Abstract
Increased expression of neurotrophins (e.g., NGF, BDNF) and chemokines (e.g., RANTES) has been observed in neurodegenerative diseases. We examined the effect of these factors on intracellular signaling cascades inducing cell cycle proteins p53, pRb, and E2F1 in human fetal mixed neuronal and glial cells. Comparing neurotrophin- and chemokine-treated cultures with untreated controls showed altered subcellular localization and expression of hyperphosphorylated retinoblastoma protein (ppRb), E2F1, and p53. Using immunofluorescent laser confocal microscopy, E2F1 and ppRb were detected exclusively in neuronal nuclei in control cultures while p53 was cytoplasmic in astrocytes and nuclear in neurons. Following treatment with neurotrophins, E2F1 and ppRb were observed in the cytoplasm of neurons, while p53 was observed in both neuronal and astrocytic nuclei. Similar findings were observed following treatment with RANTES. Semiquantitative analysis using immunoblots showed an increase in the amount of phosphorylated pRb in treated cultures. Induction of cell cycle proteins may play a role in neurodegeneration associated with neurotrophin and chemokine stimulation.
Keywords: human, in vitro, neuroglia, E2F1, p53, pRb, neurodegeneration
INTRODUCTION
Neurotrophic factors (NTF)3 and chemokines have been found to be upregulated in several neurodegenerative diseases including Alzheimer’s disease and human immunodeficiency virus encephalitis (HIVE) (10, 11, 24, 39, 52, 55). In HIVE these factors are secreted by infected/activated macrophages (17, 20, 21, 34, 35, 46, 47) and associated with neuronal loss. It has been proposed that neuronal loss in HIVE may be due to increased secretion of NTF like BDNF (55) and chemokines like MCP1 or RANTES (10, 24, 39, 52), yet how these factors may lead to neuronal death is not clear.
Neurotrophins were discovered and characterized on the basis of their function in promoting neuronal survival, maturation, neurite extension, and synaptic activity (reviewed in 11). These small, secreted peptides bind to specific members of the tyrosine kinase family of receptors, trkA, trkB, and trkC. These high-affinity receptors form heterodimers with the low-affinity neurotrophin receptor, p75NTFR, a member of the death receptor family (reviewed in 9). Activation of a neurotrophin receptor by its cognate neurotrophic factor results in initiation of the classic tyrosine kinase cascade via second messengers such as ras and phospholipase activation (22, 23). These pathways are shared with other members of the tyrosine kinase superfamily of receptors that regulate cellular proliferation (22, 23). While activation of neurotrophin receptors in developing neurons is associated with increased cell survival and differentiation little is known about their function in postmitotic, terminally differentiated neurons. In nonneuronal cells, these same receptors can induce proliferation like their mitogenic receptor family members (12, 32).
Chemokines bind receptors that are members of the seven-transmembrane, G-protein-coupled receptor superfamily (4). Chemokines act as chemoattractants for numerous cell types of the CNS during development and are believed to play a role in neuronal patterning (4). Chemokines also activate signaling cascades involved in regulating cellular proliferation such as the mitogen-activated protein kinase and adenylyl cyclase cascades (4). Receptors for chemokines are found on hematopoietic and nervous system cells including neurons, astrocytes, and macrophages. Activation of these receptors by increased chemokines produced in proximity to degenerating neurons may be partially responsible for neuronal death in various degenerative diseases. We hypothesized that activation of chemokine receptors alters activity of cell cycle regulatory proteins in neurons ultimately mediating neuronal death.
Mitogenic signaling cascades result in phosphorylation of the retinoblastoma susceptibility protein (pRb) (60, 62). Hyperphosphorylation of pRb (ppRb) disinhibits transcription factors like members of the E2F family, driving the cell into S phase of the cell cycle (1, 33). E2F1, the first member of this family to be identified, is unique among all the members in that it can not only induce cellular division, but also cell death (13, 44). In vivo evidence for this in the nervous system comes from the pRb knock out mice (57). These transgenic mice die at E14.5 of massive neuronal loss due to apoptosis. This phenotype is rescued by concomitant deletion of E2F1 (57) suggesting that E2F1 activity is regulated by pRb in developing neurons and that deregulation of E2F1 results in apoptosis in these cells.
A second protein responsive to the cellular environment that regulates cell division is the p53 tumor suppressor protein. In its active form, p53 can have two outcomes on cellular fate: cell cycle arrest or apoptosis (53). How p53 integrates cellular signals to decide between these two fates is not known, but, as a transcription factor, p53 has been shown to regulate expression of p21CDKI, which arrests cells in G1, and BAX, which induces apoptosis (16, 40). In neurons, p53 has been shown to play a role in apoptosis during hypoxia, development, excitotoxicity, neurodegeneration, and DNA damage suggesting a role for this protein in postmitotic neurons (2, 7, 15, 26, 27, 31, 51).
Neuronal death during degenerative diseases has been proposed to occur via apoptotic pathways, but the duration of these diseases suggests a prolonged mechanism as opposed to the rapid and orderly proteolytic caspase cascade of apoptosis. Recent reports have suggested that mature neurons lose caspase expression and undergo apoptosis in a p53-dependent fashion (25). Further reports have found that cell cycle regulators, specifically those that target phosphorylation of pRb, are more likely initiators of neuronal death through a mechanism with a protracted time course (56). This has led us to propose the following hypothesis: Increased neurotrophic factor and chemokine expression during neurodegenerative diseases induces abnormal expression of the cell cycle regulatory machinery in differentiated neuroglia. To test this hypothesis we treated fetal mixed neuroglial cultures with the neurotrophic factors BDNF and NGF and the chemokine RANTES. After treatment for 6 and 48 h, we stained cells for the presence of cell cycle regulatory proteins: ppRb, E2F1, and p53. We observed alteration in subcellular localization for all three of these proteins in neurons and glia. Semiquantitative analysis by immunoblotting, showed a significant increase in the amount of ppRb. These data support the hypothesis that NTF and chemokines have the ability to alter cell cycle regulatory proteins in neurons and astrocytes that may lead to neurodegeneration.
