Abstract
Interleukin 17 (IL-17) plays an important role in several autoimmune diseases. IL-17 can induce the expression of vascular cell adhesion molecule (VCAM-1) in aortic vascular smooth muscle cells (SMCs), which is important for the development of atherosclerosis. However, the signaling pathway of IL-17-induced VCAM-1 expression remains unclear. In this study, we reported that IL-17 induced expression of VCAM-1 in SMCs is dependent on NF-κB, but independent of Akt1 and TAK1. This is because knocking down Akt1 or TAK1 by siRNA did not reduce IL-17-induced activation of NF-κB and expression of VCAM-1, whereas knocking down NF-κB by siRNA markedly inhibited IL-17-mediated upregulation of VCAM-1 expression. In addition, IL-17-induced expression of VCAM-1 is partially dependent on activation of ERK1/2. Therefore, these signaling pathways of IL-17-mediated upregulation of VCAM-1 expression might be therapeutic targets for treatment of IL-17-mediated inflammation.
Keywords: IL-17, NF-κB, vascular smooth muscle cells, adhesion molecule
Introduction
It is well know that interleukin 17 (IL-17) can be produced by a number of cell types and critical for host defense against extracellular pathogens, such as T helper cells (Th17), NKT cells, and γδ T cells (1–3), all of which have been implicated in a number of autoimmune diseases (1). The signals that influence IL-17 regulation have now been studied extensively in Th17 cells revealing that high-dose antigen-loaded dendritic cells induce Th17 cell differentiation. The combination of TGF-β and IL-6 is very effective in inducing naïve CD4+ T cells to become Th17 cells (4, 5). IL-23 promotes IL-17 production by expansion of Th17 cells (6). IL-6 is sufficient to induce IL-23R expression and IL-23 further amplifies its receptor expression (7). Because IL-23, IL-21 and IL-6 induce IL-17 production by activating Stat3 (8, 9), it is not surprised that deletion of Stat3 in T cells abrogated Th17 cell differentiation (10). Stat3 directly controls the expression of many other transcription factors that participate in Th17 cell differentiation including RORγt (11). RORγt has been found to bind the IL17 gene promoter. Conversely, overexpression of RORγt promotes IL-17 expression.
IL-17 is crucial for the pathogenesis of several autoimmune diseases, such as experimental autoimmune encephalomyelitis, rheumatoid arthritis, and colitis (4, 12). IL-17 potently stimulates lung microvascular endothelial cells expressing CXCL18 and adhesion molecules, which selectively drive neutrophils to the site of inflammation (13). However, the role of IL-17 in atherosclerosis remains controversial. Although other reports have shown that IL-17 inhibits atherosclerosis when T cells lose suppressor of cytokine signaling (SOCS3) (14), increased plasma levels of IL-17 have been reported in patients with coronary atherosclerosis (15). T cells that infiltrate the atherosclerotic plaques express IL-17 in human and in mouse models (15, 16). Moreover, recent reports indicated that IL-17 promotes atherosclerosis through studies using anti-IL-17 to neutralize IL-17 or IL-17−/− mice fed with high fat diets (17, 18). Previous work showed that vascular smooth muscle cells (SMCs) are central in atherosclerosis and expression of VCAM-1 is required for the development of atherosclerosis, VCAM-1−/− mice are resistant to atherosclerosis (19, 20). Although IL-17 promotes VCAM-1 expression in vascular smooth muscle cells (13), and NF-κB-inducing kinase serves as a common mediator of IL-17-, TNF-α- and IL-1β-induced proinflammatory gene expression in intestinal epithelial cells (21), the signaling pathway for IL-17-mediated expression of VCAM-1 in SMC is still unclear.
Identifying the signaling pathway of IL-17-mediated VCAM-1 expression will help find putative therapeutic targets for treatment of autoimmune inflammation. In the current study, we showed that IL-17 induces VCAM-1 expression in SMCs dependent on NF-κB, but independent of Akt1 and TAK1 activation.
Materials and methods
Materials
Modified Eagle’s medium (MEM) and calf bovine serum were purchased from Fisher Scientific. Mouse IL-17A and human IL-17A were purchased from Peperotech. Antibodies against VCAM-1, Akt1, TAK1, Stat3, β-actin, and GAPDH were from Santa Cruz Biotechnology.
Cell culture
Rat aortic vascular smooth muscle cells were cultured as previously described (22). The cells were used at no more than 22 passages. Human aortic smooth muscle cell (HASMC) was obtained from the American Type Culture Collection (ATCC). Rat SMCs were maintained in Modified Eagle’s Medium supplemented with 10% calf bovine serum. HASMCs were maintained in Medium 231 supplemented with smooth muscle cell growth supplement (SMGS) (Invitrogen, Life Tech.). Before stimulated by cytokines, the cells were starved in serum-free MEM medium or Medium 231 supplemented with 1% fetal bovine serum for 16 hours. For some experiments, cells were pretreated with U0126 (20 µmol/L) (Calbiochem) for 60 minutes before cytokine treatment.
Western blot analysis
Whole-cell lysis and nuclear extracts were prepared and Western blot analysis was performed as previously described (23). The concentration of protein was determined with BCA protein assay reagent (Pierce). Protein samples were separated on 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane (Bi-Rad). The membranes were probed with the following antibodies against p65 (Millipore), IκBα, phospho-IκBα (Ser32, 36), Akt1, ERK1/ERK2, VCAM-1, TAK1(MAP3K7), TRAF3, TRAF6, GAPDH, β-Actin, Histone H1(Santa Cruz Biotechnology), phosphor-p65(Ser536), phosphor-Akt1(Ser473), phosphor- ERK1/ERK2(Cell Signaling). The membrane was developed using Pierce SuperSignal reagent (Pierce).
