], their consumption patterns of salt [33–35], and their propensity to hypertension [1–3] and its complications [4,5], highlight an enormous potential for prevention by a reduction of salt intake. Furthermore, ethnic diversity can be due to lifestyle, genetic variability, or both. The high heritability of proximal and distal tubular sodium reabsorption suggests that genetic determinants might play an overriding role in the regulation of sodium handling along the nephron. Further studies should address the genetic basis for the ethnic differences in renal sodium handling.
Acknowledgments
Sandra Covens and Leen Vanderhulst (Studies Coordinating Centre, Leuven, Belgium) provided expert secretarial assistance. This study would not have been possible without the voluntary collaboration of the South African and Belgian participants.
In South Africa, the study was supported by the Medical Research Council of South Africa, the National Research Foundation (Women in Research) and the Circulatory Disorders Research Trust, Johannesburg, South Africa. The International Scientific and Technological Collaboration between South Africa and Flanders (contract number BIL 01/43) supported the fellowship of South African researchers at the University of Leuven in Belgium. The Flemish Study on Environment, Genes and Health Outcomes (FLE-MENGHO) is part of the European Project on Genes in Hypertension (EPOGH), which is endorsed by the European Council for Cardiovascular Research and the European Society of Hypertension. The European Union (grants IC15-CT98-0329-EPOGH, LSMH-CT-2006-037093 InGenious HyperCare, and HEALTH-F4-2007-201550 HyperGenes), the Fonds voor Wetenschappelijk Onderzoek Vlaanderen, Ministry of the Flemish Community, Brussels, Belgium (G.0424.03 and G.0575.06), and the Katholieke Universiteit Leuven, Leuven, Belgium (OT/00/25 and OT/05/49) gave support to the FLEMENGHO study. Murielle Bochud received a PROSPER grant from the Swiss National Science Foundation (3200BO-111361/1, 3200BO-111362/1). A U.S. Public Health Service, National Institutes of Health, Bethesda, MD, Resource grant (RR03655) from the National Center for Research Resources supported the development of SAGE, and Research grant (GM28356) from the National Institute of General Medical Sciences supported Robert Elston.
Abbreviations
- FENa
fractional excretion of sodium
- h2
heritability
- RNadist
fractional renal sodium reabsorption in the distal tubules
- RNaprox
fractional enal sodium reabsorption in the proximal tubules
References
- 1.Cooper R, Rotimi C. Hypertension in blacks. Am J Hypertens. 1997;10:804–812. doi: 10.1016/s0895-7061(97)00211-2. [DOI] [PubMed] [Google Scholar]
- 2.Manatunga AK, Jones JJ, Pratt JH. Longitudinal assessment of blood pressures in Black and White children. Hypertension. 1993;22:84–89. doi: 10.1161/01.hyp.22.1.84. [DOI] [PubMed] [Google Scholar]
- 3.Geronimus AT, Bound J, Keene D, Hicken M. Black–White differences in age trajectories of hypertension prevalence among adult women and men, 1999–2002. Ethn Dis. 2007;17:40–48. [PubMed] [Google Scholar]
- 4.Kissela B, Schneider A, Kleindorfer D, Khoury J, Miller R, Alwell K, et al. Stroke in a biracial population. The excess burden of stroke among blacks. Stroke. 2004;35:426–432. doi: 10.1161/01.STR.0000110982.74967.39. [DOI] [PubMed] [Google Scholar]
- 5.Hsu C-Y, Lin F, Vittinghoff E, Shlipak MG. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003;14:2902–2907. doi: 10.1097/01.asn.0000091586.46532.b4. [DOI] [PubMed] [Google Scholar]
- 6.He FJ, Markandu ND, Sagnella GA, MacGregor GA. Importance of the renin system in determining blood pressure fall with salt restriction in black and white hypertensives. Hypertension. 1998;32:820–824. doi: 10.1161/01.hyp.32.5.820. [DOI] [PubMed] [Google Scholar]