MATERIALS AND METHODS
Cell Culture
Human telencephalic tissue between 18 and 22 weeks of gestation was collected as recommended by the guidelines of the University of Pittsburgh Human Tissue Committee. The tissue was placed in serum-and pyruvate-free DMEM and chilled on ice. Tissue was processed following a modified mouse brain dissociation protocol as previously described (61). Briefly, the meninges and debris were removed from the tissue and the tissue was minced and rinsed in ice cold Ca2+-and Mg2+ free PBS. The cells were dissociated by treatment with 0.05% trypsin/EDTA at 37°C for 5 min followed by repeated pipetting. Trypsin inhibitor was added at 10 mg/ml and the cell suspension was washed with DMEM by centrifugation at 1000 rpm for 5 min. Cells were filtered through a 70-µm nylon mesh strainer (Becton Dickinson, Franklin Lakes, NJ) and further cleaned by centrifugation through an 8% BSA (Sigma, St. Louis, MO) layer at 1000 rpm for 10 min. The cells were then filtered twice more, first through a 70-µm nylon mesh strainer then by a 40-µm nylon mesh filter. Cells were resuspended in NPMM without EGF or FGF (Clontech, Palo Alto, CA) and seeded onto laminin-coated coverslips or flasks at 1,000,000 cells/ml and grown at 37°C in 5% CO2. At the time of treatment cells had been maintained in culture for 7 days. Medium was refreshed every 3 days. After treatment, cells on laminin-coated coverslips were fixed in 4% paraformaldehyde for 20 min and washed four time in PBS prior to staining.
Immunofluorescent Laser Confocal Microscopy
Cells were fixed on coverslips, permeabilized, and blocked in 0.2% bovine serum albumin, 1% Triton X-100 in PBS. After washing the cells were incubated at 4°C overnight with p53 or E2F1 antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) diluted 1:100 in normal antibody diluent (Scytek, Logan, UT). Cells were washed three times in PBS 0.1% Tween 20 and incubated with a biotin-conjugated goat antimouse secondary antibody (Chemicon International, Temecula, CA) at 1:200 in normal antibody diluent for 30 min. Cells were washed in PBS 0.1% Tween 20 three times for 5 min. The antibody signal was then amplified using the direct tyramide amplification green system (New England Biolabs, Beverly, MA). The second primary antibody (β3-tubulin 1:80; Biogenex, San Ramon, CA; or glial fibrillary acidic protein (GFAP), 1:100; Dako, Carpinteria, CA) was incubated on the cells for 1 h at room temperature for 1 h. The second primary antibody was visualized using a Cy-3-conjugated secondary (goat anti-mouse at 1:200 for β3-tubulin and goat anti-rabbit at 1:200 for GFAP) incubated with the cells for 30 min at room temperature. Cells were washed three times and mounted on slides with gelvatol (5) and analyzed by laser confocal microscopy (Molecular Dynamics, Sunnyvale, CA), as previously described (54).
Protein Extracts and Immunoblotting
Protein extracts were prepared from mixed neurolgial cells by detergent lysis. Cells were scraped in detergent lysis buffer (0.1% NP-40, 10 mM Tris (pH 8.0), 10 mM MgCl2, 15 mM NaCl, 0.5 mM PMSF, 2 µg/ml pepstatin A, and 1 µg/ml leupeptin) and incubated on ice for 15 min. The nuclei were collected by low-speed centrifugation at 800g for 5 min. The supernatant was saved as the “S1” fraction. The pellet containing the nuclei was further extracted with high salt buffer (0.42 M NaCl, 20 mM Hepes (pH 7.9), 1.5 mM MgCl2, 0.2 mM EDTA, 20% glycerol, 0.5mM PMSF, 2 µg/ml pepstatin A, and 1 µg/ml leupeptin) on ice for 10 min. Residual insoluble material was removed by centrifugation at 14,000g for 5 min. The supernatant fraction was collected and termed the “S2” fraction, which was used for immunoblots. Protein concentrations were determined by Bio-Rad protein assay.
The proteins were transferred from the 4–16% SDS-polyacrylamide gradient gel to Immun-Blot PVDF membrane (Bio-Rad, Hercules, CA) by electrophoresis and blocked in 5% normal goat serum in TBS (10mM Tris (pH 8.0), 150 mM NaCl). All antibodies were used at 1:1000 in 0.5% milk overnight at 4°C. Blots were washed three times in TBST (TBS + 0.1% Tween 20) for 15 min. Goat anti-mouse-HRP and goat anti rabbit–HRP (1:3000; HRP (Southern Biotechnologies Inc., Birmingham, AL) were used to detect the appropriate primary antibodies. The secondary antibody was washed extensively in TBS, three times for 20 min. The antibody was then visualized using enhanced chemiluminescence (ECL) (Renaissance, NEN Life Science Products, Inc.)
