Abstract
Objective
To adapt an animal model of acute lung injury for use as a standard protocol for a screening, initial evaluation of limited function, or “surge,” ventilators for use in mass casualty scenarios.
Design
Prospective, experimental animal study.
Setting
University research laboratory.
Subjects
12 adult pigs.
Interventions
12 spontaneously breathing pigs (6 in each group) were subjected to acute lung injury/acute respiratory distress syndrome (ALI/ARDS) via pulmonary artery infusion of oleic acid. Following development of respiratory failure, animals were mechanically ventilated with a limited function ventilator (Simplified Automatic Ventilator [SAVe] I or II; Automedx) for one hour or until the ventilator could not support the animal. The limited function ventilator was then exchanged for a full function ventilator (Servo 900C; Siemens).
Measurements and Main Results
Reliable and reproducible levels of ALI/ARDS were induced. The SAVe I was unable to adequately oxygenate 5 animals, with PaO2 (52.0 ± 11.1 torr) compared to the Servo (106.0 ± 25.6 torr; p=0.002). The SAVe II was able to oxygenate and ventilate all 6 animals for one hour with no difference in PaO2 (141.8 ± 169.3 torr) compared to the Servo (158.3 ± 167.7 torr).
Conclusions
We describe a novel in vivo model of ALI/ARDS that can be used to initially screen limited function ventilators considered for mass respiratory failure stockpiles, and is intended to be combined with additional studies to defintively assess appropriateness for mass respiratory failure. Specifically, during this study we demonstrate that the SAVe I ventilator is unable to provide sufficient gas exchange, while the SAVe II, with several more functions, was able to support the same level of hypoxemic respiratory failure secondary to ALI/ARDS for one hour.
Keywords: mass casualty incidents; ventilators, mechanical; acute lung injury; oleic acid; disaster medicine; respiratory distress syndrome, adult
Introduction
Recent natural disasters, terrorist events, and the recent 2009 H1N1 influenza pandemic have compelled health care providers to consider the potential for mass respiratory failure.(1-5) No healthcare system currently has sufficient quantities of full-function critical care ventilators on hand for catastrophic needs.(5, 6) For scenarios such as a severe influenza pandemic, estimated shortfalls of mechanical ventilators are in the tens of thousands. Procurement and maintenance costs and logistical challenges prohibit stockpiles of sufficient quantities of full-function ventilators. Sophisticated transport ventilators with fewer functions than traditional intensive care unit ventilators have therefore been proposed by experts and professional societies as a “surge” ventilator supply in mass respiratory failure.(6, 7) Still, others believe that even more limited devices should be considered.(8-10) Many of these devices have technical and theoretical limitations,(11-13) yet their relatively low cost has led to early endorsement despite serious concerns regarding their capabilities.(11) To date there is not a standard approach for evaluating efficacy or effectiveness of surge mechanical ventilators.
At the minimum, a disaster mechanical ventilator should be capable of providing adequate gas exchange for the anticipated physiologic derangement. Most modern mass respiratory failure scenarios will likely lead to a surge of acute, hypoxemic respiratory failure or airflow obstruction. In most cases, hypoxemic respiratory failure due to ALI/ARDS would be the predominant conditions.(6, 14-17) The impact of a ventilator’s inability to deliver common respiratory parameters (e.g. respiratory rate, positive end expiratory pressure (PEEP), flow rate) to patients with ALI/ARDS remains uncertain.
In order to better evaluate “surge” ventilators, we describe a modification to a well-established porcine model of acute lung injury (18-21) that allows appropriate in vivo testing via simulation of acute hypoxemic respiratory failure with ALI/ARDS. We subsequently employ this model to test two new limited function ventilators that have been proposed for stockpiling. The ventilators, one of which has received Food and Drug Administration (FDA) approval for human use, have differing functional limitations. Neither has been previously tested using an animal model of lung injury.
Materials and Methods
Animal Preparation
All protocols were in accordance with the National Institutes of Health guidelines and were approved by the University of Washington Animal Care Committee and the Defense Department Animal Use Committee. Local community-bred pigs (n=12; 6 in each group; mean [±SD] weight 24.7 ± 2.9 kg) were sedated intramuscularly with ketamine (25mg/kg) and xylazine (2.5 mg/kg) to allow ear vein cannulation. A surgical plane of anesthesia was obtained with an initial bolus of thiopental sodium (20mg/kg) and maintained with a continuous infusion (10-20 mg/kg/hr). Animals were mechanically ventilated via a tracheostomy (Servo 900C; Siemens-Elema; Solna, Sweden) in the supine posture. Assist-controlled volume ventilation was adjusted to achieve a tidal volume (VT) of 8 to 10 ml/kg measured body weight, and respiratory rate was adjusted to achieve an end-tidal CO2 of 40 torr. PEEP was applied at 5 cm H2O.
Physiological Measurements
A femoral artery catheter was placed via cutdown for blood pressure measurement and arterial blood gas sampling. A femoral venous catheter was placed via cutdown for fluid and drug administration. An internal jugular catheter was placed via cutdown and a right heart catheter was advanced into the pulmonary artery to allow measurement of pulmonary artery pressure, pulmonary artery occlusion pressure (PAOP), mixed venous blood sampling, and administration of oleic acid. An esophageal balloon (Microtek Medical B.V., Zutphen, NL) was placed to measure esophageal pressure as a surrogate for pleural pressure. Body temperature was maintained between 36.0°C and 39.2°C using a heating pad.
