Summary
Delta and mu opioid receptors (DORs and MORs) are inhibitory G-protein coupled receptors that reportedly cooperatively regulate the transmission of pain messages by substance P and TRPV1-expressing pain fibers. Using a DOReGFP reporter mouse we now show that the DOR and MOR are, in fact, expressed by different subsets of primary afferents. The MOR is expressed in peptidergic pain fibers, the DOR in myelinated and nonpeptidergic afferents. Contrary to the prevailing view, we demonstrate that the DOR is trafficked to the cell surface under resting conditions, independently of substance P, and internalized following activation by DOR agonists. Finally, we show that the segregated DOR and MOR distribution is paralleled by a remarkably selective functional contribution of the two receptors to the control of mechanical and heat pain, respectively. These results demonstrate that behaviorally relevant pain modalities can be selectively regulated through the targeting of distinct subsets of primary afferent pain fibers.
Introduction
The delta and mu opioid receptors (DOR and MOR) are inhibitory G protein-coupled receptors (GPCR’s) through which endogenous opioids (endorphins and enkephalins) regulate a variety of physiological functions, including pain control, emotional tone, and reward (Kieffer and Gaveriaux-Ruff, 2002). The MOR also mediates the pain-relieving effects of some of the most clinically efficacious drugs. For example, the analgesia produced by morphine is lost in mice in which the gene that encodes the MOR is inactivated (Matthes et al., 1996; Sora et al., 1997b). The contribution of the DOR to pain processing is much less clear. Although some studies report that DOR-selective agonists exert potent analgesic effects (Narita and Suzuki, 2003; Onofrio and Yaksh, 1983; Porreca et al., 1987), others found that DOR agonists are relatively weak, particularly compared to morphine (Gallantine and Meert, 2005; Scherrer et al., 2004).
Yet another perspective is that a functional interaction between the two receptors contributes to opioid agonist-mediated pain control at the level of the spinal cord. For example, it has been reported that genetic inactivation or pharmacological blockade of the DOR can potentiate the pain-relieving effect of MOR agonists (Gomes et al., 2004; Gomes et al., 2001) and can counteract development of the tolerance that occurs with chronic morphine treatment (Zhu et al., 1999). This apparent negative cooperativity between the MOR and DOR may involve a direct interaction of the two receptors, via the formation of MOR-DOR heterodimers (Gomes et al., 2004); for review, see (Rozenfeld et al., 2007)). In fact, immunohistochemical studies demonstrated that the MOR and DOR are coexpressed in the same subpopulation of primary afferent “pain” fibers (nociceptors), namely in the small-diameter, peptidergic (substance P-(SP) and calcitonin gene-related peptide (CGRP)-containing unmyelinated afferents (Arvidsson et al., 1995a; Ji et al., 1995). As these peptidergic afferents express the heat-sensitive TRPV1 channel (Caterina et al., 2000; Caterina et al., 1997; Tominaga et al., 1998) it follows that both MOR and DOR agonists would regulate heat pain sensitivity, which is indeed what many studies have reported (Matthes et al., 1996; Narita and Suzuki, 2003; Sora et al., 1997b).
Processing of the two GPCRs in these neurons, however, is thought to be very different (Cahill et al. 2006, Zhang et al., 2006). In contrast to prototypical GPCRs, such as the MOR, the DOR is reportedly absent from the plasma membrane of the synaptic terminal of nociceptors, under resting conditions (Cahill et al., 2001; Gendron et al., 2006; Morinville et al., 2003; Patwardhan et al., 2005; Walwyn et al., 2005; Zhang et al., 1998). Rather, the DOR is transported to central terminals via the regulated secretory pathway, which results in the DOR being stored in the membrane of large, peptide-containing dense core vesicles (LDCVs; (Bao et al., 2003; Zhang et al., 1998). Functionality of the DOR only occurs when stimuli trigger exocytosis of LDCVs, resulting in their integration into the plasma membrane (Bao et al., 2003). This, in turn, renders the DOR accessible to opioid ligands. Recently, Guan et al (2005) provided insights into the mechanism through which the DOR is sorted to LDCVs. These authors discovered an interaction of SP with an extracellular loop of the DOR that is essential for proper DOR trafficking. When the SP-DOR interaction was disrupted, in mice in which the gene encoding SP was inactivated (ppt-A gene), the DOR was no longer transported to the terminals of nociceptors in the spinal cord.
Here we show that many of the existing conclusions concerning the DOR are not tenable. Using a DOReGFP reporter knockin mouse, we provide a substantially different view of the DOR and MOR distribution, function and relationship to the processing of pain messages.
Results
DOR is expressed in myelinated and nonpeptidergic unmyelinated pain fibers
We recently described a reporter knockin mouse in which a functional DOReGFP fusion receptor replaces the endogenous receptor (Scherrer et al., 2006). Here we took advantage of this mouse to address the contribution of the DOR to pain processing. We first examined the DOReGFP distribution in sensory neurons of dorsal root ganglia (DRG) immunostained with an antibody against GFP (Figure 1A) and found that 17% of sensory neurons expressed DOReGFP. Positive cells show intense labeling of the plasma membrane and the perinuclear region (Figure 1B) under resting conditions.I Intrathecal delivery of the DOR agonist SNC80 (i.e., directly into the CSF by lumbar puncture) triggered a profound internalization of DOReGFPs (Figure 1B). Based on these observations we conclude that the subcellular distribution and trafficking of DORs are characteristic of those of a prototypical GPCR, and that DORs expressed in sensory neurons can be targeted via the intrathecal route.
Figure 1.
DOR is not expressed by unmyelinated peptidergic nociceptors but predominates in myelinated and nonpeptidergic unmyelinated neurons of the dorsal root ganglia (DRG).
(A) DRG neurons from DOReGFP reporter mice immunostained with an anti-GFP antibody. 17.1% (± 2.5%, n=1236) of DRG cells express the DOReGFP.
(B) High power image shows the localization of DOReGFPs at the cell surface under resting conditions (arrow; plasmalemma/cytoplasm fluorescence density = 1.7 ± 0.1) and their profound internalization (plasmalemma/cytoplasm fluorescence density = 0.4 ± 0.1, n=12/group, p<0.001 (Student’s t-test)) 30 min after spinal delivery of SNC80 (10 nmoles).
(C)Size distribution of DOReGFP+ DRG neurons compared to those expressing SP, CGRP, IB4 or NF200. DOReGFP is expressed by two populations of cells, one of small diameter (39%) and a second of large diameter (61%).
(D) Most DOReGFP+ cells coexpress NF200 (56%), indicating that they are myelinated. Small diameter, unmyelinated cells that express DOReGFP are IB4+ (36%). Very few correspond to the peptidergic subpopulation (only 2% are SP+).
(E) Large diameter DOReGFP cells are NF200+.