RESULTS
E2F1 Translocates to the Cytoplasm of Neurons and Astrocytes Treated with NGF, BDNF, and RANTES
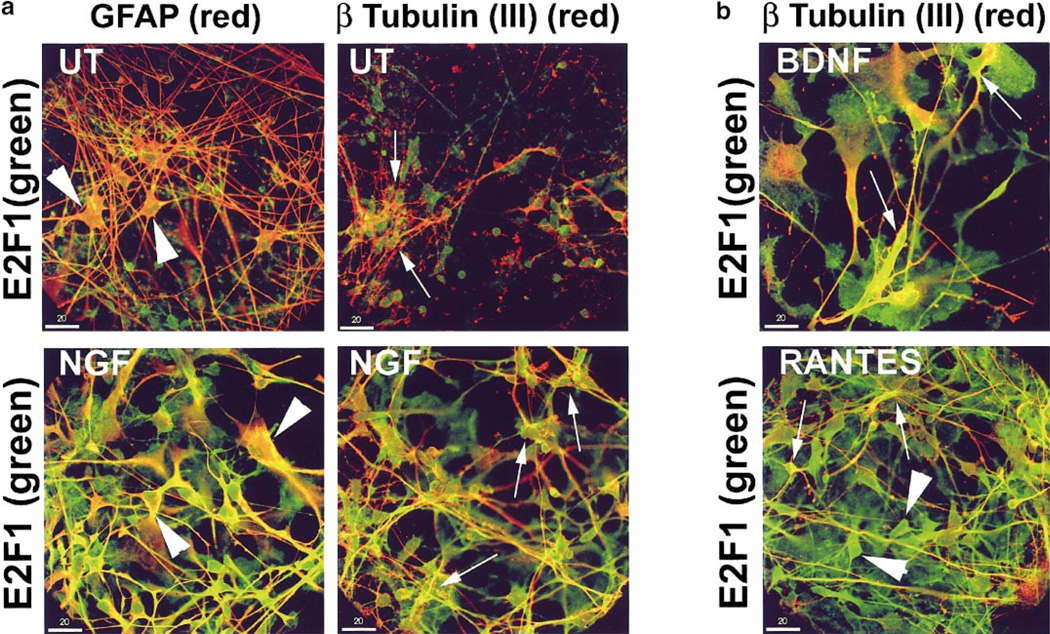
Our previous studies have demonstrated expression of NTF and chemokine receptors on neurons and microglia in human fetal neuroglial cultures (52, 61). CCR3 and CCR5 chemokine receptors were not found on astrocytes, while trk B and Trk A neurotrophin receptors were (52; Fenner and Achim, unpublished observations). In these studies, NTF treatment at 2 ng/ml increased survival and neuritic process formation in these cultures over an extended time course; however, at higher doses toxicity was observed (61; Achim, unpublished observations). Chemokine treatment of these cultures was observed to cause an increase in glial cell populations (Achim, unpublished observations). Using this model, we wanted to determine whether neurotrophic factor and chemokine signaling impact E2F1 expression patterns in neuronal and glial cells. Human fetal neuroglial cultures with subtoxic doses of NTF, BDNF, and NGF and the chemokine RANTES (50 ng/ml NGF, 50 ng/ml BDNF, or 100 ng/ml RANTES) for 6 and 48 h. Following treatment the cells were double labeled for E2F1 and phenotypic markers (class III β-tubulin) for neurons or astrocytes (GFAP). Staining was visualized by immunofluorescent laser confocal microscopy. Staining patterns at 6 and 48 h after treatment were similar; therefore, only the 6-h time point is illustrated here. In untreated cultures E2F1 is found predominantly in nuclei of class III β-tubulin-positive neurons (Fig. 1a; UT, β tubulin (III), arrows), but was not found in either cellular compartment of GFAP positive astrocytes (Fig. 1a; UT, GFAP, arrowheads). When cells were treated with NGF, E2F1 staining was observed in the cytoplasm and processes of class III β-tubulin-positive neurons (Fig. 1a; NGF, β tubulin (III), arrows) and in the cytoplasm of GFAP-positive astrocytes (Fig. 1a; NGF, GFAP, arrowheads). There is also E2F1 staining in the nuclei of these cells. Similarly, cells treated with BDNF or RANTES also changed distribution of E2F1 to the cytoplasm of neurons (Fig. 1b; BDNF and RANTES, β tubulin (III), arrows) and of nonneuronal cells, presumably astrocytes (arrowheads). While E2F1 subcellular changes were consistent among the three treatments, there were differences in the cell morphology in the treated cultures. Neuronal process staining for class III β-tubulin was increased in NGF- and BDNF-treated cultures which is consistent with their proposed roles in neuronal process guidance. RANTES-treated cultures exhibited increased numbers of astrocytes as previously reported (52).
FIG. 1.
E2F1 localizes to the cytoplasm of neurons and astrocytes treated with NGF, BDNF, and RANTES. (a) E2F1 (FITC green) was undetectable in astrocytes (GFAP; Cy3 red, arrowheads) in untreated (UT) human fetal neuroglial cultures, but observed in nuclei and cytoplasm of astrocytes treated with NGF (NGF GFAP, arrowheads). In neurons, E2F1 (FITC green) localized to nuclei (β Tubulin (III); Cy3 red, arrows) in untreated (UT) cultures and to the nuclei and cytoplasm of neurons in cultures treated with NGF (arrows). (b) In BDNF- and RANTES-treated cultures, E2F1 (FITC green) localized to the nuclei and cytoplasm of neurons (β Tubulin (III); Cy3 red, arrows) and to the nuclei and cytoplasm of nonneuronal cells similar to the NGF-treated cultures (arrowheads). Cultures shown were treated for 6 h as indicated. Colocalization of the two signals appears yellow. Bar, 20 µm.
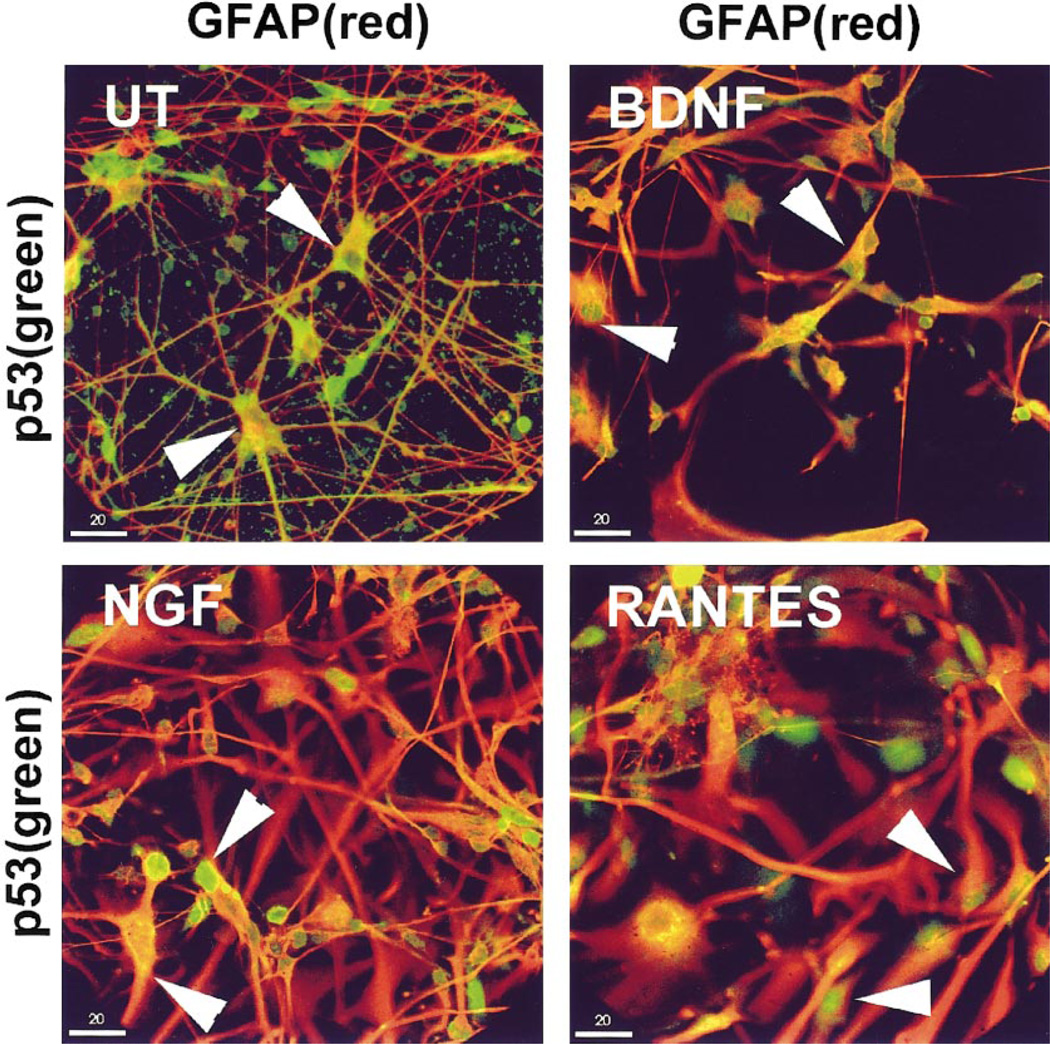
NGF, BDNF, and RANTES Treatment Alters p53 Localization in Astrocytes
In untreated neuroglial cultures p53 localized to the cytoplasm of astrocytes (Fig. 2; UT, GFAP, arrowheads) and the nuclei of neurons (class III β-tubulin, data not shown). In cultures treated with NGF or RANTES p53 staining became nuclear in astrocytes (Fig. 2; NGF and RANTES, GFAP, arrowheads), while retaining a nuclear localization in neurons. Cultures treated with BDNF stained for p53 in both the nuclei and the cell body of astrocytes with reduced process staining (Fig. 2; BDNF, GFAP, arrowheads).
