Abstract
Myocardial infarction (MI) is a major risk for ventricular arrhythmia. Pause-triggered ventricular arrhythmia can be caused by increased myofilament Ca binding due to sarcomeric mutations or Ca-sensitizing compounds. Myofilament Ca sensitivity is also increased after MI. Here we hypothesize that MI increases risk for pause-triggered ventricular arrhythmias, which can be prevented by myofilament Ca-desensitization and contractile uncoupling. To test this hypothesis, we generated a murine chronic MI model using male B6SJLF1/J mice (n=40) that underwent permanent ligation of the left anterior descending coronary artery. 4 weeks post MI, cardiac structure, function and myofilament Ca sensitivity were evaluated. Pause-dependent arrhythmia susceptibility was quantified in isolated hearts with pacing trains of increasing frequency, followed by a pause and an extra stimulus. Coronary ligation resulted in a mean infarct size of 39.6±5.7%LV and fractional shortening on echocardiography was reduced by 40% compared to non-infarcted controls. Myofilament Ca sensitivity was significantly increased in post MI hearts (pCa50: Control=5.66±0.03; MI=5.84±0.05; p<0.01). Exposure to the Ca desensitizer/contractile uncoupler blebbistatin (BLEB, 3 µM) reduced myofilament Ca sensitivity of MI hearts to that of control hearts and selectively reduced the frequency of post-pause ectopic beats (MI 0.24±0.08 vs MI+BLEB 0.02±0.01 PVC/pause; p=0.02). BLEB also reduced the incidence of ventricular tachycardia in chronic MI hearts from 59% to 10% (p<0.05). We conclude that chronic MI hearts exhibit increased myofilament Ca sensitivity and pause-triggered ventricular arrhythmias, which can be prevented by blebbistatin. Decreasing myofilament Ca sensitivity may be a strategy to reduce arrhythmia burden after MI.
Keywords: Blebbistatin, chronic MI, ventricular tachycardia, mouse hearts, myofilament Ca sensitivity, triggered arrhythmia
1. Introduction
Cardiovascular diseases are the leading cause of death and disability in the industrialized world. In particular, myocardial infarction (MI) is associated with high risk of death by arrhythmias and heart failure. After a MI, a progressive cardiac remodeling ensues with a set of neurohumoral activation and structural changes [1]. In the cellular setting, Ca handling disturbances contribute to contractility dysfunction and arrhythmogenesis [2, 3]. During the post-infarction remodeling, sudden cardiac deaths due to ventricular arrhythmias account for at least 50% of those unexpected deaths [4]. However, the pharmocotherapeutic approaches for prevention of sudden cardiac death post MI are still limited.
A common feature of post-infarct remodeling in the surviving myocardium is increased myofilament Ca sensitivity [5]. Although pharmacological approaches of increasing the myofilament Ca sensitivity are investigated as treatment of heart failure [6], myofilament Ca sensitization has also been reported to increase the susceptibility to ventricular arrhythmias [7]. For example, the increased myofilament Ca sensitivity conferred by mutations in troponin T that cause familial hypertrophic cardiomyopathy (FHC) in humans appears to be sufficient to generate a myocardial substrate for reentrant ventricular tachycardia [8]. One underlying mechanism appears to be that that myofilament Ca sensitization, either by troponin T mutations or Ca-sensitizing compounds, increases myofilament Ca binding, which in turn leads to an increased post-pause Ca transient, activation of inward current and generation of early afterdepolarizations and pause-triggered ventricular arrhythmias [9]. Moreover, in the setting of acute myocardial ischemia, pharmacological myofilament Ca sensitization increases the incidence of post-pause triggered beats and ventricular tachycardia [9]. However, whether myofilament Ca sensitization in chronic infarcted hearts contributes to the development of ventricular arrhythmias, in particular pause-dependent ventricular ectopy, remains to be determined. Here, we used a murine model of chronic induced by permanent coronary ligation to tested the hypothesis that chronic MI hearts exhibit an increases risk for pause-triggered ventricular arrhythmias, which can be prevented by myofilament Ca-desensitization and contractile uncoupling.
2. Methods
All procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the institutional Committee of Ethics in Animal Research.
2.1 Surgical coronary ligation
Male B6SJLF1/J mice (12weeks old) underwent a permanent ligation of the left anterior descending (LAD) coronary artery ligation as described [10]. Briefly, mice were anesthetized with a mixture of ketamine (90 mg/kg) plus xylazine (10 mg/kg) followed by an oropharyngeal intubation for mechanical ventilation (200 strokes/min at a tidal volume of 200 µL). A left thoracotomy was performed between the 4th and the 5th intercostal space and the left ventricular free wall and the target coronary were exposed. The LAD artery was permanently occluded with a 7.0 silk suture at approximately 1 mm of its origin. Successful occlusion was confirmed by ST-elevation in the ECG. Afterwards, the thorax was closed and pleural air was removed from the chest using plastic tubing connected to a syringe. Skin wound was closed with a 4.0 suture and pain medication was subcutaneously administered (Ketoprofen, 10 mg/kg). Aged-match mice were subjected to all above described procedures except the coronary occlusion, and were used as non-infarcted controls.
2.2 Echocardiography
Four weeks after surgery, both infarcted and control mice were subjected to transthoracic echocardiography. Animals were anesthetized with isoflurane 1.5% and placed in supine position. The electrocardiogram was continuously recorded during the experiments. The thorax was shaved to allow the best contact with the transducer. A paraesternal short-axis M-mode images were acquired at the level of the papillary muscle using a 15 MHz probe. Heart rate (HR), left ventricle (LV) internal diameter at diastole (LVIDd) and systole (LVIDs), and posterior wall thickness at diastole (LVPWd) and at systole (LVPWs), interventricular septal thickness at end-systole (IVSs) and end-diastole (IVSd) were measured. Fractional shortening was calculated according to the following equation: FS (%) = [(LVIDd − LVIDs)/ LVIDd] × 100.
2.3 Contractile force and Ca sensitivity measurements in skinned fibers
Skinned fibers were prepared from the left ventricle of mouse hearts as described [8]. Briefly, small bundles of fibers were isolated and placed in a pCa 8.0 relaxing solution (10−8 M [Ca]free, 1 mM [Mg2+]free, 7 mM EGTA, 2.5 mM MgATP, 20 mM MOPS (pH7.0), 20 mM creatine phosphate, and 15 units/ml creatine phosphokinase, I = 150 mM) containing 1% Triton X-100 at 4°C for approximately 4–6 hours. Fibers were transferred to the same solution containing 50% glycerol and stored at −20°C for up to seven days. Bundles of mouse fibers with diameters between 100 – 250 µm and ~ 1.2 mm of length were attached to hooks connected to a force transducer. To determine the Ca sensitivity of force development, the fibers were gradually exposed to solutions of increasing Ca concentration from pCa 8.0 - 4.0. Data were analyzed using the following equation: %Change in Force = 100 × [Ca]n / ([Ca]n + [Ca50]n) where “[Ca50]” is the free [Ca] that produces 50% force and “n” is the Hill coefficient. Ca sensitivity of contraction and maximal force were measured at two different sarcomere lengths. The fibers were visualized using a CCD system (Imaging Development System GmbH) connected to the side port of a Zeiss inverted microscope (Axio Observer A1) and analyzed using the 900B Sarcomere Length Detection System from Aurora Scientific Inc., Canada. The effect of blebbistatin on myofilament Ca sensitivity was tested as previously reported [8]. Briefly, the active (−/−) isomer of blebbistatin was dissolved in DMSO (100%) and tested at a final concentration of 3 µM. The stock concentration was adjusted with DMSO to achieve a final DMSO concentration of 0.05% in all experiments. All fiber experiments and handling of blebbistatin were carried out in the dark at 15°C.