Vascular pressures and esophageal pressures were measured intermittently (Mark 12 Data Management System, DMS 1000; Graphtec; Yokohama, Japan). Peak pressure, VT, respiratory rate, and minute ventilation (VE) were continuously measured with an in-line spirometer (Medical KORR Technologies; Salt Lake City, UT) and end-tidal CO2, heart rate, and transcutaneous O2 saturation were continuously monitored (CO2SMO; Novametrix Medical System Inc; Wallingford, CT). Mean arterial blood pressure, pulmonary arterial pressure, esophageal pressure, peak airway pressure and VT were digitally recorded on a personal computer (Power Lab data acquisition software; AD Instruments; Grand Junction, CO). Thermodilution cardiac outputs and core temperature were measured with a cardiac output computer (Sat-2, Baxter Edwards; Irvine, CA). Arterial and mixed-venous blood gases were analyzed at each experimental condition (ABL 5; Radiometer; Copenhagen, Denmark). Inspired O2 and mixed exhaled CO2 concentrations were measured with a spectrophotometer (MGA-1100; Perkin-Elmer Medical Instruments; Norwalk, CT).
All physiologic parameters were measured after 10 minutes of stabilization at baseline and under each experimental condition. Noninvasive measurements were monitored continuously. PaO2/FIO2 ratios were calculated. Since end-inhalation pause was not possible with the SAVe ventilators, lung compliance was estimated as VT/(Ppeak-Pesophageal). Dead space fraction (VD/VT) was estimated as (PaCO2-PĒCO2)/PaCO2.
To prevent circulatory collapse, a continuous infusion of warmed Lactated Ringers solution (LR) was maintained throughout oleic acid delivery. Evidence of hypovolemia prior to lung injury (PAOP less than 6 mm Hg) was treated with up to 250 ml of LR to achieve a PAOP greater than 6 mm Hg. Hypotension during oleic acid infusion was treated with LR administration (up to 3L, excluding LR for pre-injury hypovolemia) and boluses of epinephrine at 10-20 mcg per bolus. Oleic acid infusion was held at any point where mean arterial pressure fell below 60 mm Hg and resumed when mean arterial pressure was greater than 80 mm Hg. Circulatory collapse was treated with closed chest compressions, bag mask ventilation with FIO2 of 1.0, and intravenous fluids (up to 3L total volume, including volume administered for hypotension) and administration of epinephrine (20-40 mcg/bolus).
Ventilators
The Siemens 900C is a full feature ventilator [Table 1]. Its capabilities have been previously described.(22)
Table 1. Comparison of ventilator features.
| Feature | SAVe I | SAVe II | Servo |
|---|---|---|---|
| VT | Fixed (0.6 L) | Variable (0.2 – 1 L) | Variable (0 – 2L) |
| Respiratory Rate (breaths per minute) |
10 | 8-20 | 5-120 |
| PEEP (cm H2O) | 0 | 0-20 | 0-50 |
| PIP (cm H2O) | Up to 38 | Up to 60 | 0-100 |
| Inspiratory flow (L/min) |
16 | 10-80 | Adjustable to a maximum of 200 |
| Breath type | Volume | Volume | Volume, Pressure |
| Weight (kg) | 1.4 | 1.4 | 19 |
| Power | AC, Battery | AC, Battery | AC |
| Battery Life (hours) | 5.5 | 6.5 | NA |
| Price (US Dollars) | $1,695 | $1,995 | Not available |
Reported features for the Siemens Servo,(38) and the SAVe I & II (Personal communication, Automedx, Nov. 26, 2008)
The Simplified Automated Ventilator I (SAVe I; Automedx; Germantown, MD) is a limited function ventilator [Table 1] with manufacturer set VT and respiratory rate that is currently deployed with the United States Medical Corps and is available for purchase by medical and emergency medical services companies. The ventilator, as approved by the FDA for human use, has a set VT of 600ml and respiratory rate of 10 breaths per minute. For the purposes of this study, the ventilator was adjusted by the manufacturer to achieve a respiratory rate of 40 breaths per minute with a VT of approximately 210 ml due to the size of the animals being studied. Due to the potential risk of fire, the manufacturer recommended a limit of 6L/min oxygen entrainment. The ventilator is not capable of administering PEEP without use of an external PEEP valve.
The Simplified Automated Ventilator II (SAVe II; Automedx; Germantown, MD) is a second generation, limited function ventilator based on the SAVe I with the added capability of adjustable VT and respiratory rate, entrainment of up to 15L/minute of O2, and the ability to deliver and control PEEP (i.e. internal PEEP) [Table 1]. It has not received FDA approval at time of submission of this manuscript.
Both limited function ventilators have audible and visual alarms for circuit disconnect and high peak pressure. While the pressure alarm threshold is fixed on the SAVe I, it can be adjusted on the SAVe II. Both ventilators continue to deliver set VT despite triggering peak pressure alarms. Neither ventilator is capable of ventilator triggering by patient inspiratory effort. Both ventilators have a battery power supply and are intended to be portable mechanical ventilators. For the purposes of this study, the ventilators were powered using their provided AC power adapters. Each ventilator requires a specific set of circuit and ancillary tubing which were used in accordance with manufacturer recommendations. Neither ventilator requires a compressed gas source.
