SUMMARY
A previously unrecognized mechanism by which large ribonucleoprotein (megaRNP) granules exit the nucleus is by budding through the nuclear envelope (NE). This mechanism is akin to the nuclear egress of Herpes-type viruses and is essential for proper synapse development. However, the molecular machinery required to remodel the NE during this process is unknown. Here we identify Torsin, a AAA-ATPase that in humans is linked to dystonia, as a major mediator of primary megaRNP envelopment during NE-budding. In torsin mutants, megaRNPs accumulate within the perinuclear space and the mRNAs contained within fail to reach synaptic sites, preventing normal synaptic protein synthesis, and thus proper synaptic bouton development. These studies begin to establish the cellular machinery underlying the exit of megaRNPs via budding, offer an explanation to the “nuclear blebbing” phenotype found in dystonia models and provide an important link between Torsin and synaptic phenotypes observed in dystonia.
INTRODUCTION
Polarized assembly of cellular complexes often depends on formation of translationally silent RNA transport granules containing mRNAs and associated structural and regulatory components (e.g., proteins and miRNAs). These RNA-protein complexes (RNPs) are shuttled to distinct cellular locales where, upon specific stimuli, the mRNAs are translated into protein building blocks for local cellular architectures and macromolecular complexes (Richter, 2001). Particularly notable is RNP transport in the nervous system, where long-term changes in synaptic structure and function frame key events enabling organisms to respond to their changing environment. A special case of this adaptation is the ability of organisms to learn and remember (Wiersma-Meems et al., 2005). In these processes, localized translation of mRNAs links synaptic plasticity-inducing stimuli to the synthesis of effector proteins underlying enduring changes in synaptic structure and function (Barco et al., 2008).
Until recently, it was thought that all mRNA export occurred one molecule at a time through the nuclear pore complex (NPC) suggests that mRNAs are exported one molecule at a time (Grunwald et al., 2011; Kohler and Hurt, 2007). However, we recently uncovered a previously unrecognized mechanism by which large ribonucleoprotein (megaRNP) granules exit the nucleus via nuclear envelope- (NE) budding (Speese et al., 2012), a mechanism previously shown to be utilized for the nuclear export of large Herpes-type viral capsids (Maric et al., 2011; Mettenleiter et al., 2006). This budding process and the signaling pathway that it initiates are essential for normal synaptic bouton development at the Drosophila larval NMJ (Ataman et al., 2006; Mathew et al., 2005; Speese et al., 2012). NE-budding entails primary envelopment of viral capsids (Mettenleiter et al., 2006) or megaRNPs (Speese et al., 2012) by the inner nuclear membrane (INM); scission of this envelope from the INM creates a membrane bound particle within the perinuclear space, which subsequently fuses with the outer nuclear membrane (ONM) to allow nuclear escape of the enclosed material. However, the molecular mechanisms required for primary envelopment, INM scission and fusion were previously unknown. Here we identify Torsin, a AAA-ATPase that in humans is linked to both dystonia (Breakefield et al., 2008) and Herpes virus nuclear egress (Maric et al., 2011), as a major mediator of primary megaRNP envelopment during NE-budding, likely functioning to promote INM scission. In torsin mutants, including those mimicking genetic abnormalities in dystonia patients, megaRNPs accumulate within the perinuclear space and the mRNAs contained within fail to reach synaptic sites, preventing normal synaptic protein synthesis, and thus proper synaptic bouton development.