2.4 Experiments in isolated hearts
Isolated Langendorff-perfused heart experiments were carried out as described. Briefly, mice were deeply anesthetized using 5% isofluorane, hearts rapidly excised, aorta cannulated and perfused at constant pressure (70 mmHg) with bicarbonate buffer at a temperature of 36°C bubbled with 95% O2 and 5% CO2 containing (in mM): 130 NaCl, 4 KCl, 23 NaHCO3, 1.5 NaH2PO4, 1 MgCl2, 2 CaCl2, 10 Glucose and 0.2 µM propranolol. After thermal ablation of the atrioventricular node, hearts were paced at twice diastolic threshold using a platinum pacing electrode from the LV apex. Hearts were subjected to an electrical pacing challenge consisting of two segments. In the first segment, the hearts were subjected to a continuous pacing train for 13–15 minutes at a cycle length of 100 ms. Every 30 seconds, the pacing train was paused for 1 second, following by a post-pause S2 extra stimulus. In the second segment, following 2–3 minutes of rest, hearts were subjected to a pacing protocol of increasingly faster S1 pacing trains of 1 min duration followed by a 1 second pause and a post-pause S2 pulse. The initial S1 pacing cycle length (PCL) was 150 ms, which was sequentially reduced every minute (120 ms, 100 ms, 80 ms, and then in decrements of 10ms) until capture was lost, or arrhythmia was induced. This pacing protocol was done under basal conditions and repeated 5 min after adding 3 µM of the Ca desensitizer/contractile uncoupler (−/−) blebbistatin to the perfusate. Arrhythmia susceptibility was quantified by counting the number of premature ventricular complexes (PVC) or VT during the pacing train and after the pause. A PVC was defined as any premature ventricular beat with a coupling interval < 100 ms. Only pacing trains with full pauses (i.e., without ventricular escape beats) were used to calculate the rate of pausetriggered PVCs.
After completion of the pacing protocols, hearts were cut transversely, fixed in formaldehyde and embedded in paraffin for histological processing. Each heart was serial cut into transversal sections of 5 µm and stained with Masson trichrome to measure infarct size (Fig. 1). Infarct size was reported as the percentage of the left ventricular perimeter occupied by the infarct scar [11].
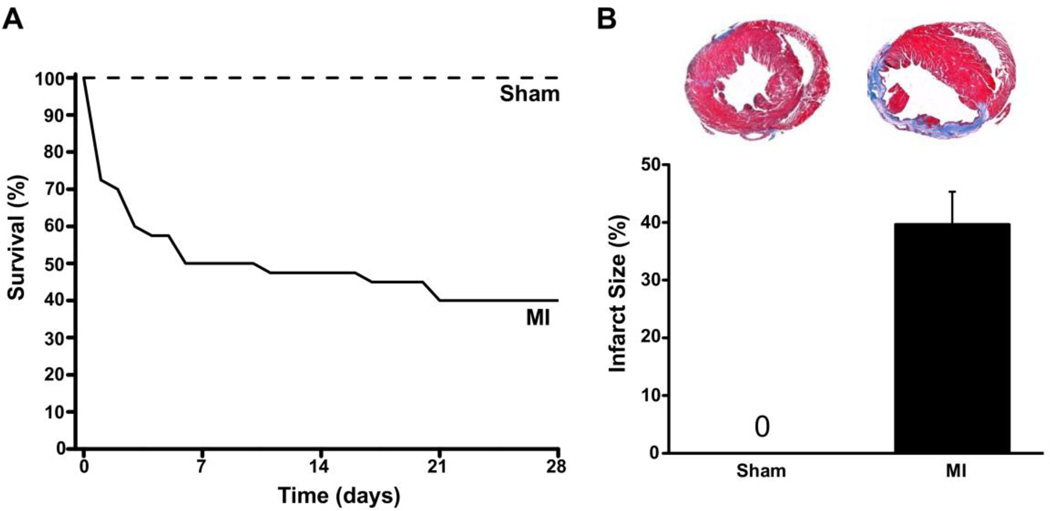
Fig. 1.
Generation of chronic MI mouse model. Compared to sham mice (n=10), the survival of mice after permanent ligation of the mid-LAD was significantly reduced, with a survival rate of only 40% after four weeks (A). Panel B shows the average infarct size of the mice that survived to the 4 week time point (n=16). Insets: representative cross-sections stained with Trichrome-blue from sham and post MI hearts. The infarct scar (blue stain) occupies the LV anterior wall and the large cavity area indicates chamber dilation.
2.5 Statistical Analysis
Normally-distributed variables were compared using the unpaired or paired student t-test as appropriate. PVC rates were compared by means of the Mann-Whitney U test. Fisher’s exact test was used to compare incidences of ventricular arrhythmia. Pearson’s correlation was done to assess the association between variables. Data are reported as the mean ± standard error of mean and considered statistical significant if P< 0.05.
3. Results
3.1 Cardiac structure and function after coronary ligation
Permanent LAD ligation resulted in a large transmural MI, with 24 out of 40 mice dying (60% mortality) during the four weeks after coronary ligation (Fig. 1A). Most deaths (20 out of 24) occurred in the first week post MI (Fig. 1A). The surviving chronic MI mice exhibited significant LV remodeling, as evidenced by chamber dilation, increased LV wall thickness and reduced LV systolic function measured by echocardiography (Table 1). On histology, LAD ligation resulted in a mean infarct size of 39.6 ± 5.7% of the LV perimeter four weeks after coronary ligation (Fig. 1B).
Table 1.
