Abstract
The envelope (E) protein of dengue virus (DENV) is composed of three domains (EDI, EDII, EDIII) and is the main target of neutralizing antibodies. Many monoclonal antibodies that bind EDIII strongly neutralize DENV. However in vitro studies indicate that anti-EDIII antibodies contribute little to the neutralizing potency of human DENV-immune serum. In this study, we assess the role of anti-EDIII antibodies in mouse and human DENV-immune serum in neutralizing or enhancing DENV infection in mice. We demonstrate that EDIII-depleted human DENV-immune serum was protective against homologous DENV infection in vivo. Although EDIII-depleted DENV-immune mouse serum demonstrated decreased neutralization potency in vitro, reduced protection in some organs, and enhanced disease in vivo, administration of increased volumes of EDIII-depleted serum abrogated these effects. These data indicate that anti-EDIII antibodies contribute to protection and minimize enhancement when present, but can be replaced by neutralizing antibodies targeting other epitopes on the dengue virion.
Keywords: Dengue, Mouse model, Envelope domain III, Protection, Enhancement
Introduction
Placing up to half of the world's population at risk, dengue virus (DENV) is the most important emerging arboviral pathogen affecting humans (WHO, 1997). Transmitted by the Aedes aegypti and Aedes albopictus mosquitoes (Effler et al., 2005; Rodhain and Rosen, 1997), DENV is comprised of 4 serotypes, DENV1-4 (Burke and Monath, 2001). A large percentage of the estimated 100 million annual infections are clinically asymptomatic; symptomatic infections range from the self-limited but debilitating dengue fever (DF) to the potentially life-threatening dengue hemorrhagic fever and dengue shock syndrome (DHF/DSS) (Halstead, 2007; Rothman, 2010).
The DENV envelope (E) protein is the major antigenic target on the surface of the virion (Roehrig et al. 1998). Initial monoclonal antibody (MAb) mapping studies (reviewed in Roehrig et al. (1998)) identified the E protein to be composed of three distinct antigenic regions: A, B and C. Subsequent analysis of the crystal structure of DENV E glycoprotein revealed three domains – I, II and III – that were directly comparable with the previously-defined antigenic regions C, A and B, respectively (Modis et al., 2003; Rey et al., 1995). Studies with mouse monoclonal antibodies (MAbs) have determined that antibodies targeting Domains I/II (EDI/II) are generally more cross-reactive among serotypes and of low to moderate neutralizing potency (Crill and Chang, 2004; Oliphant et al., 2006). In contrast, mouse MAbs binding Domain III (EDIII) are serotype-specific and highly neutralizing (Crill and Roehrig, 2001; Gromowski and Barrett, 2007; Lin et al., 1994; Lok et al., 2008; Sukupolvi-Petty et al., 2007), although both cross-reactive and non-neutralizing MAbs binding to newly identified EDIII epitopes have been identified (Shrestha et al., 2010; Sukupolvi-Petty et al., 2010, 2007).
A person exposed to a primary DENV infection develops a polyclonal antibody response that neutralizes the homologous serotype responsible for infection, while leaving the subject susceptible to a second infection with a different serotype (Endy et al., 2004; Sabin, 1952). Pre-existing cross-reactive antibodies may enhance a second DENV infection and lead to more severe disease (Halstead, 2003). Investigators are now beginning to study the binding and functional properties of human antibodies and to compare the human response to the well-studied mouse response. Studies of human sera and MAbs indicate that multiple viral antigens, including E protein, pre-membrane (prM/M) protein and non-structural protein 1 (NS1), are recognized by human antibodies (Beltramello et al., 2010; Crill et al., 2009; de Alwis et al., 2011; Dejnirattisai et al., 2010; Lai et al., 2008; Schieffelin et al., 2010). Moreover, most DENV-specific human antibodies are cross-reactive and weakly neutralizing, and a minor population of antibody appears to be responsible for the ability of immune sera to strongly neutralize the homologous serotype (Beltramello et al., 2010; Crill et al., 2009; de Alwis et al., 2011; Dejnirattisai et al., 2010; Lai et al., 2008; Schieffelin et al., 2010). Based on studies with mouse MAbs, it had been assumed that human antibodies that potently neutralize DENV also bind to EDIII. However, recent data has suggested that EDIII-specific antibodies do not constitute a large percentage of the human anti-DENV antibody repertoire and do not contribute substantially to in vitro neutralization of DENV (Midgley et al., 2010; Oliphant et al., 2007; Wahala et al., 2009). Here we investigate the contribution of EDIII-specific antibodies in mouse and human DENV-immune serum to DENV protection and enhancement using a mouse model of DENV infection and disease.
Results
Human serum depleted of anti-EDIII antibodies reduces viral load in vivo
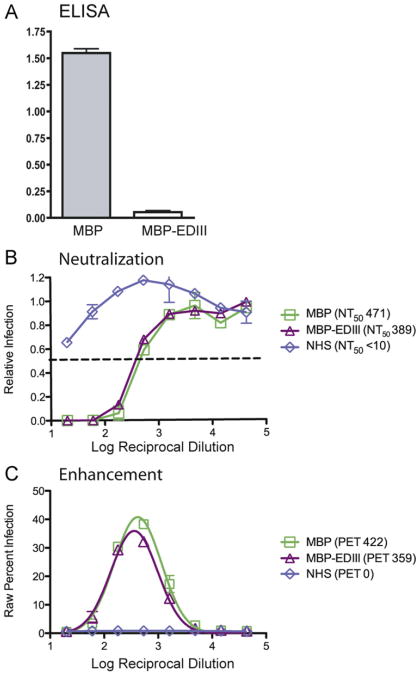
Previous studies have demonstrated that humans infected with DENV develop low levels of anti-EDIII antibodies and that these antibodies make only a minor (<15%) contribution to the neutralization potency of human immune serum in in vitro assays (Midgley et al., 2010; Wahala et al., 2009). Thus, we first asked whether anti-EDIII antibodies in human serum contribute to protection in vivo using a mouse model of DENV infection and disease we had previously developed (Balsitis et al., 2010; Kyle et al., 2008; Williams et al., 2009). We obtained a convalescent serum sample from a person exposed to a primary DENV2 infection and depleted the serum of anti-EDIII antibodies using a recombinant EDIII-maltose binding protein (MBP-EDIII) fusion protein or MBP alone as previously described (Wahala et al., 2009) (Fig. 1A). The neutralizing and enhancing abilities of the MBP-depleted and MBP-EDIII-depleted serum were assessed using U937 DC-SIGN and K562 flow-cytometry based assays, respectively. MBP-EDIII depletion led to a 17% reduction in homologous neutralization titer as compared to the MBP-depleted control (Fig. 1B). Both the MBP-depleted and MBP-EDIII-depleted samples demonstrated similar peak enhancement titers against DENV2 (422 and 359, respectively) (Fig. 1C). Taken together, these data support previously published work (Midgley et al., 2010; Wahala et al., 2009) and indicate that depletion of anti-EDIII antibodies from human serum does not drastically alter either the in vitro neutralization or enhancement profiles.
Fig. 1.