RESULTS AND DISCUSSION
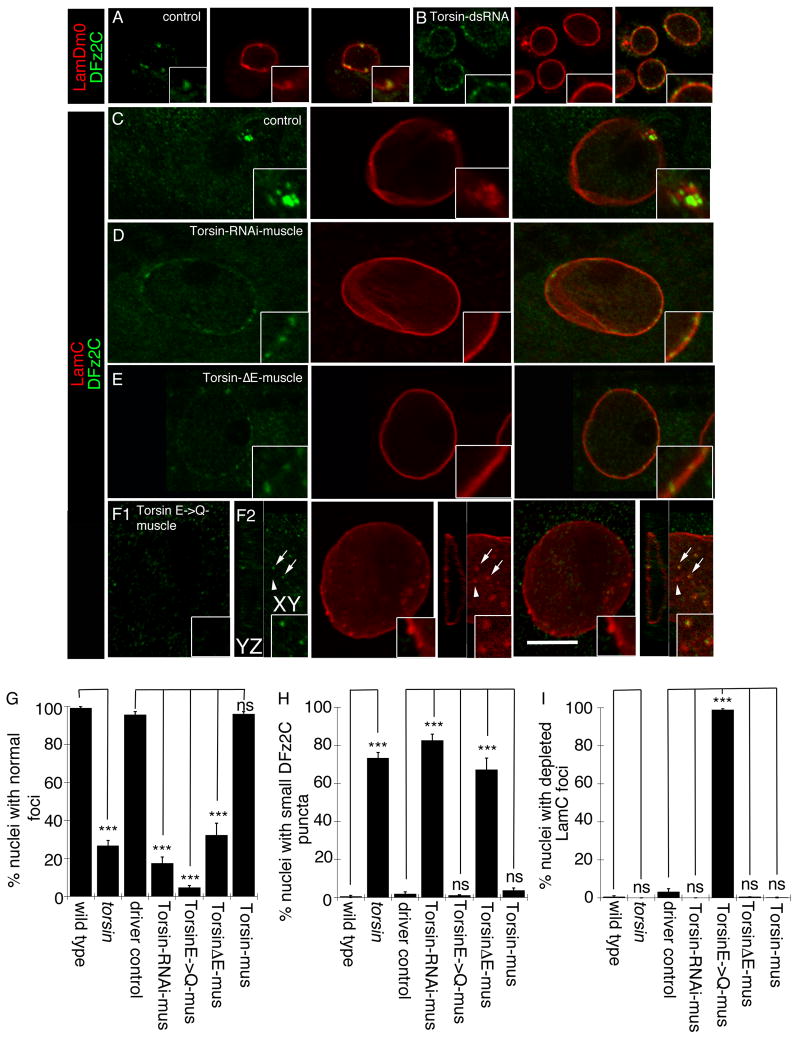
In humans, the dystonia-specific Torsin1A (TOR1A) mutation TOR1AΔE302/303 (also known as TOR1AΔGAG; referred to as TorsinΔE in this paper;) at the DYT1 gene locus is linked to early onset primary dystonia (Tanabe et al., 2009). Mouse models expressing TOR1AΔE302/303 accumulate abnormal vesicular structures at the NE (Goodchild et al., 2005; Naismith et al., 2004). These NE structures show a striking resemblance to the perinuclear megaRNPs we recently reported in Drosophila (Speese et al., 2012), raising the intriguing possibility that these structures could be related. In cultured Schneider-2 (S2) cells and Drosophila larval muscles, megaRNP clusters at the NE can be marked at the light microscopy level by antibodies to the C-terminus of the Wnt receptor, DFrizzled2 (DFz2C) and the INM-associated protein, Lamin C (LamC). DFz2C and LamC partially colocalize at NE-associated foci (DFz2C/LamC foci) (Mathew et al., 2005; Speese et al., 2012). To determine if NE defects observed in TOR1A mutant animal models reflect defects in NE-budding, S2 cells were treated with Torsin-dsRNA, targeting the sole Drosophila homolog of mammalian TOR1A (Wakabayashi-Ito et al., 2011) (see Fig. SF1 for Torsin-dsRNA efficiency). This resulted in significant abnormalities in DFz2C/LamC foci at the NE. In untreated S2 cells, NE-DFz2C foci appear as bright immunoreactive spots embedded in a thickening of the lamina, marked by LamC (Speese et al., 2012) or the B-type lamin, LamDm0 (Fig. 1A). In contrast, Torsin-dsRNA treated cells displayed small DFz2C-immunoreactive puncta dotting the NE, and thickenings of the lamina were barely visible or absent (Fig. 1B; see below for quantification of this phenotype in vivo).
Figure 1. Morphology of nuclear DFz2C/Lam foci is disrupted in Torsin mutations.