FIG. 2.
NGF, BDNF, and RANTES treatment alters p53 localization in astrocytes. p53 (FITC green) localized to the cytoplasm of astrocytes (GFAP Cy-3, arrowheads) in untreated cultures (UT). Neuroglial cultures treated with NGF, BDNF, or RANTES exhibited p53 staining in astrocytic and nonastrocytic nuclei. Cultures shown were treated for 6 h as indicated. Colocalization of the two signals appears yellow. Bar, 20 µm.
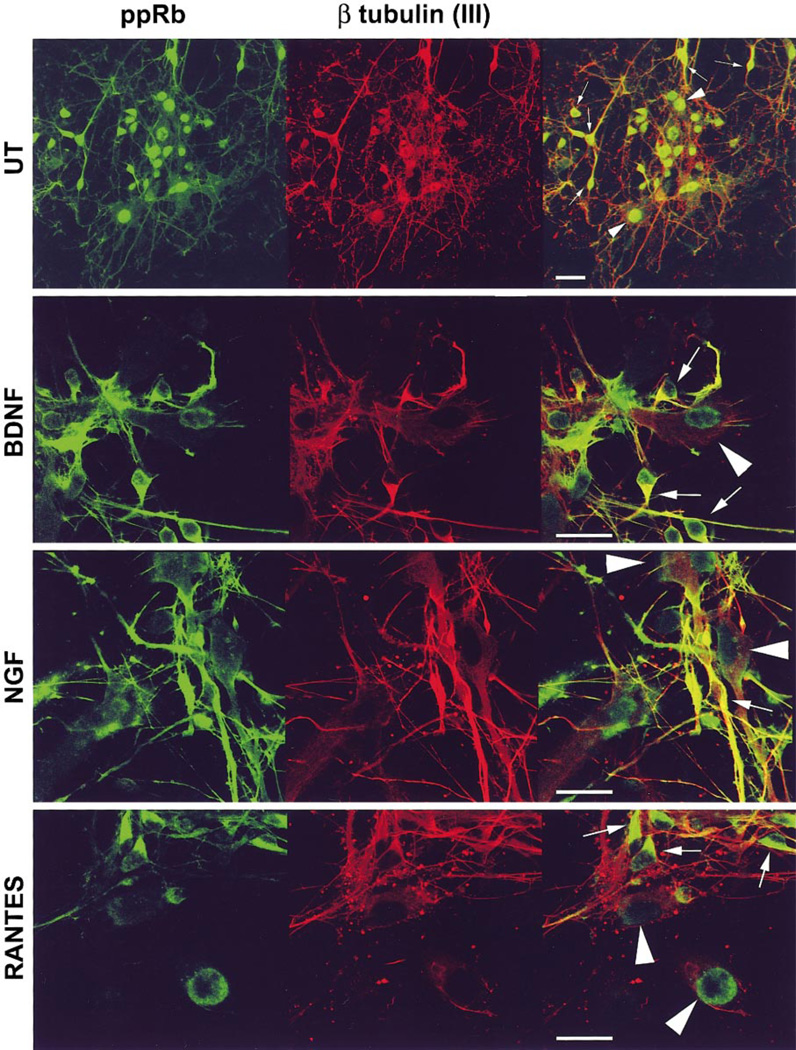
ppRb Changes from Nuclear to Cytoplasmic in Response to NGF, BDNF, and RANTES Treatment
When untreated cultures were stained using an antibody to the phosphoserine-795 isoform of ppRb, ppRb was found to be predominantly nuclear in both astrocytes and class III β-tubulin-positive neurons (Fig. 3; UT, β tubulin (III)). There was also minimal staining of processes in neurons of the untreated cultures. Treatment of the cultures with NGF or BDNF resulted in abundant ppRb staining in the cytoplasm and processes of neurons with a decrease in nuclear staining (Fig. 3; NGF, BDNF, β tubulin (III), arrows). In astrocytes, ppRb localization continued to be nuclear (Fig. 3; NGF, BDNF, β tubulin (III), arrowheads). While treatment of the cultures with RANTES also altered ppRb subcellular localization in neurons, the staining was only observed in the cytoplasm of the cell body, but not in the processes or nuclei (Fig. 3; RANTES, β tubulin (III), arrows). ppRb staining in RANTES-treated cultures appeared to be polarized in the neurons staining the cytoplasm on one side of the nucleus.
FIG. 3.
Hyperphosphorylated pRb (ppRb) localizes to the cytoplasm of neurons in response to NGF, BDNF, and RANTES treatment. In untreated neuroglial cultures (UT), ppRb (FITC green) localized to the nuclei of both neurons (β tubulin(III); Cy3 red, arrows) and astrocytes (arrowheads). Treatment with NGF and BDNF resulted in ppRb staining in the cytoplasm and processes of neurons excluding the nucleus (arrows). ppRb localized to the cytoplasm, but not processes or nuclei of neurons. Cultures were treated as indicated for 6 h. Colocalization appears yellow. Bar, 20 µm.
