Abstract
When evaluating clinical characteristics and outcomes in patients on hemodialysis, the prevalence and severity of comorbidity may change over time. Knowing whether updated assessments of comorbidity enhance predictive power will assist the design of future studies. We conducted a secondary data analysis of 846 prevalent hemodialysis patients from 5 US clinical centers enrolled in the HEMO study. Our primary explanatory variable was the Index of Coexistent Diseases score, which aggregates comorbidities, as a time-constant and time-varying covariate. Our outcomes of interest were all-cause mortality, time to first hospitalization, and total hospitalizations. We used Cox proportional hazards regression. Accounting for an updated comorbidity assessment over time yielded a more robust association with mortality than accounting for baseline comorbidity alone. The variation explained by time-varying comorbidity assessments on time to death was greater than age, baseline serum albumin, diabetes, or any other covariates. There was a less pronounced advantage of updated comorbidity assessments on determining time to hospitalization. Updated assessments of comorbidity significantly strengthen the ability to predict death in patients on hemodialysis. Future studies in dialysis should invest the necessary resources to include repeated assessments of comorbidity.
Keywords: Comorbidity, hemodialysis, HEMO study, hospitalization, mortality, Index of Coexistent Diseases (ICED)
INTRODUCTION
As the average age of the end-stage renal disease (ESRD) population increases, the burden of diabetes mellitus, cardiovascular disease, and other comorbid conditions has also increased.1 Previous studies have shown associations among baseline comorbid conditions and clinical outcomes including mortality and hospitalization,2–5 underscoring the importance of accounting for these factors in observational studies and clinical trials. Unlike some relatively straightforward confounding variables such as age or sex, the assessment of comorbidity can be a complex process.
Numerous indices have been used to aggregate comorbidity information, thereby providing comprehensive inclusion of multiple conditions while reducing the number of dimensions in an analytical model. Comorbidity indices have been shown to change over time, and these changes are strongly and independently associated with mortality.6,7 Despite this association, most published observational studies adjust for baseline comorbidity2–5 without updating the information over the follow-up period.
Detailed information on comorbidity was collected as part of the HEMO study, a randomized clinical trial conducted in 1846 prevalent hemodialysis patients. We conducted a secondary analysis of data from the HEMO study to test the hypothesis that when evaluating clinical characteristics and outcomes in patients on hemodialysis, the prevalence of comorbidity changes over time and that updated assessments of comorbidity materially enhance the predictive power.
MATERIALS AND METHODS
Study population
Details of the HEMO study protocol have been published previously.8 Briefly, the HEMO study was a randomized clinical trial of prevalent hemodialysis patients between 18 and 80 years of age from 15 US clinical centers and 72 dialysis units. Subjects were enrolled between March 1995 and October 2000 and randomly assigned in a 2 × 2 factorial design to standard-dose or high-dose equilibrated Kt/Vurea, and low-flux or high-flux dialyzer membranes. Subjects were followed until death or the administrative end of study (December 2001) and censored at the time of kidney transplant.
Explanatory variables
The primary explanatory variable in our analysis was the Index of Coexistent Diseases (ICED) score. The ICED score reflects a combination of scores from the Index of Disease Severity (IDS) and the Index of Physical Impairment (IPI).2 Scores from the IDS range from 0 (none) to 3 (moderate, severe manifestations despite treatment) and assess the following 19 disease categories: congestive heart failure, ischemic heart disease, arrhythmias and conduction problems, other heart disease and conditions such as valve disease or syncope, cerebral vascular disease, peripheral vascular disease, hypertension, ophthalmologic conditions, musculoskeletal and connective tissue disease, nonvascular nervous system disease, gastrointestinal disease, diabetes mellitus, respiratory disease, hepatobiliary disease, hematologic conditions, anticoagulation, urinary tract disease, malignancy, and HIV/AIDS. Scores from the IPI range from 0 (none) to 2 (serious impairment) and assess the following 11 categories of physical impairment: circulation, neurological, mental status, ambulation vision, respiratory, urinary, fecal, feeding, hearing and speech.