Lung Injury Protocol
In order to model acute respiratory failure, once surgical manipulation was complete and baseline values were obtained, continuous thiopental infusion was reduced until the animal was noted to breathe spontaneously while maintaining a deep plane of anesthesia (corresponding to a Richmond Agitation and Sedation Score (RASS) of −5). Baseline measurements were obtained and lung injury was accomplished via administration of 0.06 to 0.09 ml/kg oleic acid (O1630-25G; Sigma-Aldrich; St Louis, MO) via the proximal port of the right heart catheter with a goal to achieve lung injury as defined by a PaO2/FIO2 ratio of less than 300. Within each group, animals were randomly selected to receive oleic acid at either high, moderate, or low range of oleic acid to achieve a range of lung injury.
Due to change in availability of oleic acid during the course of the study, a purer form of oleic acid (O1008-25G; Sigma-Aldrich; St Louis, MO) was substituted for the second half of the study. Following this substitution of oleic acid, we witnessed a decrease in lung injury obtained (as measured by PaO2/FIO2 ratio); therefore, the dose of oleic acid was increased to 1.0-1.25 ml/kg for the second set of 6 pigs. Oleic acid infusion was performed in the same manner.
To minimize systemic hypotension during oleic acid infusion, oleic acid was diluted into saline to achieve 20 ml total infused volume. Due to the hydrophobic nature of oleic acid, 1 ml of air was also introduced into the glass syringe to facilitate mixing of the oleic acid with saline. A vortex machine (Maxi Mix II; Thermolyne; Dubuque, IA) kept the oleic acid mixed with the saline while being administered. Oleic acid was administered by hand injection over a 10 to 20 minute period. Following infusion, the catheter was flushed with saline to ensure all of the oleic acid was administered.
Experimental Protocol
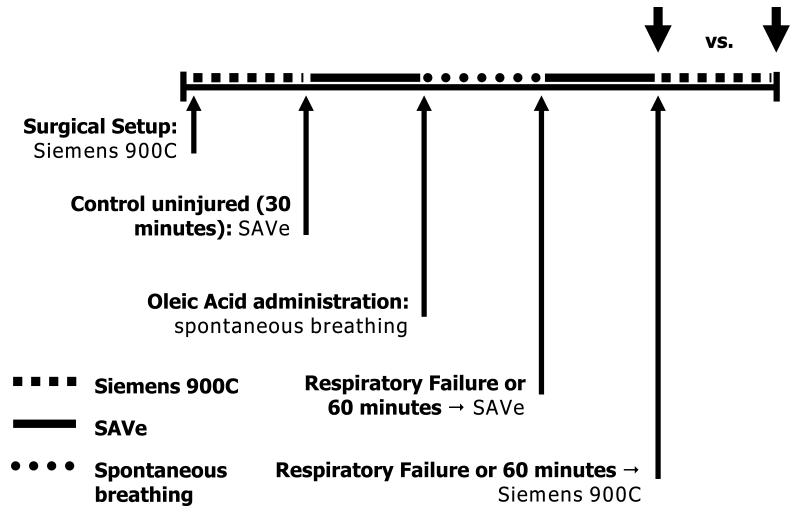
Each animal was ventilated with the Siemens 900C, followed by the SAVe I (first 6 animals) or SAVe II (second 6 animals) prior to lung injury. The ventilators were tested independently with sequential groups of pigs. Following baseline data acquisition, anesthesia was decreased until sustained spontaneous ventilation was noted, and lung injury was established [Figure].
Figure. Flow diagram of experimental model.
Each animal was initially ventilated while baseline data was obtained. Anesthesia was then lightened to allow for spontaneous respirations. Lung injury was then induced. Pigs continued to breathe spontaneously until respiratory failure when they were moved to SAVe ventilator. Upon failure of the SAVe ventilator, or after a one hour period, they were ventilated with the Siemens Servo 900C. Comparisons of ventilator capability to support each pig was made at the end of each time period (Large arrows).
Following lung injury, spontaneous breathing animals were monitored for evidence of respiratory failure: respiratory rate greater than 50 or less than 20 breaths per minute, SpO2 less than 80% despite administration of 100% O2, apnea, or cardiac arrest. Following spontaneous respiratory failure, the SAVe ventilator was used to provide respiratory support and anesthesia was returned to a surgical plane (RASS −5) by increasing the infusion rate of thiopental.
Support was continued with the SAVe ventilator until evidence of respiratory failure was noted: SpO2 less than 80% despite maximal oxygen and PEEP delivery (if PEEP was able to be delivered), PaCO2 greater than 80 torr, or PaO2 less than 45 torr. Once respiratory failure was noted, the Servo 900C was substituted and adjustments were made to correct any noted physiologic deficits while targeting VT of 6ml/kg. As peak injury from oleic acid occurs one hour following infusion,(23) the animal was supported with the SAVe for one hour if respiratory failure did not develop, and then transitioned to the Servo 900C.