Phosphorylation of pRb Increases after Treatment with NTF
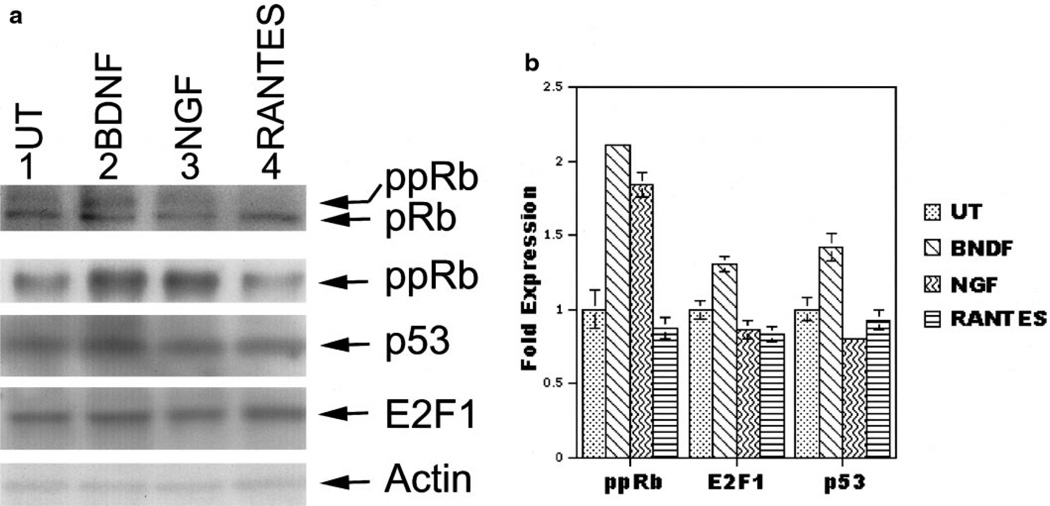
To determine if the changes in subcellular distribution were accompanied by increased protein expression, we assessed changes in protein expression by immunoblot analysis. Protein extracts were prepared from human neuroglial cultures treated with 50 ng/ml BDNF, 50 ng/ml NGF, 100 ng/ml RANTES, or medium alone for 48 h and 50 µg of extract was run on a 4–16% gradient Tris–glycine, SDS–polyacrylamide gel. Immunoblots for the pRb protein revealed two bands of approximately 105–110 kDa. Presumably the upper band is the hyperphosphorylated form of pRb (Fig. 4, pRb and ppRb, top row). To confirm this, we used an antibody generated to the phosphoserine-795 isoform of pRb and found that ppRb was increased in neurotrophin-treated cultures (Figs. 4A and 4B, ppRb lanes 2 and 3, second row). The amount of p53 and E2F1 protein was not altered by the various treatments (Figs. 4A and 4B, third and fourth rows). Actin is shown as a control for loading. Quantification of these data (Fig. 4B) suggests that while p53 and E2F1 change subcellular localization in response to NTF and chemokines, their expression levels remain constant.
FIG. 4.
Phosphorylation of pRb increases after treatment with NTF. (A) Protein levels of pRb (top panel), ppRb (panel 2), p53 (panel 3), E2F1 (panel 4), and actin (panel 5) comparing protein extracts from untreated (UT) (lane 1) neuroglial cultures or cultures treated with BDNF, NGF, or RANTES (lanes 2–4). The slower migrating band on the pRb immunoblot (top panel) is a hyperphosphorylated form of pRb (ppRb). An antibody to the serine 795 phosphoisoform of pRb is shown in panel 2. Immunoblot for actin was used to normalize for loading. (B) Bands detected by ppRb, E2F1, p53, and actin were quantified by NIH Image 1.5.8. All bands were normalized to the amount of actin. Shown is the fold increase in protein expression over untreated cultures. Standard deviations between three quantification trials are indicated by error bars.
DISCUSSION
During progression of neurodegenerative diseases, especially those that involve an inflammatory component, neurotrophins and chemokines have been observed to be expressed at an increased level (4, 11, 52, 55). The impact of this change in the composition of the extracellular neuronal milieu is not clearly defined. To begin to determine the potential mechanisms that contribute to neuronal loss during neurodegenerative diseases, we hypothesized that the neuronal and astrocytic response to neurotrophins and chemokines might include alteration in the activity and expression of cell cycle regulatory machinery intricately associated with the state of differentiation. To test this hypothesis we treated human neuroglial cultures with NGF, BDNF, and RANTES. We report here that cell cycle proteins, E2F1, p53, and hyperphosphorylated pRb exhibit altered subcellular localization in response to NTF and chemokines.
In our culture system, E2F1 is predominantly nuclear in untreated neurons. After treatment with neurotrophins or RANTES, E2F1 localizes to the cytoplasm and processes of both neurons and astrocytes. This is in contrast to previous reports that E2F1 is a nuclear protein that functions as a transcription factor (1, 33). Recently, E2F1 has been shown to cause apoptosis by a transcription-independent mechanism. Increased expression of E2F1 caused increased degradation of the TRAF2 protein (45). Since TRAF2 is a cytoplasmic protein that binds death receptors like TNF-α receptor in the plasma membrane, destabilization of TRAF2 is likely to occur in the cytoplasm (14). Further, TRAF6, a TRAF2 family member, has been shown to bind the p75NTFR, the low-affinity receptor bound by NGF and BDNF (30). Taken together, these data and our findings suggest that E2F1 may play a role in the cytoplasm regulating receptor activity that can directly impact cell survival in neurons and astrocytes.
The fact that we do not see an increase in E2F1 protein expression in response to NTF or chemokine treatments, but do see dramatic changes in subcellular distribution, suggests an alteration in E2F1 activity. E2F1 activity is regulated by interactions with the pRb family of proteins (1, 33). The antibody used to detect E2F1 in this study specifically recognizes the pRb interaction domain of E2F1. Since we do not use harsh detergents that would disrupt this interaction in our staining protocol, we believe that in untreated cultures, E2F1 is present, but in complex with pocket proteins, and therefore not detected by our antibody. In the NTF and chemokine treated cultures, we observe changes in localization and phosphorylation of pRb concomitant with changes in E2F1 subcellular distribution. Since hyperphosphorylated pRb does not interact with E2F1, our findings are consistent with detection of free E2F1 in the cytoplasm as opposed to newly produced E2F1. Future experimentation is directed at addressing the source of the cytoplasmic E2F1.
Treatment of neuroglial cultures with neurotrophins results in increased phosphorylation of pRb. In the hyperphosphorylated state, ppRb becomes localized to the cytoplasm and processes of neurons. This change in subcellular localization suggests that E2F1 and ppRb are not localized so as to interact and regulate transcription in the nucleus, their most well characterized activity. The presence of ppRb in the cytoplasm of neurons is a novel observation that is consistent with our in vivo observations in the cortex of simians with lentiviral induced encephalitis (29). We also observed an increase in hyperphosphorylated pRb when cells are treated with neurotrophins, but not with chemokines. This is interesting in light of the difference in staining pattern for ppRb in response to NTF and chemokines. In future experiments, we plan to study the differential response of ppRb to chemokines and NTF and determine the role of ppRb in neuronal cell death. The role of ppRb in cell death is likely to involve changes in E2F1 activity, which will be studied in the same context.
p53 appears to have a role that is distinct from E2F1 and ppRb. Changes in p53 subcellular localization occur in astrocytes where p53 becomes nuclear in response to neurotrophins and RANTES. These changes would be consistent with the stabilization of p53 in these cells. Normally p53 is produced and degraded in the cytoplasm, but in response to appropriate signals, p53 becomes stabilized and accumulates in the nucleus where it acts as a transcription factor (53). p53 induces several target genes that can cause a cell to arrest or to undergo apoptosis (16, 40). Our observations suggest that p53 is being stabilized in astrocytes where it may arrest the cells or induce apoptosis. The presence of p53 in the nuclei of neurons suggests that these cells are already postmitotic as expected. Since p53 can be stabilized by increased expression of p19ARF or phosphorylation by kinases like DNA protein kinase, we will characterize the expression of p19ARF and the phosphorylation of p53 in response to neurotrophins and chemokines.