An algorithm combines the IDS and IPI scores to yield an ICED score ranging from 0 to 3, with higher scores representing more extensive and severe comorbid conditions. We used a modified ICED score as in the original HEMO study,9 which excluded diabetes mellitus, including it instead as a separate covariate in all analyses. Trained study coordinators compiled the ICED score at baseline and annually thereafter. Because < 0.1 % of patients had an ICED score of 0, we combined these scores with ICED=1 into a single category.
In multivariable models, we adjusted for age (per 10-year increment), sex, race (black vs. non-black), vintage (years on dialysis at time of enrollment), diabetes mellitus, mean baseline serum albumin concentration (per 0. 1g/dL increment), and intervention group (high-dose or standard-dose dialysis and high-flux or low-flux dialyzer membrane), stratified by the clinical center. We chose these covariates because they were prespecified and shown to be significant independent predictors of mortality in the HEMO study.9
Outcomes
The primary outcome was time from randomization to all-cause mortality. We additionally assessed time from randomization to first hospitalization and total hospitalizations. If a patient was discharged and readmitted the same day, we counted this as a single hospitalization event. All deaths were reported to the Data Coordinating Center (DCC) within 6 weeks of the event, and cause of death was adjudicated by the committee after review of the medical records. All hospitalizations were reported to the DCC within 2 weeks of the event, and hospital admission and discharge diagnoses were based on review of the discharge summary or telephone call to the attending physician. Because hospitalizations primarily for vascular access may not have the same risk factors as other types of hospitalizations, we conducted a companion analysis excluding vascular access-related hospitalizations.
Statistical methods
Baseline characteristics for the cohort are described as mean ± standard deviation for normally distributed variables, median with interquartile range for skewed variables, or frequencies for categorical variables. We compared differences among groups divided by the baseline ICED score using analysis of variance (ANOVA). Kruskal-Wallis tests and chi-square tests.
We evaluated the association of the ICED score with time from randomization to all-cause mortality using Cox proportional hazards regression in 2 ways. First, we analyzed the baseline ICED score as a fixed, time-constant variable. Second, we analyzed the ICED score as a time-varying variable. If the closest ICED score to an event was missing, we used the next closest available preceding ICED score as the explanatory variable, in a last observation carried forward (LOCF) manner. To assess the sensitivity of our assumption in the LOCF analysis, we conducted 2 separate companion analyses: one analysis included only those subjects with complete ICED scores (complete case analysis); the other analysis was performed after multiple imputation using a Markov chain Monte Carlo method10 to account for missing ICED scores.
We analyzed time from randomization to first hospitalization in an analogous fashion as all-cause mortality using Cox proportional hazards regression, described above. Total hospitalizations were analyzed using an extended Cox regression method as described by Andersen and Gill (AG).11 In this model, the subject’s time at risk begins from randomization to the first hospital admission day, followed by the day of discharge to the next hospital admission day, etc., until death or a censoring event; therefore, we did not count the duration of the hospital stay as time at risk.12 We considered 2 models: one with the ICED score as a baseline, time-constant predictor and the other with the ICED score as a time-varying predictor of total hospitalizations. When the ICED score is a time-varying predictor, the AG model relates the hazard for hospitalization at any given time point to the most recently measured ICED score.
We tested proportionality assumptions with log-negative-log and Schoenfeld residual plots.13 We calculated hazard ratios (HR) and 95% confidence intervals (95% CI) from model parameter estimates and standard errors, respectively. We conducted 2-sided tests using a 0.05 level of significance. All analyses were conducted with SAS Enterprise Guide 4.2 (Cary, NC, USA)
RESULTS
The HEMO study enrolled 1846 prevalent patients on hemodialysis; demographic and other baseline characteristics by baseline ICED score are shown in table 1. Subjects with higher baseline ICED scores were older and more often women, non-black, had diabetes mellitus, and lower baseline serum albumin concentrations. Subjects with higher baseline ICED scores experienced more deaths and hospitalizations than those with lower baseline ICED scores. Subjects were followed for a median of 2.5 years (interquartile range 1.3–4.3 years)
Table 1.