Reverse transcription-PCR
Resting Rat SMCs were treated with or without IL-17 as described above for 12 hrs. RNA was isolated using TRIZOL according to the manufacturer’s instructions (Invitrogen, Life Tech., USA). Total mRNA was converted to cDNA by MMLV reverse transcriptase (Perkin–Elmer Cetus, Branchburg, NJ, USA) and oligo(dT) 12–18 primers. The following primers were used. Rat VCAM-1 forward: 5’-ACACCTCCCCCAAGAATACAG-3’, reverse: 5’-GCTCATCCTCAACACCCACAG-3’; Rat GAPDH forward: 5’-GCCATCAACGACCCCTTCAT-3’, reverse: 5’-CGCCTGCTTCACCACCTTCT-3’. PCR was performed as described previously (24). PCR products were separated by electrophoresis in 3% agarose gels and visualized by UV light following ethidium bromide staining. Densitometry analysis was performed using a ChemiDoc™ MP System (Bio-Rad, USA). Samples within the linear relationship between input cDNA and final PCR products were collected, and the densitometric units for each cytokine band were normalized to that for the corresponding GAPDH band. In the figures a ratio of 100 indicates a 1:1 ratio between VCAM-1 and GAPDH.
SiRNA infection
All the siRNA duplexes used in this project were purchased from Applied Bioscience. SMCs were transfected with siRNA for NF-κB, Akt1, ATK1, GAPDH and siRNA transfection medium (Fisher Sci, Thermo) according to manufacturer’s instructions. Negative control siRNA was used as control. Before performing experiments, we used 3 different concentrations of siRNA (5 pmol, 7.5 pmol and 10 pmol) for infection to get the optimal concentration of siRNA for knocking down specific target protein. The results showed that 5pmol siRNA could knock down 60% of target protein and 7.5 pmol siRNA for around 95% target protein without affecting other proteins such as actin, but 10 pmol siRNA reduced both target proteins and non-target protein actin due to cytotoxicity. Therefore, we used 7.5 pmol concentration of siRNA in most infection experiments. The assay experiments were carried out at 48 hours after transfection. Western blots were used to detect the efficacy of knocking down specific proteins.
EMSA assay
DNA-Protein binding assays were carried out with nuclear extract from IL-17 treated rat SMCs. A total of 5 µg of nuclear protein was used for each sample. NF-κB consensus double-stranded oligonucleotides were purchased from Applied Biosciences Inc. Synthetic complementary oligonucleotides were labeled at the 3’ end with digoxigenin (DIG)-11-dUTP according to the user manual (Roche). The probe sequence is 5’-TCTGCCCTGGGTTTCCCCTTGAAGGGATTTCCCTCCG-3’, corresponding to the region between −116 and −79 upstream of transcriptional start site (+1) of rat VCAM-1 gene (25, 26). Binding reactions were carried out with the DIG Gel Shift Kit (Roche). For the supershift assay, the reaction mixture was pre-incubated with specific antibody for 10 minutes at room temperature before the labeled probe was added. After electrophoresis, the separated components on the native gel were electro-transferred to a nylon membrane. Transferred DNAs were cross-linked to the membrane at 120 mJ/cm2 and detected using anti-Digoxigenin-AP conjugated antibody according to the manufacturer’s instruction (Roche).
Statistics
The data are expressed as the mean ± SD. Each experiment was repeated at least three times. Student’s t-test was used to analyze the results.
Results
IL-17 induces VCAM-1 expression in SMCs dependent on NF-κB
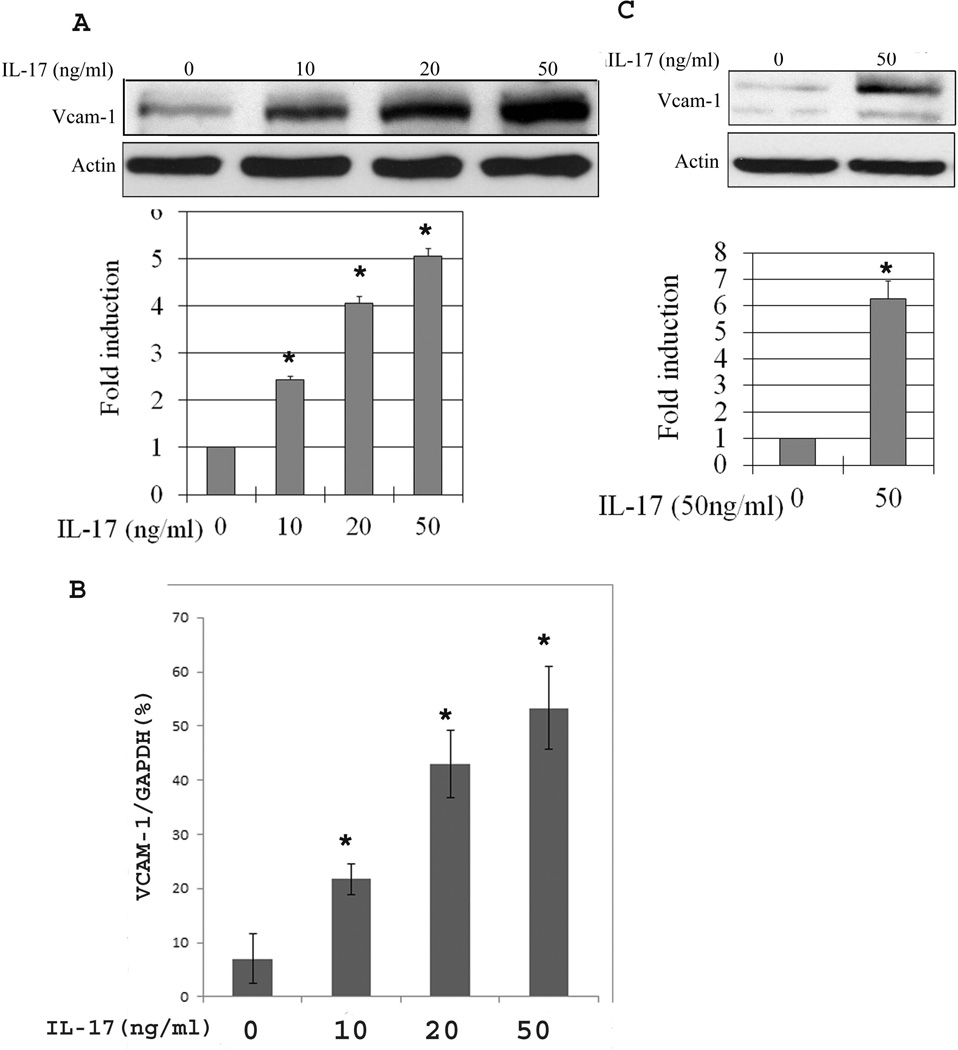
To determine the signaling pathway involved in IL-17-mediated VCAM-1 expression in SMCs, growth arrested SMCs were treated with IL-17 for 12–24 hours. The expression of VCAM-1 was measured by Western blot. Compared to control SMCs cultured with medium alone, IL-17 induced VCAM-1 expression in SMCs in a dose-dependent manner (Fig. 1A). To further determine if IL-17 promoted VCAM-1 mRNA expression in rat SMCs, we performed RT-PCR with results showing that IL-17 enhanced expression of VCAM-1 mRNA (Fig. 1B). In addition, we demonstrated that IL-17 upregulated VCAM-1 expression in human SMCs by western blots (Fig.1 C).