Reactivation of cell cycle proteins has been implicated in other neurodegenerative diseases including Alzheimer’s disease and amyotrophic lateral sclerosis (3, 6, 8, 28, 37, 38, 43, 48–50, 58, 59). Increased p53 and pRb staining have been reported (36, 41, 42) as well as regulators of pRb activity, the cyclin-dependent kinases and their inhibitors (3, 6, 37, 43). Cyclin-dependent kinases, pRb, and E2F activity have been implicated in β-amyloid toxicity in an in vitro model of Alzheimer’s disease (18, 19). These data suggest that further experiments defining the role of cell cycle proteins in neuronal loss may have implications for other neurodegenerative diseases.
Because we used mixed neuroglial cultures we cannot rule out indirect effects mediated by paracrine and autocrine responses to our treatments. We are currently testing the effects of NTF and chemokines on cell cycle proteins in pure neuronal cultures compared to pure astrocytic cultures to address this issue. Future experiments will focus on the mechanisms employed by cell cycle proteins to cause neuronal death. The observation that cell cycle proteins are not only present in neurons and astrocytes, but responsive to neurotrophin and chemokine signaling, suggests that cell cycle regulators have functions beyond proliferation in these postmitotic cells. The extracellular environment of several neurodegenerative diseases including Alzheimer’s disease and HIV encephalitis is consistent with activation of cell cycle proteins shown here (10, 11, 24, 39, 52). We have recently reported a similar change in subcellular localization of E2F1 and ppRb in the simian model of HIV encephalitis further suggesting that our in vitro model is mimicking in vivo events (29). Determining how these proteins respond to such signaling in CNS cell types, what signaling pathways are involved, and whether they contribute to cell death will give us insight into the molecular mechanisms behind neurodegenerative disorders that involve inflammatory cells of the CNS.
ACKNOWLEDGMENTS
We thank our collaborators Dr. Simon Watkins of the Department of Cell Biology and Physiology and Dr. Mitchell Creinin at Magee-Women’s Hospital at the University of Pittsburgh Medical Center. We also thank Richard Whitehead and Sarah Brown for technical support.
Footnotes
This work was supported by Grants MH 58528 to C.L.A., NS 35731 to C.A.W., and NS10572 to K.L.J.S.
Abbreviations used: BDNF, brain derived neurotrophic factor; CNS, central nervous system; DMEM, Dulbecco’s modified essential medium; GFAP, glial fibrillary acidic protein; HIV, human immunodeficiency virus; HIVE, human immunodeficiency virus encephalitis; NGF, nerve growth factor; NTF, neurotrophic factor; NTFR, neurotrophic factor receptor; PBS, phosphate-buffered saline; pRb, retinoblastoma susceptibility gene product; TBS, Tris-buffered saline.
REFERENCES
- 1.Adams P, Kaelin WJ. Transcriptional control by E2F. Semin. Cancer Biol. 1995;6:99–108. doi: 10.1006/scbi.1995.0013. [DOI] [PubMed] [Google Scholar]
- 2.Aloyz R, Bamji S, Pozniak C, Toma J, Atwal J, Kaplan D, Miller F. p53 is essential for developmental neuron death as regulated by the TrkA and p75 neurotrophin receptors. J. Cell Biol. 1998;143:1691–1703. doi: 10.1083/jcb.143.6.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arendt T, Rodel L, Gartner U, Holzer M. Expression of the cyclin-dependent kinase inhibitor p16 in Alzheimer’s disease. NeuroReport. 1996;7:3047–3049. doi: 10.1097/00001756-199611250-00050. [DOI] [PubMed] [Google Scholar]
- 4.Asensio V, Campbell I. Chemokines in the CNS: Plurifunctional mediators of diverse states. Trends Neurosci. 1999;22:504–512. doi: 10.1016/s0166-2236(99)01453-8. [DOI] [PubMed] [Google Scholar]
- 5.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. In: Current Protocols in Molecular Biology. Janssen K, editor. Vol. 2. New York: Wiley; 1994. pp. 12.11.1–12.11.8. [Google Scholar]
- 6.Bajaj N, Al-Sarraj S, Leigh P, Anderson V, Miller C. Cyclin dependent kinase-5 (CDK5) phosphorylates neurofilament heavy (NF-H) chain to generate epitopes for anitbodies that label neurofilament accumulations in amyotrophic lateral sclerosis (ALS) Prog. Neuro-Psychopharmacol. Biol. Psychiat. 1999;23:833–850. doi: 10.1016/s0278-5846(99)00044-5. [DOI] [PubMed] [Google Scholar]
- 7.Banasiak K, Haddad G. Hypoxia-induced apoptosis: effect of hypoxic severity and role of p53 in neuronal death. Brain Res. 1998;797:295–304. doi: 10.1016/s0006-8993(98)00286-8. [DOI] [PubMed] [Google Scholar]
- 8.Busser J, Gelmacher D, Herrup K. Ectopic cell cycle proteins predict the sites of neuronal cell death in Alzheimer’s disease brain. J. Neurosci. 1998;18:2801–2807. doi: 10.1523/JNEUROSCI.18-08-02801.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter B, Lewin G. Neurotrophins live or let die: Does p75NTR decide? Neuron. 1997;18:187–190. doi: 10.1016/s0896-6273(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 10.Cinque P, Vago L, Mengozzi M, Torri V, Ceresa D, Vicenzi E, Transidico P, Vagani A, Sozzani S, Mantovani A, Lazzarin A, Poli G. Elevated cerebrospinal fluid levels of monocyte chemotactic protein-1 correlate with HIV-1 encephalitis and local viral replication. AIDS. 1998;12:1327–1332. doi: 10.1097/00002030-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Connor B, Dragunow M. The role of neuronal growth factors in neurodegenerative disorders of the human brain. Brain Res. Rev. 1998;27:1–39. doi: 10.1016/s0165-0173(98)00004-6. [DOI] [PubMed] [Google Scholar]