(Also see Fig. SF1). (A, B) Localization and morphology of DFz2C/Lam foci at the nuclei of S2 cells in (A) untreated cells, and (B) cells treated with Torsin-dsRNA. (C–F) Localization and morphology of nuclear DFz2C/LamC foci in larval muscles of (C) wild type and (D–F) larvae expressing (D) Torsin RNAi, (E) TorsinΔE, and (F) TorsinE→Q in muscles. F1 is a low magnification view. F2 shows a high magnification view of DFz2 puncta in the YZ and XY planes. (A–F) correspond to singe confocal slices. (G–I) Percentage of nuclear foci showing (G) normal organization of DFz2C/LamC, (H) the presence of small DFz2C puncta associated with the lamina (see text), and (I) the presence of thickenings of the lamina devoid of DFz2C signal. mus= muscle. N([number of nuclei;number of larvae])= [908;6],[731;6],[639;6],[802;6],[846;6],[733;6],[693;6]. Error bars represent ± SEM (***: p<0.0001). Calibration scale (μm)=A,B:14, (4μm for insets); C–F:10 (6μm for insets).
In mammals, Torsin isoforms are derived from 4 genes, Tor1A, Tor1B, Tor2A and Tor3A. The DYT1 mutation in Tor1A specifically affects the neuronal NE (Goodchild et al., 2005), consistent with the belief that dystonia is a disease of the nervous system. This neuronal specificity is likely due to compensation by expression of torsinB in non-neuronal tissues, as knockdown of TOR1B in a DYT mutant background caused NE defects in non-neuronal cells (Kim et al., 2010). In Drosophila, there is a single torsin gene, thus overcoming difficulties associated with redundancy. Moreover, we previously showed that NE-budding occurs in several cell types, including larval body wall muscle cells wherein the large nuclei are particularly suitable for high resolution studies (Speese et al., 2012). In addition, the glutamatergic larval NMJ is a powerful model system to understand mechanisms of synapse development and function.
To determine the significance of the S2 cell NE phenotype upon Torsin downregulation, DFz2C/LamC foci were examined in torsinKO78 null mutants (Wakabayashi-Ito et al., 2011), and in larvae in which Torsin was specifically downregulated in muscles by expressing Torsin-RNAi using the muscle-specific Gal4 driver, C57-Gal4 (Budnik et al., 1996). As in untreated S2 cells, NE DFz2C/LamC foci were observed in wild type larvae as DFz2C immunoreactive spots surrounded by a thickening of LamC immunoreactivity (Speese et al., 2012) (Fig. 1C). In contrast, in larvae expressing Torsin-RNAi in muscles (Fig. 1D) or in torsin null mutants, DFz2C foci were observed as small puncta decorating the NE, but lacking any detectable thickening of the lamina. These phenotypes were quantified by determining the percentage of nuclei containing DFz2C spots surrounded by a thickening of the lamina (normal foci; Fig. 1G) and the percentage of nuclei containing small NE-associated DFz2C puncta lacking LamC thickening (Fig. 1H). There were highly significant differences between wild type controls and both torsin null mutants as well as larvae expressing Torsin-RNAi in muscles (Fig. 1G,H).
Typical of AAA-ATPases, Torsin contains Walker A and Walker B domains involved in ATP binding and ATP hydrolysis, respectively (Neuwald et al., 1999; Wakabayashi-Ito et al., 2011; Walker et al., 1982), as well as Sensor1 and Sensor2 domains also involved in ATP hydrolysis (Iyer et al., 2004). A conserved amino acid deletion in the Sensor2 domain (TorsinΔE; TorsinΔE306 in Drosophila) is dominantly linked to dystonia (Ozelius et al., 1997). In addition, an amino acid substitution in the Walker B domain (TorsinE→Q; TorsinE177Q in Drosophila), leads to a Torsin protein that can dominantly bind to its substrate, but being unable to hydrolyze ATP, remains bound to this substrate, thus constituting a substrate trap (Goodchild et al., 2005; Wakabayashi-Ito et al., 2011). To determine if TorsinΔE or TorsinE→Q transgene expression would also disrupt DFz2C/LamC foci morphology, we expressed these proteins in larval muscles. Expressing TorsinΔE mimicked the torsin null and Torsin-RNAi phenotypes (Fig. 1E,G,H). In contrast, TorsinE→Q expression resulted in formation of numerous NE LamC foci, most of which were devoid of DFz2C immunoreactivity (Fig. 1F1,G–I). Careful examination of these depleted LamC foci by confocal microscopy demonstrated that many contained a small DFz2C puncta, but this signal was barely visible (Fig. 1F2 arrows). The above phenotypes observed upon expressing TorsinΔE and TorsinE→Q were the specific results of the mutations in the Torsin transgenes, as larvae expressing a wild type Torsin transgene were indistinguishable from wild type not expressing this transgene (Fig. 1G–I).