Human E Domain III (EDIII) antibodies constitute a small portion of the polyvalent serum neutralizing antibody response. Human DENV2-immune serum was obtained from a DENV-immune individual enrolled in the University of North Carolina dengue traveler study and was subsequently depleted of anti-EDIII antibodies using either MBP-EDIII (383D) or MBP-control beads. (A) ELISA plates were coated with DENV2 MBP-EDIII (383D) or MBP protein (negative control). Undiluted MBP-EDIII-383D- and MBP-depleted human sera were tested for reactivity against with DENV2 MBP-EDIII (383D), and the absolute OD value at 405 nm is shown on the y-axis. (B) Neutralization titer against the DENV2 D2S10 strain used for in vivo infections was measured by a neutralization assay using human U937 DC-SIGN cells. Relative infection is shown on the x-axis, log reciprocal dilution of the serum is shown on the y-axis, and a dashed line indicates 50% infection. (C) Enhancement against the same DENV2 D2S10 strain was measured using K562 cells, where raw percent infection is measured on the y-axis and log reciprocal serum dilution is on the x-axis. Each panel is representative of two or three separate experiments.
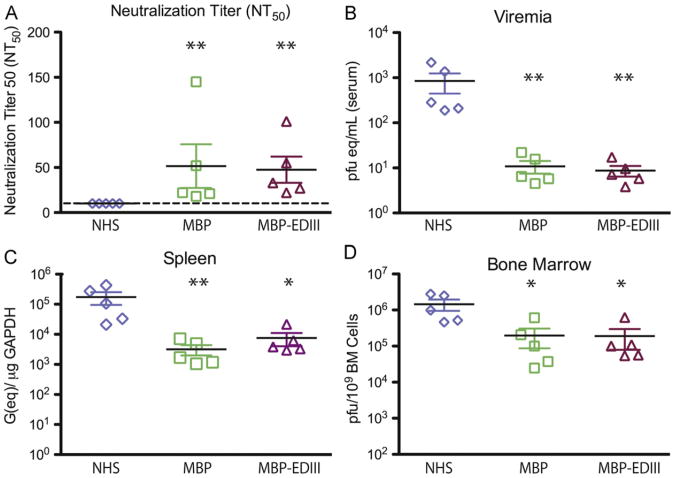
While depletion of anti-EDIII antibodies only decreased the in vitro neutralization titer of the human immune serum by 17%, it is conceivable these antibodies might be required for protection in vivo. To test this possibility, experiments were performed using a recently developed mouse model of DENV infection and disease (Balsitis et al., 2010; Kyle et al., 2008; Williams et al., 2009). Seventy microliters of MBP-depleted or MBP-EDIII-depleted serum was transferred into AG129 mice (n=5 per group) 24 h prior to an infection with 103 pfu of DENV2 D2S10, a dose at which morbidity is not observed but reduction in viremia and tissue viral load can be measured (Fig. 2A). Non-terminal bleeds were collected 4–6 h prior to infection to measure circulating anti-DENV antibody titers. As demonstrated in Fig. 2A, the average NT50 titer for MBP-depleted and MBP-EDIII-depleted groups were not significantly different from each other, but both were significantly higher than the normal human serum (NHS) recipient mice. Serum viremia and tissue viral load levels were subsequently measured 4 days post-infection by either plaque assay or quantitative RT-PCR. Mice receiving either MBP-depleted or MBP-EDIII-depleted serum displayed viremia levels that were at or slightly above the limit of detection for the assay (1 pfu(eq)/mL) and were significantly lower than the NHS-recipient group (Fig. 2C). Viral load levels were further measured in primary and secondary lymphoid organs; significant reductions in the spleen and bone marrow were observed in both experimental groups, as compared to the NHS control (Fig. 2D and E). Importantly, across all organs tested, there was no difference in serum viremia or tissue viral load between the MBP-depleted and MBP-EDIII- depleted groups. These data indicate that anti-EDIII antibodies in human serum were not required for protection observed in vivo.
Fig. 2.
Human anti-EDIII antibodies are not required for protection in vivo. AG129 mice (n=5 per group) were administered 70 μl of either human MBP-depleted (“MBP”) or MBP-EDIII-depleted (“MBP-EDIII”) DENV2 immune serum or non-immune human serum (“NHS”) 24 h prior to infection with 103 pfu of D2S10 iv. (A) Retro-orbital eye-bleeds were taken 4–6 h prior to DENV infection, and circulating neutralizing antibodies were measured against the infecting virus. The dashed line indicates the limit of detection for the assay. (B–D) Animals were sacrificed 4 days post-infection, and viral load was measured in the serum (B), spleen (C), and bone marrow (D). The data points presented here are combined from two separate experiments. In each graph B–D, the x-axis is the limit of detection. *p-value <0.05 as compared to NHS; **p-value <0.01 as compared to NHS.
Mice infected with DENV develop anti-EDIII antibodies that contribute to neutralization but not enhancement in vitro
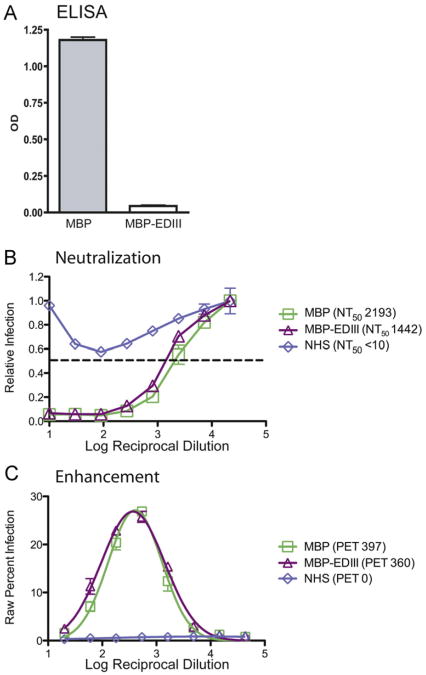
Previous data have suggested that most mouse MAbs that strongly neutralize DENV bind to EDIII (Sukupolvi-Petty et al., 2007). Thus, we next examined whether mice exposed to DENV infection develop a polyclonal neutralizing antibody response that is mainly directed against epitopes on EDIII. We depleted pooled anti-DENV2 mouse serum with MBP-EDIII protein using the same approach described for human immune sera (Wahala et al., 2009). We confirmed depletion of anti-EDIII antibodies using a MBP-EDIII-specific ELISA and demonstrated that there was negligible measurable binding to the MBP-EDIII protein in depleted serum (Fig. 3A).
Fig. 3.
Mouse anti-EDIII antibodies contribute to serotype-specific neutralization but not enhancement in vitro. Pooled anti-DENV2 PL046 serum was collected 6–8 weeks following intra-peritoneal infection of AG129 mice and was depleted of anti-EDIII antibodies using MBP-EDIII (383E) or control MBP protein. (A) ELISA plates were coated with DENV2 MBP-EDIII (383E) and MBP protein (negative control). Undiluted MBP-EDIII- and MBP-depleted mouse sera were tested for reactivity with DENV2 MBP-EDIII (383E), and absolute OD value at 405 nm is shown on the y-axis. (B) Neutralization titer against the DENV2 D2S10 strain used for in vivo infections was measured by neutralization assay in U937 DC-SIGN cells. Relative infection is shown on the x-axis, the log reciprocal dilution of the serum is shown on the y-axis, and a dashed line indicates 50% infection. (C) Enhancement against DENV2 D2S10 was measured in K562 cells, where raw percent infection is shown on the y-axis and log reciprocal serum dilution is shown on the x-axis. Each panel is representative of two or three separate experiments.
Having removed the EDIII-specific antibodies from the anti-DENV2 serum, we measured the contribution of anti-EDIII antibodies to homologous neutralization and enhancement in vitro. A 34% reduction in homologous neutralization titer was observed comparing MBP-EDIII-depleted serum to MBP-depleted serum (Fig. 3B). The MBP-depleted and MBP-EDIII-depleted serum demonstrated similar peak enhancement titers (397 and 360, respectively) against DENV2 (Fig. 2C). Our data indicate that mice make EDIII-specific antibodies that contribute more significantly to in vitro neutralization than do human anti-EDIII antibodies. Nonetheless, more than half the neutralizing antibodies in mice are not directed against EDIII.