Since the SAVe I was not capable of delivering PEEP, increased FIO2 was used as the primary method for treating hypoxemia with all ventilators; PEEP could be added for refractory hypoxia, however, while on the Servo. When transitioning from the SAVe II to the Servo, PEEP was initially applied at the same level provided by the SAVe II. This could then be titrated to maintain appropriate oxygen saturations.
Statistical Analysis
Values are reported as mean ± standard deviation. Statistical analyses were performed using STATA version 10 for Windows (StataCorp LP, College Station, TX, USA). T-tests were used to compare continuous variables. A p value <0.05 was used to determine statistical significance.
Results
Save I
There was no difference in PaO2 or PaO2/FIO2 ratio at baseline whether ventilated by Servo or SAVe I [Table 2]. However, PaCO2 was lower while undergoing baseline ventilation with the SAVe I and VE was greater. Lung compliance tended to be greater during baseline ventilation on the Servo, though this difference was not statistically significant. There was no difference in baseline mean systemic blood pressure, pulmonary artery pressure, or cardiac output.
Table 2. Baseline measured physiologic parameters for ventilation with the SAVe I, SAVe II, or Siemens Servo 900C.
| SAVe I(n=6) | SAVe II (n=6) | |||
|---|---|---|---|---|
| Variable | Servo | SAVe I | Servo | SAVe II |
| PaO2, torr | 92.5 ± 9.1 | 92.5 ± 14.1 | 96.3 ± 3.7 | 98.5 ± 6.3 |
| PaCO2, torr | 37.7 ± 1.8* | 27.7 ± 4.2* | 38.3 ± 2.9 | 36.5 ± 3.1 |
| VE, L/min | 5.7 ± 1.5† | 8.3 ± 0.8† | 5.3 ± 1.6 | 5.1 ± 1.3 |
|
Peak airway
pressure, cm H2O |
16.7 ± 2.1 | 14.7 ± 3.4 | 16.9 ± 2.3§ | 20.5 ± 2.5§ |
|
Compliance,
ml/cm H2O |
21.3 ± 9.0 | 15.6 ± 5.2 | 27.8 ± 10.4‡ | 16.8 ± 3.0‡ |
| PaO2/FIO2 ratio | 440.5 ± 43.2 | 440.5 ± 67.1 | 458.7 ± 17.5 | 463.9 ± 24.0 |
|
Mean BP, mm
Hg |
106.5 ± 9.8 | 99.8 ± 13.9 | 97.7 ± 10.2 | 97.7 ± 7.3 |
|
Mean
pulmonary artery pressure, mm Hg |
17.5 ± 3.0 | 15.6 ± 3.3 | 15.6 ± 2.6 | 15.9 ± 2.3 |
|
Cardiac
Output, L/min |
2.47 ± 0.40 | 2.83 ± 0.60 | 2.58 ± 0.62 | 2.79 ± 0.57 |
| PAOP | 7.63 ± 0.98+ | 5.83 ± 0.98+ | 7.50 ± 1.87 | 7.42 ± 2.11 |
Values are mean ± SD. Comparisons are made between Siemens Servo and SAVe ventilator in each group. p>0.05 unless specified.
p=0.001
p=0.007
p=0.02
p=0.048
p=0.01.
One of six animals suffered cardiac arrest during oleic acid infusion but was resuscitated and the experiment was completed. All animals were included in the final analysis. Doses of oleic acid ranged from 0.06 ml/kg to 0.09 ml/kg with a median dose of 0.08 ml/kg. The degree of lung injury varied between animals with PaO2/FIO2 ratio ranging from 91 to 288 (median 129). Measured dead space fraction (measured while ventilated on the Siemens) increased from 0.49 ± 0.06 pre-injury to 0.68 ± 0.09 after injury (p=0.003), while measured compliance decreased from 21.3 ± 9.0 ml/cm H2O to 8.8 ± 1.9 ml/cm H2O (p=0.03) following lung injury.
The SAVe I ventilator was capable of supporting only one of the six animals after development of acute lung injury. For the remaining five animals, the SAVe I was not capable of maintaining measured oxygen saturation greater than 80% for at least one hour after completion of oleic acid infusion. In contrast, the Siemens Servo 900C was capable of maintaining specified clinical variables in all 6 animals. PaO2 was lower while being ventilated with the SAVe I ventilator compared to the Servo [Table 3]. The decrease in PaO2 coincided with a lower FIO2 and PEEP, although the difference in FIO2 delivered was not statistically significant. There was no statistically significant difference in PaO2/FIO2 ratio, PaCO2, VE, pulmonary compliance, peak airway pressure, mean systemic blood pressure, mean pulmonary artery pressure, PAOP, or cardiac output when ventilated with the SAVe I or Siemens Servo.