(F) DOReGFP and TRPV2 are often co-expressed in large diameter neurons.
(G) DOReGFP and SP do not colocalize in DRG neurons.
(H) DOReGFP and TRPV1 almost never colocalize in DRG neurons.
(I) Small DOReGFP+ neurons bind IB4 and thus belong to the nonpeptidergic subset of unmyelinated nociceptors.
(J) DOReGFP and CGRP colocalization only occurs in some large diameter neurons.
All scale bars equal 20 μm. Arrows indicate cells where costaining occurs.
Based on previous studies using antibodies against the DOR, we expected that the DOReGFP+ DRG cell bodies would overlap with the peptide-containing subpopulation of unmyelinated nociceptors. This was not the case (Figures 1C and 1D; supplementary table 1). Instead, more than 61% of DOReGFP cells were of medium to large size and expressed NF200 (Figures 1D and 1E), a neurofilament marker of neurons with myelinated axons. In fact, 65% of the DOReGFP myelinated neurons coexpressed TRPV2, a channel that is restricted to myelinated afferents (Figure 1F and TableS1).
Strikingly, when we directly assessed the extent of colocalization of DOReGFP with markers of peptidergic unmyelinated nociceptors, SP, CGRP and the capsaicin and heat-sensitive channel, TRPV1, we found no overlap (Figures 1G, H). Rather, all small diameter DOReGFP neurons, which represent 39% of all DOReGFP DRG cells, bound the lectin IB4 (Figure 1I) and coexpressed the purinergic receptor, P2X3 (Figure S1), two features of the nonpeptidergic population of unmyelinated nociceptors. We found some colocalization of CGRP and DOReGFP, predominantly in the NF200+ neurons (Figure 1J). Together, our results indicate that DOReGFP is expressed in myelinated and nonpeptidergic unmyelinated DRG neurons, not in peptidergic unmyelinated nociceptors.
Dissociation of DOReGFP and substance P
Given the large literature reporting coexpression and functional interactions of the DOR and SP, our finding that DOReGFP almost never colocalizes with SP+ DRG neurons was completely unexpected. Because rapid transport of the DOReGFP from the cell body to the terminals of SP+ cells could have made detection of the DOReGFP in DRG cell bodies difficult, we next costained for SP and DOReGFP in both the central and peripheral terminals of primary sensory neurons. As expected, we found that SP+ terminals in the spinal cord are concentrated in the most superficial laminae (I and outer II) of the dorsal horn (Hökfelt et al., 1977) (Figure 2A). By contrast, the DOReGFP predominates in terminals in the most inner part of lamina II, a region defined by its large number of PKCγ interneurons (Figures 2C and 2D). That result is of particular interest as we recently reported that the PKCγ layer of interneurons, which has been implicated in the development of injury-induced persistent pain (Malmberg et al., 1997) receives a myelinated primary afferent input (Neumann et al., 2008).
Figure 2.
DOR and substance P do not colocalize in the central or peripheral terminals of sensory neurons.
(A) SP and DOReGFP+ terminals are located in different laminae of the superficial dorsal horn (I-IIo: laminae I and outer II).
(B) High power image of (A) shows the lack of colocalization of DOReGFP and SP in laminae I and outer II.
(C) DOReGFP signal is concentrated in the inner part of lamina II (IIi), a region that contains many PKCγ+ interneurons.
(D) High power image of (C).
(E) Double immunolabeling of the glabrous skin illustrates the dense innervation of the epidermis (e) and dermis (d) by DOReGFP+ fibers and the absence of colocalization with SP+ terminals.
(F) High power image shows that DOReGFP and SP fibers are distinct, but intertwined in the dermis of the glabrous skin.
(G) Many neurons of the nodose ganglion, which contains the cell bodies of visceral (vagal) afferents, are SP+, but almost none are DOReGFP+.
(H) There is intense SP staining in the nucleus of the solitary tract (NTS), which is the major medullary target of visceral afferents. By contrast, the NTS is devoid of DOReGFP.
(I) The bladder contains a dense innervation by SP+ terminals, but there is an almost complete absence of DOReGFP. Quantitative analysis indicated a mean of 34.4 ± 3.4 substance P positive fibers/mm versus 2.9 ± 0.7 DOReGFP positive fibers/mm, n=8/group, p < 0.001 (Student’s t-test).
We also observed a less dense band of DOReGFP staining in lamina I, but even here confocal analysis showed that the SP+ and DOReGFP+ terminals in lamina I do not overlap (Figure 2B). Figures 2A-C also show that there is light, relatively uniform DOReGFP staining throughout the grey matter (dorsal and ventral horns), which agrees with the distribution pattern of the DOR revealed in radioligand binding studies (Mennicken et al., 2003, Figure S2).
In the skin, we observed a dense plexus of DOReGFP axons that course through the dermis and epidermis, but no colocalization with SP axon terminals (Figures 2E and 2F). We conclude that there is no overlap of the DOR and SP, in either the central or peripheral terminals of nociceptors. Finally, we show that the segregated expression of DOR and SP is not restricted to somatic afferent nociceptors, but is particularly apparent for afferents that innervate viscera (Supplemental results, Figures 2 G-I, FigureS3). Together these results not only indicate that the DOR is not expressed in SP+ nociceptors, but also that the DOR is largely excluded from the innervation of visceral organs.
The DOR is a prototypical GPCR that is trafficked via the non-regulated pathway, independently of SP
Our finding that the DOR is expressed in myelinated and nonpeptidergic unmyelinated primary afferents and localized at the cell surface under resting conditions differs greatly from the prevailing view that is based on studies using anti-DOR antibodies. We therefore reexamined the specificity of the immunoreactivity generated with DOR antisera. The staining pattern that we obtained was identical to that reported in the literature, however, it did not change in tissues from two different mice strains with a deletion of the dor gene (Filliol et al., 2000; Zhu et al., 1999). See supplementary results, Figures S4 and S5). We conclude that this anti-DOR antibody, which is the most widely used, does not recognize the DOR in immunohistochemical preparations, but rather must cross-react with an as yet unidentified molecule. Additionally, we have tested several others commonly used anti-DOR antibodies, all of which equally immunostain tissue from wild-type and dor null mice (Figure S6).