EDIII-depleted anti-DENV2 mouse serum can protect against DENV2 infection
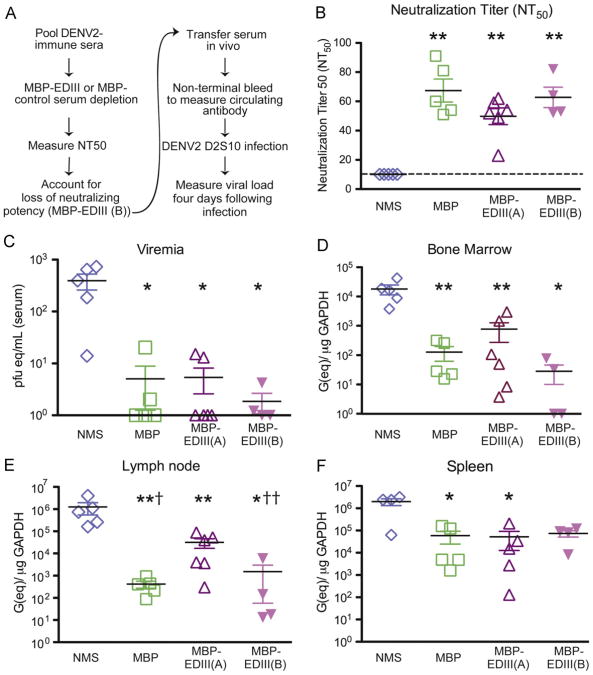
As our in vitro analysis of the MBP-EDIII-depleted serum indicated that EDIII-specific mouse antibodies contributed 34% to the overall neutralizing potency of DENV2-immune serum, we next asked whether MBP-EDIII-depleted serum would be as protective in vivo as control-depleted serum. Similarly to the experiments described using human immune serum, anti-DENV immune mouse serum was transferred 24 h prior to a sublethal 103 pfu infection, and non-terminal bleeds were taken 4–6 h prior to infection to measure the neutralizing titer of the circulating anti-DENV antibodies present at the time of infection (Fig. 4A). We first transferred equivalent volumes of MBP-depleted (MBP) and MBP-EDIII-depleted (MBP-EDIII-A) serum (n=4–6 per group) such that the average neutralization titer of the transferred serum in vivo was 67.4 and 47, respectively (Fig. 4B) and significantly higher than NMS-recipient mice (p < 0.01 for both comparisons). As the difference between the MBP-depleted and MBP-EDIII-depleted serum was 34% in vitro, we expected to see a comparable difference following transfer in vivo; indeed, comparing the average NT50 titers between the MBP (67.4) and MBP-EDIII-A (47) groups yielded a difference of 30%. Serum viremia levels in mice administered either MBP or MBP-EDIII-A serum were significantly reduced as compared to NMS-recipient mice (p=0.015, MBP and p=0.014, MBP-EDIII-A) (Fig. 4C); however, the viremia levels were not significantly different from each other. In the primary and secondary lymphoid organs, the mice administered either MBP or MBP-EDIII-A immune serum showed significant reductions in viral load as compared to the NMS-recipient groups (Fig. 4D–F). In the lymph node, the animals receiving MBP-EDIII-A depleted serum had statistically elevated titers as compared to the MBP group (p=0.0285) while both groups demonstrated reduced viral loads as compared to NMS-recipient mice (Fig. 4E).
Fig. 4.
Murine EDIII-specific antibodies contribute slightly to protection in vivo. (A) Schematic of the anti-DENV2 MBP-EDIII-depletion and in vivo experimental design. DENV2-immune serum was generated 6 weeks post-infection and serum pooled prior to depletion. AG129 mice (n=3–5/group) were administered either 250 μl of MBP-depleted serum (MBP), 250 μl (MBP-EDIII (A)) or 400 μl (MBP-EDIII (B)) of EDIII-depleted serum, or 400 μl non-immune mouse serum (NMS) 24 h prior to infection with 103 pfu of D2S10 iv. (B) Sub-mandibular bleeds were taken 4–6 h prior to infection, and circulating anti-DENV neutralizing antibodies were measured against the infecting virus. The dashed line indicates the limit of detection for the assay. (C–E) Animals were sacrificed 4 days post-infection, and viral load was measured in the serum (C), bone marrow (D), lymph node (E), and spleen (F). The data points presented are combined from two separate experiments except for MBP-EDIII (B) which could only be performed once due to limited volumes of serum available. In each graph C–F, the x-axis indicates the limit of detection. *p-value <0.05 as compared to NMS; **p-value <0.01 as compared to NMS; †p-value <0.05 as compared to MBP-EDIII (A); ††p-value=0.055 as compared to MBP–EDIII (A).
We next assessed whether MBP-EDIII-depleted serum transferred at equivalent neutralizing titer (MBP-EDIII-B) as MBP-depleted serum (MBP) would be more protective than MBP-EDIII-depleted serum of lower in vivo neutralizing titer (MBP-EDIII-A). From the in vitro neutralization titers, we determined that in order to transfer MBP-EDIII-depleted serum of equivalent neutralizing capacity, we had to increase the volume of MBP-EDIII-depleted serum administered prior to D2S10 infection by approximately one third. Thus, we transferred 400 μL of MBP-EDIII-depleted (MBP-EDIII-B) serum such that the average NT50 titer of the circulating antibodies was 63 (Fig. 4B) and comparable to animals receiving MBP-depleted serum (MBP, average NT50=67). Animals receiving MBP-EDIII-B serum had significantly reduced viral loads as compared to NMS-recipient mice, and a reduction in viral load was observed in the lymph node (p=0.055; Fig. 4E) and bone marrow (p=0.13, Fig. 4F) as compared to MBP-EDIII-A recipient mice. There was no difference in viral load comparing the MBP-EDIII-B to the MBP recipient mice. These data demonstrate that even in the absence of anti-EDIII antibodies, homologous serum is still potently neutralizing and capable of serotype-specific reduction in viral load. Taken together, we conclude that anti-EDIII antibodies, when present, contribute to neutralization in vitro and protection in vivo, but are not required.