Figure 1.
IL-17 induces expression of VCAM-1 in SMCs. SMCs were treated with IL-17 (10–50ng/ml) for 12 hours. The VCAM-1 in whole cell extracts was identified by Western blots. Actin was used as equal loading. Western blots showed that IL-17 induces VCAM-1 expression (A), which is consistent with RT-PCR results that IL-17 promote VCAM-1 mRNA expression in SMCs (B). In addition, IL-17 is able to induce VCAM-1 expression in human aortic smooth muscle cells (C). The data is presented as the mean ± SE from three independent experiments. *, P < 0.05.
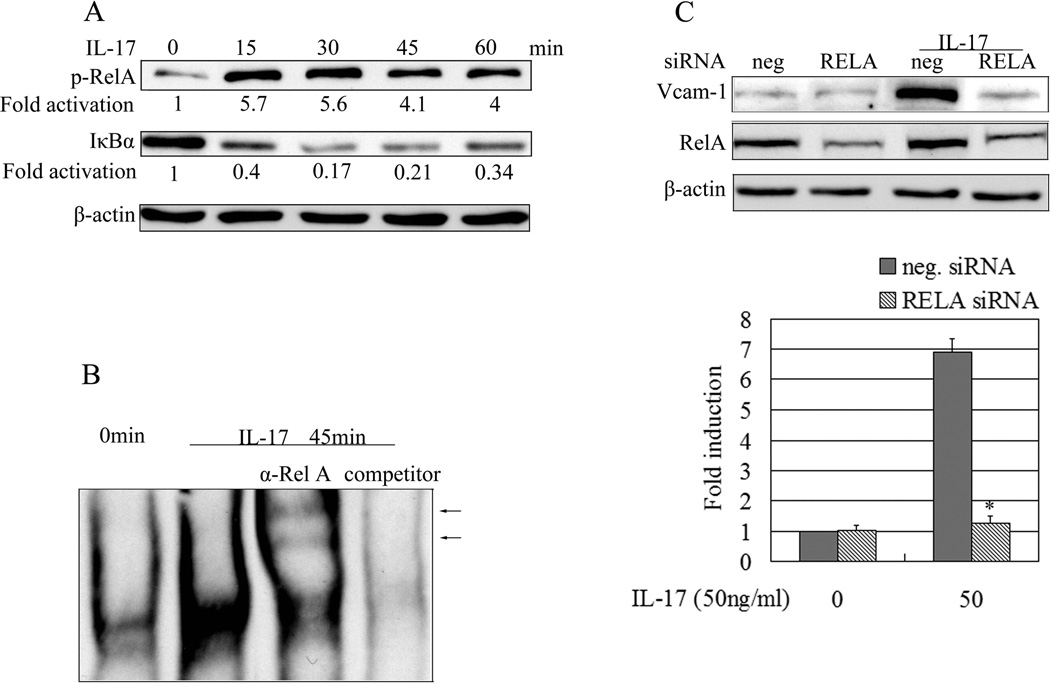
To determine the signaling pathway involved in IL-17-mediated effects, the cell extracts were collected at different time points after IL-17 treatment. Western blots were done to examine the activation of NF-κB. Activation of NF-κB involves phosphorylation and subsequent proteolytic degradation of IκB through the specific IκB kinase complex (27). The results showed that at 15 minutes after IL-17 treatment, we could detect degradation of Ikβα, indicating the activation of NF-κB stimulated by IL-17 (Fig. 2A). Consistent with this notion, the results of anti-phospho-p65 (anti-p-RelA) immunoblots correlated well with the degradation of Ikβα (Fig. 2A).
Figure 2.
IL-17 induces expression of VCAM-1 in rat SMCs via activation of NF-kB. (A) The Rat SMCs were stimulated with IL-17 for different times. The whole cell lyses were blotted for phosphorylation of RelA and the degradation of cytoplasmic inhibitor IκBα. (B) NF-κB activity was determined by EMSA. The arrowhead indicated the band supershifted by antibody against Rel A. The probe sequence corresponds to the region between −116 and −79 upstream of transcriptional start site (+1) of Rat VCAM-1 gene. (C) The Rat SMCs were transfected with 7.5pmol/ml RELA or negative siRNA. After 2 days the cells were incubated with IL-17 (50 ng/ml) for 12 hours. Whole cell lyses was blotted with anti-RelA or anti-VCAM-1. The data presented as the mean ± SE from three independent experiments. *, P < 0.05 for the suppression of IL-17-induced VCAM-1 by RELA siRNA.