Table 3. Physiologic variables at failure of mechanical ventilation or peak lung injury while being supported with SAVe I or Siemens 900C. (n=6).
| Variable | Servo | SAVe I |
|---|---|---|
| PaO2, torr | 106.0 ± 25.6* | 52.0 ± 11.1* |
| PaCO2, torr | 57.8 ± 11.3 | 56.3 ± 19.7 |
| VE, L/min | 5.9 ± 2.0 | 5.5 ± 1.1 |
| Compliance, ml/cm H2O | 8.8 ± 1.9 | 5.9 ± 2.9 |
| PaO2/FIO2 ratio | 147.2 ± 71.1 | 93.2 ± 33.2 |
| Mean BP, mm Hg | 97.3 ± 18.4 | 106.7 ± 25.9 |
|
Mean pulmonary artery
pressure, mm Hg |
35.1 ± 9.1 | 46.7 ± 17.2 |
| Cardiac Output, L/min | 1.55 ± 0.53 | 1.71 ± 0.51 |
| PAOP, mm Hg | 12.50 ± 4.04 | 14.30 ± 6.06 |
| PEEP, cm H2O | 6.7 ± 3.2† | 0† |
| FIO2 | 0.80 ± 0.25 | 0.58 ± 0.06 |
|
Peak airway pressure,
cm H2O |
30.8 ± 7.2 | 29.5 ± 6.9 |
Values are mean ± SD. Comparisons are made between Siemens Servo and SAVe I ventilator. p>0.05 unless specified.
p=0.002
p=0.003.
SAVe II
There was no difference in PaO2, PaCO2, VE, or PaO2/FIO2 ratio at baseline whether ventilated by Servo or SAVe II [Table 2]. Measured lung compliance tended to be greater during baseline ventilation on the Servo while peak airway pressures were greater while being ventilated with the SAVe II. There was no difference in baseline mean systemic blood pressure, pulmonary artery pressure, PAOP or cardiac output.
Doses of oleic acid ranged from 0.090 ml/kg to 0.125 ml/kg with a median dose of 0.124 ml/kg. The degree of lung injury varied between animals with PaO2/FIO2 ratio ranging from 97 to 303 (median 193), with one animal not achieving lung injury. Measured dead space (measured while ventilated on the Servo) increased from 0.47 ± 0.04 pre-injury to 0.64 ± 0.13 after injury (p=0.02), while measured compliance decreased from 27.8 ± 10.4 ml/cm H2O to 10.4 ± 4.1 ml/cm H2O (p=0.008) following lung injury.
The SAVe II ventilator was capable of supporting all six animals for at least one hour after development of acute lung injury based on pre-specified clinical requirements using lung protective ventilation and permissive hypercapnia. There was no statistically significant difference in measured PaO2, PaCO2, VE, compliance, PaO2/FIO2 ratio, PAOP, cardiac output, peak airway pressure, PEEP, FIO2, mean systemic blood pressure, or pulmonary artery pressure when ventilated with the SAVe II or the Servo [Table 4].
Table 4. Physiologic variables at failure of mechanical ventilation or peak lung injury while being supported with SAVe II or Siemens 900C. (n=6).
| Variable | Servo | SAVe II |
|---|---|---|
| PaO2, torr | 158.3 ± 167.7 | 141.8 ± 169.3 |
| PaCO2, torr | 58.5 ± 17.0 | 52.5 ± 10.4 |
| VE, L/min | 5.2 ± 1.4 | 5.0 ± 1.3 |
| Compliance, ml/cm H2O | 10.4 ± 4.1 | 8.6 ± 3.8 |
| PaO2/FIO2 ratio | 244.2 ± 140.8 | 224.8 ± 145.8 |
| Mean BP, mm Hg | 94.5 ± 22.5 | 90.8 ± 22.0 |
|
Mean pulmonary artery
pressure, mm Hg |
32.2 ± 5.4 | 32.7 ± 4.0 |
| Cardiac Output, L/min | 2.04 ± 0.72 | 2.03 ± 0.75 |
| PAOP, mm Hg | 10.58 ± 3.77 | 11.50 ± 4.85 |
| PEEP, cm H2O | 7.7 ± 2.6 | 5.7 ± 1.2 |
| FIO2 | 0.61 ± 0.30 | 0.58 ± 0.30 |
|
Peak airway pressure,
cm H2O |
31.7 ± 7.3 | 36.2 ± 10.8 |
Values are mean ± SD. Comparisons are made between Siemens Servo and SAVe II ventilator. p>0.05 for all comparisons.
There was no difference in lung compliance, PaO2/FIO2 ratio, measured dead space fraction, or peak airway pressure between the two groups of pigs at the start of the experiment or at the end of the experiment (as measured while being ventilated with the Siemens Servo 900C).
Discussion
We demonstrated the utility of an oleic acid model of acute lung injury modified to replicate acute hypoxemic respiratory failure allowing initial screening of limited function ventilators that might be stockpiled for mass respiratory failure events. Using this model, we demonstrated that the limited-feature device (SAVe I) was unable to adequately oxygenate animals even for a short period of time, while the additional features of the SAVe II allowed successful short-term ventilation and oxygenation despite severe lung injury. The SAVe I’s failure was likely due to ventilator limitations rather than extreme levels of respiratory failure, as the same animals were able to be “rescued” using a Servo 900C and supplemental oxygen. Although there was a wide range of variability in the degree of acute lung injury obtained, by using a cross-over design we were able to demonstrate that the SAVe I was not capable of supporting any level of lung injury for one hour, while the Servo was able to support a similar degree of lung injury. No significant difference in PaO2/FiO2 ratio was present in the animals at peak lung injury or respiratory failure when ventilated by the SAVe I ventilator or Servo; the non-significant difference that was observed likely reflects the difference in PEEP between the two groups more than a difference in the severity of lung injury.