Echocardiographic characteristics four weeks after MI.
| SHAM (n=10) | MI (n=16) | |
|---|---|---|
| Sample size | 10 | 16 |
| Heart rate (bpm) | 641 ± 10 | 669 ± 28 |
| IVSd (mm) | 0.682 ± 0.01 | 0.678 ± 0.02 |
| IVSs (mm) | 1.214 ± 0.02 | 0.991 ± 0.04* |
| LVIDd (mm) | 3.017 ± 0.06 | 4.412 ± 0.14* |
| LVIDs (mm) | 1.542 ± 0.04 | 3.125 ± 0.16* |
| LVPWd (mm) | 0.603 ± 0.02 | 0.727 ± 0.03* |
| LVPWs (mm) | 0.816 ± 0.02 | 1.008 ± 0.02* |
| FS (%) | 48.9 ± 0.5 | 29.7 ± 1.5* |
P<0.05
3.2 Myofilament Ca sensitivity and developed force of cardiac muscle after chronic MI
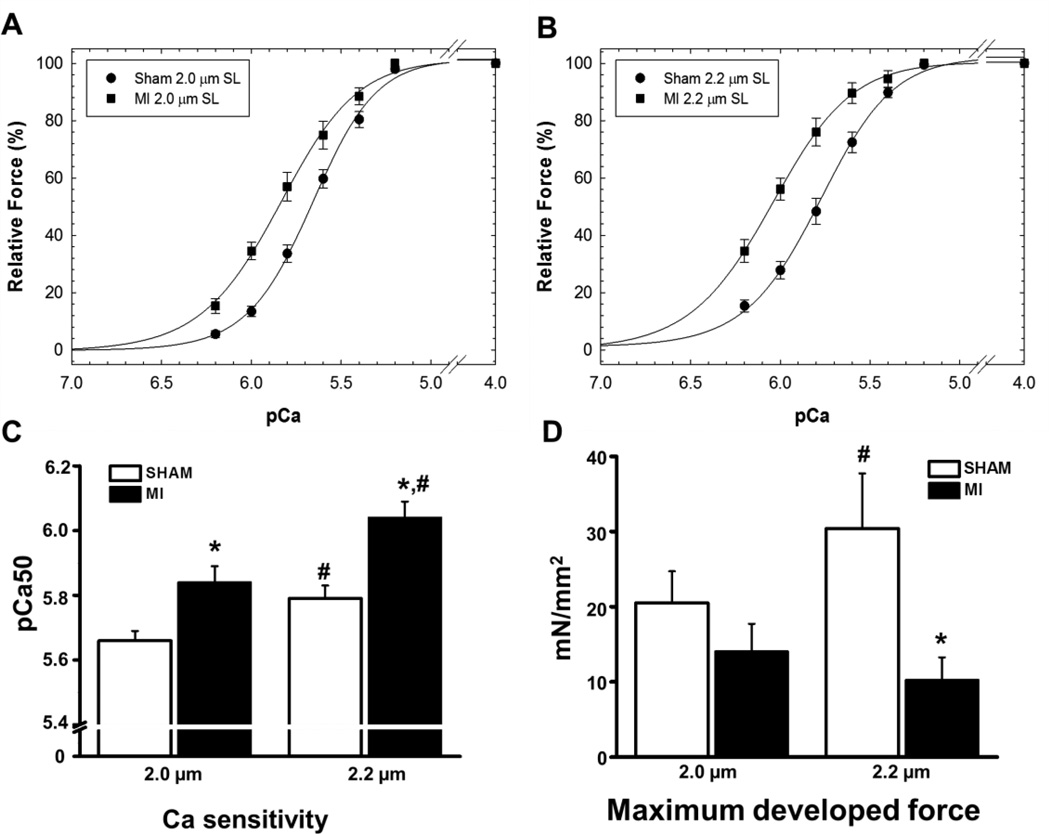
To assess the function of the surviving LV cardiac muscle, we next measured Ca sensitivity and maximal developed force in skinned papillary muscle fibers harvested from the LV. Consistent with literature reports [5], myofilament Ca sensitivity was significantly increased in post MI fibers compared to sham fibers, as evidenced by the leftward shift in the relationship between pCa and force development (Figs. 2A, B). The increased Ca sensitivity was present at both sarcomere lengths tested in post MI fibers (pCa50 at 2.0 µm: 5.84 ± 0.05 vs. 5.66 ± 0.03, P< 0.01; pCa50 at 2.2µm: 6.04 ± 0.05 vs. 5.79 ± 0.04, P< 0.05, Fig. 2C). Furthermore, the maximal force developed by skinned fibers from the chronic MI group was significantly lower than that of fibers harvested from sham hearts at 2.2 µM sarcomere length (Sham = 30.4±7.3 vs. MI = 10.3±3.0 mN/mm2, P< 0.05, Fig. 2D). Although the length-dependent increase in myofilament Ca sensitivity (= Frank-Starling response) was intact in both groups (i.e., increased pCa50 values at 2.2 compared to 2.0 µM, Fig. 2C), fibers from post MI hearts lacked the physiological increase in maximal developed force upon increasing the sarcomere length from 2.0 to 2.2 µM (Fig. 2D).
Fig. 2.
Comparison of myofilament Ca sensitivity (A, B, C) and maximal developed force (D) of skinned fibers obtained from sham and post MI hearts. Measurements were obtained at 2.0 µm and 2.2 µm sarcomere length. The Hill coefficients were: Sham 2.0 µm = 2.49 ± 0.11; MI 2.0 µm = 2.14 ± 0.14; Sham 2.2 µm = 2.21 ± 0.09; and MI 2.2 µm = 2.20 ± 0.21. *indicates P< 0.05 vs. Sham, #indicates P<0.05 vs. 2.0 µm. N=5–6 fibers per group from 4 sham and 4 MI hearts.
3.3 Chronic MI hearts exhibit pause-triggered ventricular arrhythmias
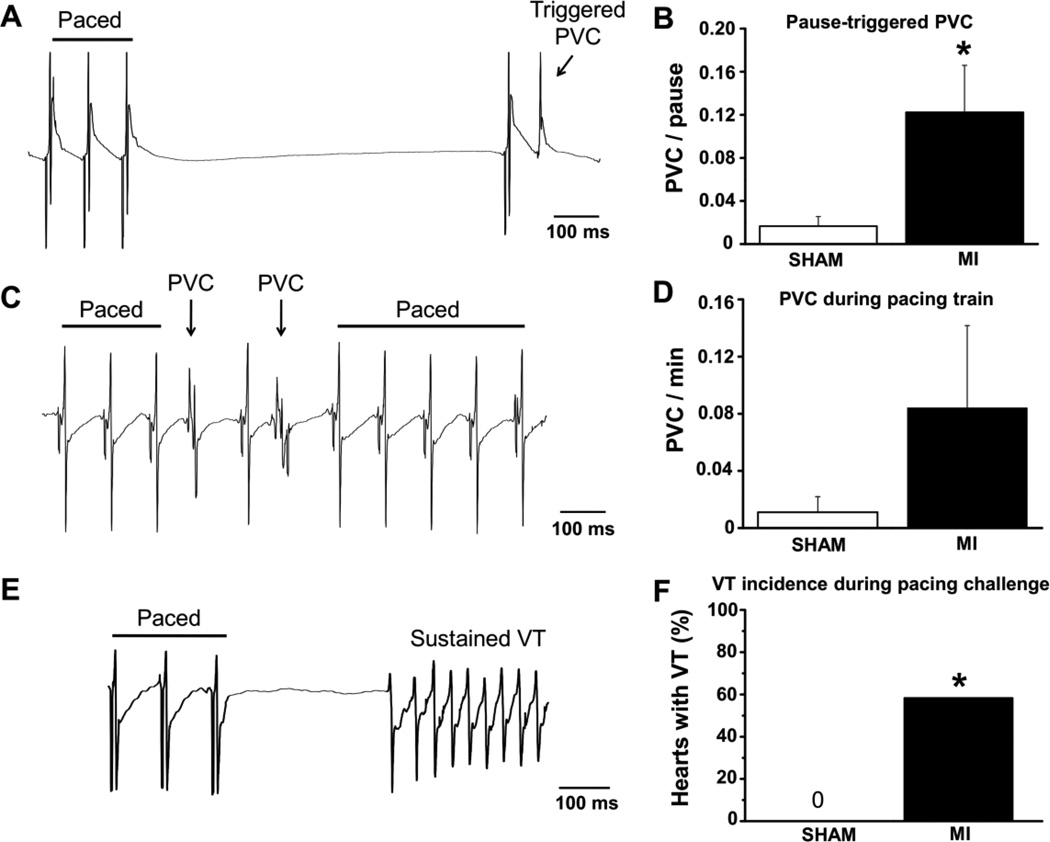
We previously reported that myofilament Ca sensitization either by TnT mutations or by Ca sensitizing compounds generates susceptibility to pause-triggered ventricular ectopy [9]. In contrast, acute myocardial ischemia induced by coronary ligation does not increase the incidence of post-pause triggered beats [9]. Hence, in order to evaluate the effect of myofilament Ca sensitization in the setting of a chronic MI, we used the previously established pacing challenge protocol [9] consisting of pacing trains of successively shorter PCL followed by brief (1s) pauses (Fig. 3A). Whereas non-infarcted sham hearts rarely exhibited PVCs after pauses (1 out of 50 pauses, Fig. 3B), the post-pause S2 stimulus frequently triggered PVCs in chronic MI hearts (1 out of 7 pauses, Fig. 3A, B). The average coupling interval between the triggered PVC and the post-pause S2 stimulus was 75±10 ms. There was a trend towards higher rates of PVCs during the pacing train in post MI hearts that did not reach statistical significance (Fig. 3C,D). VT was induced in 59% of post MI hearts, but never observed in control hearts (Fig. 3F).