Demographic and other characteristics of the HEMO cohort at time of entry into the study by baseline ICED score
| Baseline ICED score
|
||||
|---|---|---|---|---|
| 0 or 1, N = 657 | 2, N = 577 | 3, N=612 | P | |
| Age (years), mean ± SD | 53.5 ± 14.9 | 60.7 ± 12.9 | 59.1 ± 13.1 | < 0.001 |
| Female sex (%) | 50.7 | 60.0 | 58.7 | 0.002 |
| Black race (%) | 66.8 | 63.6 | 57.4 | 0.002 |
| Cause of ESRD (%) | ||||
| Diabetic nephropathy | 22.8 | 38.3 | 51.5 | < 0.001 |
| Hypertension | 38.7 | 33.1 | 23.0 | |
| Glomerular disease | 17.8 | 14.0 | 9.2 | |
| PKD | 4.4 | 2.8 | 2.1 | |
| Other/unknown | 16.3 | 11.8 | 14.2 | |
| Diabetes (%) | 28.6 | 47.7 | 58.8 | < 0.001 |
| Vintage (years), median (IQR) | 2.0 (0.9–4.8) | 2.2 (1.0–4.7) | 2.2 (1.0–4.5) | 0.6 |
| Baseline serum albumin (g/dL), mean ± SD | 3.7 ± 0.4 | 3.6 ± 0.3 | 3.5 ± 0.4 | < 0.001 |
| Baseline single pool (Kt/VUrea), mean ± SD | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.2 | 0.9 |
| Deaths (N) | 198 | 303 | 370 | < 0.001 |
| Total hospitalizations (N) | 2348 | 2644 | 2874 | < 0.001 |
| Patients with N hospitalizations (%) | ||||
| 0 | 23.1 | 16.6 | 14.4 | < 0.001 |
| 1 | 17.8 | 12.3 | 15.0 | |
| 2 | 10.8 | 13.2 | 13.9 | |
| 3 | 10.7 | 9.0 | 10.0 | |
| 4 | 8.8 | 11.8 | 9.2 | |
| 5 | 7.0 | 7.6 | 7.7 | |
| 6 | 3.8 | 7.1 | 5.7 | |
| 7 | 4.0 | 3.1 | 3.8 | |
| 8 | 2.9 | 2.8 | 4.7 | |
| 9 | 2.6 | 2.4 | 2.0 | |
| 10 | 1.7 | 3.8 | 3.3 | |
| ≥11 | 6.7 | 10.2 | 10.5 | |
P values calculated using the W2 test, ANOVA, or the Kruskal-Wallis test.
ESRD=end-stage renal disease; ICED = Index of Coexistent Diseases; IQR=interquartile range; PKD-polycystic kidney diease.
ICED measurements
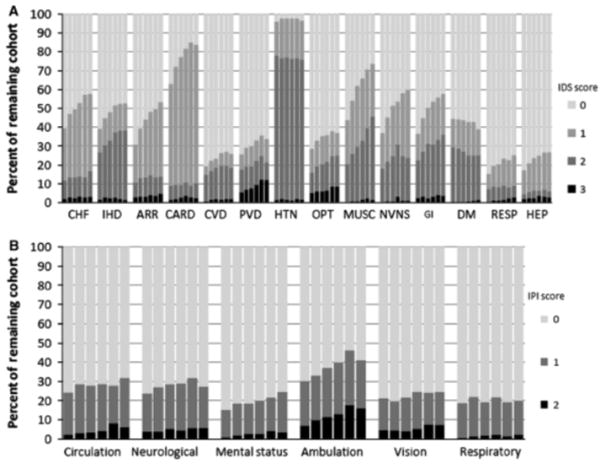
At each year of follow-up, over 92% of the remaining cohort had an ICED assessment (Table 2) While a large proportion of the remaining cohort had an increase in the ICED score, 9% to 15% of the remaining cohort had a decrease in the ICED score as compared with the baseline assessment (Figure 1). The HEMO study cohort had a high baseline prevalence of comorbid conditions, which generally increased during follow-up as reflected in increasing proportions of surviving patients with IDS scores 4.0 (Figure 2). For example, ischemic heart disease increased in prevalence from 39.3% at baseline to 52.7% at 5-year follow-up. The largest increase was observed in musculoskeletal and connective tissue diseases, which increased in prevalence from 43.8% at baseline to 73.6% at 5-year follow-up. Exceptions to the trend were hypertension, which had a relatively constant high prevalence of 4.95%, and diabetes mellitus, which decreased in prevalence by 12.2% from baseline to 5-year follow-up. Physical impairments, as indicated by the IPI score, tended to remain relatively constant during follow-up. One notable exception was impairment in ambulation, which increased in prevalence from 30.1% to 41.1% from baseline to 5-year follow-up.