Neutralization titer, rather than antibody repertoire of DENV-immune serum, is a better in vitro correlate of enhancement in vivo
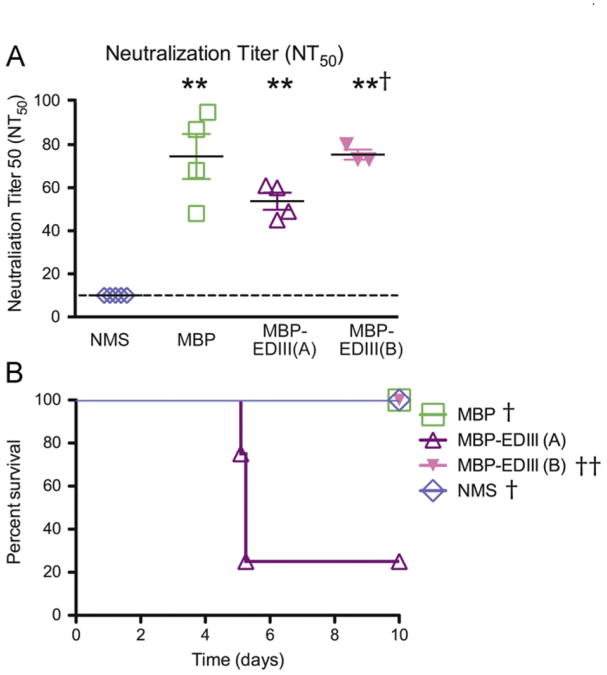
Infection with one serotype of DENV is expected to confer life-long immunity against the same serotype (Innis, 1997; Sabin, 1952). In contrast, a second infection with a different serotype is the greatest risk factor associated with the development of severe disease (Halstead, 2003). As the anti-EDIII antibodies do not appear to contribute substantially to in vivo protection, we next hypothesized that they may be a key component in preventing homologous enhancement. To test this hypothesis, AG129 mice (n=3–5 per group) were administered equivalent volumes of either MBP-depleted or MBP-EDIII-depleted serum or NMS. Pre-infection bleeds were taken 4–6 h prior to infection to measure the circulating antibody titers by neutralization assay (NT50 titer). As in the protection experiment, the average NT50 titer of the MBP-EDIII-A group (54) was approximately one-third (28%) lower than that of the MBP group (74.5). A fourth group of mice received MBP-EDIII-depleted serum of equivalent neutralizing potency (MBP-EDIII-B, average NT50=75) as the MBP group. The control group of mice receiving NMS did not have a measurable NT50 titer (Fig. 5A, Table 1).
Fig. 5.
Anti-EDIII antibodies prevent enhancement in vivo. AG129 mice (n=3–5/group) were administered 250 μl of MBP-depleted serum (MBP) or 250 μl (MBP-EDIII (A)) or 400 μl (MBP-EDIII (B)) of EDIII-depleted serum, or 400 μl of non-immune mouse serum (NMS) prior to a sublethal infection with 104 pfu of DENV2 D2S10 iv. See schematic in Fig. 4A for details. (A) Twenty-four hours prior to infection, sub-mandibular bleeds were taken and the serum was used to measure neutralization titer in U937 DC-SIGN cells. The dashed line indicates the limit of detection for the assay. (B) Animals were followed for morbidity and mortality for 10 days following infection. Survival outcome is depicted in a Kaplan–Meier survival curve. The data points presented are combined from two separate experiments, except for the MBP–EDIII (A) and MBP-EDIII (B) conditions, which could only be tested once due to limited volumes of serum available. *p-value <0.05 as compared to NMS; **p-value <0.01 as compared to NMS; †p-value <0.05 as compared to MBP-EDIII (A); ††p-value=0.075 as compared to MBP-EDIII (A).
Table 1.
| Neutralizing Titer (NT50 Mean ± sd) | Morbidity | Mortality | Mortality p-value* | |
|---|---|---|---|---|
| NMS | 10±0 | 0/5 | 0/5 | 0.025 |
| MBP | 74.5±21 | 1/4 | 0/4 | 0.043 |
| EDIII-A | 53.8±8 | 3/4 | 3/4 | – |
| EDIII-B | 75±4 | 0/3 | 0/3 | 0.075 |
p-value calculated in comparison to EDIII-A.
Mice were infected 24 h following serum transfer with 104 pfu DENV2 D2S10, a sublethal dose that can be lethal under enhancing antibody conditions, and were followed for morbidity and mortality. Only 1/4 of the animals receiving the MBP-depleted serum developed transient signs of disease (0.5 day in duration). In contrast, 3/4 of the animals receiving the same volume of MBP-EDIII-depleted serum (MBP-EDIII-A) all developed severe signs of disease and died by Day 5 (p=0.043 as compared to MBP-depleted serum control) (Fig. 5B, Table 1). These data validate previously published results identifying a reciprocal relationship between neutralization titer and risk of homologous enhancement in vivo (Balsitis et al., 2010).
To determine whether an increase in neutralization titer of the MBP-EDIII-depleted serum would be sufficient to abrogate this enhancement response, we transferred sufficient volumes of MBP-EDIII-depleted serum such that the average NT50 titer of the transferred serum in vivo was 75 (MBP-EDIII-B) and not significantly different from the MBP-depleted serum recipient mice (MBP) (Fig. 5A, Table 1). Following a 104 sub-lethal D2S10 infection, none of the three animals receiving the increased volume of MBP-EDIII-depleted serum demonstrated signs or symptoms of illness (p=0.075 as compared to the MBP-EDIII-A group) (Fig. 5B, Table 1). These data support a relationship between decreased neutralization titer and increased risk of enhancement and further confirm that the risk of enhancement is minimized upon transfer of either control-depleted or MBP-EDIII-depleted serum of equivalent neutralizing titer. Further, as the increased volume of MBP-EDIII-B serum was determined based on the neutralization titer measured in vitro prior to transfer in vivo, these data further validate the relationship between in vitro neutralization titer and homologous enhancement in vivo.
Discussion
Many mouse MAbs that strongly neutralize DENV and other flaviviruses bind to well-defined epitopes on Domain III of the viral envelope protein. The objective of this study was to determine whether anti-EDIII antibodies were responsible for potent neutralizing activity in immune sera from mice and humans exposed to DENV infection. Thus, the role of anti-EDIII antibodies in mouse and human immune sera were studied using in vitro cell culture models and an in vivo mouse model of DENV infection and disease.
Our initial observations studying human serum confirm previously published results that anti-EDIII antibodies constitute between 5% and 15% of the serotype-specific neutralizing titer measured in vitro (Midgley et al., 2010; Wahala et al., 2009). As suggested by the in vitro neutralization studies, anti-EDIII-depleted human serum was as protective as control-depleted serum when tested in vivo. These data additionally validate the ability of human immune serum to mediate robust protection against DENV infection in a mouse model. To further investigate the role of anti-EDIII antibodies in modulating protection in vivo, we turned to anti-DENV-immune mouse serum, where we found that anti-EDIII antibodies contributed ∼34% to in vitro serotype-specific neutralization. Following in vivo transfer, the difference in average neutralizing potency between the control-depleted and MBP-EDIII-depleted (MBP-EDIII-A) groups (measured ex vivo) was 30%, thus mirroring the difference in serum NT50 titer measured in vitro prior to transfer. While we observed a significant increase in replicating virus in the lymph node in animals receiving MBP-EDIII-depleted (MBP-EDIII-A) as compared to control-depleted serum, there was little to no difference in viral burden between the two groups in the serum or spleen.
The organ-specific differences observed while measuring protection may be explained in that initial interactions between antibody and virus would first occur in the serum and quickly thereafter in the spleen, which acts as a direct filter for the blood. However, trafficking of antibody to and DENV infection of the lymph node and bone marrow is more complicated. Different trafficking routes required for both the antibody (transferred i.p.) and virus (infected i.v.) to cross the blood-tissue barrier, as well as the potential requirement for active migration of antigen-presenting cells containing infectious virus may jointly contribute to the differences observed in the lymph node and bone marrow (Alvarez et al., 2008; von Andrian and Mempel, 2003). For instance, studies of the infection kinetics of DENV2 D2S10 demonstrate that replicating virus appears 24 h earlier in the spleen (day 2) than lymph node (day 3) (Kyle et al., 2008) these organ-specific infection kinetics may further explain the differences in protection observed between the MBP- and MBP-EDIII-A-depleted experimental groups. Robust protection in all organs was re-established when MBP-EDIII-depleted (MBP-EDIII-B) serum was transferred at an increased volume (400 μl) to compensate for the marginal loss in neutralizing titer, indicating that the differences observed between viral titers in lymph node and spleen are due to differences in the neutralizing potency, rather than EDIII-binding specificity, of the polyvalent serum. Taken together, these data indicate that mouse anti-EDIII antibodies are responsible for approximately one third of in vitro serum neutralizing potency, but the reduction in neutralization potency resulting from depletion of anti-EDIII antibodies confers only a small loss of protection in vivo. Furthermore, complete protection can be re-established following transfer of an increased volume of additional MBP-EDIII-depleted serum, indicating that anti-EDIII antibodies perse are not required for homologous protection in vivo.