Fig. 3.
Arrhythmogenesis in the chronic MI model. Representative volume-conducted ECG traces show PVCs triggered by a pause (A) and during the pacing train (C), and a triggered sustained VT during the pause (E). The rate of PVCs triggered by a pause (B) was significantly increased in the MI compared to the sham group. PVCs also occurred during the pacing train (D) in the MI group but the difference was not statistically significant. VT was only seen in MI hearts (F). N=23 (sham) and N=12 (MI) hearts, *P< 0.05 vs. Sham.
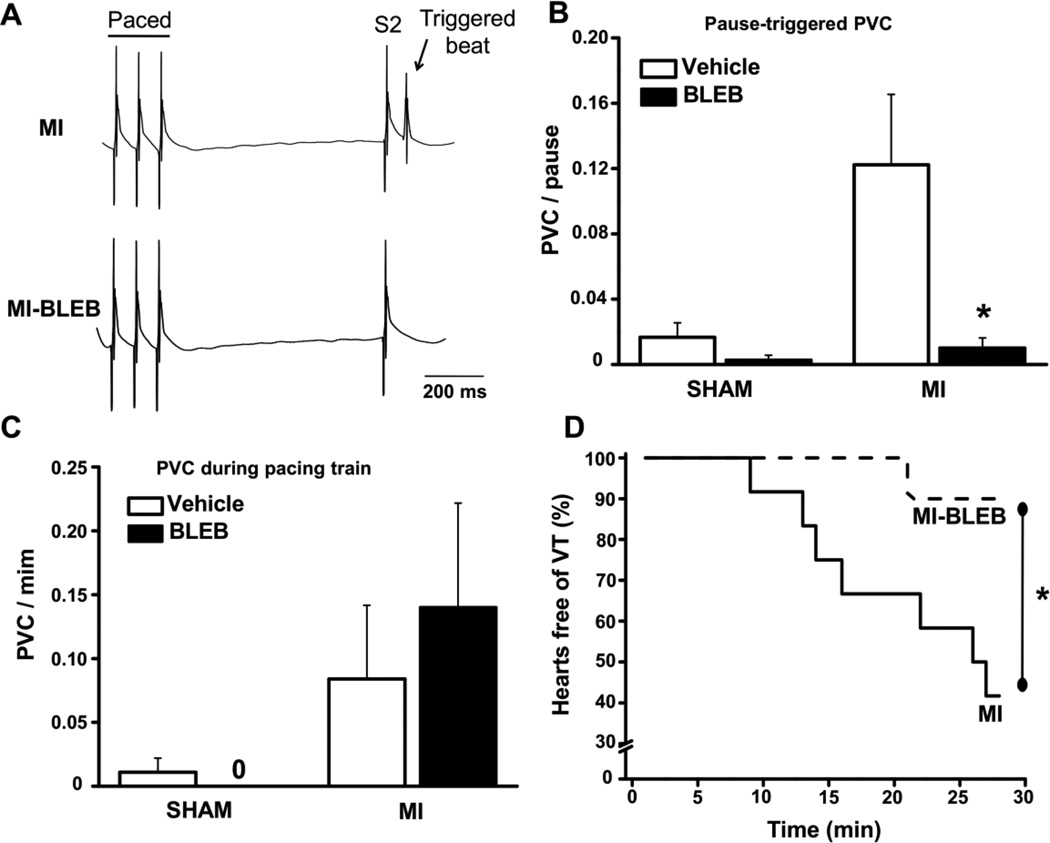
3.4 Blebbistatin decreases myofilament Ca sensitivity and selectively reduces pause-triggered ventricular ectopy in chronic MI hearts
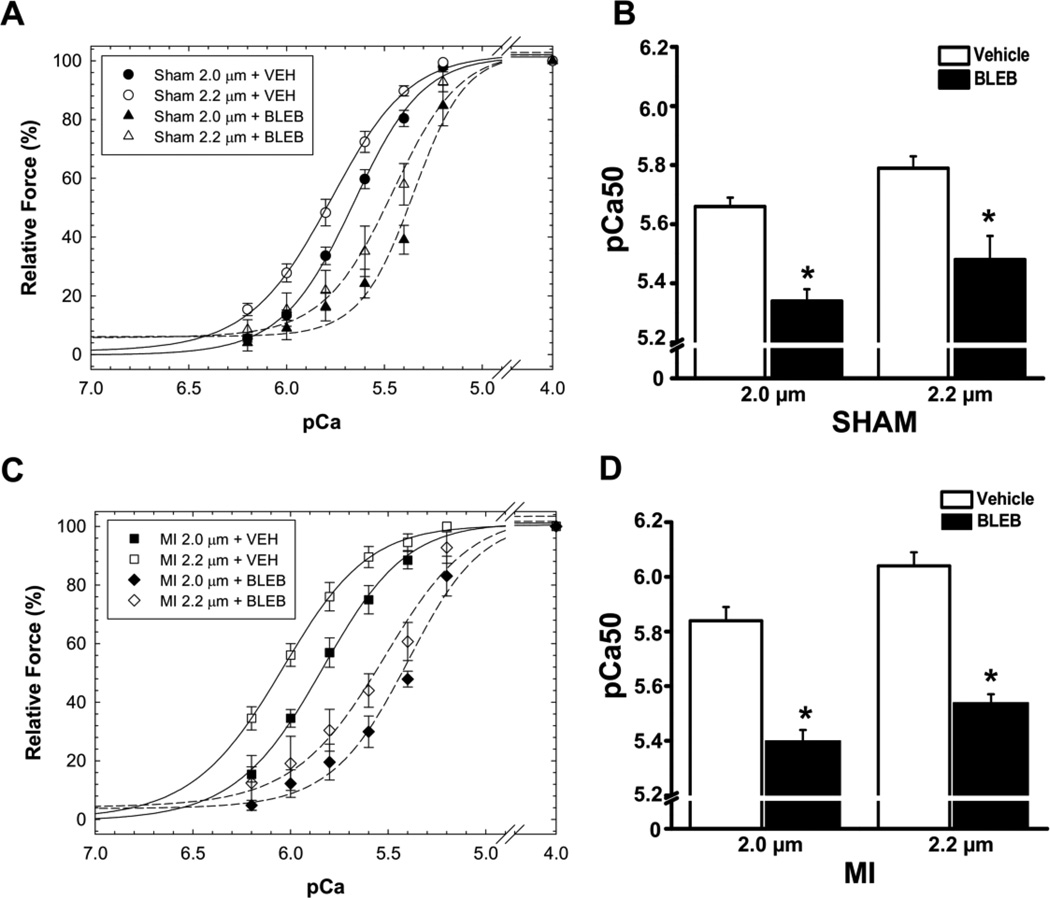
To find an agent that decreases myofilament Ca sensitivity without affecting membrane ion channels, we evaluated the selective myosin ATPase inhibitor (−/−) blebbistatin in skinned fibers. Blebbistatin has no known direct effects on cardiac ion channels and/or action potential duration in mouse or rat ventricular muscle at concentrations below 10 µM [8, 12, 13]. Blebbistatin at 3 µM significantly reduced myofilament Ca sensitivity both in sham and in post MI fibers (Fig. 4). To test whether a reduction of myofilament Ca sensitivity can prevent the pause-triggered arrhythmia in post MI hearts, we next repeated the pacing challenge after treating post MI hearts with 3 µM blebbistatin. As shown in Fig. 5B, the rate of pause-triggered PVCs was drastically reduced by blebbistatin (0.02 ± 0.01 vs. 0.24 ± 0.08 PVC/pause before blebbistatin; P=0.02). The effect of blebbistatin was selective for pause-triggered PVCs, because blebbistatin did not affect the rate of PVCs during the pacing train (Fig. 5C). Finally, pause-triggered VT where almost completely prevented by myofilament Ca de-sensitization with blebbistatin (Fig. 5D).
Fig. 4.
Blebbistatin (BLEB, 3 µM) reduces myofilament Ca sensitivity of skinned papillary muscle fibers harvested from sham (A, B) and post-MI hearts (C, D). Myofilament Ca sensitivity was evaluated from the normalized pCa-force relationships for sham (A) and post-MI hearts (C) and pCa50 values calculated for each fiber. Measurements were obtained at 2.0 µm and 2.2 µm sarcomere length. The Hill coefficients in the presence of BLEB were: Sham 2.0 µm = 3.92 ± 0.60; Sham 2.2 µm = 3.18 ± 0.75; MI 2.0 µm = 2.35 ± 0.50; and MI 2.2 µm = 2.09 ± 0.54. The relative force (P/P0) measured at pCa 4.0 in the presence of BLEB was: Sham 2.0 µm = 7.48 ± 0.76 %; Sham 2.2 µm = 6.94 ± 1.27 %; MI 2.0 µm = 10.23 ± 1.33 %; and MI 2.2 µm = 10.60 ± 3.18 %. * indicates P< 0.05 vs. Vehicle. N=4–6 fibers per group from 4 sham and 4 MI hearts
Fig. 5.
Effect of the myofilament Ca de-sensitizer blebbistatin (BLEB, 3 µM) on the rate of PVC’s and VT’s in sham and post-MI hearts. Panel A shows representative examples of ECG traces before (upper) and after (bottom) BLEB treatment. BLEB reduced the number of PVC’s per pause (B), but did not affect the ectopy during the pacing train (C). The incidence of VT was significantly reduced after BLEB (D). N=7 (Sham) and N=12 (MI) hearts, *P< 0.05 vs vehicle (DMSO).