By using the probe of NF-κB binding site in VCAM-1 promoter and doing EMSA assays, we found that IL-17 promotes NF-κB migration to the nucleus of SMCs (Fig. 2B) and anti-p65 (anti-RelA) delayed the shift of NF-κB probe-protein complex band (Fig. 2B line 3), suggesting that the band is NF-κB p65 specific binding to VCAM-1 promoter DNA.
siRNA knocking down p65 abolished IL-17-induced expression of VCAM-1 in SMCs
To further determine if activation of NF-κB is necessary for IL-17-induced VCAM-1 expression, we utilized RELA (p65) siRNA transfection to knock down p65 and determine whether knock-down p65 would abolish IL-17-induced VCAM-1 expression in SMCs. Rested SMCs were transfected with RELA or negative siRNA according to the procedure described in Materials and Methods. Transfection was performed by using different dosages of RELA siRNA (5–10 pmol) to find the optimal dosage of siRNA for knocking down p65. We found that 7.5 pmol RELA siRNA transfection could effectively knock down RELA protein without cytotoxicity to the transfected cells. 12 hours after siRNA transfection, SMCs were treated with IL-17 for another 12 hours. Later, cells extracts were used for Western blots. The results showed that knocking down p65 by siRNA reverses IL-17-induced VCAM-1 expression (Fig. 2C), which further indicated that NF-κB is indispensible for IL-17-induced VCAM-1 expression in SMCs.
Akt1 is not required for IL-17-induced VCAM-1 expression
Previous work by others has shown that IL-17-mediated multiple biological effects dependent on Akt1 (28, 29). Our results also showed that IL-17-induced Akt1 activation in SMCs (Fig. 3A). To further determine the role of Akt1 in IL-17-induced expression of VCAM-1, we performed Akt1 siRNA transfection to knock down Akt1 in SMCs (Supplementary Fig. 1B). The results showed that knocking down Akt1 did not reduce IL-17-induced expression of VCAM-1 in SMCs (Supple. Fig. 1B), suggesting that Akt1 is not required for IL-17-induced VCAM-1 expression. Consistent with this observation, IL-17 induced comparable level of activation of NF-κB (phospho-p65) in SMCs with or without knocking down of Akt1 (Supple. Fig. 1C, D). In fact, knocking down Akt slightly enhanced activation of NF-κB as characterized by increased phosphorylation of IκBα (Supple. Fig. 1D).
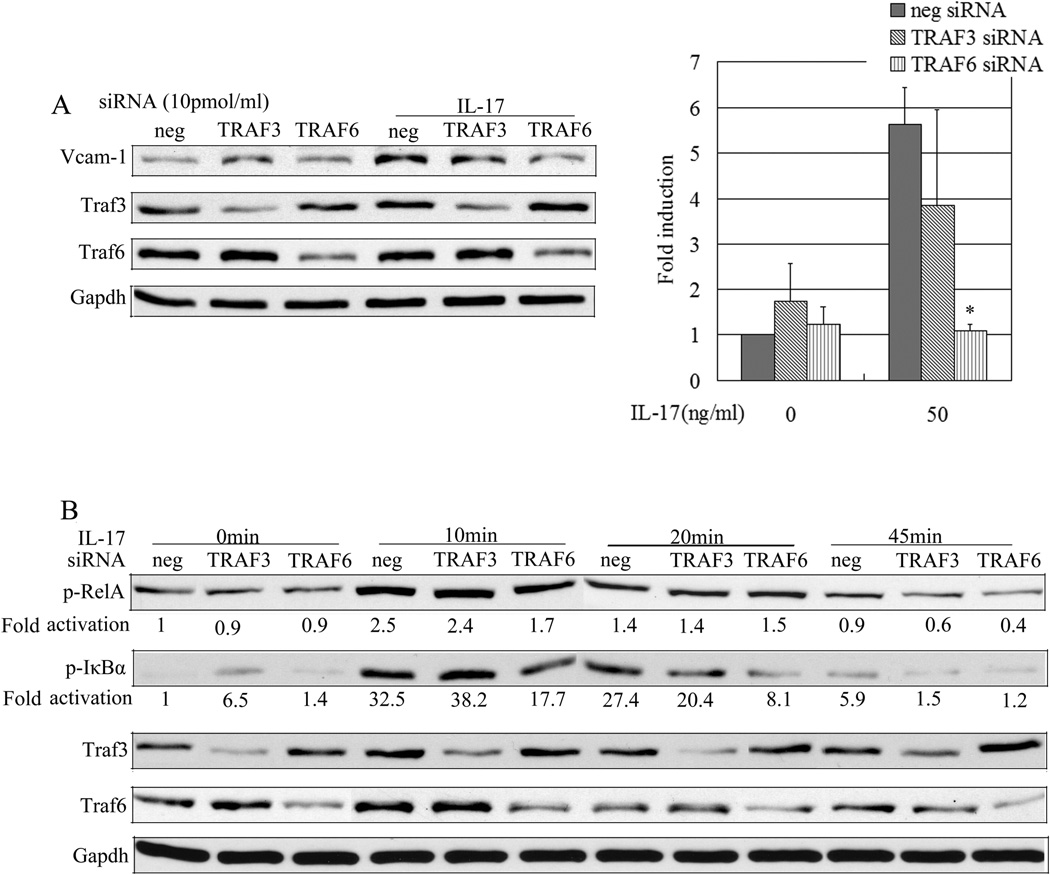
Figure 3.
TRAF3 does not negatively regulate IL-17-mediated NF-κB activation and the expression of VCAM-1 in Rat SMCs. Rat SMCs transfected with 7.5pmol/ml negative control (neg), TRAF3, and TRAF6 siRNA were treated with or without IL-17 (50ng/ml) for different times (B) or 12 hours(A). Whole cell lyses were immunoblotted with anti-p-IκBα, anti-p-p65, anti-Traf3, anti-Traf6, anti-Gapdh (B) or anti-VCAM-1(A). Data are representative of five independent experiments. *, P < 0.05.
Knocking down TRAF3 did not enhance IL-17-induced VCAM-1 expression
TRAF3 and TARF6 regulate NF-κB activation through different means (30, 31). Previous work showed TRAF6 is required for IL-17-induced chemokine expression in mouse embryonic fibroblast cells and airway epithelial cells (32), whereas more recent work has shown that TRAF3 is a negative regulator of IL-17 signaling (33). To determine if TRAF3 and TRAF6 regulated IL-17-induced VCAM-1 expression in SMCs, SMCs were transfected with negative control, TRAF3, and TRAF6 siRNA. Later, the cells were stimulated with IL-17 for 12 hours. Cytoplasmic protein and/or cell nuclear protein were isolated for Western blots. The results showed that knocking down TRAF6 reduced IL-17-induced VCAM-1 expression (Fig. 3A). However, knocking down TRAF3 did not promote IL-17-induced VCAM-1 expression, but enhanced the basal level of VCAM-1 expression in SMCs without IL-17 stimulation (Fig. 3A). P-IKα and P-p65 Western blot analysis was performed. Knocking down TRAF3 slightly enhanced the basal level of P-IKα, indicating activation of NF-κB in SMCs without IL-17 stimulation (Fig. 3B line 2). Clearly these results suggested that knocking down TRAF3 did not enhance IL-17-induced VCAM-1 expression.