These new observations clearly called into question the proposed influence of SP on trafficking of the DOR. We, therefore, crossed DOReGFP mice with mice in which the ppt-A gene encoding SP has been deleted (Cao et al., 1998). We confirm that the staining obtained with the anti-DOR antibody is lost in the spinal cord of ppt-A null mice (Figures 3A and 3B) but further show that the loss of staining also occurs in the DRG (Figure S7A), despite persistence of dor transcript (Figure S7B). On the other hand, we found no change in the DOReGFP signal in the spinal cord (Figures 3C and 3D). We also used radioligand binding on spinal cord sections to examine independently the distribution of the DOR in the ppt-A null mice. Figures 3E-H show that the pattern of DOR binding in the spinal cord is unchanged in the ppt-A null mice, consistent with the lack of change in the DOReGFP mice. Finally, high power confocal imaging revealed that DOReGFP is not only present at the plasma membrane in DRG neurons in ppt-A null mice (Figure S7D) but has the same location in the DRG neurons that coexpress DOReGFP and substance P (Figure S7C). Taken together, we conclude that transport of the DOR from DRG cell bodies to their central terminals occurs independently of SP. We propose that the DOR behaves as a prototypical GPCR that is continuously trafficked to the terminals and present at the cell surface in the resting state, where it can be activated and internalized by agonists, a hallmark of GPCRs.
Figure 3.
The DOR is transported to the central terminals of sensory neurons in the spinal cord, independently of substance P.
(A) Immunoreactivity obtained with the anti-DOR antibody is lost in mice with a deletion of the ppt-A gene.
(B) Quantification of results in A.
(C) DOReGFP in the spinal cord is not altered in ppt-A null mice, indicating that DOR transport from the cell body to the central terminals of sensory neurons is independent of SP.
(D) Quantification of results in C.
(E, G) The binding pattern obtained with the DOR selective ligands, [125I]-DPDPE (E) and [125I]-deltorphin II (G), is not altered in ppt-A null mice.
(F, H) Quantification of results in E, G.
Results are expressed as mean ± SEM, n=8/group, *** p < 0.001.
Segregation of the DOR and the MOR in nociceptors
As our new results bear directly on others that have implicated interactions of the DOR and MOR in the regulation of pain, we reexamined the relationship of the DOR and the MOR in primary afferents, by staining tissues from the DOReGFP mouse with an antiserum directed against the MOR. The specificity of this anti-MOR antibody is demonstrated by the loss of immunostaining in tissues from mor null mice (Figure S8). We found an almost complete segregation of the expression of the two opioid receptors in DRG neurons (Figure 4C) and in the spinal cord (Figure S9). Most importantly and in distinct contrast with the pattern of DOReGFP expression, the MOR is concentrated in the SP+ and TRPV1+ unmyelinated peptidergic nociceptors (Figures 4A and 4B). In fact, less than 5% of DOReGFP+ and/or MOR+ cells coexpress the two receptors. Interestingly, these rare neurons, in which the two receptors could dimerize, were mostly myelinated (NF200+) and of large diameter (Figure 4C). Consistent with its expression pattern in DRG neurons, MOR staining in the spinal cord is restricted to laminae I and IIo, where it partially colocalizes with SP and TRPV1 (Figures 4D and 4E).
Figure 4.
MOR, but not DOR, is expressed by TRPV1+ peptidergic nociceptors.
(A) Almost all DRG cells that express SP coexpress the MOR.
(B) MOR is expressed in almost all TRPV1+ nociceptors, in distinct contrast with the DOR (Figure 1).
(C) Less than 5% (4.8 ± 0.7%, n=1180) of the opioid receptor-expressing DRG cells coexpress the DOR and MOR.
(D) There is significant colocalization of MOR and SP in lamina I of the spinal cord. MOR staining extends ventrally to lamina II where SP and TRPV1 (E) are absent.
(E) MOR and TRPV1 staining colocalize in the superficial dorsal horn.
(F) DOReGFP and TRPV1 staining predominate in different laminae of the spinal cord.
(G) Intrathecal injection of a high dose of the TRPV1 agonist, capsaicin, destroys the central terminals of TRPV1+ nociceptors and eliminates TRPV1 staining in the spinal cord.
(H) Quantification of results in G.
(I) Reduction of MOR staining after intrathecal capsaicin (compare with B).
(J) Quantification of results in I.
(K) Intrathecal administration of capsaicin does not alter DOReGFP.
(L) Quantification of results in H.
Results are expressed as mean ± SEM, n=8/group, ** p < 0.01, *** p < 0.001.
To delve further into the association of DOR and MOR with TRPV1 afferents, we used lumbar puncture to make an intrathecal injection of the TRPV1-binding neurotoxin, capsaicin, which selectively destroys all central terminals of DRG neurons that express TRPV1 (Figures 4G and 4H). As predicted, we found a significant decrease of MOR staining in lamina I of the spinal cord (Figures 4I and 4J). There was residual MOR staining in the spinal cord, likely arising from the large number of MOR+ postsynaptic neurons (Aicher et al., 2000b; Arvidsson et al., 1995b; Trafton et al., 2000). By contrast, when we made intrathecal capsaicin injections in the DOReGFP mice, there was no decrease of DOReGFP in the spinal cord dorsal horn (Figures 4F, 4K and 4L), confirming that the MOR, but not the DOR, is expressed in TRPV1+, unmyelinated peptidergic afferents. Based on these experiments, we conclude that the MOR and DOR are differentially distributed in primary sensory neurons. The MOR is concentrated in peptidergic nociceptors; the DOR predominates in myelinated and nonpeptidergic unmyelinated primary afferents.
MOR and DOR differentially regulate heat and mechanical pain
As described above, previous studies reported comparable effects of MOR and DOR agonists in tests of pain sensitivity. Indeed, the great majority found that MOR and DOR agonists reduce heat pain (Matthes et al., 1996; Narita and Suzuki, 2003; Sora et al., 1997b). Given our new observations concerning the differential expression of the DOR and the MOR in heat sensitive peptidergic TRPV1+ nociceptors we hypothesized that a functional correlate of this remarkable segregation might exist. In part, this hypothesis is based on the fact that many myelinated afferents are mechanosensitive (Cain et al., 2001; Koltzenburg et al., 1997), suggesting that they may contribute more to mechanical than to heat sensibility (Sun et al., 2001). To address this question, we compared the analgesic profile of the DOR selective agonist SNC80 to that of DAMGO, a full and selective agonist, with nanomolar affinity, for the MOR (Zadina et al., 1997). We tested the effect of intrathecal injections of low doses of DAMGO or SNC80 on heat and mechanical pain in mice. Consistent with the expression of the MOR in TRPV1-afferents, we found that DAMGO dose-dependently reduced heat pain responsiveness (Figure 5A). However, Figure 5B also shows that the same doses of DAMGO were without significant effect against mechanical pain.
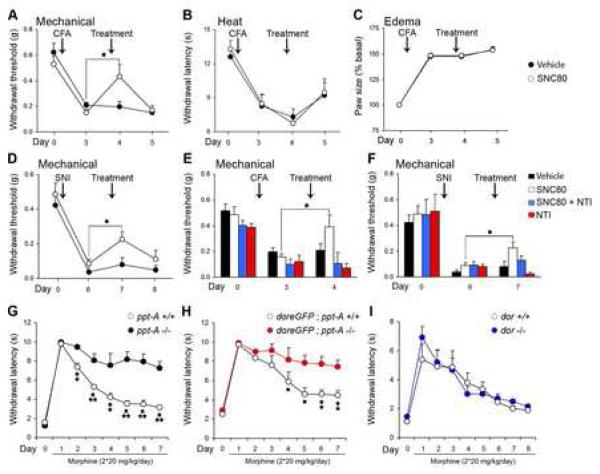
Figure 5.