To test if differences in the infarct size would account for the observed ventricular ectopy, we performed a Pearson’s correlation analysis. No significant correlation was found between the rate of pause-triggered ectopy and infarct size (r=0.047, P=0.897). Also, although myocardial hypertrophy is a risk factor for arrhythmias, we did not find significant correlation between IVSd and the rate of post-pause ectopy (r=0.405, P=0.245), indicating that in isolated mouse hearts post MI the increased myofilament Ca sensitivity appears to be a major contributor to the pause-triggered arrhythmogenesis.
4. Discussion
The main finding reported here is that chronic MI hearts exhibit increased myofilament Ca sensitivity (Fig. 2) and pause-triggered ventricular arrhythmia (Fig. 3), which can be prevented by pharmacological myofilament Ca de-sensitization and contractile uncoupling (Figs. 4 and 5). These results suggest that increased myofilament Ca sensitivity and contractile dysfunction importantly contribute to the increased incidence of ventricular arrhythmia in chronic MI hearts.
4.1 Myofilament Ca sensitivity increase and pause-trigged ventricular ectopy
We recently reported that pharmacological myofilament sensitization can cause increased post-pause ectopy and VT incidence [9]. What is a possible underlying mechanism for the pause-triggered ventricular ectopy in Ca sensitized hearts? The increased myofilament Ca sensitivity results in increased myofilament Ca binding, which in turn leads to Ca accumulation in the cytosol during physiological heart rates [9]. The accumulated Ca is mobilized and taken up by the sarcoplasmic reticulum (SR) during longer diastolic intervals or pauses. The resulting post-pause excessive Ca release causes action potential (AP) prolongation, early afterdepolarizations (EADs) and triggered ventricular beats. Pause-dependent SR Ca overload and triggered arrhythmias can be reproduced with the Ca-sensitizing agent EMD 57033 and prevented by myofilament Ca desensitization with blebbistatin [9]. The results of our studies reported here suggest the same pause-dependent triggering mechanism may also operational in post MI hearts. Chronic infarcted hearts also exhibit increased myofilament Ca sensitivity (Fig. 2), are pause-triggered ectopy (Fig. 3), which can be largely prevented by blebbistatin (Fig. 5). At the same time, blebbistatin had no effect on ventricular ectopy during the pacing train (Fig. 5). While not directly tested here, literature reports are consistent with the myofilament Ca binding mechanism. For example, ventricular myocytes isolated from chronic MI rat hearts also exhibit excessive AP prolongation and EADs of the first post-pause beat caused by an increased Ca transients [14]. The excessive post-pause action potential prolongation in turn can generate EADs and triggered beats. Others have reported that the incidence of delayed afterdepolarizations is also increased in chronic MI hearts, which was related to the hypertrophic status of the survival tissue [14]. However, we were unable to confirm this hypothesis in our chronic MI mouse model, since we did not find a significant association between hypertrophy and post-pause ectopy, corroborating the hypothesis that increased myofilament Ca sensitivity primarily causes pause-dependent EADs and pause-triggered arrhythmias.