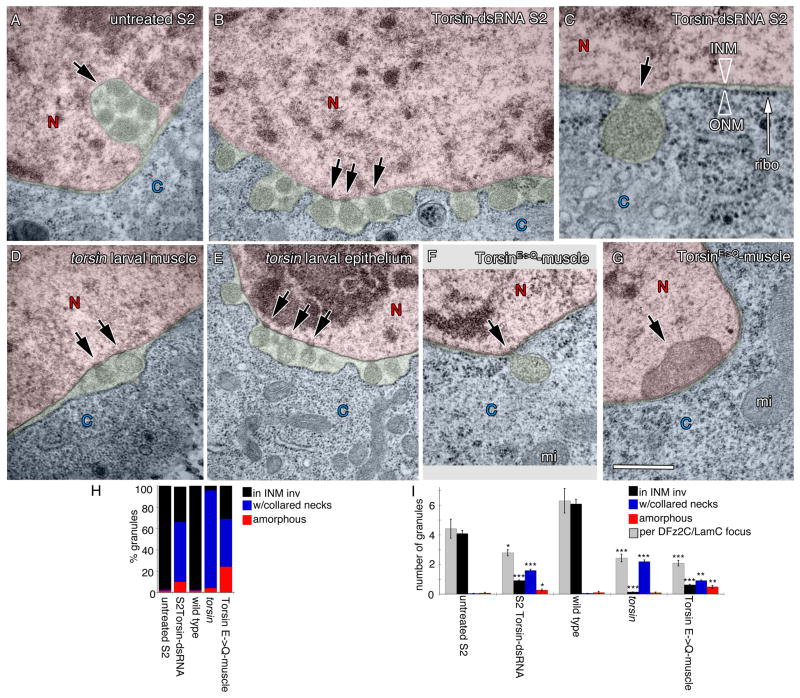
To determine ultrastructural correlates of the above phenotypes, untreated and Torsin-dsRNA-treated S2 cells were examined by transmission electron microscopy (TEM). As previously described (Speese et al., 2012), untreated S2 cells displayed local singlets or clusters of megaRNPs within INM invaginations at discrete regions of the NE (Fig. 2A,H,I), paralleling light microscopy observations (Fig. 1A). In contrast, in Torsin-dsRNA-treated cells these local megaRNPs at the NE were reduced by ~75% (Fig. 2H,I) and instead many mega- RNP granules were often observed in rows of singlets lining the perinuclear space (Fig. 2B,C). In these regions the perinuclear space appeared distended (green in Fig. 2B,C) and the ribosome-decorated ONM appeared to evaginate (Fig. 2B, C). However, NPCs and the rest of the NE appeared normal (Fig. SF2A–D). In addition, the distribution of a number of nuclear proteins, such as the fly Emerin homolog, Bocksbeutel (Fig. SF2E,I), dMan1 (Fig. SF2F,J), Otefin, a protein required for NE assembly (Fig. SF2G,K), and the Drosophila homolog of Hsap, Squid, a ribonuclear protein (Fig. SF2H,L) were normally distributed in the mutants. About half of megaRNPs appeared attached to the INM through a collared neck (arrows in Fig. 2B,C,H,I; see experimental procedures for definition). Thus, downregulating Torsin results in abnormal attachment of megaRNPs to the INM, raising the possibility that Torsin could be involved in INM scission after primary megaRNP envelopment.
Figure 2. Ultrastructural organization of NE-associated megaRNPs is disrupted in Torsin mutations.
(Also see Fig. SF2)
(A–G) Electron micrographs of nuclear regions in (A–C) S2 cells, (D,F,G) larval body wall muscles, and (E) larval epithelial cells, showing NE-associated megaRNPs. Red=nucleus; blue=cytoplasm; green=perinuclear space. N=nucleus; C=cytoplasm.
(A) Untreated S2 cell showing a normal nuclear focus (arrow) containing electron dense megaRNP granules.
(B, C) NE of a Torsin-dsRNA-treated S2 cells, displaying megaRNPs tethered to the INM by collared necks (arrows), shown at (B) low and (C) high magnification. ONM=outer nuclear membrane, INM=inner nuclear membrane, ribo=ribosome.