The respiratory alkalosis observed in the animals on the SAVe I ventilator prior to lung injury is a consequence of the fixed respiratory rate and tidal volume on the device. These parameters were set by the manufacturer in anticipation of the ventilatory needs of the animal after lung injury. This highlights the importance of adjustable rate and tidal volume on a limited-function surge ventilator, which should be capable of providing ventilation to patients with varying degrees of lung injury.
In this experiment, the FDA-approved SAVe I, a device with a compressor capable of flows of 16 L/min and with a fixed respiratory rate and tidal volume, was unable to adequately oxygenate a porcine model of oleic acid induced acute lung injury. In contrast, the SAVe II ventilator, which incorporates a compressor capable of flow at 80 L/min, adjustable VT and respiratory rate, and the ability to deliver FIO2 up to 1.0 and maintain PEEP, was able to both oxygenate and ventilate the pigs for up to one hour. Despite similar lung injury between the SAVe I and the SAVe II groups, there was no evidence of failure of the SAVe II over the course of one hour. This difference is likely attributable to the SAVe II’s ability to provide higher levels of FIO2 and PEEP than could be provided by the SAVe I. This provides experimental support for expert statements that “surge” ventilators should be able to provide PEEP.(7, 15, 16)
Some disaster planners have proposed that limited feature ventilators be used as an initial device until patients with worsening respiratory failure require ventilators with additional features. The epidemiology of mechanical ventilation requirements for critically ill medical patients does not support this stepwise expectation. For such patients, where respiratory failure progresses to require mechanical ventilation, the initial settings are usually quite severe.(24, 25) The protocol in this study was developed to accurately mimic the typical early course of ALI/ARDS in previously spontaneously breathing subjects.(26, 27)
Although some have argued that few disaster victims who require respiratory failure will have ALI/ARDS, this has not born out in clinical studies or recent experience.(28) The majority of H5N1 patients with respiratory failure have ARDS,(29, 30) as do patients with seasonal flu(31) or H1N1 influenza.(32-36) Hence, we believe that our model, which allows for titration of PaO2/FIO2 abnormalities across a range of lung injury, has utility for initially evaluating surge mechanical ventilators. In fact, in light of reports of very severe ARDS with PaO2/FIO2 less than 100 in patients with respiratory failure due to novel H1N1, this model could be titrated to screen out inadequate devices. We acknowledge that some of the limited function devices will be allocated to lesser ill patients, patients improving and patients ventilated for reasons other than pulmonary pathology (e.g. traumatic brain injury). Yet the ability for these devices to have some utility for the predominant cause of the mass respiratory failure remains a logical requirement for stockpiling.
Of note, while the features of the SAVe II ventilator are more compatible with those of the ARDSnet ventilatory protocol (24) than the SAVe I device (permitting greater respiratory rate, PEEP settings, and adjustable tidal volumes), the device is not fully capable of the protocol’s requirements. Specifically, its maximum respiratory rate, its maximum PEEP, the lack of adjustable inspiratory:expiratory duration or flow and the lack of plateau pressure monitors all limit its ability to match the protocol’s requirements. Moreover, the device is capable only of Controlled Mechanical Ventilation, not Volume Assist-Control Ventilation as directed by the protocol; this limitation would likely mandate either paralysis or high levels of sedation for patients.
Currently, there is no standard against which to evaluate limited function or “surge” ventilators. While expert opinion has offered suggestions for minimal functional capabilities, there is no consensus. Some ventilators with fewer features and limited clinical data continue to be endorsed for use in stockpiling.(8, 37) Animal models of acute lung injury such as ours can provide a rapid assessment of minimal ventilator capabilities to target which limited function ventilators warrant further testing for use in clinical settings.
Although limited function ventilators are less expensive than full function ventilators, we believe that clinical requirements must determine the correct testing standards. While some have offered the polio epidemic of the 1950’s as evidence that patients suffering respiratory failure can be supported with limited supportive techniques, such patients suffered from neuromuscular failure rather than parenchymal lung disease.(16) Similarly, groups have published data suggesting a single ventilator could ventilate several patients,(8) although this method has not been adequately tested in the setting of lung injury.(10)
Our in vivo model has limitations. The ventilators were only tested for a short duration, a function of the natural course of oleic acid-induced lung injury. The animals had limited spontaneous breathing once respiratory failure ensued (RASS −5); they were therefore not challenged with ventilator dysynchrony as commonly seen in humans with respiratory failure who retain a spontaneous ventilatory drive. This model is better equipped to assess ventilators’ ability to support hypoxemic respiratory failure than hypercapneic respiratory failure. Lastly, there was no noticeable airflow obstruction, which could require higher flow rates than achievable by some limited feature devices. Although our model may have utility in excluding proposed limited function ventilators for stockpiling, further testing for a longer duration is warranted prior to decisions to procure such devices on a large scale. Additionally, we believe that in vivo protocols should be established to mimic anticipated needs for spontaneously breathing patients, airflow obstruction, and severely ill pediatric patients in addition to longer term testing of ventilators that pass an initial evaluation.
Our protocol can be used as a screen so that devices with very limited utility are not purchased for mass respiratory failure stockpiles. However, further evaluation, including human testing, would still be necessary to prove clinical utility for devices that passed initial screening tests.