Table 2.
Number of patients and ICED assessments at each year of follow-up and number of deaths and transplants that occurred before the next ICED assessment
| Year of follow-up | Patients in cohort | ICED assessments | Events before next ICED assessment
|
|
|---|---|---|---|---|
| Deaths | Transplants | |||
| Baseline | 1846 | 1846 | 223 | 78 |
| 1 | 1505 | 1422 | 228 | 35 |
| 2 | 989 | 940 | 151 | 36 |
| 3 | 708 | 656 | 116 | 20 |
| 4 | 458 | 430 | 100 | 7 |
| 5 | 279 | 270 | 47 | 2 |
| 6 | 112 | 106 | 6 | 1 |
ICED=Index of Coexistent Diseases.
Figure 1.
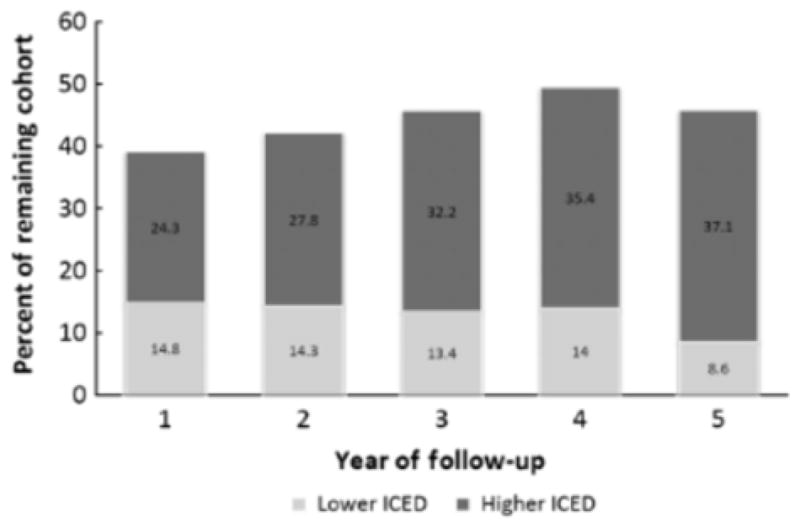
Percent of the remaining cohort at each year of follow-up with either a lower or a higher ICED score compared with the baseline assessment. ICED=Index of Coexistent Diseases.
Figure 2.
Prevalence and severity of (A) IDS and (B) IPI conditions per year of follow-up (from baseline to 5 years). Within each category, bars from left to right represent follow-up at baseline, year 1, year 2, year 3, year 4, and year 5. ARR=arrhythmias and conduction problems; CARD=other heart disease and conditions; CHP=congestive heart failure; CVD=cerebral vascular disease; DM = diabetes mellitus; GI = gastrointestinal; HEP=hepatobiliary disease; HTN=hypertension; IDS=the Index of Disease Severity; IHD=ischemic heart disease; IPI=Index of Physical Impairment; MUSC=musculoskeletal and connective tissue disease; NVNS=nonvascular nervous system disease; OPT ophthalmologic conditions; PVD = peripheral vascular disease; RESP = respiratory disease. Hematologic conditions, anticoagulation, urinary tract disease, malignancy, HIV/AIDS, were omitted in (A) and urinary, fecal, feeding, hearing and speech impairment categories were omitted in (B) because prevalence of these conditions were < 20% at any point of follow-up.