IL-17 regulating VCAM-1 expression may be independent of TAK1
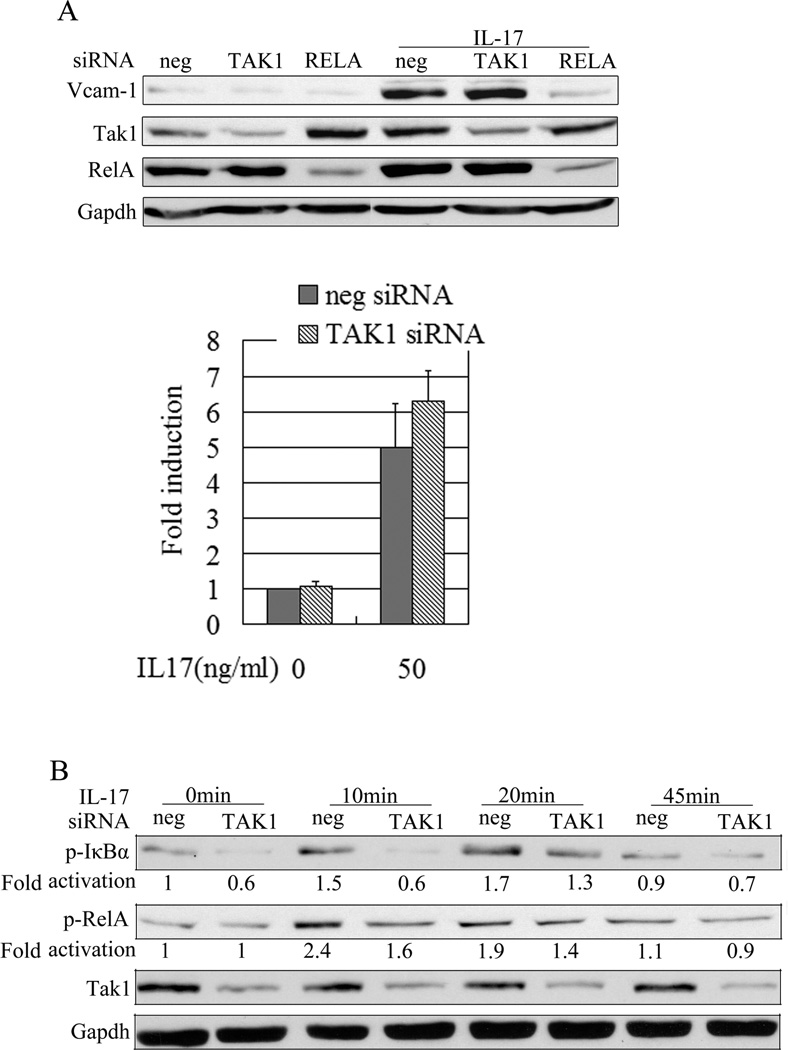
TAK1 is an important signaling molecule in IL-17-mediated effects (29, 34). To determine if TAK1 is involved in IL-17-induced VCAM-1 expression in SMCs, we used TAK1 or negative control siRNA to transfect SMCs following by stimulation with IL-17. 12 hours after incubation with IL-17, cells were harvested for Western blots. The results showed that TAK1 siRNA knocked down TAK1 protein as expected (Fig. 4A). However, knocking down TAK1 did not reduce IL-17-induced VCAM-1 expression in SMCs. Because IL-17 regulates VCAM-1 expression dependent on activation of NF-κB, we examined NF-κB in TAK1-knocking down SMCs stimulated by IL-17. Consistent with the expression of VCAM-1, knocking down TAK1 did not reduce activation of NF-κB (Fig. 4B), suggesting IL-17-activated NF-κB may be through TAK1-independent pathway.
Figure 4.
Knocking down TAK1 (MAP3K7) inhibits the phosphorylation of IκBα, but does not reduce the expression of VCAM-1 stimulated by IL-17 in Rat SMCs. The rat SMCs transfected with TAK1 siRNA (7.5pmol/ml) were treated with or without IL-17 for different times (B) or 12 hours (A). Whole cell lysates were immunoblotted with (A) anti-VCAM-1, (B) anti-TAK1, anti-p- IκBα, anti-p65 or Gapdh. The data are representative of three independent experiments.