- 12.Coulier F, Kumar RME, Klein R, Martin-Zanaca D, Barbacid M. Human trk oncogenes activated by point mutation, in frame deletion and duplication of the tyrosine kinase domain. Mol. Cell. Biol. 1990;10:4202–4210. doi: 10.1128/mcb.10.8.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeGregori J, Leone G, Miron A, Jakoi L, Nevins J. Distinct roles for E2F proteins in cell growth control and apoptosis. Proc. Natl. Adad. Sci. USA. 1997;94:7245–7250. doi: 10.1073/pnas.94.14.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duckett C, Thompson C. CD-30-dependent degradation of TRAF-2: implications for negative regulation of TRAF signaling and the control of cell survival. Genes Dev. 1997;11:2810–2821. doi: 10.1101/gad.11.21.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eizenberg O, Faber-Elman A, Gottlieb E, Oren M, Rotter V, Schwartz M. p53 plays a regulatory role in differentiation an apoptosis of central nervous system-associated cells. Mol. Cell. Biol. 1996;16:5178–5185. doi: 10.1128/mcb.16.9.5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Deiry W, Tokino T, Velculescu V, Levy D, Parsons R, Trent J, Lin D, Mercer W, Kinzler K, Bogelstein B. Waf1 a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 17.Gelbard HA, Nottet HSLM, Swindells S, Jett M, Dzenko KA, Genis P, White R, Wang L, Choi Y-B, Zhang D, Lipton SA, Tourtellotte WW, Epstein LG, Gendelman HE. Platelet activating factor: A candidate HIV-1-induced neurotoxin. J. Virol. 1994;68:4628–4635. doi: 10.1128/jvi.68.7.4628-4635.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giovanni A, Keramaris E, Morris E, Hou S, O’Hare M, Dyson N, Robertson G, Slack R, Park D. E2F1 mediates death of B-amyloid-treated cortical neurons in a manner independent of p53 and dependent on Bax and caspase 3. J. Biol. Chem. 2000;275:11553–11560. doi: 10.1074/jbc.275.16.11553. [DOI] [PubMed] [Google Scholar]
- 19.Giovanni A, Wirtz-Bruger F, Keramaris E, Slack R, Park D. Involvement of cell cycle elements, cyclin-dependent kinases, pRb, and E2F-DP, in B-amyloid-induced neuronal death. J. Biol. Chem. 1999;274:19011–19016. doi: 10.1074/jbc.274.27.19011. [DOI] [PubMed] [Google Scholar]
- 20.Giulian D, Yu JH, Li X, Tom D, Li J, Wendt E, Lin SN, Schwarcz R, Noonan C. Study of receptormediated neurotoxins released by HIV-1-infected mononuclear phagocytes found in human brain. J. Neurosci. 1996;16:3139–3153. doi: 10.1523/JNEUROSCI.16-10-03139.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heyes PM, Saito K, Lackner A, Wiley CA, Achim CL, Markey SP. Sources of the neurotoxin quinolinic acid in the brain of HIV-1 infected patients and retrovirus-infected macaques. FASEB J. 1998;12:881–896. doi: 10.1096/fasebj.12.10.881. [DOI] [PubMed] [Google Scholar]
- 22.Ip N, Yancopoulos G. Neurotrophic factor receptors: Just like other growth factor and cytokine receptors? Curr. Opin. Neurobiol. 1994;4:400–405. doi: 10.1016/0959-4388(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 23.Ip N, Yancopoulos G. The neurotrophins and CNTF: Two families of collaborative neurotrophic factors. Annu. Rev. Neurosci. 1996;19:491–515. doi: 10.1146/annurev.ne.19.030196.002423. [DOI] [PubMed] [Google Scholar]
- 24.Janabi N, Di Stefano M, Wallon C, Hery C, Chiodi F, Tardieu M. Induction of human immunodeficiency virus type 1 replication in human neuroglial cells after proinflammatory cytokines stimulation: Effect of IFN gamma, IL1 beta, and TNF alpha on differentiation and chemokine production in glial cells. Glia. 1998;23:304–315. [PubMed] [Google Scholar]
- 25.Johnson M, Kinoshita Y, Xiang H, Ghatan S, Morrison R. Contribution of p53-dependent caspase activation ot neuronal cell death declines with neuronal maturation. J. Neurosci. 1999;19:2996–3006. doi: 10.1523/JNEUROSCI.19-08-02996.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson M, Xiang H, London S, Kinoshita Y, Knudson M, Mayberg M, Korsmeyer S, Morrison R. Evidence for involvement of Bax and p53, but not caspases, in radiationinduced cell death of cultured postnatal hippocampal neurons. J. Neurosci. Res. 1998;54:721–733. doi: 10.1002/(SICI)1097-4547(19981215)54:6<721::AID-JNR1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 27.Jordan J, Galindo M, Prehn J, Weichselbaum R, Beckett M, Ghadge G, Roos R, Leiden J, Miller R. p53 expression induces apoptosis in hippocampal pyramidal neuron cultures. J. Neurosci. 1997;17:1397–1405. doi: 10.1523/JNEUROSCI.17-04-01397.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jordan-Sciutto K, Bowser R. Alzheimer’s disease and brain development: Common molecular pathways. Front. Biosci. 1998;3:100–112. doi: 10.2741/a267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jordan-Sciutto K, Wang G, Murphy-Corb M, Wiley C. Induction of cell cycle regulators in simian immunodeficiency virus encephalitis. Am. J. Pathol. 2000;157:497–507. doi: 10.1016/S0002-9440(10)64561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khursigara G, Orlinick J, Chao M. Association of the p75 neurotrophin receptor with TRAF6. J. Biol. Chem. 1999;274:2579–2600. doi: 10.1074/jbc.274.5.2597. [DOI] [PubMed] [Google Scholar]
- 31.Kitamura Y, Shimohama S, Kamoshima W, Matsuoka Y, Nomura Y, Taniguchi T. Changes in p53 in the brains of patients with Alzheimer’s disease. Biochem. Biophys. Res. Commun. 1997;232:418–421. doi: 10.1006/bbrc.1997.6301. [DOI] [PubMed] [Google Scholar]
- 32.Klein R, Jing S, Nanduri B, O’Rourke E, Barbacid M. The trk proto-oncogene encodes the receptor for nerve growth factor. Cell. 1991;65:189–197. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- 33.Kouzarides T. Transcriptional control by the retinoblastoma protein. Semin. Cancer Biol. 1995;6:91–98. doi: 10.1006/scbi.1995.0012. [DOI] [PubMed] [Google Scholar]
- 34.Lipton SA, Sucher NJ, Kaiser PK, Dreyer EB. Synergistic effects of HIV coat protein and NMDA receptor-mediated neurotoxicity. Neuron. 1991;7:111–118. doi: 10.1016/0896-6273(91)90079-f. [DOI] [PubMed] [Google Scholar]
- 35.Lo TM, Fallert CJ, Piser TM, Thayer SA. HIV-1 envelope protein evokes intracellular calcium oscillations in rat hippocampal neurons. Brain Res. 1992;594:189–196. doi: 10.1016/0006-8993(92)91125-x. [DOI] [PubMed] [Google Scholar]