Taken together, these results suggest that myofilament sensitization selectively increases the susceptibility to pause-triggered ectopy, which is consistent with the results obtained with Ca sensitizing compounds in structurally normal hearts [9]. Others have reported that strategies that reduce myofilament Ca sensitivity can be anti-arrhythmic and increase survival: Chronic exercise training post MI reduces myofilament Ca sensitivity [5] and also reduces sudden cardiac deaths [15]. On the other hand, the Ca sensitizer levosimendan increases VT incidence in heart failure patients [16].
4.2 Cardiac muscle remodeling and arrhythmia mechanisms in chronic MI
The mouse model of coronary artery ligation has been widely used as a model of ischemic heart failure [17]. Experimental MI produced by permanent coronary ligation is associated with intense inflammatory reaction and cell death, followed by increased neurohumoral activation. Cardiac structure is severely affected and, as a consequence of the acute loss of significant amount of contractile myocardium, cardiac dilation occurs early at the 2nd week after coronary ligation in mice, with a progressive increase in the cardiac mass in the surviving myocardium [17]. At the same time, fractional shortening is reduced early after myocardial infarction [18]. Consistent with previous reports, four weeks after permanent coronary occlusion, our infarcted mice showed evidence of marked LV remodeling, with significant LV chamber dilation, compensatory hypertrophy and reduced contractile function (Fig. 1 and Table 1).
At the cellular level, a number of changes in the Ca handling proteins contribute to the impairment in excitation-contraction coupling post MI. The sarcoplasmic reticulum Ca reuptake is reduced, the expression and activity of Na+/Ca exchanger is increased, RyR2 Ca release channels become leaky, resulting in a net reduction in SR Ca content, reduced Ca release, and reduced contractile function [19–22]. Further contributing to impaired contractile function is an intrinsic dysfunction of the myofilaments, with decreased force development reported in isolated cell [23] as well as in skinned fibers [24] reported after chronic MI in pigs. Our data corroborate these results in the mouse chronic MI model. Post MI fibers not only exhibited a reduction of maximal developed force, but also a loss of the physiological increase in maximum force with increased sarcomere length (Fig. 2D). Nevertheless, the sarcomere length dependence of Ca sensitivity, which is thought to underlie the Frank-Starling-effect in the heart [25], remained intact in the post MI muscle fibers (Fig. 2C).
The force development during cardiac muscle contraction depends on several variables. Variations in the sarcomere length as stated by the Frank-Starling law of the heart and the amount of cytosolic Ca binding to TnC during systole are both important mechanisms of beat-to-beat force regulation [26]. Another important way to regulate cardiac contractility is to change the myofilament Ca sensitivity. We observed that, even with a reduced force generation in skinned fibers and in vivo left ventricular dysfunction, myofilament Ca sensitivity was increased in chronic infarcted hearts. Our results are in accordance with literature reports of increased myofilament Ca sensitivity in a chronic MI pig model [24] and in mouse MI mouse models, where Ca sensitivity was increased as early as 3 days after coronary ligation [27] up to at least 8 weeks after coronary ligation [5]. The underlying mechanism is unclear, with several different mechanisms being reported. Avner et al. attribute Ca sensitivity increase post MI to increased oxidation of sarcomere proteins [27]. Others have suggested reduced β-adrenergic responsiveness in failing hearts [28] accompanied by a reduction in protein kinase A (PKA) levels in the surviving myocardium [24]. Reduced PKA levels could explain why de Waard et al. [5] find that myofilament Ca sensitivity in chronic MI skinned mouse myocytes was normalized after in vitro treatment with exogenous PKA. Since phosphorylation of TnI appears to be unaltered in myofibrils harvested from chronic MI mouse hearts [5, 29], the PKA target responsible for normalizing Ca sensitivity in chronic MI hearts remains unknown.
Ventricular remodeling after MI provides an arrhythmogenic substrate for ventricular tachycardia and sudden cardiac death. Several pro-arrhythmic features such as arrhythmogenic Ca release due to ryanodine receptor phosphorylation [19] and/or oxidation [22], prolongation of action potential duration, increased repolarization dispersion and decreased conduction velocity have been reported in chronic MI hearts [30–32]. This post-MI electrophysiological remodeling together with the scar formation and structural remodeling support reentrant excitation as a common cause of ventricular tachycardia in infarcted hearts [33–35]. Our group has previously reported that increased myofilament Ca sensitivity commonly found in mouse models of human FHC generates susceptibility to reentrant excitation even in the absence of structural heart disease [8]. Mice carrying human FHC-linked TnT mutation show increased propensity to development of reentrant ventricular tachycardia, which was attributed to an increase in the dispersion of conduction velocity, action potential alternans and action potential triangulation [8]. The new pause-dependent triggering mechanism reported here due to increased myofilament Ca sensitivity likely further increases the risk for ventricular arrhythmia in chronic MI.
4.3 Study limitations
An important limitation of our study is that blebbistatin is also a contractile uncoupler [13]. As a result, we cannot exclude that blebbistatin conferred its anti-arrhythmic action by mechanisms unrelated to changes in Ca sensitivity. For example, contractile uncoupling by blebbistatin will reduce non-uniform muscle contraction in the scar borderzone and thereby could prevent PVC’s caused by stretch-activated ion channels [36] or by stretch-induced Ca dissociation from the myofilaments [37]. Reduced ATP utilization due to myosin ATPase inhibition [38] may also contribute to blebbistatin’s antiarrhythmic effect post MI. While blebbistatin currently is the only Ca desensitizer [9, 12, 39] that has no known direct effects on cardiac ion channels [13, 40], as with any pharmacological agent, we cannot exclude that other, as of yet unknown properties of blebbistatin contribute to its anti-arrhythmic action in post MI hearts. Finally, since in MI the cardiac function is already depressed, any treatment that decreases myofilament Ca sensitivity, while preventing arrhythmia, may have a profound effect on further depressing cardiac function. On the other hand, reducing myofilament Ca sensitivity (e.g., via TnI phosphorylation [41]) will also improve myocardial relaxation, diastolic filling and, based on our data with blebbistatin [9], enhance diastolic SR Ca uptake, which may more than offset the reduced systolic function produced by the reduced myofilament Ca sensitivity. Unfortunately, since a specific Ca de-sensitizing agent presently does not exist, the net effect cannot be tested experimentally at present time.
4.4 Conclusion
We show here that chronic infarcted hearts exhibit increased myofilament Ca sensitivity and high propensity to the development of ventricular arrhythmias after brief pauses. Importantly, pharmacological de-sensitization and contractile uncoupling with blebbistatin is associated with a selective reduction of pause-triggered arrhythmogenesis. Our results provide evidence that myofilament Ca de-sensitization could be a strategy to prevent sudden cardiac death in post-MI heart failure patients. Moreover, our data also draw attention to a potential pro-arrhythmic liability of using Ca sensitizer drugs in heart failure patients.
Highlights.
Ventricular arrhythmia (VA) are common after a myocardial infarction (MI).