These results indicate that while the neutralizing antibody response may be more skewed towards EDIII-specific antibodies in mice than in humans, the anti-EDIII antibodies are not critical for in vivo protection. This finding is intriguing, as MAb studies have frequently isolated strongly neutralizing, EDIII-specific antibodies from mice (Lin et al., 1994; Roehrig et al., 1998; Shrestha et al., 2010; Sukupolvi-Petty et al., 2010), whereas such MAbs are present but rare in humans (Beltramello et al., 2010; Crill et al., 2009; de Alwis et al., 2011; Dejnirattisai et al., 2010; Lai et al., 2008; Schieffelin et al., 2010). These observations may be explained in that the strongly neutralizing anti-EDIII murine antibodies often resulted from a multi-tiered vaccination strategy using recombinant E protein or EDIII alone as the primary immunogen or as a final boost (Shrestha et al., 2010; Sukupolvi-Petty et al., 2010). In contrast, our DENV-immune mouse serum was generated following only one infection (i.p. route) with live DENV, similar to a natural primary DENV infection in humans. Further, the serum was collected between 6 and 8 weeks postinfection, consistent with a time frame when the anti-EDIII antibody response should be fully developed (Oliphant et al., 2007). In agreement with observations that both murine and human antibodies can bind outside EDIII and potently neutralize DENV and other flaviviruses (Gromowski et al., 2008; Roehrig et al., 1998; Sukupolvi-Petty et al., 2010), our data supports a model in which non-EDIII antibodies are sufficient to mediate robust protection in the absence of anti-EDIII antibodies.
Although only small changes in protection were observed when equivalent volumes of intact versus MBP-EDIII-depleted serum were transferred in vivo, clear phenotypic differences in in vivo outcome were observed when the animals were challenged with an enhancing dose of DENV2. Similar to the protection experiment, a ∼30% reduction was observed when comparing the average NT50 titer between the MBP- and MBP-EDIII-depleted (MBP-EDIII-A) groups; however, this difference was not statistically significant. Nonetheless, 75% of animals receiving MBP-EDIII-depleted (MBP-EDIII-A) serum succumbed, while none of the mice receiving MBP-depleted control serum died. These data suggest that both antibody composition and neutralizing potency of transferred serum may be important factors in preventing enhancement of homologous infection. However, increasing the NT50 titer of the MBP-EDIII-depleted (MBP-EDIII-B) serum to an average titer identical to control, MBP-depleted serum eliminated enhancement in vivo, implying that neutralizing potency, rather than antibody composition of the transferred serum, was the more important correlate of enhancement in vivo. These data support previous observations that anti-DENV2 mouse serum enhanced a DENV2 D2S10 infection in the AG129 mouse model once diluted below a pivotal NT50 threshold (Balsitis et al., 2010). Further, observations in human maternal—infant clinical studies have similarly suggested a relationship between reduced neutralization titers against the infecting serotype and development of severe disease (Chau et al., 2009; Kliks et al., 1988; Libraty et al., 2009).
Given the ability of anti-EDIII antibodies to contribute robustly to homologous protection with minimal cross-reactive binding, recombinant EDIII protein has been proposed as a vaccine candidate (Block et al., 2010; Valdes et al., 2009, 2011). In initial studies using a novel capsid-EDIII recombinant fusion protein, Valdes et al. (2009) demonstrated a measurable humoral and cellular immune response; when the protein was used to boost a homologous infection, the antibody response was both potent and long-lasting (Valdes et al., 2011). In a separate set of experiments, Block et al. (2010) tested a similar hypothesis using an insect cell-derived, recombinant EDIII-based protein vaccine and found that vaccination of mice with tetravalent EDIII protein produced antibodies that exhibited both neutralizing and enhancing activity. However, the enhancing activity of the antibodies generated following EDIII vaccination spanned a much smaller range of serum dilutions than comparable polyvalent mouse serum generated from a DENV infection (Block et al., 2010).
One potential limitation of this study is the passive transfer of human immune serum into mice. While the interactions between human IgG and mouse Fcγ receptor (FcγR) are not identical in either specificity or strength of binding (Nimmerjahn and Ravetch, 2008), human IgG has been shown to bind to relevant mouse FcγRs (Ravetch and Anderson, 1990). Further, passive transfer into mice of human serum generated following vaccination with the 1976 swine flu vaccine has been employed to study cross-reactive protection against H1N1 influenza infection (Xie et al., 2011). Additionally, experiments studying the development of active immunity to tick-borne encephalitis virus (TBEV) in the presence of either human or mouse TBEV-immune serum demonstrated equivalent survival rates and provided further evidence that human IgG could interact with mouse FcγR in a similar fashion as mouse IgG (Kreil et al., 1998). Finally, we have previously shown that human IgG1 monoclonal antibodies 82.11 and 87.1 can enhance sub-lethal infections and cause lethal disease in the AG129 mouse model, whereas aglycosylated variants incapable of interacting with the FcγR are not (Beltramello et al., 2010). These two human IgG1 antibodies enhanced disease at equivalent concentrations and with similar kinetics to chimeric antibodies generated using the mouse IgG2a Fcγ constant region in place of the human IgG1 Fcγ constant region (K. Williams, M. Beltramello, A. Lanzavecchia, F. Sallusto, E. Harris, unpublished data). Together, these findings demonstrate that certain properties of human sera can be evaluated in a mouse model. Another potential limitation could be mis-folding of the recombinant MBP-EDIII used to deplete the sera; however, proper folding of the recombinant protein used for depletion was confirmed using a panel of MAbs directed towards critical EDIII epitopes (Wahala et al., 2009). Nonetheless, it is possible that some antibodies may bind to oligomeric epitopes that straddle either EDII and EDIII or EDI and EDIII, such as the recently identified 5H2 MAb that includes contact residues in the EDI-EDIII linker (Cockburn et al., 2011); such antibodies would not be depleted using recombinant MBP-EDIII.
Using a murine model of DENV infection and disease, we analyzed the relationship between DENV-immune serum and disease severity in the absence of additional contributing factors such as long-lived plasma cells, memory B cells, or the T cell-mediated immune response, and our findings indicate that the components of the antibody repertoire (anti-EDI/II or anti-virion antibodies) remaining after depletion of EDIII-specific antibodies are protective at a sufficiently high neutralizing titer. While neither mice nor humans appear to make large amounts of functional anti-EDIII antibodies in response to natural infection, our data suggest that when present, anti-EDIII antibodies help to reduce viral load and minimize the risk of enhancement. Vaccines designed to skew the immune response towards highly neutralizing and serotype-specific epitopes on the surface of EDIII would be predicted to be quite efficient in protecting against homotypic re-infection and minimizing cross-reactive enhancement. Further evaluation of the correlation between neutralization titer and disease severity in the context of prospective pediatric and maternal-antibody cohort studies will complement this work and contribute to improving the long-term safety and efficacy of tetravalent DENV vaccines.