Relation of ICED score to mortality
There were a total of 871 deaths of any cause during the follow-up period. When analyzed as a time-constant variable, baseline ICED score was significantly associated with risk of all-cause mortality, but did not explain as much variability as age or serum albumin (Table 3). When we analyzed ICED score as a time-varying variable, the ICED score explained the highest fraction of variation of all parameters in the multivariable model (Table 3). Analyses conducted using complete case analysis or multiple imputation of missing ICED scores yielded nearly identical results (data not shown)
Table 3.
Adjusted Cox proportional hazards regression showing the relation of baseline and time-varying ICED score with risk of all-cause mortalitya
| Baseline ICED score
|
Time-Varying ICED score
|
|||||
|---|---|---|---|---|---|---|
| W2 | HR (95% CI) | P | W2 | HR (95% CI) | P | |
| ICED score (per 1 unit) | 48.0 | 1.37 (1.26–1.50) | <0.001 | 112.7 | 1.70 (1.54–1.88) | < 0.001 |
| Age (per 10 y) | 120.8 | 1.41 (1.33–1.50) | <0.001 | 104.8 | 1.38 (1.30–1.47) | < 0.001 |
| Female sex | 5.4 | 0.84 (0.73–0.97) | 0.02 | 6.2 | 0.83 (0.72–0.96) | 0.01 |
| Black race | 9.7 | 0.77 (0.66–0.91) | 0.002 | 8.2 | 0.79 (0.67–0.93) | 0.004 |
| Diabetes | 11.3 | 1.29 (1.11–1.50) | 0.001 | 6.8 | 1.22 (1.05–1.41) | 0.01 |
| Vintage (per 1 y) | 19.9 | 1.04 (1.02–1.06) | <0.001 | 18.7 | 1.04 (1.02–1.06) | < 0.001 |
| Baseline serum albumin (per 0.1 g/dL) | 67.8 | 0.92 (0.90–0.94) | <0.001 | 57.6 | 0.92 (0.90–0.94) | < 0.001 |
Models adjusted for each of the other listed variables and intervention group and stratified by clinical center
CI=confidence interval; HR=hazard ratio; ICED = Index of Coexistent Diseases.
Relation of ICED score to hospitalization
There were 7866 hospitalizations during the follow-up period. Only 18.3% of the HEMO study cohort had no hospitalizations during follow-up (Table 1). Both the baseline ICED score and the time-varying ICED score were both significantly associated with risk of first hospitalization and yielded similar HR (Table 4). Lack of significant increase in the magnitude or the association using the time-varying ICED score was expected, as the median time to first hospitalization was 233 days (interquartile range 83–516). Therefore, most patients had their first hospitalization between the baseline and 1-year ICED assessment. As with the analysis of first hospitalization, baseline and the time-varying ICED score yielded similar HR when we analyzed total hospitalizations (Table 4).
Table 4.