Arrhythmia mechanisms were studied 4 weeks post MI in mice.
MI increased myofilament Ca2+ sensitivity and caused VA after brief pauses.
Reducing Ca2+ sensitivity and force with blebbistatin prevents pause-triggered VA.
Myofilament Ca2+ de-sensitization could be a new strategy to prevent VA after MI.
Acknowledgments
Financial Support
This work was supported in part by the US National Institutes of Health grants HL71670 (to BCK & FJB), HL88635 (to BCK), HL103840 (to JRP), by the Australian National Health and Medical Research Council Project Grant 10005974 (to BCK), by the American Heart Association Established Investigator Award 0840071N (to BCK) and Scientist Development Grant 12050597 (to HSH). Marcelo P. Baldo is the recipient of a fellowship from CNPq (202347/2011-7).
Glossary of abbreviations
- DAD
delayed afterdepolarization
- EAD
early afterdepolarization
- FHC
Familial hypertrophic cardiomyopathy
- HR
Heart rate
- IVSd
interventricular septal thickness at end-diastole
- IVSs
interventricular septal thickness at end-systole
- LVIDd
LV internal diameter at diastole
- LVIDs
LV internal diameter at systole
- LVPWd
posterior wall thickness at diastole
- LVPWs
posterior wall thickness at systole
- MI
Myocardial infarction
- PCL
pacing cycle length
- PVC
premature ventricular complex
- VT
ventricular tachycardia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
All authors declare no conflict of interest
References
- 1.Gajarsa JJ, Kloner RA. Left ventricular remodeling in the post-infarction heart: a review of cellular, molecular mechanisms, and therapeutic modalities. Heart Fail Rev. 2011;16:13–21. doi: 10.1007/s10741-010-9181-7. [DOI] [PubMed] [Google Scholar]
- 2.Bers DM. Altered cardiac myocyte Ca regulation in heart failure. Physiology (Bethesda) 2006;21:380–387. doi: 10.1152/physiol.00019.2006. [DOI] [PubMed] [Google Scholar]
- 3.Belevych AE, Terentyev D, Terentyeva R, Ho HT, Gyorke I, Bonilla IM, et al. Shortened Ca2+ signaling refractoriness underlies cellular arrhythmogenesis in a postinfarction model of sudden cardiac death. Circ Res. 2012;110:569–577. doi: 10.1161/CIRCRESAHA.111.260455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomaselli GF, Marban E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res. 1999;42:270–283. doi: 10.1016/s0008-6363(99)00017-6. [DOI] [PubMed] [Google Scholar]
- 5.de Waard MC, van der Velden J, Bito V, Ozdemir S, Biesmans L, Boontje NM, et al. Early exercise training normalizes myofilament function and attenuates left ventricular pump dysfunction in mice with a large myocardial infarction. Circ Res. 2007;100:1079–1088. doi: 10.1161/01.RES.0000262655.16373.37. [DOI] [PubMed] [Google Scholar]
- 6.Edes I, Kiss E, Kitada Y, Powers FM, Papp JG, Kranias EG, et al. Effects of Levosimendan, a cardiotonic agent targeted to troponin C on cardiac function and on phosphorylation and Ca2+ sensitivity of cardiac myofibrils and sarcoplasmic reticulum in guinea pig heart. Circ Res. 1995;77:107–113. doi: 10.1161/01.res.77.1.107. [DOI] [PubMed] [Google Scholar]
- 7.Huke S, Knollmann BC. Increased myofilament Ca2+-sensitivity and arrhythmia susceptibility. J Mol Cell Cardiol. 2010;48:824–833. doi: 10.1016/j.yjmcc.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baudenbacher F, Schober T, Pinto JR, Sidorov VY, Hilliard F, Solaro RJ, et al. Myofilament Ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. J Clin Invest. 2008;118:3893–3903. doi: 10.1172/JCI36642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schober T, Huke S, Venkataraman R, Gryshchenko O, Kryshtal D, Hwang HS, et al. Myofilament Ca sensitization increases cytosolic Ca binding affinity, alters intracellular Ca homeostasis, and causes pause-dependent Ca-triggered arrhythmia. Circ Res. 2012;111:170–179. doi: 10.1161/CIRCRESAHA.112.270041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh YS, Jong AY, Kim DT, Li H, Wang C, Zemljic-Harpf A, et al. Spatial distribution of nerve sprouting after myocardial infarction in mice. Heart Rhythm. 2006;3:728–736. doi: 10.1016/j.hrthm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Takagawa J, Zhang Y, Wong ML, Sievers RE, Kapasi NK, Wang Y, et al. Myocardial infarct size measurement in the mouse chronic infarction model: comparison of area- and length-based approaches. J Appl Physiol. 2007;102:2104–2111. doi: 10.1152/japplphysiol.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dou Y, Arlock P, Arner A. Blebbistatin specifically inhibits actin-myosin interaction in mouse cardiac muscle. Am J Physiol Cell Physiol. 2007;293:C1148–C1153. doi: 10.1152/ajpcell.00551.2006. [DOI] [PubMed] [Google Scholar]
- 13.Fedorov VV, Lozinsky IT, Sosunov EA, Anyukhovsky EP, Rosen MR, Balke CW, et al. Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm. 2007;4:619–626. doi: 10.1016/j.hrthm.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 14.Fauconnier J, Pasquie JL, Bideaux P, Lacampagne A, Richard S. Cardiomyocytes hypertrophic status after myocardial infarction determines distinct types of arrhythmia: role of the ryanodine receptor. Prog Biophys Mol Biol. 2010;103:71–80. doi: 10.1016/j.pbiomolbio.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Billman GE, Schwartz PJ, Stone HL. The effects of daily exercise on susceptibility to sudden cardiac death. Circulation. 1984;69:1182–1189. doi: 10.1161/01.cir.69.6.1182. [DOI] [PubMed] [Google Scholar]
- 16.Flevari P, Parissis JT, Leftheriotis D, Panou F, Kourea K, Kremastinos DT. Effect of levosimendan on ventricular arrhythmias and prognostic autonomic indexes in patients with decompensated advanced heart failure secondary to ischemic or dilated cardiomyopathy. Am J Cardiol. 2006;98:1641–1645. doi: 10.1016/j.amjcard.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 17.Houser SR, Margulies KB, Murphy AM, Spinale FG, Francis GS, Prabhu SD, et al. Animal models of heart failure: a scientific statement from the American Heart Association. Circ Res. 2012;111:131–150. doi: 10.1161/RES.0b013e3182582523. [DOI] [PubMed] [Google Scholar]
- 18.Yang F, Liu YH, Yang XP, Xu J, Kapke A, Carretero OA. Myocardial infarction and cardiac remodelling in mice. Exp Physiol. 2002;87:547–555. doi: 10.1113/eph8702385. [DOI] [PubMed] [Google Scholar]