- 7.Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8:II-127–II-134. doi: 10.1161/01.hyp.8.6_pt_2.ii127. [DOI] [PubMed] [Google Scholar]
- 8.Wright JT, Rahman M, Scarpa A, Fatholahi M, Griffin V, Jean-Baptiste R, et al. Determinants of salt sensitivity in black and white normotensive and hypertensive women. Hypertension. 2003;42:1087–1092. doi: 10.1161/01.HYP.0000101687.89160.19. [DOI] [PubMed] [Google Scholar]
- 9.Vollmer WM, Sacks FM, Ard J, Appel LJ, Bray GA, Simons-Morton DG, et al. Effects of diet and sodium intake on blood pressure: subgroup analysis of the DASH-sodium diet. Ann Intern Med. 2001;135:1019–1028. doi: 10.7326/0003-4819-135-12-200112180-00005. [DOI] [PubMed] [Google Scholar]
- 10.Burnier M, Bochud M, Maillard M. Proximal tubulae function and salt sensitivity. Curr Hypertens Rep. 2006;8:8–15. doi: 10.1007/s11906-006-0035-6. [DOI] [PubMed] [Google Scholar]
- 11.Thomsen K, Shirley DG. The validity of lithium clearance as an index of sodium and water delivery from the proximal tubules. Nephron. 1997;77:125–138. doi: 10.1159/000190264. [DOI] [PubMed] [Google Scholar]
- 12.Chiolero A, Maillard M, Nussberger J, Brunner HR, Burnier M. Proximal sodium reabsorption. An independent determinant of blood pressure response to salt. Hypertension. 2003;36:631–637. doi: 10.1161/01.hyp.36.4.631. [DOI] [PubMed] [Google Scholar]
- 13.Shiburi CP, Staessen JA, Maseko M, Wojciechowska W, Thijs L, Van Bortel LM, et al. Reference values for SphygmoCor measurements in South Africans of African ancestry. Am J Hypertens. 2006;19:40–46. doi: 10.1016/j.amjhyper.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Staessen JA, Wang JG, Brand E, Barlassina C, Birkenhäger WH, Herrmann SM, et al. Effects of three candidate genes on prevalence and incidence of hypertension in a Caucasian population. J Hypertens. 2001;19:1349–1358. doi: 10.1097/00004872-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Seidlerová J, Staessen JA, Maillard M, Nawrot T, Zhang H, Bochud M, et al. Association between arterial properties and renal sodium handling in a general population. Hypertension. 2006;48:609–615. doi: 10.1161/01.HYP.0000240516.60040.ba. [DOI] [PubMed] [Google Scholar]
- 16.Magnin JL, Decosterd LA, Centeno C, Burnier M, Diezi J, Biollaz J. Determination of trace lithium in biological fluids using graphite funace atomic absorption spectrophotometry: variability of urine matrices circumvented by cation exchange solid phase extraction. Pharm Acta Helv. 1996;71:237–246. doi: 10.1016/s0031-6865(96)00020-9. [DOI] [PubMed] [Google Scholar]
- 17.Barbato A, Cappuccio FP, Folkerd EJ, Strazullo P, Sampson B, Cook DG, et al. Metabolic syndrome and renal sodium handling in three ethnic groups living in England. Diabetologia. 2004;47:40–46. doi: 10.1007/s00125-003-1260-z. [DOI] [PubMed] [Google Scholar]
- 18.Pratt JH, Rebhun JF, Zhou L, Ambrosius WT, Newman SA, Gomez-Sanchez CE, et al. Levels of mineralocorticoids in whites and blacks. Hypertension. 1999;34:315–319. doi: 10.1161/01.hyp.34.2.315. [DOI] [PubMed] [Google Scholar]
- 19.Pratt JH, Ambrosius WT, Agarwal R, Eckert GJ, Newman S. Racial differences in the activity of the amiloride-sensitive epithelial sodium channel. Hypertension. 2002;40:903–908. doi: 10.1161/01.hyp.0000039749.75068.f4. [DOI] [PubMed] [Google Scholar]
- 20.Staessen JA, Wang J, Bianchi G, Birkenhäger WH. Essential hypertension. Lancet. 2003;361:1629–1641. doi: 10.1016/S0140-6736(03)13302-8. [DOI] [PubMed] [Google Scholar]