(D, E) NE in torsin null mutants also showing megaRNPs tethered to the INM (arrows).
(F, G) NE in muscle cells expressing TorsinE→Q showing the presence of (F) a megaRNP (arrow) tethered to the INM, and (G) a large amorphous megaRNP (arrow) tightly apposed to the INM. mi=mitochondria.
(H) Percentage of megaRNP granules present in INM invaginations (black), with collared necks (blue) and being large and amorphous (red).
(I) Average number of megaRNP granules in INM invaginations (black), with collared necks (blue), being large and amorphous (red), per focus (white) N[number of granules;foci])= [159;36],[366;122],[207;33],[166;68],[181;88]. Error bars represent ±SEM. (*= p<0.05,**= p<0.001,***= p<0.0001). Calibration scale (μm)= A,B,D–G:0.5; and C:0.2.
Corroboration of the above results in vivo was obtained by examining larval body wall muscles of torsin null mutants. As in S2 cells, megaRNPs tethered to the INM by a collared neck were observed in torsin null mutant muscles and epithelial cells (Fig. 2D,E,H,I), suggesting this pathway functions in even more tissues than previously characterized. Similarly, muscles expressing TorsinE→Q displayed INM tethered megaRNPs (Fig. 2F,H,I). In ~30% of cases, megaRNPs in muscles expressing TorsinE→Q appeared as large (>250nm) amorphous dense structures directly apposed to the INM (Fig. 2G,H,I). Thus, disruption of Torsin function in vivo leads to abnormal megaRNP tethering to the INM.
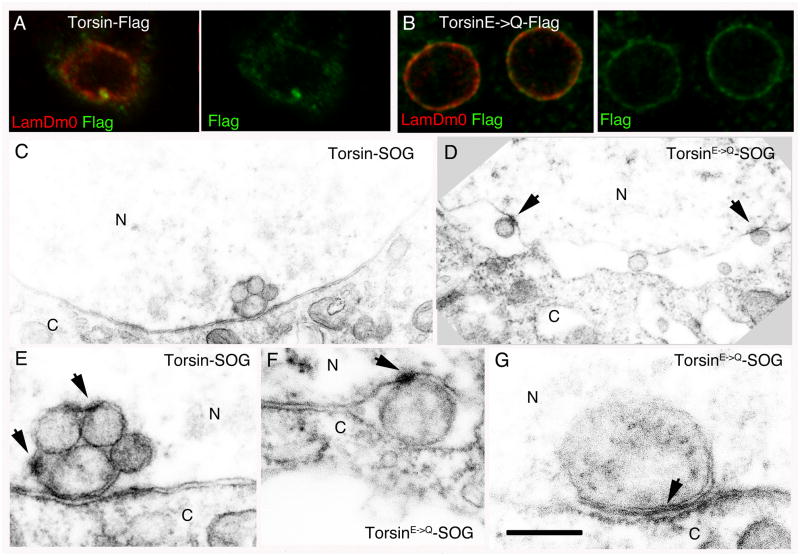
If Torsin is involved in INM scission during megaRNP primary envelopment, the substrate trap TorsinE→Q should accumulate at the electron dense collared necks. This prediction was tested by generating wild type and TorsinE→Q variants fused to a mini-SOG tag (Shu et al., 2011) at their C-termini. Mini-SOG is a flavoprotein derived from Arabidopsis Phototropin 2 that when illuminated by blue light, produces oxygen species that can photoconvert diaminobenzidine into an electron dense precipitate (Shu et al., 2011).
We first determined if C-terminally tagged Torsin was localized to the NE. Although mini-SOG excitation results in fluorescence emission, its rapid bleaching upon illumination prevented high-resolution acquisition of images. Therefore, we generated Flag-tagged Torsin constructs and expressed them in S2 cells. Wild type Torsin-Flag signal localized to bright spots coinciding with Lamin foci at the NE; low levels were also observed at the NE and in the cytoplasm (Fig. 3A). In contrast, TorsinE→Q-Flag was observed in a punctate pattern lining the NE (Fig. 3B).
Figure 3. The TorsinE→Q protein accumulates at megaRNP collared necks.