Materials and methods
Viruses and cell lines
All viruses was propagated in Ae. albopictus cell line C6/36 (American Type Culture Collection) and titered by plaque assay on baby hamster kidney cells (BHK21, clone 15) (Shresta et al., 2004). DENV2 D2S10 was derived as described in Shresta et al. (2006). PL046 was obtained from H.-Y. Lei (National Cheng Kung University, Taiwan). U937 DC-SIGN (A. de Silva, University of North Carolina, Chapel Hill) and K562 cells were used for neutralization and enhancement assays, respectively. Both cells lines were grown in RPMI media (Invitrogen) at 37 °C in 5% CO2.
DENV immune human serum
Convalescent DENV immune sera were obtained from volunteers who had experienced natural DENV infections during travel abroad. The protocol for recruiting and collecting blood samples from people was approved by the Institutional Review Board of the University of North Carolina at Chapel Hill. Written informed consent was obtained from all subjects before collecting blood.
AG129 mouse infections
AG129 mice (van den Broek et al., 1995) were bred at the University of California, Berkeley. All procedures were pre-approved and conducted according to UC Berkeley Animal Care and Use Committee.
Production of mouse anti-DENV serum
AG129 mice were infected intra-peritoneally (i.p.) with 105 pfu of DENV2 PL046. Six to eight weeks post-infection, mice were sacrificed and whole blood collected by terminal cardiac puncture. Serum was isolated from whole blood by centrifugation, heat inactivated, and stored at − 80 °C.
In vivo protection experiments
AG129 mice were administered either mouse or human anti-DENV2 immune serum in a final volume of 400 ml 24 h prior to infection with an intra-venous (i.v.) sub-lethal, 103 pfu dose of DENV2 D2S10. Mice assigned to either the MBP-control or the MBP-EDIII-A experimental group received 250 μl of anti-DENV2 serum diluted into a final volume of 400 μl, and mice assigned to the MBP-EDIII-B group received 400 μl of undiluted EDIII-depleted serum. Four to six hours prior to infection, a small retro-orbital or sub-mandibular bleed was performed and 100 μl of whole blood obtained and processed to generate serum by high speed centrifugation. Four days following infection, animals were sacrificed and multiple organs obtained to measure serum viremia and tissue viral load as described below.
In vivo enhancement experiments
AG129 mice were administered either mouse anti-DENV2 immune serum or normal mouse serum (NMS) in a final volume of 400 μl 24 h prior to infection with an i.v. sub-lethal, 104pfu dose of DENV2 D2S10. As in the protection experiments, the mice assigned to the MBP-EDIII-A or MBP-EDIII-B groups received either 250 or 400 μl of MBP-EDIII-depleted serum, respectively in a final volume of 400 μl. Sub-mandibular bleeds were performed prior to infection as described above to collect serum for further analysis ex vivo.
Depletion of mouse and human DENV2 immune serum
Recombinant EDIII protein fused to maltose binding protein (MBP-EDIII) was made as previously described using DENV2 S16803. However, when DENV2 strains S16803 and PL046 (used to generate anti-DENV serum in vivo) were aligned, we observed 1 amino acid difference in EDIII at residue 383 (Suppl. Fig. 1A), which has been previously identified as a putative residue in the lateral ridge epitope (Sukupolvi-Petty et al., 2007; Trirawatanapong et al., 1992). Therefore, we generated the single D383E point mutation in 16803 backbone and re-expressed MBP-EDIII protein. No difference in binding was observed using an anti-EDIII non-lateral ridge MAb 12C1 (Wahala et al., 2010) (Suppl. Fig. 1B), but lateral ridge-specific MAb 3H5 bound more efficiently to MBP-EDIII-383E than MBP-EDIII-383D (Suppl. Fig. 1C). Human DENV2-immune serum depleted with MBP-EDIII-383D did not demonstrate differences in binding ability to either MBP-EDIII-383D or MBP-EDIII-383E protein (Suppl. Fig. 1D). However, mouse DENV2-immune serum, raised against DENV2 PL046 (383E) but depleted with DENV2 S16803 (383D), did not bind to EDIII-383D but did demonstrate ∼60% residual binding to EDIII-383E (Suppl. Fig. 1E). Therefore, we used MBP-EDIII-383D to deplete human DENV2-immune serum and MBP-EDIII-383E to deplete mouse DENV2-immune serum (raised against DENV2 PL046). Anti-EDIII antibodies from mouse and human sera were depleted according to Wahala et al. (2009) with minor modifications. Briefly, 250 μl of amylase resin was coated with 350 μg of MBP-EDIII or MBP control protein in column buffer. After washing, the resin was blocked with 8% NHS or NMS and undiluted DENV2 immune sera were incubated with MBP-EDIII resin for 1.5 h. Repeated depletion cycles were performed until all of the EDIII-reactive antibodies were removed. The same serum was depleted in parallel with MBP-coated resin as a control. Complete depletion of EDIII-reactive antibodies was confirmed by ELISA as previously described (Wahala et al., 2009).
Quantification of virus in tissue by plaque assay and quantitative RT-PCR
Tissue viral load was measured by plaque assay as previously described (Shresta et al., 2004) and was expressed as pfu/g in solid tissue and as pfu/109 cells in bone marrow. Samples of all tissues were saved in RNA later (Ambion) and RNA extracted using an RNeasy Mini kit (Qiagen). Serum was generated from whole blood by centrifugation, and RNA was extracted using a Qia-Amp Viral Recovery RNA kit (Qiagen). Serum viremia levels and tissue viral load were measured as described in Balsitis et al. (2010). Viral load was expressed as either plaque-forming unit equivalents/μg GAPDH (tissue) or pfu (eq)/mL (serum).
In vitro neutralization and enhancement assays
Pre-transfer serum (control or EDIII-depleted serum prior to administration to mice) or serum obtained from the small bleeds taken from each mouse 4–6 h prior to infection was subsequently analyzed for neutralization titer using U937 DC-SIGN cells and enhancing activity using K562 cells (pre-transfer serum only). In both assays, serum was diluted either 1:5 (small bleeds) or 1:10 (pre-transfer serum) and titrated using eight 3-fold dilutions prior to the addition of DENV2 D2S10 virus, followed by a 45-min incubation. U937 or K562 cells were then infected for 2 h, washed, and resuspended in RPMI media. Twenty-four hours (U937 DC-SIGN cells) or 48 h (K562 cells) following infection, the cells were washed, fixed in 2% paraformaldehyde (Ted Pella, Inc.), permeabilized with saponin (Sigma Aldrich) and intracellularly stained with 4G2-Alexa488 (Invitrogen). Percent infection was determined by flow cytometry using the BD LSR-Fortessa flow cytometer. Relative infection was calculated using the last dilution of each serum sample (1:21,870) as its own denominator. NT50 titers were calculated as described in Balsitis et al. (2010). Peak enhancement titer (PET) was calculated by plotting raw percent infection on the y-axis and log serum reciprocal dilution on the x-axis. A Gaussian distribution was used to fit each enhancement curve, and the amplitude identified. This data point was then used to derive the log-reciprocal serum dilution corresponding to the amplitude and was reported as the PET.
Statistical analysis
Nonparametric analysis of neutralization titers, PET, tissue viral load and serum viremia data were compared using a Wilcoxon Rank Sum analysis. Time to onset of morbidity was assessed using a logrank analysis. All data was analyzed in STATA v10 (College Station, TX).
Supplementary Material
Acknowledgments
This work was supported by NIH grants R01 AI085607 (E.H.) and U54 AI057157 from the Southeastern Regional Center of Excellence for Emerging Infections and Biodefense (A.d.S.).
Footnotes
Appendix A. Supplementary material Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.virol.2012.03.003.