IL-17 promotes expression of VCAM-1 in SMCs partially through ERK1/2 MAPK signaling pathway
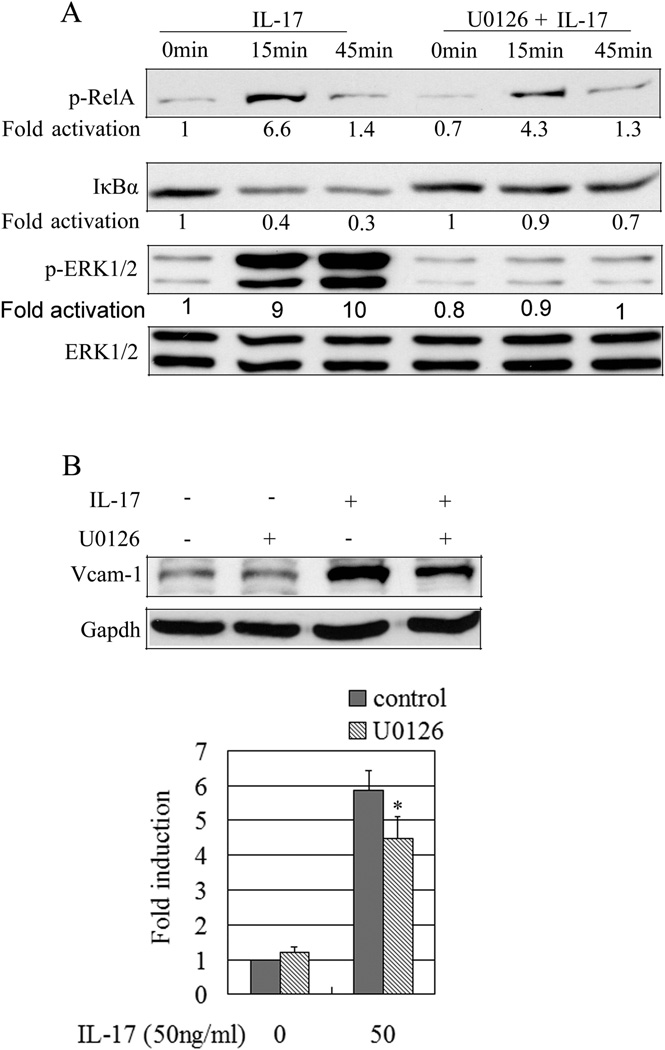
Previous work showed that IL-17-mediated C-reactive protein expression in SMCs depends on the activation of ERK1/2 (35). In cultured SMCs treated with IL-17, we performed Western blotting and examined the phosphorylation of ERK1/2. The results showed that IL-17 induces activation of ERK1/2 and expression of VCAM-1 (Fig. 5A, B). ERK1/2 inhibitor U0126 (20µM) was able to completely inhibit phosphorylation of ERK1/2 in SMCs treated with IL-17 (Fig. 5), but IL-17-induced expression of VCAM-1 was only partially inhibited (Fig. 5B), suggesting that IL-17 induces expression of VCAM-1 in SMCs partially through ERK1/2 signaling pathway.
Figure 5.
IL-17 regulates VCAM-1 expression partially dependent on MAPK ERK1/2. The rested Rat SMCs were treated with inhibitor U0126 (25 µM) for 1 hour in advance and then incubated with or without IL17 for 15 min, 45 min (A) or 12 hours (B). Whole cell lyses were immunoblotted with anti-p-RelA, anti-IκBα, and anti-p-ERK1/2 (A) or with anti-VCAM-1 (B). *, P = 0.045.
Discussion
In this study, we showed that IL-17 induces expression of VCAM-1 in SMCs, providing a potential explanation for IL-17 in promoting development of atherosclerosis. In addition, we identified the signaling pathway by which IL-17 enhances VCAM-1 expression. Our data indicates that IL-17-induced expression of VCAM-1 is through augmented activation of NF-κB, but not Akt1 and TAK1.
Stimulation of murine SMCs with IL-17 also induces expression of several chemokines such as CCL20 and CCL5, as well as adhesion molecules such as ICAM-1 and VCAM-1 (36). In our current study, we showed that IL-17 induced VCAM-1 expression in rat and human SMCs. However, earlier studies (14) showed that in vivo administration of IL-17 to LDLR−/− mice resulted in reduced endothelial VCAM-1 expression, as well as reduced vascular T cell infiltration and atherosclerotic lesion development, which contradicts the recent reports about pathogenic role of IL-17 in atherosclerosis (15, 17, 18).
In contrast to previous work which indicated that IL-17 reduces TNF-α-induced rates and expression of VCAM-1 in human synovial fibroblasts (37), our results showed that IL-17 enhances TNF-α-induced VCAM-1 expression in SMCs (data not shown). We do not know the reason why IL-17 has different effects on TNF-α-mediate effects. This discrepancy may be due to differences in cell lines used in the studies.
An earlier report indicated that IL-17 induced ICAM-1 expression by NF-κB and TRAF-6 is indispensible for the gene regulatory activities of IL-17 (31, 32). Similarly, we showed that IL-17 regulates VCAM-1 expression, and is dependent on TRAF-6 and NF-κB. Knocking down TRAF6 or RELA (p65) by siRNA significantly reduced IL-17-mediated expression of VCAM-1 in SMCs. TRAF3 has been shown to act as a suppressing signal in activation of NF-κB by IL-17 (33). However, when we used siRNA to knock down TRAF3, it did not enhance IL-17-induced activation of NF-κB and expression of VCAM-1 (Fig. 3), suggesting that TARF3 does not negatively regulate IL-17-induced expression of VCAM-1 in SMCs.
IL-17 induces IL-23p19 expression in rheumatoid arthritis synovial fibroblasts through PI3-kinase- and P38 MAPK-dependent signaling pathways (38), whereas our results showed that IL-17-induced VCAM-1 expression in SMCs is independent of the PI3-kinase–Akt axis, since knocking down Akt1 by siRNA did not interrupt up-regulation of VCAM-1 by IL-17 in SMCs (Supple. Fig. 1). However, siRNA knocking down Akt1 slightly increased NF-κB activation (Supple. Fig. 1), suggesting activation of Akt1 by IL-17 is parallel to NF-κB activation by IL-17.
Transforming growth factor-β–activated kinase 1 (TAK1) is a pivotal upstream mitogen-activated protein kinase-kinase-kinase acting as a mediator of cytokine expression (39). The previous work by others showed that IL-17 induced cytokine expression dependent on JAK-mediated PI3K signaling and ACT1/TRAF6/TAK1-dependent NF-κB activation in human airway epithelial cells (29). To determine if TAK1 is required for IL-17-mediated VCAM-1 expression in SMCs, we used TAK1 siRNA to knock down TAK1. To our surprise, knocking down TAK1 did not reduce IL-17-induced VCAM-1 expression. Suggesting TAK1 may be dispensable for IL-17-induced VCAM-1 expression in SMCs. Knocking down TAK1 did not reduce activation of NF-κB (Fig. 4), this implied that IL-17 promoting activation of NF-κB may be by TAK1-independent pathway. Consistent with this result, a recent work indicated that TAK1 negatively regulates NF-κB and p38 MAP kinase activation (40). Notably, TAK1 is involved in TARF6-depedent NF-κB activation pathways in some cell types (29, 41). However, in other cell types, TAK1 does not seem to have a clear role in NF-κB activation (41). Because TAK1 siRNA could not completely knock down TAK1, we cannot rule out the possibility that the small residual TAK1 molecules could deliver signals to activate NF-κB. This question could be further addressed by using genetic mutation of TAK1in SMCs in a future study.