Conclusion
We describe a novel in vivo model of ALI/ARDS that can be used to initially screen limited function ventilators considered for mass respiratory failure stockpiles. We demonstrate that the SAVe I ventilator is unable to provide sufficient gas exchange, while the SAVe II ventilator is able to support a comparable level of hypoxemic respiratory failure secondary to ALI/ARDS for one hour. We recommend further study assessing longer duration and different etiologies and aspects of respiratory failure prior to large-scale procurement of these devices.
Acknowledgements
Robert Dickson performed the experiments and was a writer of the manuscript.
David Hotchkin developed the protocol, performed the experiments and was a writer of this manuscript.
Wayne Lamm developed the protocol, performed the experiments, and helped with manuscript preparation.
Carl Hinkson developed the protocol and helped with manuscript preparation.
David Pierson developed the protocol and helped with manuscript preparation.
Robb Glenny developed the protocol and helped with manuscript preparation.
Lewis Rubinson conceived of the project, developed the protocol, assisted with some of the experiments and was a major contributor to the manuscript.
Funding:
This work was supported by the National Institute of Health Grant HL07287; and Defense Department DARPA DSO Defense Sciences Research and Technology Grant 07-21-Open-BAA-WP-151 Dr. Hotchkin received funding from NIH. Dr. Dickson, Dr. Glenny, and Dr. Lamm received funding and a Defense Sciences Research and Technology Grant from the Department of Defense – DARPA. The other authors have not disclosed any potential conflicts of interest.
Footnotes
Disclosures:
Dr. Dickson has no conflicts of interest to disclose.
Dr. Hotchkin has no conflicts of interest to disclose.
Mr. Lamm has no conflicts of interest to disclose.
Mr. Hinkson has no conflicts of interest to disclose.
Dr. Pierson has no conflicts of interest to disclose.
Dr. Glenny has no conflicts of interest to disclose.
Dr. Rubinson has no conflicts of interest to disclose.
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the Department of Health and Human Services or its components
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.deBoisblanc BP. Black Hawk, please come down: reflections on a hospital’s struggle to survive in the wake of Hurricane Katrina. Am J Respir Crit Care Med. 2005;172(10):1239–1240. doi: 10.1164/rccm.2509004. [DOI] [PubMed] [Google Scholar]
- 2.Daugherty EL, Branson R, Rubinson L. Mass casualty respiratory failure. Curr Opin Crit Care. 2007;13(1):51–56. doi: 10.1097/MCC.0b013e3280129979. [DOI] [PubMed] [Google Scholar]
- 3.Rubinson L, Nuzzo JB, Talmor DS, et al. Augmentation of hospital critical care capacity after bioterrorist attacks or epidemics: recommendations of the Working Group on Emergency Mass Critical Care. Crit Care Med. 2005;33(10):2393–2403. doi: 10.1097/01.ccm.0000173411.06574.d5. [DOI] [PubMed] [Google Scholar]
- 4.Rubinson L, O’Toole T. Critical care during epidemics. Crit Care. 2005;9(4):311–313. doi: 10.1186/cc3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipsitch M, Riley S, Cauchemez S, et al. Managing and Reducing Uncertainty in an Emerging Influenza Pandemic. N Engl J Med. 2009;361(2):112–115. doi: 10.1056/NEJMp0904380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubinson L, Hick JL, Curtis JR, et al. Definitive care for the critically ill during a disaster: medical resources for surge capacity: from a Task Force for Mass Critical Care summit meeting, January 26-27, 2007, Chicago, IL. Chest. 2008;133(5 Suppl):32S–50S. doi: 10.1378/chest.07-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Association for Respiratory Care Guidelines for acquisition of ventilators to meet demands for pandemic flu and mass casualty incidents. 2006 [cited 2009 Feb. 5, 2009] Available from: http://www.aarc.org/resources/vent_guidelines.pdf.