The DOR agonist SNC80 reduces acute mechanical, but not heat pain.
(A) DAMGO dose-dependently increases tail withdrawal latency to noxious heat.
(B) DAMGO does not significantly alter the mechanical pain threshold at doses that are analgesic for heat pain.
(C) The analgesic effect of DAMGO in the heat pain test is not affected by the selective DOR antagonist, naltrindole (NTI).
(D) SNC80 dose-dependently increases mechanical pain threshold.
(E) SNC80 is not effective against heat pain, at doses that are analgesic against mechanical pain.
(F) Low doses of naltrindole (NTI) reverse the analgesic activity of SNC80, indicating that DORs are targeted by SNC80 in vivo.
(G) Intrathecal administration of capsaicin does not change the mechanical threshold of mice but results in a dramatic reduction of heat pain responsiveness.
(H) The analgesic effect of SNC80 on mechanical pain is independent of TRPV1+, peptidergic nociceptors; it is not affected by intrathecal injection of capsaicin.
For all experiments, the dose of naltrindole was 0.2 nmoles. Results are expressed as mean ± SEM, n=8-12/group, * p < 0.05, ** p < 0.01, *** p < 0.001.
The profile of SNC80 was completely complementary to that of DAMGO, under the same experimental conditions (Figures 5D and 5E). Thus, SNC80 dose-dependently increased mechanical pain thresholds (Figure 5D), but was ineffective against heat pain (Figure 5E). The analgesic action of SNC80 against mechanical pain, but not that of DAMGO against heat pain (Figure 5C), was blocked by coadministration a low dose of the DOR selective antagonist naltrindole (Portoghese et al., 1990)Figure 5F), indicating that the DOR mediates the SNC80 analgesia. Because intrathecal administration of analgesic doses of SNC80 in DOReGFP mice also produced a profound internalization of the receptor in vivo, SNC80 likely exerts its analgesic effect via an action on DRG neurons that express the DOR (Figure 1B).
Together, these results indicate that there is not only a differential localization of the DOR and MOR in DRG cells, but also that these differences underlie qualitatively distinct pain relieving profiles. We suggest that the reports of DOR agonist-mediated regulation of heat pain were generated at agonist doses that interacted with the MOR. We conclude that spinal administration of DOR agonists produces a selective reduction of mechanical pain via an action at myelinated nociceptors that respond to noxious mechanical stimuli and perhaps on mechanosensitive nonpeptidergic afferents (including the subset that expresses the Mrgprd (Cavanaugh et al, 2009).
DOR mediated mechanical analgesia does not require TRPV1+ nociceptors
Given that the MOR, but not the DOR, is expressed by TRPV1+ nociceptors, we next assayed for mechanical and heat pain responsiveness and for the analgesic effect of SNC80 in mice in which the central terminals of TRPV1+ nociceptors were destroyed by prior intrathecal administration of capsaicin. We found that heat, but not mechanical pain responsiveness was abolished (Figure 5G), confirming our recent report that TRPV1+ nociceptors are absolutely required for normal heat pain sensibility (Cavanaugh et al., 2009). In this respect, deletion of the central terminals of the TRPV1+ nociceptors is functionally equivalent to the action of MOR agonists on these nociceptors: both treatments produce a selective reduction of heat pain. However, the potency of SNC80 against mechanical pain was not altered by loss of the TRPV1+ nociceptors (Figure 5H). Based on these results, we conclude that SNC80-mediated reduction of mechanical pain does not require TRPV1+ nociceptors and that the lack of expression of the DOR in these afferents accounts for the inability of intrathecal DOR agonists to regulate heat pain.
DORs control tissue and nerve injury-induced mechanical hypersensitivity
How general is the utility of DOR agonists against mechanical pain? As treating mechanical hypersensitivity represents one of the major current clinical challenges, we next asked whether an action at the DOR can regulate the mechanical hypersensitivity produced in two different models of chronic pain (inflammatory and neuropathic). To model the inflammatory pain induced by tissue injury, we injected complete Freund’s adjuvant (CFA) into the hindpaw of the mouse. To model neuropathic pain, we used the spared nerve injury model, in which two of the three branches of the sciatic nerve are transected, resulting in a prolonged mechanical hypersensitivity of the partially denervated hind paw (Shields et al., 2003)
Four days after the CFA injection, the mice displayed both mechanical (Figure 6A) and heat (Figure 6B) hypersensitivity, as well as paw edema (Figure 6C). Comparable to its effect on acute mechanical pain, intrathecal SNC80 significantly decreased the mechanical hypersensitivity (Figure 6A) but SNC80 was without effect on the hypersensitivity to heat (Figure 6B) and did not reduce paw edema (Figure 6C).
Figure 6.
SNC80 significantly reduces mechanical hypersensitivity in models of inflammatory and neuropathic pain (tissue or nerve injury, respectively), without affecting heat hyperalgesia.
(A) A single intrathecal injection of SNC80 reverses the mechanical hypersensitivity observed 4 days after intraplantar injection of complete Freund’s adjuvant (CFA).
(B) SNC80 does not reduce the heat hyperalgesia produced by CFA.
(C) SNC80 did not reduce the edema generated by CFA.
(D) Intrathecal injection of SNC80 reduces the mechanical hypersensitivity induced in the spared nerve injury (SNI) model of neuropathic pain. Animals were tested 7 days after the nerve transection.
(E) The analgesic effect of SNC80 in the CFA model of inflammatory pain is abolished by co-administration of the DOR selective antagonist, naltrindole (NTI).
(F) Naltrindole blocks the analgesic effect of SNC80 in the SNI model of neuropathic pain.
(G, H) The development of analgesic tolerance to chronic morphine is significantly reduced in ppt-A null mice, both on C57Bl6 (G) or doregfp (H) genetic backgrounds.
(I) Tolerance to the analgesic effect of morphine develops normally in dor null mice, indicating that SP, but not the DOR, is critical to the development of morphine tolerance.
For all experiments the doses of SNC80 and naltrindole were 10 nmoles and 0.2 nmoles, respectively. Results are expressed as mean ± SEM, n=9-14/group, * p < 0.05, ** p < 0.01, *** p < 0.001.