Consistent with the effects of IL-17 on inducing expression of chemokines, adhesion molecules on other epithelial cells (29) and increasing MMP-9 on vascular SMCS (42) by activation of ERK1/2, the IL-17-induced expression of VCAM-1 is partially via the activation of ERK-1/2, because inhibition of ERK-1/2 MAPK activity by U0126 only partially reduced expression of VCAM-1 in SMCs treated with IL-17 (Fig. 5).
IL-17 exhibits pleiotropic biological activities on various cell types, including macrophages, fibroblasts, and endothelial and epithelial cells (36). However, few studies have been done on the role of IL-17 in atherosclerosis. Only recently was IL-17 detected in the serum of patients with coronary atherosclerosis and a significant population of artery-infiltrating T helper cells produce IL-17 (15), suggesting that IL-17 could be crucial for the pathogenesis of atherosclerosis (17, 18).
The VCAM-1 is expressed in endothelial cells and intimal SMCs in the atherosclerotic lesions in humans and in animal models, and is essential for the recruitment of leukocytes and vascular inflammation (20). Inflammation is a critical mechanism in the development and progression of cardiovascular diseases. Current studies showed that IL-17 induced VCAM-1 expression in a dose-dependent manner in SMCs (Fig. 1), suggesting that this could be one of the mechanisms by which IL-17 play a pathogenic role in atherosclerosis (17, 18). Therefore, targeting IL-17 signal pathways should provide a novel therapeutic intervention for treatment of vascular disease.
Supplementary Material
Supplementary Fig. 1. IL-17 regulates VCAM-1 expression in SMCs independent of Serine/Threonine-protein kinase Akt1 in Rat SMCs. (A) Rested Rat SMCs were incubated with IL-17 for the indicated time, and the whole cell lyses were detected with anti-p-Akt1 and anti-Akt1. (B) Rat SMCs transfected with 7.5 pmol/ml AKT1 or negative siRNA were treated with or without IL-17 for 12 hours. Knocking down Akt1 did not reduce IL-17-induced VCAM-1 expression. (C, D) Rat SMCs transfected with 7.5 pmol/ml AKT1 or negative siRNA were treated with or without IL-17 (50ng/ml) for 15 or 45 minutes. The nuclear fractions were immunoblotted with antibodies against phosphor-RelA (Ser536) or total RelA. The cytoplasmic fractions were detected with antibodies against the phosph- IkBα, total IkBα and Akt1. Blotting nuclear histone H1 and Gapdh were used for equal loading.
Acknowledgments
The project described was supported by NIH Grant Number p20GM103429 from the IDeA Networks of Biomedical Research Excellence (INBRE) Program of the National Center for Research Resources, and Arkansas Biosciences Institute New Faculty startup package. LA was a NSF RISE recipient.
Footnotes
Disclosures: The authors have no financial conflicts of interest.
References
- 1.Hemdan NY, Birkenmeier G, Wichmann G, et al. Interleukin-17-producing T helper cells in autoimmunity. Autoimmun. Rev. 2010;9:785–792. doi: 10.1016/j.autrev.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Cua DJ, Tato CM. Innate IL-17-producing cells: The sentinels of the immune system. Nat. Rev. Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 3.Iwakura Y, Ishigame H, SAijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–158. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4+ T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee YK, Turner H, Maynard CL, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGeachy MJ, Bak-Jensen KS, Chen Y, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 7.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, et al. IL-6 programs Th-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Laurence A, Kanno M, et al. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc. Natl. Acad. Sci. USA. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei LA, Laurence A, Elias KM, O’Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a ATAt3-dependent manner. J. Biol. Chem. 2007;282:34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathur AN, Chang HC, Zisoulis DG, et al. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178:4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 11.Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 12.Zheng Y, Danilenko DM, Valdez P, Kasman I, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 13.Roussel L, Houle F, Chan C, et al. IL-17 promotes p38 MAPK-dependent endothelial activation enhancing neutrophil recruitment to sites of inflammation. J Immunol. 2010;184:4531–4537. doi: 10.4049/jimmunol.0903162. [DOI] [PubMed] [Google Scholar]
- 14.Taleb S, Romain M, Ramkhelawon B, et al. 2009. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J. Exp. Med. 2009;206:2067–2077. doi: 10.1084/jem.20090545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eid RE, Rao DA, Zhou J, et al. Interliukin-17 and interferon-γ are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119:1424–1432. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng X, Yu X, Ding YJ, et al. The Th17/Treg functional imbalance in patients with acute coronary syndrome. Clin. Immunol. 2008;127:89–97. doi: 10.1016/j.clim.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Chen S, Shimada K, Zhang W, Huang G, Crother TR, Arditi M. 2010. IL-17A is proatherogenic inhigh-fat diet-induced and Chlamydia pneumonide infection-accelerated atherosclerosis in mice. J. Immunol. 2010;185:5619–5627. doi: 10.4049/jimmunol.1001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao Q, Jiang Y, Ma T, et al. A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J. Immunol. 2010;185:5820–5827. doi: 10.4049/jimmunol.1000116. [DOI] [PubMed] [Google Scholar]
- 19.Cybulsky MI, Iiyama K, Li H, et al. 2001. A major role for VCAM-1, but, not ICAM-1, in early atherosclerosis. J. Clin. Invest. 2001;107:1255–1262. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2008;28:812–819. doi: 10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Awane M, Andres PG, Li DJ, Reinecker HC. NF-κB-inducing kinase is a common mediator of IL-17-, TNF-α-, and IL-1β-induced chemokine promoter activation in intestinal epithelial cells. J Immunol. 1999;162:5337–5344. [PubMed] [Google Scholar]