- 8.Neyman G, Irvin CB. A Single Ventilator for Multiple Simulated Patients to Meet Disaster Surge. Acad Emerg Med. 2006;13(11):1246–1249. doi: 10.1197/j.aem.2006.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graansma C. Pandemic Ventilator Project. 2009 2008 April 26. [cited 2009 June 22, 2009] Available from http://panvent.blogspot.com/
- 10.Paladino L, Silverberg M, Charchaflieh JG, et al. Increasing ventilator surge capacity in disasters: ventilation of four adult-human-sized sheep on a single ventilator with a modified circuit. Resuscitation. 2008;77(1):121–126. doi: 10.1016/j.resuscitation.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Branson RD, Rubinson L. A single ventilator for multiple simulated patients to meet disaster surge. Acad Emerg Med. 2006;13(12):1352–1353. doi: 10.1197/j.aem.2006.10.002. author reply 1353-1354. [DOI] [PubMed] [Google Scholar]
- 12.Babic MD, Chatburn RL, Stoller JK. Laboratory evaluation of the Vortran Automatic Resuscitator Model RTM. Respir Care. 2007;52(12):1718–1727. [PubMed] [Google Scholar]
- 13.Vortran VAR. gas-powered resuscitators (also referred to as the Surevent) may spontaneously stop delivering breaths. Health Devices. 2007;36(12):404–406. [PubMed] [Google Scholar]
- 14.ECRI Preparing for Avian Influenza. Healthcare Hazard Management Monitor. 2006 Aug;19(12):1–8. [PubMed] [Google Scholar]
- 15.Rubinson L, Branson RD, Pesik N, et al. Positive-pressure ventilation equipment for mass casualty respiratory failure. Biosecur Bioterror. 2006;4(2):183–194. doi: 10.1089/bsp.2006.4.183. [DOI] [PubMed] [Google Scholar]
- 16.Branson RD, Johannigman JA, Daugherty EL, et al. Surge capacity mechanical ventilation. Respir Care. 2008;53(1):78–88. discussion 88-90. [PubMed] [Google Scholar]
- 17.McCracken J. Noninvasive ventilation during a mass-casualty event. Respir Care. 2008;53(7):916–917. author reply 917-920. [PubMed] [Google Scholar]
- 18.Schuster DP. ARDS: clinical lessons from the oleic acid model of acute lung injury. Am J Respir Crit Care Med. 1994;149(1):245–260. doi: 10.1164/ajrccm.149.1.8111590. [DOI] [PubMed] [Google Scholar]
- 19.Grotjohan HP, van der Heijde RM, Jansen JR, et al. A stable model of respiratory distress by small injections of oleic acid in pigs. Intensive Care Med. 1996;22(4):336–344. doi: 10.1007/BF01700456. [DOI] [PubMed] [Google Scholar]
- 20.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295(3):L379–399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang HM, Bodenstein M, Markstaller K. Overview of the pathology of three widely used animal models of acute lung injury. Eur Surg Res. 2008;40(4):305–316. doi: 10.1159/000121471. [DOI] [PubMed] [Google Scholar]
- 22.Jonson B, Richard J-C, Straus C, et al. Pressure-Volume Curves and Compliance in Acute Lung Injury. Evidence of Recruitment Above the Lower Inflection Point. Am J Respir Crit Care Med. 1999;159(4):1172–1178. doi: 10.1164/ajrccm.159.4.9801088. [DOI] [PubMed] [Google Scholar]
- 23.Sum-Ping ST, Symreng T, Jebson P, et al. Stable and reproducible porcine model of acute lung injury induced by oleic acid. Crit Care Med. 1991;19(3):405–408. doi: 10.1097/00003246-199103000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 25.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 26.Lobo SM, Lobo FRM, Lopes-Ferreira F, et al. Initial and Delayed Onset of Acute Respiratory Failure: Factors Associated with Development and Outcome. Anesth Analg. 2006;103(5):1219–1223. doi: 10.1213/01.ane.0000237433.00877.5a. [DOI] [PubMed] [Google Scholar]
- 27.Vincent J-L, Akça S, de Mendonça A, et al. The Epidemiology of Acute Respiratory Failure in Critically Ill Patients*. Chest. 2002;121(5):1602–1609. doi: 10.1378/chest.121.5.1602. [DOI] [PubMed] [Google Scholar]
- 28.Vincent JL, Sakr Y, Ranieri VM. Epidemiology and outcome of acute respiratory failure in intensive care unit patients. Crit Care Med. 2003;31(4 Suppl):S296–299. doi: 10.1097/01.CCM.0000057906.89552.8F. [DOI] [PubMed] [Google Scholar]
- 29.Yu H, Gao Z, Feng Z, et al. Clinical Characteristics of 26 Human Cases of Highly Pathogenic Avian Influenza A (H5N1) Virus Infection in China. PLoS ONE. 2008;3(8):e2985. doi: 10.1371/journal.pone.0002985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gruber PC, Gomersall CD, Joynt GM. Avian influenza (H5N1): implications for intensive care. Intensive Care Med. 2006;32(6):823–829. doi: 10.1007/s00134-006-0148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho YC, Wang JL, Wang JT, et al. Prognostic factors for fatal adult influenza pneumonia. J Infect. 2009;58(6):439–445. doi: 10.1016/j.jinf.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Chowell G, Bertozzi SM, Colchero MA, et al. Severe Respiratory Disease Concurrent with the Circulation of H1N1 Influenza. N Engl J Med. 2009 doi: 10.1056/NEJMoa0904023. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease C, Prevention Update: novel influenza A (H1N1) virus infections - worldwide, May 6, 2009. MMWR Morb Mortal Wkly Rep. 2009;58(17):453–458. [PubMed] [Google Scholar]
- 34.Dominguez-Cherit G, Lapinsky SE, Macias AE, et al. Critically Ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009;302(17):1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 35.Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302(17):1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 36.Ramsey CD, Funk D, Miller RR, 3rd, Kumar A. Ventilator management for hypoxemic respiratory failure attributable to H1N1 novel swine origin influenza virus. Crit Care Med. 2010 Apr;38(4 Suppl):e58, 65. doi: 10.1097/CCM.0b013e3181cde600. [DOI] [PubMed] [Google Scholar]
- 37.Graansma C. A Proposal for an Open Source Design to Assemble Ventilators to Meet Pandemic Surge Demand. Pandemic Ventilator Project [Blog] 2007 March 11. 2007 [cited 2009 June 22, 2009] Available from: http://panvent.blogspot.com/
- 38.ZigmaMeditech Siemens Servo Ventilator 900C. 2009 [cited 2010 Feb. 26, 2010] Available from: www.zigmameditech.com/Tech/900C.pdf.