Also see Fig. SF1. (Also see Fig. SF3)
(A, B) S2 cells expressing (A) wild type Torsin-Flag and (B) the TorsinE→Q-Flag, showing that Torsin-Flag accumulates at foci, and TorsinE→Q is punctate at the NE.
(C–G) Electron micrographs of nuclear regions of S2 cells expressing (C, E) Torsin-SOG showing electron dense signal surrounding megaRNPs, or (D, F–G) TorsinE→Q-SOG showing that signal accumulates at (D, F) megaRNP collared necks or (G) at appositions of amorphous megaRNPs with the INM.
Calibration scale (μm)= A,B:7; C,D:0.7; E–G:0.3.
Consistent with the above observations, S2 cells expressing Torsin-SOG displayed electron dense signal at sites of megaRNP occurrence in the NE (Fig. 3C,E; see Fig. SF3A,B for specificity control). Electron dense SOG-induced signal surrounded each megaRNP (Fig. 3C,E) in a relatively homogenous fashion, but local accumulations of the signal were also apparent (arrows in Fig. 3E). SOG-specific signal was also observed at the INM and ONM in proximity to megaRNPs (Fig. 3E). In contrast, in cells expressing the substrate trap, TorsinE→Q-SOG, SOG-induced signal was concentrated at collared necks of INM- associated megaRNPs and little SOG signal surrounded the megaRNPs (Fig. 3D,F). In cases where large amorphous megaRNPs were tightly apposed to the INM in TorsinE→Q, SOG signal was considerably denser at the sites of contact between the megaRNP and the INM (Fig. 3G). These observations suggest that Torsin is present at sites of NE-budding. Further, accumulation of the TorsinE→Q substrate trap protein at collared necks of megaRNPs suggests that these necks represent the normal site of Torsin action and provide evidence that Torsin is involved in scission of the INM during primary envelopment.
Our previous study revealed that in Drosophila larval muscles, megaRNPs contain transcripts encoding postsynaptic proteins, including the PDZ scaffolding proteins, Par6 and MAGI (Speese et al., 2012). In the case of Par6, interfering with megaRNP formation by inhibiting the Frizzled Nuclear Import (FNI) pathway or LamC expression results in decreased in NMJ localization of par6 mRNA (Speese et al., 2012), decreased postsynaptic Par6 protein levels (Speese et al., 2012), and marked defects in NMJ structure (Ataman et al., 2006; Ataman et al., 2008; Packard et al., 2002; Speese et al., 2012). In particular, under these conditions, NMJs fail to expand normally as muscles grow in size during larval development, and a subset of synaptic boutons (called ghost boutons) remain in an immature state. These ghost boutons fail to recruit postsynaptic proteins and to organize postsynaptic specializations, such as the postsynaptic density and subsynaptic reticulum (SSR) (Ataman et al., 2006; Ataman et al., 2008; Packard et al., 2002; Speese et al., 2012).
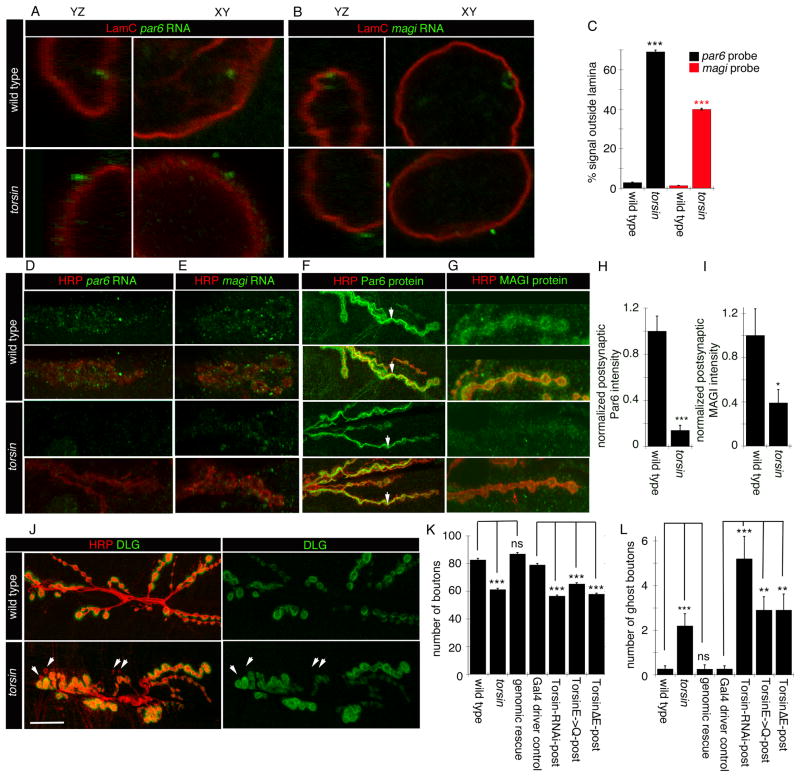