In the neuropathic pain model, the mice showed a profound reduction of the mechanical withdrawal threshold of the partially denervated hindpaw (Figure 6D), and as generally reported for this model, no hypersensitivity to heat (data not shown). Similar to its effects in the inflammatory pain model, intrathecal SNC80 significantly decreased the mechanical hypersensitivity produced by nerve injury (Figure 6D). In both models of persistent pain, the SNC80 effect was transient; the mechanical hypersensitivity was restored after 24h (Figures 6A and 6D). Confirming that these effects were mediated via an action at the DOR, we found that a low dose of the DOR antagonist naltrindole blocked the analgesic effect of SNC80 against mechanical hypersensitivity (Figures 6E and 6F). Together, these results suggest that DOR+ mechanoreceptive DRG neurons can be selectively targeted, so as to control both acute mechanical pain and the prolonged mechanical hypersensitivity that occurs after tissue or nerve injury.
Substance P, rather than the DOR, is a major contributor to the development of tolerance to the analgesic effect of morphine
As noted above, several studies reported that tolerance to the analgesic action of MOR agonists can be regulated by an action at the DOR (Zhang et al., 2006; Zhu et al., 1999). Specifically, presence of the DOR in the central terminals of nociceptors in the spinal cord is considered critical to the development of morphine tolerance. Because ppt-A null mice do not develop analgesic tolerance to morphine, Guan et al (2005) concluded that disruption of the transport of the DOR to the central terminals of SP+ unmyelinated nociceptors was the causal factor. As we find that DOR trafficking to central terminals is, in fact, intact in the DOReGFP mice crossed with ppt-A null mice, our next studies dissociated the functional contribution of the DOR and SP.
Here we reexamined the impact of deleting the ppt-A or the dor gene on the development of morphine tolerance (Supplementary results, Figures G-I). In agreement with Guan et al (2005), we found that morphine tolerance was decreased in ppt-A null mice, however, contrary to the literature (Zhu et al., 1999), tolerance developed normally in dor null mice. It follows that regulation of the MOR in pain processing is not a primary function of the DOR. The two receptors are differentially distributed in primary afferent pain fibers and selectively regulate different pain modalities. We conclude that an interaction of the two receptors is not required for the development of analgesic tolerance with chronic morphine.
Discussion
Morphine and other MOR agonists remain the drugs of choice for the treatment of severe pain, despite the fact they have significant adverse side effects and limited efficacy against the mechanical hypersensitivity that occurs postoperatively or after nerve injury (neuropathic pain). Using a DOReGFP mouse, we now provide a reappraisal of the distribution and functional relevance to pain processing of the DOR and a mechanistic rationale for the development of novel therapeutics for chronic pain.
DOR-mediated control of heat and mechanical pain
The MOR and DOR are highly homologous proteins, with 64% amino acid identity (Lacoste and Evans, 2003). Many of the shared amino acids are distributed among the transmembrane domains that form the binding pocket of the receptors. As a result, DOR agonists, including the widely used DPDPE (Mosberg et al., 1983) and deltorphin (Erspamer et al., 1989) offer only modest selectivity for the DOR compared to the MOR, and can activate the MOR at high doses. Furthermore, because of the very high intrinsic efficacy of the MOR in blocking the transmission of pain messages at the level of the nociceptor (Jessell and Iversen, 1977; Taddese et al., 1995), cross-reactivity in in vivo studies is particularly difficult to avoid and its extent difficult to estimate.
There is, in fact, considerable evidence that DOR agonists can block heat pain via an action at the MOR. For example, several groups reported either a decrease or a total loss of the activity of DOR agonists in tests of heat pain in mor null mice (Gendron et al., 2007; Matthes et al., 1996; Scherrer et al., 2004; Sora et al., 1997a). Other studies found that the activity of DOR agonists against heat pain is only partially reduced, or even preserved in DOR null mice (Scherrer et al., 2004; Zhu et al., 1999). To some authors these data are not explained by cross-reactivity of DOR agonists with the MOR, but rather support the idea that presence of the MOR is required for DOR agonists to have full activity. Indeed, a functional interaction between the two receptors, including dimerization, which is readily demonstrated in heterologous expression systems, is presumed to occur in SP+ nociceptors (Rozenfeld et al., 2007). Given our new finding that the MOR and DOR do not cooccur in these neurons, we suggest that cross reactivity of DOR agonists at the MOR, rather than loss of cooperativity, is the most likely explanation for the decreased activity of DOR agonists in the MOR null mice.
To differentiate between the contribution of the DOR and the MOR to pain processing, we delivered low doses of SNC80 (at least 10 times lower that what is typically used) or DAMGO, directly to the spinal cord, and then gradually increased these doses until we reached a maximal analgesic effect, either in the heat or mechanical pain tests. We found that intrathecal SNC80 dose-dependently increased the mechanical pain threshold, with no change in the response to noxious heat. The mechanical hypersensitivity that occurs in models of inflammatory and neuropathic pain was also blocked by SNC80, but the heat hypersensitivity was not. These behavioral results correlate well with the predominant expression of the DOR in myelinated primary afferents and TRPV1-negative nociceptors and its absence from heat-sensitive, TRPV1+ nociceptors.
As even these low doses of DOR agonist produced a massive internalization of the receptor in DRG neurons, we are confident that the pain-relieving effects occur at doses that induce activity at the DOR. We also showed that a low dose of the high affinity and selective DOR antagonist, naltrindole, completely blocked SNC80-induced analgesia in the mechanical pain test. At the same dose, naltrindole did not alter the effect of the MOR agonist, DAMGO, in tests of heat pain, demonstrating both that selective activity at the DOR and MOR can be achieved and that these distinct opioid receptor subtypes regulate two very different modalities of pain. We, of course, recognize the possibility that systemic or intracerebral administration of DOR agonists could exert antinociceptive effects on heat pain via an action at supraspinal sites (Fraser et al., 2000; Hurley and Hammond, 2000; Ossipov et al., 1995; Scherrer et al., 2004).
DOR is a prototypical GPCR
In the DOReGFP mice, the GFP is covalently bound to DOR through a knockin strategy that left the 3′ and 5′ regulatory sequences intact. In fact, the DOReGFP distribution patterns in brain (Goody et al., 2002) and spinal cord (Mennicken et al., 2003) are identical to those revealed in autoradiographic binding studies using DOR ligands. This result not only indicates that DOReGFPs are expressed in cells that endogenously express the DOR in wild-type mice, but also that DOReGFPs are properly trafficked to subcellular domains.