- 21.Zhu H, Sagnella GA, Dong Y, Miller MA, Onipinla A, Markandu ND, et al. Contrasting associations between aldosterone synthase and gene polymorphisms and essential hypertension in blacks and in whites. J Hypertens. 2003;21:87–95. doi: 10.1097/00004872-200301000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Staessen JA, Li Y, Thijs L. Meta-analysis of blood pressure and the CYP11B2 polymorphism highlights the need for better designed studies. J Hypertens. 2007;25:37–39. doi: 10.1097/HJH.0b013e32801143b3. [DOI] [PubMed] [Google Scholar]
- 23.McNamara DM, Tam SW, Sabolinski ML, Tobelmann P, Janosko K, Taylor AL, et al. Aldosterone synthase promoter polymorphism predicts outcome in African Americans with heart failure. Results from the A-HeFT Trial. J Am Coll Cardiol. 2006;48:1277–1282. doi: 10.1016/j.jacc.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 24.Lohmueller KE, Wong LJC, Mauney MM, Jiang L, Felder RA, Jose PA, et al. Patterns of genetic variation in the hypertension candidate gene GRK4: ethnic variation and haplotype structure. Ann Hum Genet. 2005;70:27–41. doi: 10.1111/j.1529-8817.2005.00197.x. [DOI] [PubMed] [Google Scholar]
- 25.Strazzullo P, Barba G, Cappuccio FP, Siani A, Trevisan M, Farinaro E, et al. Altered renal sodium handling in men with abdominal adiposity: a link to hypertension. J Hypertens. 2001;19:2157–2164. doi: 10.1097/00004872-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Luft FC, Fineberg NS, Sloan RS. Estimating dietary sodium intake in individuals receiving a randomly fluctuating intake. Hypertension. 1982;4:805–808. doi: 10.1161/01.hyp.4.6.805. [DOI] [PubMed] [Google Scholar]
- 27.Shirley DG, Walter SJ. Renal tubular lithium reabsorption in potassium-depleted rats. J Physiol. 1997;503:663–670. doi: 10.1111/j.1469-7793.1997.663bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atherton JC, Green R, Hughes S, McFall V, Sharples JA, Solomon LR, et al. Lithium clearance in man: effect of dietary salt intake, acute changes in extracellular fluid volume, amiloride and frusemide. Clin Sci. 1987;73:645–651. doi: 10.1042/cs0730645. [DOI] [PubMed] [Google Scholar]
- 29.Boer WH, Koomans HA, Dorhout Mees EJ, Gaillard CA, Rabelink AJ. Lithium clearance during variations in sodium intake in man: effects of sodium restriction and amiloride. Eur J Clin Invest. 1988;18:279–283. doi: 10.1111/j.1365-2362.1988.tb01259.x. [DOI] [PubMed] [Google Scholar]
- 30.Aviv A, Hollenberg NK, Weder A. Urinary potassium excretion and sodium sensitivity in blacks. Hypertension. 2004;43:707–713. doi: 10.1161/01.HYP.0000120155.48024.6f. [DOI] [PubMed] [Google Scholar]
- 31.Chapman AB, Schwartz GL, Boerwinkle E, Turner ST. Predictors of antihypertensive response to standard dose of hydrochlorothiazide for essential hypertension. Kidney Int. 2002;61:1047–1055. doi: 10.1046/j.1523-1755.2002.00200.x. [DOI] [PubMed] [Google Scholar]
- 32.Preston RA, Materson BJ, Reda DJ, Williams DW, Hamburger RJ, Cushman WC, et al. Age-race subgroup compared with renin profile as predictors of blood pressure response to antihypertensive therapy. Department of Veterans Affairs Cooperative Study Group for essential hypertension. J Am Med Assoc. 1998;280:1168–1172. doi: 10.1001/jama.280.13.1168. [DOI] [PubMed] [Google Scholar]
- 33.Kerr GR, Amante P, Decker M, Callen PW. Ethnic patterns of salt purchase in Houston, Texas. Am J Epidemiol. 1982;115:906–916. doi: 10.1093/oxfordjournals.aje.a113378. [DOI] [PubMed] [Google Scholar]
- 34.Kollipara UK, Mo V, Toto KH, Nelson LL, Schneider RA, Neily JB, et al. High-sodium food choices by southern, urban African Americans with heart failure. J Card Fail. 2006;12:144–148. doi: 10.1016/j.cardfail.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Poulter NR, Khaw KT, Hopwood BEC, Mugambi M, Peart WS, Rose G, et al. The Kenyan Luo migration study: observations on the initiation of a rise in blood pressure. Br Med J. 1990;300:967–972. doi: 10.1136/bmj.300.6730.967. [DOI] [PMC free article] [PubMed] [Google Scholar]