- 22.Liu XM, Peyton KJ, Ensenat D, et al. Nitric oxide stimulates heme oxygenase-1 gene transcription via the Nrf2/ARE complex to promote vascular smooth muscle cell survival. Cardiovasc. Res. 2007;175:381–389. doi: 10.1016/j.cardiores.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee H, Hermann A, Deng J, et al. Persistently activated Stat3 maintains constitutive NF-κB activity in tumors. Cancer Cell. 2009;15:283–293. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu S, Fang Y, Sharp GC, Braley-Mullen H. Transgenic expression of TGF-beta on thyrocytes inhibits development of spontaneous autoimmune thyroiditis and increases regulatory T cells in thyroids of NOD.H-2h4 mice. J Immunol. 2010;184:5352–5359. doi: 10.4049/jimmunol.0903620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iademarco MF, McQuillan JJ, Rosen GD, Dean DC. Characterization of the promoter for vascular cell adhesion molecule-1 (VCAM-1) J. Biol. Chem. 1992;267:16323–16329. [PubMed] [Google Scholar]
- 26.Neish AS, Williams AJ, Palmer HJ, Whitley MZ, Collins T. Functional analysis of human vascular cell adhesion molecule 1 promoter. J Exp. Med. 1992;176:1583–1593. doi: 10.1084/jem.176.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayden MS, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 28.Gaffen SL. Structure and signaling in the IL-17 receptor family. Nat. Rev. Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang F, Kao CY, Wachi S, Thai P, Ryu J, Wu R. Requirement for both JAK-mediated PI3K signaling and ACT1/TRF6/TAK1-dependent NF-kappaB activation by IL-17A in enhancing cytokine expression in human airway epithelial cells. J. Immunol. 2007;179:6504–6513. doi: 10.4049/jimmunol.179.10.6504. [DOI] [PubMed] [Google Scholar]
- 30.He JQ, Saha SK, Kang JR, Zamegar B, Cheng G. Specificity of TARF3 in its negative regulation of the noncanonical NF-kappa B pathway. J. Biol. Chem. 2007;282:3688–3694. doi: 10.1074/jbc.M610271200. [DOI] [PubMed] [Google Scholar]
- 31.Lamothe B, Besse A, Campos AD, Webster WK, Wu H, Darnay BG. Site-specific Lys-63-linked tumor necrosis factor receptor-associated factor 6 auto-ubiquitination is a critical determinant of I kappa B kinase activation. J Biol. Chem. 2007;282:4102–4112. doi: 10.1074/jbc.M609503200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwandner R, Yamaguchi K, Cao H. Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. J. Exp. Med. 2000;191:1233–1239. doi: 10.1084/jem.191.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu S, Pan W, Shi P, et al. Modulation of experimental autoimmune encephalomyelitis through TARF3-mediated suppression interleukin 17 receptor signaling. J. Exp. Med. 2010;207:2647–2662. doi: 10.1084/jem.20100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zepp J, Wu L, Li X. IL-17 receptor signaling and T helper 17-mediated autoimmune demyelinating disease. Trends in Immunol. 2011;32:232–239. doi: 10.1016/j.it.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel DN, King CA, Bailey SR, et al. Interliukin-17 stimulates C-reactive protein expression in hepatocytes and smooth muscle cells via p38 MAPK and ERK1/2-dependent NF-kappaB and C/EBPbeta. J. Biol. Chem. 2007;282:27229–27238. doi: 10.1074/jbc.M703250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erbel C, Chen L, Bea F, et al. Inhibition of IL-17A attenuates atherosclerosis lesion development in ApoE-deficient mice. J Immunol. 2009;183:8167–8175. doi: 10.4049/jimmunol.0901126. [DOI] [PubMed] [Google Scholar]
- 37.Schnyder B, Schnyder-Candrian S, Pansky A, et al. IL-17 reduces TNF-induced rantes and VCAM-1 expression. Cytokine. 2005;31:191–202. doi: 10.1016/j.cyto.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Kim KW, Cho ML, Park MK, et al. Increased interleukin-17 production via phosphoinostide 3-kinase/Akt and nuclear factor kappa B-dependent pathway in patients with rheumatoid arthritis. Arthritis Res. Ther. 2005;7:R139–R148. doi: 10.1186/ar1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Courties G, Seiffart V, Presumey J, et al. In vivo RNAi-mediated silencing of TAK1 decreases inflammatory TH1 and Th17 cells through targeting of myeloid cells. Blood. 2010;116:3505–3516. doi: 10.1182/blood-2010-02-269605. [DOI] [PubMed] [Google Scholar]
- 40.Alagbala AA, Wang Q, Cui J, et al. TAK1 negatively regulates NF-κB and p38 MAP kinase activation in Gr-1(+) CD11b(+) neutrophils. Immunity. 2012;36:43–54. doi: 10.1016/j.immuni.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, et al. Essential function for the kinase TAK1 in innate and adaptive immune response. Nature Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- 42.Cheng G, Wei L, Xiurong W, et al. IL-17 stimulates migration of carotid artery vascular smooth muscle cells in an MMP-9 depdendent mannar via p38 MAPK and ERK1/2-dependent NF-kappa B and AP-1 activation. Cell Mol Neurobiol. 2009;29:1161–1168. doi: 10.1007/s10571-009-9409-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. IL-17 regulates VCAM-1 expression in SMCs independent of Serine/Threonine-protein kinase Akt1 in Rat SMCs. (A) Rested Rat SMCs were incubated with IL-17 for the indicated time, and the whole cell lyses were detected with anti-p-Akt1 and anti-Akt1. (B) Rat SMCs transfected with 7.5 pmol/ml AKT1 or negative siRNA were treated with or without IL-17 for 12 hours. Knocking down Akt1 did not reduce IL-17-induced VCAM-1 expression. (C, D) Rat SMCs transfected with 7.5 pmol/ml AKT1 or negative siRNA were treated with or without IL-17 (50ng/ml) for 15 or 45 minutes. The nuclear fractions were immunoblotted with antibodies against phosphor-RelA (Ser536) or total RelA. The cytoplasmic fractions were detected with antibodies against the phosph- IkBα, total IkBα and Akt1. Blotting nuclear histone H1 and Gapdh were used for equal loading.