- 36.Masliah E, Mallory M, Alford M, Hansen L, Saitoh T. Immunoreactivity of the nuclear antigen p105 is associated with plaques and tangles in Alzheimer’s disease. Lab. Invest. 1993;69:562–569. [PubMed] [Google Scholar]
- 37.McShea A, Harris P, Webster K, Wahl A, Smith M. Abnormal expression of the cell cycle regulators p16 and cdk4 in Alzheimer’s disease. Am. J. Pathol. 1997;150:1933–1939. [PMC free article] [PubMed] [Google Scholar]
- 38.McShea A, Wahl A, Smith M. Reentry into the cell cycle: A mechanism for neurodegeneration in Alzheimer’s disease. Med. Hypoth. 1999;52:525–527. doi: 10.1054/mehy.1997.0680. [DOI] [PubMed] [Google Scholar]
- 39.Mengozzi M, De Filippi J, Transidico P, Biswas PMC, Ghezzi S, Vecenzi E, Mantovani A, Sozzani S, Poli G. Human immunodeficiency virus replication induces monocyte chemotactic protein-1 in human macrophages and U937 promonocytic cells. Blood. 1999;93:1851–1857. [PubMed] [Google Scholar]
- 40.Miyashita T, Reed J. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 41.Nagy Z, Esiri M, Cato AM, Smith AD. Cell cycle markers in the hippocampus in Alzheimer’s disease. Acta Neuropathol. 1997;94:6–15. doi: 10.1007/s004010050665. [DOI] [PubMed] [Google Scholar]
- 42.Nagy Z, Esiri M, Smith A. Expression of cell division markers in the hippocampus in Alzheimer’s disease and other neurodegenerative conditions. Acta Neuropathol. 1997;93:294–300. doi: 10.1007/s004010050617. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura S, Kawamoto Y, Nakano S, Ikemoto A, Akiguchi I, Kimura J. Cyclin-dependent kinase 5 in Lewy body-like inclusions in anterior horn cells of patients with sporadic amyotrophic lateral sclerosis. Neurology. 1997;48:267–270. doi: 10.1212/wnl.48.1.267. [DOI] [PubMed] [Google Scholar]
- 44.Nevins J. Towards an understanding of the functional complexity of the E2F and retinoblastoma families. Cell Growth Differ. 1998;9:585–593. [PubMed] [Google Scholar]
- 45.Phillips A, Ernst M, Bates S, Rice N, Vousden K. E2F-1 potentiates cell death by blocking antiapoptotic signaling pathways. Mol. Cell. 1999;4:771–781. doi: 10.1016/s1097-2765(00)80387-1. [DOI] [PubMed] [Google Scholar]
- 46.Pulliam L, Clarke JA, McGrath MS, Moore D, McGuire D. Monokine products as predictors of AIDS dementia. AIDS. 1996;10:1495–1500. doi: 10.1097/00002030-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Pulliam L, Clarke JA, McGuire D, McGrath MS. Investigation of HIV-infected macrophage neurotoxin production from patients with AIDS dementia. Adv. Neuroimmunol. 1994;4:195–198. doi: 10.1016/s0960-5428(06)80257-3. [DOI] [PubMed] [Google Scholar]
- 48.Raina A, Monteiro M, McShea A, Smith M. The role of cell cycle-mediated events in Alzheimer’s disease. Int. J. Exp. Pathol. 1999;80:71–76. doi: 10.1046/j.1365-2613.1999.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ranganathan S, Scudiere S, Bowser R. Hyperphosphorylation of the retinoblastoma gene product and altered subcellular distribution of E2F1 in Alzheimer’s disease and amyotrophic lateral sclerosis. J. Alzheimer’s Dis. in press doi: 10.3233/jad-2001-3403. [DOI] [PubMed] [Google Scholar]
- 50.Ross M. Cell division and the nervous system: Regulating the cycle from neural differentiation to death. Trends Neurosci. 1996;19:62–68. doi: 10.1016/0166-2236(96)89622-6. [DOI] [PubMed] [Google Scholar]
- 51.Sakhi S, Bruce A, Sun N, Tocco G, Baudry M, Schreiber S. p53 induction is associated with neuronal damage in the central nervous system. Proc. Natl. Acad. Sci. USA. 1994;90:7525–7529. doi: 10.1073/pnas.91.16.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanders V, Pittman C, White M, Wang G, Wiley C, Achim C. Chemokines and receptors in HIV encephalitis. AIDS. 1998;12:1021–1026. [PubMed] [Google Scholar]
- 53.Scherr C. Tumor surveillance via the ARF-p53 pathway. Genes Dev. 1998;12:2984–2991. doi: 10.1101/gad.12.19.2984. [DOI] [PubMed] [Google Scholar]
- 54.Soontornniyomkij V, Wang G, Kapadia S, Achim C, Wiley C. Confocal assessment of lymphoid tissues with follicular hyperplasia from patients infected with human immunodeficiency virus type 1. Arch. Pathol. Lab. Med. 1998;122:534–538. [PubMed] [Google Scholar]
- 55.Soontornniyomkij V, Wang G, Pittman C, Wiley C, Achim C. Expression of brain-derived neurotrophic factor protein in activated microglia of human immunodeficiency virus type1 encephalitis. Neuropathol. Appl. Neurobiol. 1998;24:453–460. doi: 10.1046/j.1365-2990.1998.00134.x. [DOI] [PubMed] [Google Scholar]
- 56.Stefanis L, Park D, Friedman W, Greene L. Caspased-ependent and -independent death of camptothecintreated embryonic cortical neurons. J. Neurosci. 1999;19:6235–6247. doi: 10.1523/JNEUROSCI.19-15-06235.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsai K, Hu Y, Macleod K, Crowley D, Yamasaki L, Jacks T. Mutation of E2F1 suppresses apoptosis and inappropriate S phase entry and extends survival of Rb-eficient mouse embryos. Mol. Cell. 1998;2:293–304. doi: 10.1016/s1097-2765(00)80274-9. [DOI] [PubMed] [Google Scholar]
- 58.Vincent I, Rosado M, Davies P. Mitotic mechanisms in Alzheimer’s disease? J. Cell Biol. 1996;132:413–425. doi: 10.1083/jcb.132.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vincent I, Zheng J, Dickson D, Kress Y, Davies P. Mitotic phosphoepitopes precede paired helical filaments in Alzheimer’s disease. Neurobiol. Aging. 1998;19:287–296. doi: 10.1016/s0197-4580(98)00071-2. [DOI] [PubMed] [Google Scholar]
- 60.Weinberg R. The Retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 61.White M, Hammond R, Sanders V, Bonaroti E, Mehta A, Wang G, Wiley C, Achim C. Neuron-enriched second trimester human cultures: Growth factor response and in vivo graft survival. Cell Transplant. 1999;8:59–73. doi: 10.1177/096368979900800115. [DOI] [PubMed] [Google Scholar]
- 62.Whyte P. The retinoblastoma protein and its relatives. Semin. Cancer Biol. 1996;6:83–90. doi: 10.1006/scbi.1995.0011. [DOI] [PubMed] [Google Scholar]