Adjusted Cox proportional hazards regression showing the relation of baseline and time-varying ICED score with risk of first and total hospitalizationsa
| First hospitalizations
|
Repeated hospitalizations
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline ICED score
|
Time-varying ICED score
|
Baseline ICED score
|
Time-varying ICED score
|
|||||
| b (SE) | HR (95% CI) | b (SE) | HR (95% CI) | b (SE) | HR (95% CI) | b (SE) | HR (95% CI) | |
| ICED score (per 1 unit) | 0.14 (0.03) | 1.15 (1.08–1.23) | 0.15 (0.03) | 1.17 (1.09–1.25) | 0.17 (0.02) | 1.19 (1.14–1.24) | 0.21 (0.02) | 1.24 (1.18–1.29) |
| Age (per 10 y) | 0.06 (0.02) | 1.06 (1.02–1.10) | 0.06 (0.02) | 1.06 (1.02–1.10) | 0.05 (0.01) | 1.05 (1.02–1.08) | 0.04 (0.01) | 1.04 (1.01–1.07) |
| Female sex | 0.13 (0.06) | 1.14 (1.02–1.27) | 0.13 (0.06) | 1.14 (1.02–1.27) | 0.20 (0.04) | 1.22 (1.14–1.31) | 0.20 (0.04) | 1.22 (1.13–1.31) |
| Black race | −0.12 (0.06) | 0.89 (0.79–1.01) | −0.12 (0.06) | 0.89 (0.79–1.01) | −0.008 (0.04) | 0.99 (0.91–1.08) | −0.004 (0.04) | 1.00 (0.99–1.01) |
| Diabetes | 0.16 (0.06) | 1.17 (1.04–1.30) | 0.14 (0.06) | 1.16 (1.04–1.30) | 0.16 (0.04) | 1.18 (1.09–1.27) | 0.16 (0.04) | 1.18 (1.09–1.26) |
| Vintage (per 1 y) | 0.008 (0.006) | 1.01 (1.00–1.02) | 0.007 (0.006) | 1.01 (1.00–1.02) | 0.002 (0.005) | 1.00 (0.99–1.01) | 0.001 (0.004) | 1.00 (0.99–1.01) |
| Serum albumin (per 0.1 g/dL) | −0.05 (0.008) | 0.95 (0.94–0.97) | −0.05 (0.008) | 0.95 (0.94–.0.97) | −0.04 (0.005) | 0.96 (0.95–0.97) | −0.04 (0.005) | 0.96 (0.95–0.97) |
Models adjusted for each of the other listed variables and intervention group and stratified by clinical center.
HR=hazard ratio; CI=confidence interval; ICED=Index of Coexistent Diseases; SE=standard error
In adjusted models, age (per 10-year increment) remained weakly associated with risk of first and total hospitalizations, but race and vintage were not significantly associated with risk of hospitalization (Table 4). Although female sex was associated with a reduced risk for death, it was associated with an increased risk for hospitalization. The results did not change materially when we excluded vascular access-related hospitalizations (data not shown).
Of note, a majority of patients (66.1%) in the HEMO study cohort had 4.1 hospitalization (Table 1) By considering total hospitalizations, we captured a 6-fold higher number of events than when only the first hospitalization was considered. In addition, we increased the power and precision of our analysis, as indicated by the smaller standard errors and subsequently narrower confidence intervals in the analysis of total vs. first hospitalization (Table 4).
DISCUSSION
The present analysis shows that in a cohort of prevalent hemodialysis patients, accounting for an updated comorbidity assessment allows us to explain a greater fraction of variability in mortality than accounting for baseline comorbidity alone. In models predicting time to death, the time-varying ICED score explained a greater fraction of variability than age, baseline serum albumin, diabetes, or any of the other model covariates. These results are consistent with those of 2 other studies examining change in comorbidity in patients on dialysis. Miskulin et al.6 analyzed incident dialysis patients enrolled in the Choices for Healthy Outcomes in Caring for ESRD (CHOICE) Study and showed that a 1-unit and 2-unit increase in ICED score from the previous year conferred an additional independent 48% (95% CI 4–110%) and 99% (95% CI 6–276%) increased risk of death, respectively. Similarly, Plantinga et al.,7 also using data from the CHOICE study, observed a significant, independent relation of ICED score with death across all time intervals when it was analyzed as a time-varying predictor, but not as a time-constant, baseline predictor.
Both baseline and time-varying ICED scores were among the strongest predictors of first and total hospitalizations in their respective models. Therefore, updating comorbidity over time did not confer much additional predictive power over baseline comorbidity. One possible explanation for our observed results may be that the ICED score is more sensitive in detecting conditions associated with death than with hospitalizations.