References
- Alvarez D, Vollmann EH, von Andrian UH. Mechanisms and consequences of dendritic cell migration. Immunity. 2008;29(3):325–342. doi: 10.1016/j.immuni.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsitis SJ, Williams KL, Lachica R, Flores D, Kyle JL, Mehlhop E, Johnson S, Diamond MS, Beatty PR, Harris E. Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification. PLoS Pathog. 2010;6(2):e1000790. doi: 10.1371/journal.ppat.1000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramello M, Williams KL, Simmons CP, Macagno A, Simonelli L, Quyen NT, Sukupolvi-Petty S, Navarro-Sanchez E, Young PR, de Silva AM, Rey FA, Varani L, Whitehead SS, Diamond MS, Harris E, Lanzavecchia A, Sallusto F. The human immune response to dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe. 2010;8(3):271–283. doi: 10.1016/j.chom.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block OK, Rodrigo WW, Quinn M, Jin X, Rose RC, Schlesinger JJ. A tetravalent recombinant dengue domain III protein vaccine stimulates neutralizing and enhancing antibodies in mice. Vaccine. 2010;28(51):8085–8094. doi: 10.1016/j.vaccine.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Burke DS, Monath TP. Flaviviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 4th. Lippincott Williams and Wilkins; Philadelphia: 2001. p. 1. [Google Scholar]
- Chau TN, Hieu NT, Anders KL, Wolbers M, Lien LB, Hieu LT, Hien TT, Hung NT, Farrar J, Whitehead S, Simmons CP. Dengue virus infections and maternal antibody decay in a prospective birth cohort study of vietnamese infants. J Infect Dis. 2009;200(12):1893–1900. doi: 10.1086/648407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn JJ, Navarro Sanchez ME, Goncalvez AP, Zaitseva E, Stura EA, Kikuti CM, Duquerroy S, Dussart P, Chernomordik LV, Lai CJ, Rey FA. Structural insights into the neutralization mechanism of a higher primate antibody against dengue virus. Embo J. 2011 doi: 10.1038/emboj.2011.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crill WD, Chang GJ. Localization and characterization of flavivirus envelope glycoprotein cross-reactive epitopes. J Virol. 2004;78(24):13975–13986. doi: 10.1128/JVI.78.24.13975-13986.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crill WD, Hughes HR, Delorey MJ, Chang GJ. Humoral immune responses of dengue fever patients using epitope-specific serotype-2 viruslike particle antigens. PLoS One. 2009;4(4):e4991. doi: 10.1371/journal.pone.0004991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crill WD, Roehrig JT. Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J Virol. 2001;75(16):7769–7773. doi: 10.1128/JVI.75.16.7769-7773.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Alwis R, Beltramello M, Messer WB, Sukupolvi-Petty S, Wahala WM, Kraus A, Olivarez NP, Pham Q, Brian J, Tsai WY, Wang WK, Halstead S, Kliks S, Diamond MS, Baric R, Lanzavecchia A, Sallusto F, de Silva AM. In-depth analysis of the antibody response of individuals exposed to primary dengue virus infection. PLoS Negl Trop Dis. 2011;5(6):e1188. doi: 10.1371/journal.pntd.0001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, Puttikhunt C, Edwards C, Duangchinda T, Supasa S, Chawansuntati K, Malasit P, Mongkolsapaya J, Screaton G. Cross-reacting antibodies enhance dengue virus infection in humans. Science. 2010;328(5979):745–748. doi: 10.1126/science.1185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effler PV, Pang L, Kitsutani P, Vorndam V, Nakata M, Ayers T, Elm J, Tom T, Reiter P, Rigau-Perez JG, Hayes JM, Mills K, Napier M, Clark GG, Gubler DJ. Dengue fever, Hawaii, 2001–2002. Emerg Infect Dis. 2005;11(5):742–749. doi: 10.3201/eid1105.041063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endy TP, Nisalak A, Chunsuttitwat S, Vaughn DW, Green S, Ennis FA, Rothman AL, Libraty DH. Relationship of preexisting dengue virus (DV) neutralizing antibody levels to viremia and severity of disease in a prospective cohort study of DV infection in Thailand. J Infect Dis. 2004;189(6):990–1000. doi: 10.1086/382280. [DOI] [PubMed] [Google Scholar]
- Gromowski GD, Barrett AD. Characterization of an antigenic site that contains a dominant, type-specific neutralization determinant on the envelope protein domain III (ED3) of dengue 2 virus. Virology. 2007;366(2):349–360. doi: 10.1016/j.virol.2007.05.042. [DOI] [PubMed] [Google Scholar]
- Gromowski GD, Barrett ND, Barrett AD. Characterization of dengue virus complex-specific neutralizing epitopes on envelope protein domain III of dengue 2 virus. J Virol. 2008;82(17):8828–8837. doi: 10.1128/JVI.00606-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead SB. Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res. 2003;60:421–467. doi: 10.1016/s0065-3527(03)60011-4. [DOI] [PubMed] [Google Scholar]
- Halstead SB. Dengue. Lancet. 2007;370(9599):1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- Innis BL. Antibody responses to dengue virus infection. In: Gubler DJ, Kuno G, editors. Dengue and Dengue Hemorrhaggic Fever. CAB Int; New York: 1997. pp. 221–243. [Google Scholar]
- Kliks SC, Nimmanitya S, Nisalak A, Burke DS. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg. 1988;38(2):411–419. doi: 10.4269/ajtmh.1988.38.411. [DOI] [PubMed] [Google Scholar]
- Kreil TR, Maier E, Fraiss S, Eibl MM. Neutralizing antibodies protect against lethal flavivirus challenge but allow for the development of active humoral immunity to a nonstructural virus protein. J Virol. 1998;72(4):3076–3081. doi: 10.1128/jvi.72.4.3076-3081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle JL, Balsitis S, Zhang L, Beatty PR, Harris E. Antibodies play a greater role than immune cells in heterologous protection against secondary dengue virus infection in a mouse model. Virology. 2008;380:296–303. doi: 10.1016/j.virol.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CY, Tsai WY, Lin SR, Kao CL, Hu HP, King CC, Wu HC, Chang GJ, Wang WK. Antibodies to envelope glycoprotein of dengue virus during the natural course of infection are predominantly cross-reactive and recognize epitopes containing highly conserved residues at the fusion loop of domain II. J Virol. 2008;82(13):6631–6643. doi: 10.1128/JVI.00316-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libraty DH, Acosta LP, Tallo V, Segubre-Mercado E, Bautista A, Potts JA, Jarman RG, Yoon IK, Gibbons RV, Brion JD, Capeding RZ. A prospective nested case-control study of dengue in infants: rethinking and refining the antibody-dependent enhancement dengue hemorrhagic fever model. PLoS Med. 2009;6(10):e1000171. doi: 10.1371/journal.pmed.1000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Parrish CR, Murray JM, Wright PJ. Localization of a neutralizing epitope on the envelope protein of dengue virus type 2. Virology. 1994;202(2):885–890. doi: 10.1006/viro.1994.1410. [DOI] [PubMed] [Google Scholar]
- Lok SM, Kostyuchenko V, Nybakken GE, Holdaway HA, Battisti AJ, Sukupolvi-Petty S, Sedlak D, Fremont DH, Chipman PR, Roehrig JT, Diamond MS, Kuhn RJ, Rossmann MG. Binding of a neutralizing antibody to dengue virus alters the arrangement of surface glycoproteins. Nat Struct Mol Biol. 2008;15(3):312–317. doi: 10.1038/nsmb.1382. [DOI] [PubMed] [Google Scholar]