Our results raise significant questions concerning two of the major conclusions derived from studies using antisera directed against the DOR, namely that DOR immunoreactivity is concentrated in the SP-containing LDCVs of the nociceptors rather than on the plasma membrane (Bao et al., 2003; Cahill et al., 2001; Gendron et al., 2006; Morinville et al., 2003; Zhang et al., 1998) and second that DOR staining is lost in the central terminals of nociceptors in the spinal cord of mice with a deletion of the ppt-A gene (Guan et al., 2005). Most importantly, the elegant cell biological illustration of an interaction between a G-protein coupled receptor and a peptide neurotransmitter, even if readily demonstrated in heterologous expression systems with tagged DORs, is clearly untenable if the two molecules do not co-occur in the SP+ neurons. For the same reason, our results cannot support the view that DORs decrease heat pain more efficiently in the setting of tissue injury because there is enhanced translocation of the receptor from the LDCVs to the plasma membrane of the central terminals of unmyelinated peptidergic receptors (Cahill et al., 2007; Cahill et al., 2001). Indeed, not only did we find that the DOR is absent from SP+ and TRPV1+ nociceptors, which agrees with earlier in situ hybridization and ligand binding studies performed in rats and primates (Minami et al., 1995), but we also showed that functional DORs are located at the plasma membrane of unmyelinated nonpeptidergic or myelinated nociceptors, in the resting state, where they can be activated by SNC80 to reduce mechanical pain.
Our findings also do not support the hypothesis that loss of morphine tolerance in ppt-A null mice results from the absence of the DOR in the SP+ nociceptor terminals (Guan et al., 2005). Indeed, because a SP receptor antagonist can prevent the development of morphine tolerance (Powell et al., 2003), it appears that absence of SP alone is sufficient to explain the phenotype of the ppt-A null mice. Whether the protein recognized by the traditional DOR antibodies contributes to these processes remains to be determined.
Specificity in the processing and regulation of pain messages
There are two diametrically opposed views as to how noxious stimuli evoke qualitatively distinct pain sensations. One view holds that a particular subset of nociceptors generates a particular modality of pain, e.g. heat vs. mechanical, despite the fact that nociceptors are polymodal, i.e. respond to multiple pain stimuli. This is the labeled line hypothesis. Another view holds that circuits within the CNS interpret activity generated by polymodal nociceptors, i.e. there is no peripheral specificity in the processing of pain messages. In agreement with recent studies (Abrahamsen et al., 2008; Cavanaugh et al., 2009), our results indicate that at least at the level of the peripheral afferent, there is behaviorally relevant specificity, despite the polymodal nature of the nociceptors, and that selective regulation of mechanical vs heat pain can be produced by interfering with the activity of subsets of nociceptors. Figure 7 schematizes the differential contribution of subpopulations of nociceptors to heat and mechanical pain as well as the selective MOR and DOR-mediated controls that can be exerted upon these nociceptors. Heat pain behavior is produced by activity of the TRPV1+ population of nociceptors and can be blocked by MOR selective agonists. By contrast, mechanical pain is generated by activity in different populations (myelinated and unmyelinated nonpeptidergic nociceptors) and can be selectively blocked by DOR agonists. Of course, high doses of MOR agonists could reduce mechanical pain, possibly through a direct action on the MOR that is expressed in some mechano-sensitive pain fibers (such as the rare afferents in which there is coexpression of the MOR and DOR), or through a cross-reactivity at the DOR. Unfortunately, the use of such doses also triggers unacceptable MOR-mediated adverse side effects, many of which involve visceral organ dysfunction (Aicher et al., 2000a). It is thus particularly significant that we find that the DOR, in contrast to the MOR, is largely excluded from visceral sensory afferents (See Figure 7). It follows that peripherally-restricted DOR agonists might be useful in order to avoid their supraspinally-mediated locomotor and convulsive side effects (Jutkiewicz et al., 2004). Finally, because we now have a tool to identify sensory neurons with mechanoreceptive properties, our results point to the use of the DOReGFP mouse to define the molecular make-up of these neurons, including identification of the as yet elusive mechanical pain transducer, which is a likely to be a valuable therapeutic target.
Figure 7.
The differential expression of the DOR and MOR in primary sensory neurons allows for the selective pharmacological control of distinct pain modalities.
The MOR is expressed by heat-sensitive (TRPV1+), peptidergic (SP+ and CGRP+) unmyelinated nociceptors that innervate the skin (peripheral terminals; red box). The DOR, in contrast, predominates in the mechanosensitive myelinated and nonpeptidergic unmyelinated nociceptors. The MOR+ and DOR+ sensory neurons project to different laminae of the spinal cord (laminae I and outer II (I-IIo) and I and inner II (IIi), respectively), where they activate neurons that transmit painful heat and mechanical messages to the brain. Because of the segregated opioid receptor expression in nociceptors, MOR agonists preferentially reduce heat pain, while DOR agonists can relieve mechanical pain.
The MOR, but not the DOR, is also expressed in a subpopulation of sensory neurons that innervate the heart, lungs and abdominal viscera (green box). The cells bodies of these afferents are located in the DRG or in the nodose ganglia and project to the spinal cord (visceral pain) or to the nucleus of the solitary tract (NTS) in the brainstem (yellow box), respectively. Thus, only MOR agonists will generate visceral organ adverse side affects.
Experimental procedures
Immunohistochemistry
In these experiments we used standard procedures as described previously (Neumann et al., 2008). Briefly, we incubated sections of formalin-fixed DRG (20 μm), spinal cord or brain (40 μm) in primary antibody solution overnight at 4°C. Tissues were then placed in fluorescent-tagged secondary antibody solution for 2 hours at room temperature. A detailed protocol and list of antibodies used can be found in Supplemental Data.
Autoradiography
Sixteen μm frozen spinal cord sections were incubated in 40 pM [125I]-deltorphin II or [125I]-DPDPE and autoradiography was performed subsequently as described (Mennicken et al., 2003). See Supplemental Data for details.
Behavioral analysis
Behavioral testing was performed in adult, male C57Bl6 mice (20-30 g; Charles River, Hollister, CA, USA) after a week of acclimation to the housing facility. Detailed procedures for intrathecal injections and pain testing can be found in the Supplemental Data. To assess heat pain we measured the latency (sec) to tail withdrawal from 55°C water. For mechanical pain, we determined the withdrawal threshold (g) of the hindpaw when stimulating the plantar surface with calibrated von Frey hairs. All animal experiments were approved by the Institutional Animal Care and Use Committee at UCSF and were conducted in accordance with the NIH Guide for the Care and use of Laboratory animals.
Statistical analysis
Analysis was performed with StatView software using the Student’s t-test for paired comparisons, or ANOVA for multiple comparisons followed by a Fisher post hoc test. When mice were tested at different time points we used a repeated measures ANOVA. Differences between groups at each time point were analyzed with a Fisher post-hoc test. All results are expressed as mean ± SEM.