Our analysis confirms the high prevalence of comorbidity in patients on hemodialysis and shows that for a significant proportion of patients, the comorbidity index generally worsens over time. For the small proportion of patients who showed a decrease in the ICED score during follow-up, this may stem from the fact that several of the questions in the ICED address symptoms or events that occurred in the past year rather than at any time. Thus, a decrease in the ICED score over time possibly reflects fewer recent events associated with the comorbid condition rather than actual recovery from the comorbid condition.
Most categories of disease in the IDS increased in prevalence at each year of follow-up. Interestingly, in the CHOICE study, the IDS scores tended to increase at the 1-year follow-up time, but then decreased across several categories at the 2-year follow-up time. The authors observed that this possibly represented survival bias, with the sickest patients dying rather than recovering from disease. In general, mortality rates are higher in the first year after initiation of hemodialysis than in the second or third years of hemodialysis.14 The discrepancy between their analyses and ours may stem from the fact that the CHOICE study enrolled incident dialysis patients, while the HEMO study enrolled patients who had already survived hemodialysis for a median of 2.2 years at study entry. Our analysis therefore did not capture the ICED score of most patients during the first year of hemodialysis.
Our analysis has several limitations. First, because of death or a censoring event, we had smaller sample sizes at each year of ICED assessment. However, the HEMO study had excellent follow-up in the remaining cohort, as 4.92%) of eligible patients had comorbidity assessments at the annual study visit. Second, most of the HEMO study subjects were followed for a relatively short duration. Having longer follow-up for a greater proportion of patients would enhance our ability to study changes in comorbidity over time. Third, while the HEMO study subjects appeared to be similar to the general hemodialysis population, the HEMO study was a randomized clinical trial and subjects may be systematically different (and generally healthier) in ways that may not have been measured. Fourth, the HEMO study utilized the ICED, which was originally developed in a non-ESRD population. Although the ICED was later adapted for use in patients on dialysis,15–18 there could still be limitations in the validity of applying it to the hemodialysis population. Strengths of the ICED are its comprehensiveness, level of detail, and assessment of severity, rather than just the presence of disease. However, compilation of the ICED score requires specially trained personnel to accurately abstract information from the medical record, which can be cumbersome and resource intensive. Other comorbidity assessments such as the Charleson Comorbidity Index19 have been shown to be associated significantly with mortality in patients on dialysis20,21 and may be easier to administer than the ICED. Recently, Liu et al.22 developed a comorbidity index specific to patients on dialysis based on administrative data from the US Renal Data System. Although the index developed by Liu et al. outperformed the Charlson Comorbidity, it is unclear how this index might compare with the ICED
In summary, we have shown that updated assessments of comorbidity augment the capacity to predict death in patients on hemodialysis compared with baseline comorbidity assessment. Using baseline comorbidity assessments may be appropriate if studying long-term effects of putative risk factors on outcomes. However, the strong association of time-varying ICED score with mortality demonstrated in our analysis indicates that updating comorbidity assessments may be preferable for improved case-mix adjustment. Future cohort studies and clinical trials in dialysis should consider investing the necessary resources to include repeated assessments of comorbidity as often as is feasible
Acknowledgments
Dr Chang is supported by a Ruth L. Kirschstein National Research Service Award (NRSA) Institutional Research Training Grant (5 T32 DK7357)
References