- Midgley CM, Bajwa-Joseph M, Vasanawathana S, Limpitikul W, Wills B, Flanagan A, Waiyaiya E, Tran HB, Cowper AE, Chotiyarnwon P, Grimes JM, Yoksan S, Malasit P, Simmons CP, Mongkolsapaya J, Screaton GR. An in-depth analysis of original antigenic sin in dengue virus infection. J Virol. 2010;85(1):410–421. doi: 10.1128/JVI.01826-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modis Y, Ogata S, Clements D, Harrison SC. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc Natl Acad Sci USA. 2003;100(12):6986–6991. doi: 10.1073/pnas.0832193100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8(1):34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- Oliphant T, Nybakken GE, Austin SK, Xu Q, Bramson J, Loeb M, Throsby M, Fremont DH, Pierson TC, Diamond MS. Induction of epitope-specific neutralizing antibodies against West Nile virus. J Virol. 2007;81(21):11828–11839. doi: 10.1128/JVI.00643-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliphant T, Nybakken GE, Engle M, Xu Q, Nelson CA, Sukupolvi-Petty S, Marri A, Lachmi BE, Olshevsky U, Fremont DH, Pierson TC, Diamond MS. Antibody recognition and neutralization determinants on domains I and II of West Nile Virus envelope protein. J Virol. 2006;80(24):12149–12159. doi: 10.1128/JVI.01732-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch JV, Anderson CL. FcγR family: proteins, transcripts and genes. In: Metzger H, editor. Fc Receptors and the Action of Antibodies. American Society for Microbiology; Washington, DC: 1990. pp. 211–235. [Google Scholar]
- Rey FA, Heinz FX, Mandl C, Kunz C, Harrison SC. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature. 1995;375(6529):291–298. doi: 10.1038/375291a0. [DOI] [PubMed] [Google Scholar]
- Rodhain F, Rosen L. Mosquito vectors and dengue virus–vector relationships. In: Gubler DJ, Kuno G, editors. Dengue and Dengue Hemorrhagic Fever. CAB Int; New York: 1997. pp. 45–60. [Google Scholar]
- Roehrig JT, Bolin RA, Kelly RG. Monoclonal antibody mapping of the envelope glycoprotein of the dengue 2 virus, Jamaica. Virology. 1998;246(2):317–328. doi: 10.1006/viro.1998.9200. [DOI] [PubMed] [Google Scholar]
- Rothman AL. Cellular immunology of sequential dengue virus infection and its role in disease pathogenesis. Curr Top Microbiol Immunol. 2010;338:83–98. doi: 10.1007/978-3-642-02215-9_7. [DOI] [PubMed] [Google Scholar]
- Sabin AB. Research on dengue during World War II. Am J Trop Med Hyg. 1952;1(1):30–50. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- Schieffelin JS, Costin JM, Nicholson CO, Orgeron NM, Fontaine KA, Isern S, Michael SF, Robinson JE. Neutralizing and non-neutralizing monoclonal antibodies against dengue virus E protein derived from a naturally infected patient. Virol J. 2010;7:28. doi: 10.1186/1743-422X-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shresta S, Kyle JL, Snider HM, Basavapatna M, Beatty PR, Harris E. Interferon-dependent immunity is essential for resistance to primary dengue virus infection in mice, whereas T- and B-cell-dependent immunity are less critical. J Virol. 2004;78(6):2701–2710. doi: 10.1128/JVI.78.6.2701-2710.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shresta S, Sharar KL, Prigozhin DM, Beatty PR, Harris E. A murine model for dengue virus-induced lethal disease with increased vascular permeability. J Virol. 2006;80:10208–10217. doi: 10.1128/JVI.00062-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha B, Brien JD, Sukupolvi-Petty S, Austin SK, Edeling MA, Kim T, O'Brien KM, Nelson CA, Johnson S, Fremont DH, Diamond MS. The development of therapeutic antibodies that neutralize homologous and heterologous genotypes of dengue virus type 1. PLoS Pathog. 2010;6(4):e1000823. doi: 10.1371/journal.ppat.1000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukupolvi-Petty S, Austin SK, Engle M, Brien JD, Dowd KA, Williams KL, Johnson S, Rico-Hesse R, Harris E, Pierson TC, Fremont DH, Diamond MS. Structure and function analysis of therapeutic monoclonal antibodies against dengue virus type 2. J Virol. 2010;84(18):9227–9239. doi: 10.1128/JVI.01087-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukupolvi-Petty S, Austin SK, Purtha WE, Oliphant T, Nybakken GE, Schlesinger JJ, Roehrig JT, Gromowski GD, Barrett AD, Fremont DH, Diamond MS. Type- and subcomplex-specific neutralizing antibodies against domain III of dengue virus type 2 envelope protein recognize adjacent epitopes. J Virol. 2007;81(23):12816–12826. doi: 10.1128/JVI.00432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trirawatanapong T, Chandran B, Putnak R, Padmanabhan R. Mapping of a region of dengue virus type-2 glycoprotein required for binding by a neutralizing monoclonal antibody. Gene. 1992;116(2):139–150. doi: 10.1016/0378-1119(92)90509-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes I, Bernardo L, Gil L, Pavon A, Lazo L, Lopez C, Romero Y, Menendez I, Falcon V, Betancourt L, Martin J, Chinea G, Silva R, Guzman MG, Guillen G, Hermida L. A novel fusion protein domain III-capsid from dengue-2, in a highly aggregated form, induces a functional immune response and protection in mice. Virology. 2009;394(2):249–258. doi: 10.1016/j.virol.2009.08.029. [DOI] [PubMed] [Google Scholar]
- Valdes I, Gil L, Romero Y, Castro J, Puente P, Lazo L, Marcos E, Guzman MG, Guillen G, Hermida L. The chimeric protein domain III-capsid of dengue virus serotype 2 (DEN-2) successfully boosts neutralizing antibodies generated in monkeys upon infection with DEN-2. Clin Vaccine Immunol. 2011;18(3):455–459. doi: 10.1128/CVI.00382-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek MF, Muller U, Huang S, Aguet M, Zinkernagel RM. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J Virol. 1995;69(8):4792–4796. doi: 10.1128/jvi.69.8.4792-4796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3(11):867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- Wahala WM, Donaldson EF, de Alwis R, Accavitti-Loper MA, Baric RS, de Silva AM. Natural strain variation and antibody neutralization of dengue serotype 3 viruses. PLoS Pathog. 2010;6(3):e1000821. doi: 10.1371/journal.ppat.1000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahala WM, Kraus AA, Haymore LB, Accavitti-Loper MA, de Silva AM. Dengue virus neutralization by human immune sera: role of envelope protein domain III-reactive antibody. Virology. 2009;392(1):103–113. doi: 10.1016/j.virol.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention, and Control. WHO 1997 [Google Scholar]
- Williams KL, Zompi S, Beatty PR, Harris E. A mouse model for studying dengue virus pathogenesis and immune response. Ann N Y Acad Sci. 2009;1171(Suppl 1):E12–E23. doi: 10.1111/j.1749-6632.2009.05057.x. [DOI] [PubMed] [Google Scholar]
- Xie H, Li X, Gao J, Lin Z, Jing X, Plant E, Zoueva O, Eichelberger MC, Ye Z. Revisiting the 1976 “swine flu” vaccine clinical trials: cross-reactive hemagglutinin and neuraminidase antibodies and their role in protection against the 2009 H1N1 pandemic virus in mice. Clin Infect Dis. 2011;53(12):1179–1187. doi: 10.1093/cid/cir693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.