- 19.van Oort RJ, McCauley MD, Dixit SS, Pereira L, Yang Y, Respress JL, et al. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation. 2010;122:2669–2679. doi: 10.1161/CIRCULATIONAHA.110.982298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshiyama M, Takeuchi K, Hanatani A, Kim S, Omura T, Toda I, et al. Differences in expression of sarcoplasmic reticulum Ca2+-ATPase and Na+-Ca2+ exchanger genes between adjacent and remote noninfarcted myocardium after myocardial infarction. J Mol Cell Cardiol. 1997;29:255–264. doi: 10.1006/jmcc.1996.0270. [DOI] [PubMed] [Google Scholar]
- 21.Gomez AM, Schwaller B, Porzig H, Vassort G, Niggli E, Egger M. Increased exchange current but normal Ca2+ transport via Na+-Ca2+ exchange during cardiac hypertrophy after myocardial infarction. Circ Res. 2002;91:323–330. doi: 10.1161/01.res.0000031384.55006.db. [DOI] [PubMed] [Google Scholar]
- 22.Belevych A, Kubalova Z, Terentyev D, Hamlin RL, Carnes CA, Gyorke S. Enhanced ryanodine receptor-mediated calcium leak determines reduced sarcoplasmic reticulum calcium content in chronic canine heart failure. Biophys J. 2007;93:4083–4092. doi: 10.1529/biophysj.107.114546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duncker DJ, Boontje NM, Merkus D, Versteilen A, Krysiak J, Mearini G, et al. Prevention of myofilament dysfunction by beta-blocker therapy in postinfarct remodeling. Circ Heart Fail. 2009;2:233–242. doi: 10.1161/CIRCHEARTFAILURE.108.806125. [DOI] [PubMed] [Google Scholar]
- 24.van der Velden J, Merkus D, Klarenbeek BR, James AT, Boontje NM, Dekkers DH, et al. Alterations in myofilament function contribute to left ventricular dysfunction in pigs early after myocardial infarction. Circ Res. 2004;95:e85–e95. doi: 10.1161/01.RES.0000149531.02904.09. [DOI] [PubMed] [Google Scholar]
- 25.Bers DM. Excitation and Contraction Coupling and Cardiac Contractile Force. 2nd ed. Dordrecht/Boston/London: Kluwer Academic Publishers; 2001. [Google Scholar]
- 26.Wannenburg T, Heijne GH, Geerdink JH, Van Den Dool HW, Janssen PM, De Tombe PP. Cross-bridge kinetics in rat myocardium: effect of sarcomere length and calcium activation. Am J Physiol Heart Circ Physiol. 2000;279:H779–H790. doi: 10.1152/ajpheart.2000.279.2.H779. [DOI] [PubMed] [Google Scholar]
- 27.Avner BS, Shioura KM, Scruggs SB, Grachoff M, Geenen DL, Helseth DL, Jr, et al. Myocardial infarction in mice alters sarcomeric function via post-translational protein modification. Mol Cell Biochem. 2012;363:203–215. doi: 10.1007/s11010-011-1172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perrino C, Schroder JN, Lima B, Villamizar N, Nienaber JJ, Milano CA, et al. Dynamic regulation of phosphoinositide 3-kinase-gamma activity and beta-adrenergic receptor trafficking in end-stage human heart failure. Circulation. 2007;116:2571–2579. doi: 10.1161/CIRCULATIONAHA.107.706515. [DOI] [PubMed] [Google Scholar]
- 29.Walker LA, Walker JS, Ambler SK, Buttrick PM. Stage-specific changes in myofilament protein phosphorylation following myocardial infarction in mice. J Mol Cell Cardiol. 2010;48:1180–1186. doi: 10.1016/j.yjmcc.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker NL, Burton FL, Kettlewell S, Smith GL, Cobbe SM. Mapping of epicardial activation in a rabbit model of chronic myocardial infarction. J Cardiovasc Electrophysiol. 2007;18:862–868. doi: 10.1111/j.1540-8167.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 31.Li YG, Wang QS, Israel CW, Gronefeld G, Lu SB, Ehrlich JR, et al. Quantitative analysis of the duration of slow conduction in the reentrant circuit of ventricular tachycardia after myocardial infarction. J Cardiovasc Electrophysiol. 2008;19:920–927. doi: 10.1111/j.1540-8167.2008.01155.x. [DOI] [PubMed] [Google Scholar]
- 32.Pouliopoulos J, Thiagalingam A, Eipper VE, Campbell C, Ross DL, Kovoor P. Transmural mapping of myocardial refractoriness and endocardial dispersion of repolarization in an ovine model of chronic myocardial infarction. Pacing Clin Electrophysiol. 2009;32:851–861. doi: 10.1111/j.1540-8159.2009.02399.x. [DOI] [PubMed] [Google Scholar]
- 33.Mills WR, Mal N, Forudi F, Popovic ZB, Penn MS, Laurita KR. Optical mapping of late myocardial infarction in rats. Am J Physiol Heart Circ Physiol. 2006;290:H1298–H1306. doi: 10.1152/ajpheart.00437.2005. [DOI] [PubMed] [Google Scholar]
- 34.Haqqani HM, Marchlinski FE. Electrophysiologic substrate underlying postinfarction ventricular tachycardia: characterization and role in catheter ablation. Heart Rhythm. 2009;6:S70–S76. doi: 10.1016/j.hrthm.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 35.Ripplinger CM, Lou Q, Li W, Hadley J, Efimov IR. Panoramic imaging reveals basic mechanisms of induction and termination of ventricular tachycardia in rabbit heart with chronic infarction: implications for low-voltage cardioversion. Heart Rhythm. 2009;6:87–97. doi: 10.1016/j.hrthm.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vermeulen JT. Mechanisms of arrhythmias in heart failure. J Cardiovasc Electrophysiol. 1998;9:208–221. doi: 10.1111/j.1540-8167.1998.tb00902.x. [DOI] [PubMed] [Google Scholar]
- 37.Miura M, Nishio T, Hattori T, Murai N, Stuyvers BD, Shindoh C, et al. Effect of nonuniform muscle contraction on sustainability and frequency of triggered arrhythmias in rat cardiac muscle. Circulation. 2010;121:2711–2717. doi: 10.1161/CIRCULATIONAHA.109.907717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kovacs M, Toth J, Hetenyi C, Malnasi-Csizmadia A, Sellers JR. Mechanism of blebbistatin inhibition of myosin II. J Biol Chem. 2004;279:35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- 39.Farman GP, Allen EJ, Schoenfelt KQ, Backx PH, de Tombe PP. The role of thin filament cooperativity in cardiac length-dependent calcium activation. Biophys J. 2010;99:2978–2986. doi: 10.1016/j.bpj.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lou Q, Li W, Efimov IR. The role of dynamic instability and wavelength in arrhythmia maintenance as revealed by panoramic imaging with blebbistatin vs. 2,3-butanedione monoxime. Am J Physiol Heart Circ Physiol. 2012;302:H262–H269. doi: 10.1152/ajpheart.00711.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Layland J, Grieve DJ, Cave AC, Sparks E, Solaro RJ, Shah AM. Essential role of troponin I in the positive inotropic response to isoprenaline in mouse hearts contracting auxotonically. J Physiol. 2004;556:835–847. doi: 10.1113/jphysiol.2004.061176. [DOI] [PMC free article] [PubMed] [Google Scholar]