- 1.USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2009. (US Renal Data System). [Google Scholar]
- 2.Athienites NV, Miskulin DC, Fernandez G, et al. Comorbidity Assessment in Hemodialysis and Peritoneal Dialysis Using the Index of Coexistent Disease [Article] Blackwell Publishing Limited; 2000. [DOI] [PubMed] [Google Scholar]
- 3.Khan IH, Catto GRD, MacLeod AM, Edward N, Fleming LW, Henderson IS. Influence of coexisting disease on survival on renal-replacement therapy. Lancet. 1993;341:415–418. doi: 10.1016/0140-6736(93)93003-j. [DOI] [PubMed] [Google Scholar]
- 4.Nicolucci A, Cubasso D, Labbrozzi D, et al. Effect of coexistent diseases on survival of patients undergoing dialysis. ASAIO J. 1992;38:M291–M295. doi: 10.1097/00002480-199207000-00040. [DOI] [PubMed] [Google Scholar]
- 5.van Manen JG, Korevaar JC, Dekker FW, Boeschoten EW, Bossuyt PM, Krediet RT. How to adjust for comorbidity in survival studies in ESRD patients: A comparison of different indices. Am J Kidney Dis. 2002;40:82–89. doi: 10.1053/ajkd.2002.33916. [DOI] [PubMed] [Google Scholar]
- 6.Miskulin DC, Meyer KB, Martin AA, et al. Comorbidity and its change predict survival in incident dialysis patients. Am J Kidney Dis. 2003;41:149–161. doi: 10.1053/ajkd.2003.50034. [DOI] [PubMed] [Google Scholar]
- 7.Plantinga LC, Fink NE, Levin NW, et al. Early intermediate, and long-term risk factors for mortality in incident dialysis patients: The Choices for Healthy Outcomes in Caring for ESRD (CHOICE) Study. Am J Kidney Dis. 2007;49:831–840. doi: 10.1053/j.ajkd.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 8.Greene T, Beck GJ, Gassman JJ, et al. Design and statistical issues of the hemodialysis (HEMO) study. Control Clin Trials. 2000;21:502–525. doi: 10.1016/s0197-2456(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 9.Eknoyan G, Beck GJ, Cheung AK, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347:2010–2019. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 10.SAS Institute Inc. SAS OnlineDoc(™): Version 8. Chapter 9. The MI Procedure. Cary, NC: SAS Institute Inc; 1999. pp. 130–200. [Google Scholar]
- 11.Andersen PK, Gill RD. Cox’s Regression Model for Counting Processes: A Large Sample Study. Ann Stat. 1982;10:1100–1120. [Google Scholar]
- 12.Guo Z, Gill TM, Allore HG. Modeling repeated time-to-event health conditions with discontinuous risk intervals. An example of a longitudinal study of functional disability among older persons. Methods Inf Med. 2008;47:107–116. [PMC free article] [PubMed] [Google Scholar]
- 13.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–241. [Google Scholar]
- 14.USRDS. 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2008. [Google Scholar]
- 15.Greenfield S, Aronow HU, Flashoff RM, Watanabe D. Flaws in mortality data: The hazards of ignoring comorbid disease. JAMA. 1988;260:2253–2255. [PubMed] [Google Scholar]
- 16.Greenfield S, Sullivan L, Silliman RA, Dukes K, Kaplan SH. Principles and practice of case mix adjustment Applications to end-stage renal disease. Am J Kidney Dis: Official J National Kidney Found. 1994;24:298–307. doi: 10.1016/s0272-6386(12)80195-8. [DOI] [PubMed] [Google Scholar]
- 17.Kravitz RL, Greenfield S, Rogers W, et al. Differences in the Mix of Patients Among Medical Specialties and Systems of Care: Results From the Medical Outcomes Study. JAMA. 1992;267:1617–1623. [PubMed] [Google Scholar]
- 18.Miskulin DC, Athienites NV, Yan G, et al. Comorbidity assessment using the Index of Coexistent Diseases in a multicenter clinical trial. Kidney Int. 2001;60:1498–1510. doi: 10.1046/j.1523-1755.2001.00954.x. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Beddhu S, Bruns FJ, Saul M, Seddon P, Zeidel ML. A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. Am J Med. 2000;108:609–613. doi: 10.1016/s0002-9343(00)00371-5. [DOI] [PubMed] [Google Scholar]
- 21.Miskulin DC, Martin AA, Brown R, et al. Predicting 1 year mortality in an outpatient haemodialysis population: A comparison of comorbidity instruments. Nephrol Dial Transplant. 2004;19:413–420. doi: 10.1093/ndt/gfg571. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Huang Z, Gilbertson DT, Foley RN, Collins AJ. An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int. 2010;77:141–151. doi: 10.1038/ki.2009.413. [DOI] [PubMed] [Google Scholar]