Supplementary Material
Acknowledgments
We thank S.D. Shields, D. Julius, D. Cavanaugh and J. Braz for helpful discussions, J.E. Maggio, C.J. Evans and D. Julius for providing the SP, MOR and TRP antibodies, J. Pintar for providing tissue from dor null mice and J. Butterworth and D. Hubatsch for providing excellent technical support. This work was supported by NS48499, NS14627 and Fondation pour la Recherche Médicale. DOD and FM are employees of AstraZeneca and declare that they have competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321:702–705. doi: 10.1126/science.1156916. [DOI] [PubMed] [Google Scholar]
- Aicher SA, Goldberg A, Sharma S, Pickel VM. mu-opioid receptors are present in vagal afferents and their dendritic targets in the medial nucleus tractus solitarius. J Comp Neurol. 2000a;422:181–190. doi: 10.1002/(sici)1096-9861(20000626)422:2<181::aid-cne3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Aicher SA, Punnoose A, Goldberg A. mu-Opioid receptors often colocalize with the substance P receptor (NK1) in the trigeminal dorsal horn. J Neurosci. 2000b;20:4345–4354. doi: 10.1523/JNEUROSCI.20-11-04345.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson U, Dado RJ, Riedl M, Lee JH, Law PY, Loh HH, Elde R, Wessendorf MW. delta-Opioid receptor immunoreactivity: distribution in brainstem and spinal cord, and relationship to biogenic amines and enkephalin. J Neurosci. 1995a;15:1215–1235. doi: 10.1523/JNEUROSCI.15-02-01215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, Loh HH, Law PY, Wessendorf MW, Elde R. Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J Neurosci. 1995b;15:3328–3341. doi: 10.1523/JNEUROSCI.15-05-03328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Jin SX, Zhang C, Wang LH, Xu ZZ, Zhang FX, Wang LC, Ning FS, Cai HJ, Guan JS, et al. Activation of delta opioid receptors induces receptor insertion and neuropeptide secretion. Neuron. 2003;37:121–133. doi: 10.1016/s0896-6273(02)01103-0. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Holdridge SV, Morinville A. Trafficking of delta-opioid receptors and other G-protein-coupled receptors: implications for pain and analgesia. Trends Pharmacol Sci. 2007;28:23–31. doi: 10.1016/j.tips.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Lee MC, Vincent JP, Collier B, Beaudet A. Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception. J Neurosci. 2001;21:7598–7607. doi: 10.1523/JNEUROSCI.21-19-07598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain DM, Khasabov SG, Simone DA. Response properties of mechanoreceptors and nociceptors in mouse glabrous skin: an in vivo study. J Neurophysiol. 2001;85:1561–1574. doi: 10.1152/jn.2001.85.4.1561. [DOI] [PubMed] [Google Scholar]
- Cao YQ, Mantyh PW, Carlson EJ, Gillespie AM, Epstein CJ, Basbaum AI. Primary afferent tachykinins are required to experience moderate to intense pain. Nature. 1998;392:390–394. doi: 10.1038/32897. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci (USA) 2009 doi: 10.1073/pnas.0901507106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erspamer V, Melchiorri P, Falconieri-Erspamer G, Negri L, Corsi R, Severini C, Barra D, Simmaco M, Kreil G. Deltorphins: a family of naturally occurring peptides with high affinity and selectivity for delta opioid binding sites. Proc Natl Acad Sci (USA) 1989;86:5188–5192. doi: 10.1073/pnas.86.13.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, et al. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Fraser GL, Gaudreau GA, Clarke PB, Menard DP, Perkins MN. Antihyperalgesic effects of delta opioid agonists in a rat model of chronic inflammation. Br J Pharmacol. 2000;129:1668–1672. doi: 10.1038/sj.bjp.0703248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallantine EL, Meert TF. A comparison of the antinociceptive and adverse effects of the mu-opioid agonist morphine and the delta-opioid agonist SNC80. Basic Clin Pharmacol Toxicol. 2005;97:39–51. doi: 10.1111/j.1742-7843.2005.pto_97107.x. [DOI] [PubMed] [Google Scholar]
- Gendron L, Pintar JE, Chavkin C. Essential role of mu opioid receptor in the regulation of delta opioid receptor-mediated antihyperalgesia. Neuroscience. 2007;150:807–817. doi: 10.1016/j.neuroscience.2007.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci (USA) 2004;101:5135–5139. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Jordan BA, Gupta A, Rios C, Trapaidze N, Devi LA. G protein coupled receptor dimerization: implications in modulating receptor function. J Mol Med. 2001;79:226–242. doi: 10.1007/s001090100219. [DOI] [PubMed] [Google Scholar]
- Goody RJ, Oakley SM, Filliol D, Kieffer BL, Kitchen I. Quantitative autoradiographic mapping of opioid receptors in the brain of delta-opioid receptor gene knockout mice. Brain Res. 2002;945:9–19. doi: 10.1016/s0006-8993(02)02452-6. [DOI] [PubMed] [Google Scholar]
- Guan JS, Xu ZZ, Gao H, He SQ, Ma GQ, Sun T, Wang LH, Zhang ZN, Lena I, Kitchen I, et al. Interaction with vesicle luminal protachykinin regulates surface expression of delta-opioid receptors and opioid analgesia. Cell. 2005;122:619–631. doi: 10.1016/j.cell.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Ljungdahl A, Terenius L, Elde R, Nilsson G. Immunohistochemical analysis of peptide pathways possibly related to pain and analgesia: enkephalin and substance P. Proc Natl Acad Sci (USA) 1977;74:3081–3085. doi: 10.1073/pnas.74.7.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RW, Hammond DL. The analgesic effects of supraspinal mu and delta opioid receptor agonists are potentiated during persistent inflammation. J Neurosci. 2000;20:1249–1259. doi: 10.1523/JNEUROSCI.20-03-01249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell TM, Iversen LL. Opiate analgesics inhibit substance P release from rat trigeminal nucleus. Nature. 1977;268:549–551. doi: 10.1038/268549a0. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Eller EB, Folk JE, Rice KC, Traynor JR, Woods JH. Delta-opioid agonists: differential efficacy and potency of SNC80, its 3-OH (SNC86) and 3-desoxy (SNC162) derivatives in Sprague-Dawley rats. J Pharmacol Exp Ther. 2004;309:173–181. doi: 10.1124/jpet.103.061242. [DOI] [PubMed] [Google Scholar]
- Kieffer BL, Gaveriaux-Ruff C. Exploring the opioid system by gene knockout. Prog Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Stucky CL, Lewin GR. Receptive properties of mouse sensory neurons innervating hairy skin. J Neurophysiol. 1997;78:1841–1850. doi: 10.1152/jn.1997.78.4.1841. [DOI] [PubMed] [Google Scholar]
- Lacoste A, Evans CJ. Cloning of Delta Opioid Receptors. In: Chang K-J, Porreca F, Woods J, editors. The Delta Receptor. Informa Healthcare; USA: 2003. pp. 15–30. [Google Scholar]
- Malmberg AB, Chen C, Tonegawa S, Basbaum AI. Preserved acute pain and reduced neuropathic pain in mice lacking PKCγ. Science. 1997;278:279–283. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Mennicken F, Zhang J, Hoffert C, Ahmad S, Beaudet A, O’Donnell D. Phylogenetic changes in the expression of delta opioid receptors in spinal cord and dorsal root ganglia. J Comp Neurol. 2003;465:349–360. doi: 10.1002/cne.10839. [DOI] [PubMed] [Google Scholar]
- Minami M, Maekawa K, Yabuuchi K, Satoh M. Double in situ hybridization study on coexistence of mu-, delta- and kappa-opioid receptor mRNAs with preprotachykinin A mRNA in the rat dorsal root ganglia. Brain Res Mol Brain Res. 1995;30:203–210. doi: 10.1016/0169-328x(94)00290-u. [DOI] [PubMed] [Google Scholar]
- Mosberg HI, Hurst R, Hruby VJ, Gee K, Yamamura HI, Galligan JJ, Burks TF. Bis-penicillamine enkephalins possess highly improved specificity toward delta opioid receptors. Proc Natl Acad Sci (USA) 1983;80:5871–5874. doi: 10.1073/pnas.80.19.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Suzuki T. Delta opioid receptor-mediated antinociception/analgesia. In: Chang K-J, Porreca F, Woods J, editors. The Delta Receptor. 2003. pp. 331–354. [Google Scholar]
- Neumann S, Braz JM, Skinner K, Llewellyn-Smith IJ, Basbaum AI. Innocuous, not noxious, input activates PKCγ interneurons of the spinal dorsal horn via myelinated afferent fibers. J Neurosci. 2008;28:7936–7944. doi: 10.1523/JNEUROSCI.1259-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onofrio BM, Yaksh TL. Intrathecal delta-receptor ligand produces analgesia in man. Lancet. 1983;1:1386–1387. doi: 10.1016/s0140-6736(83)92170-0. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Kovelowski CJ, Nichols ML, Hruby VJ, Porreca F. Characterization of supraspinal antinociceptive actions of opioid delta agonists in the rat. Pain. 1995;62:287–293. doi: 10.1016/0304-3959(94)00231-3. [DOI] [PubMed] [Google Scholar]; J Neurosci. 25:8825–8832. doi: 10.1523/JNEUROSCI.0160-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F, Heyman JS, Mosberg HI, Omnaas JR, Vaught JL. Role of mu and delta receptors in the supraspinal and spinal analgesic effects of [D-Pen2, D-Pen5]enkephalin in the mouse. J Pharmacol Exp Ther. 1987;241:393–400. [PubMed] [Google Scholar]
- Portoghese PS, Sultana M, Takemori AE. Naltrindole 5′-isothiocyanate: a nonequilibrium, highly selective delta opioid receptor antagonist. J Med Chem. 1990;33:1547–1548. doi: 10.1021/jm00168a004. [DOI] [PubMed] [Google Scholar]
- Powell KJ, Quirion R, Jhamandas K. Inhibition of neurokinin-1-substance P receptor and prostanoid activity prevents and reverses the development of morphine tolerance in vivo and the morphine-induced increase in CGRP expression in cultured dorsal root ganglion neurons. Eur J Neurosci. 2003;18:1572–1583. doi: 10.1046/j.1460-9568.2003.02887.x. [DOI] [PubMed] [Google Scholar]
- Rozenfeld R, Abul-Husn NS, Gomez I, Devi LA. An emerging role for the delta opioid receptor in the regulation of mu opioid receptor function. ScientificWorldJournal. 2007;7:64–73. doi: 10.1100/tsw.2007.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Befort K, Contet C, Becker J, Matifas A, Kieffer BL. The delta agonists DPDPE and deltorphin II recruit predominantly mu receptors to produce thermal analgesia: a parallel study of mu, delta and combinatorial opioid receptor knockout mice. Eur J Neurosci. 2004;19:2239–2248. doi: 10.1111/j.0953-816X.2004.03339.x. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Laustriat D, Cao YQ, Basbaum AI, Dierich A, Vonesh JL, Gaveriaux-Ruff C, et al. Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc Natl Acad Sci (USA) 2006;103:9691–9696. doi: 10.1073/pnas.0603359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields SD, Eckert WA, 3rd, Basbaum AI. Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. J Pain. 2003;4:465–470. doi: 10.1067/s1526-5900(03)00781-8. [DOI] [PubMed] [Google Scholar]
- Sora I, Funada M, Uhl GR. The mu-opioid receptor is necessary for [D-Pen2,D-Pen5]enkephalin-induced analgesia. Eur J Pharmacol. 1997a;324:R1–2. doi: 10.1016/s0014-2999(97)10016-4. [DOI] [PubMed] [Google Scholar]
- Sora I, Takahashi N, Funada M, Ujike H, Revay RS, Donovan DM, Miner LL, Uhl GR. Opiate receptor knockout mice define mu receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc Natl Acad Sci (USA) 1997b;94:1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Ren K, Zhong CM, Ossipov MH, Malan TP, Lai J, Porreca F. Nerve injury-induced tactile allodynia is mediated via ascending spinal dorsal column projections. Pain. 2001;90:105–111. doi: 10.1016/s0304-3959(00)00392-4. [DOI] [PubMed] [Google Scholar]
- Taddese A, Nah SY, McCleskey EW. Selective opioid inhibition of small nociceptive neurons. Science. 1995;270:1366–1369. doi: 10.1126/science.270.5240.1366. [DOI] [PubMed] [Google Scholar]
- Trafton JA, Abbadie C, Marek K, Basbaum AI. Postsynaptic signaling via the [mu]-opioid receptor: responses of dorsal horn neurons to exogenous opioids and noxious stimulation. J Neurosci. 2000;20:8578–8584. doi: 10.1523/JNEUROSCI.20-23-08578.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walwyn W, Maidment NT, Sanders M, Evans CJ, Kieffer BL, Hales TG. Induction of delta opioid receptor function by up-regulation of membrane receptors in mouse primary afferent neurons. Mol Pharmacol. 2005;68:1688–1698. doi: 10.1124/mol.105.014829. [DOI] [PubMed] [Google Scholar]
- Zadina JE, Hackler L, Ge LJ, Kastin AJ. A potent and selective endogenous agonist for the mu-opiate receptor. Nature. 1997;386:499–502. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bao L, Arvidsson U, Elde R, Hökfelt T. Localization and regulation of the delta-opioid receptor in dorsal root ganglia and spinal cord of the rat and monkey: evidence for association with the membrane of large dense-core vesicles. Neuroscience. 1998;82:1225–1242. doi: 10.1016/s0306-4522(97)00341-2. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bao L, Guan JS. Role of delivery and trafficking of delta-opioid peptide receptors in opioid analgesia and tolerance. Trends Pharmacol Sci. 2006;27:324–329. doi: 10.1016/j.tips.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, Elde RP, Unterwald E, Pasternak GW, Pintar JE. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.