The above observations support a model that alterations in Torsin function inhibit nuclear megaRNP exit by slowing or blocking INM scission during primary envelopment. As a consequence, such alterations should result in abnormal transcript localization both in the nucleus and at synaptic sites, as well as decreased synaptic protein synthesis, abnormal NMJ expansion, and an accumulation of ghost boutons. To ascertain the localization of megaRNP transcripts known to be present in megaRNPs at the NE, we carried out fluorescent in situ hybridization (FISH) using par6 and magi RNA probes. As previously described (Speese et al., 2012), in wild type muscles par6 and magi mRNAs are enriched at NE foci associated with LamC foci or nuclear folds marked by antibodies to LamC (Fig. 4A,B, top row panels). In contrast, in torsin null mutants, par6 and magi FISH signals appeared as foci that, while associated with the NE, were on the cytoplasmic side of the LamC signal (Fig. 4A,B, bottom row panels; Fig. 4C). This is in agreement with the light and electron microscopy studies, showing that altering Torsin function prevents megaRNP nuclear egress and results in megaRNPs remaining attached to the INM within the perinuclear space.
Figure 4. The distribution of mRNAs at the NE and synaptic sites in disrupted in torsin mutants.
(Also see Fig. SF4).
(A,B) FISH to body wall muscles showing the nuclear distribution of (A) par6 and (B) magi transcripts in wild type and torsin mutants.
(C) Quantification of FISH signal outside the nucleus. N [nuclei;larvae]= [18;6],[18;6],[18;6],[14;6].
(D, E) FISH to body wall muscles showing the distribution of (D) par6 and (E) magi transcript at the NMJ in wild type and torsin null mutants.
(F, G) Distribution of (F) Par6 and (G) Magi immunoreactivity at the NMJ in wild type and torsin mutants.
(H, I) Quantification of postsynaptic (H) Par6 and (I) Magi immunoreactive signal, normalized to wild type control. N([NMJs;larvae])= [16;6],[18;6] for H and [17;6],[15;6] for I.
(J) NMJs in wild type and torsin mutants labeled with anti-HRP and anti-DLG showing reduced size and increased ghost boutons (arrowheads) in torsin mutants.
(K, L) Quantification of the number of (K) synaptic boutons and (L) ghost boutons. N([NMJs;larvae])= [19;10],[18;10],[19;10],[19;10],[20;10],[19;10],[19;10] for K,L. Error bars represent ±SEM (**= p<0.001, ***= p<0.0001).
Calibration scale (μm)= A,B:3; D–G:10; J:20.
When we examined FISH signals at the NMJ in wild type controls, par6 mRNA was concentrated at subsynaptic sites as previously reported (Fig. 4D; top panels)(Speese et al., 2012). However, this synaptic par6 FISH signal was virtually eliminated in torsin null mutants (Fig. 4D; bottom panels). Similarly, the synaptic localization of magi mRNA was significantly decreased in the torsin mutants (Fig. 4E). The marked decrease in par6 and magi mRNA levels at the NMJ appeared specific for the NE-budding pathway, as no significant differences around the NMJ in torsin mutants were observed upon FISH of discs-large (dlg) RNA, which is not associated with nuclear DFz2C/LamC foci (Fig. SF4A-C)(Speese et al., 2012). Thus, in the absence of Torsin function, synaptic mRNAs known to be present in megaRNPs exhibit reduced localization at the NMJ, but a non-megaRNP mRNA does not.
We also examined Par6 and MAGI protein levels, using antibodies specific to Drosophila Par6 (Ruiz-Canada et al., 2004) and MAGI (this report). In wild type larvae, Par6 and MAGI immunoreactivity localized primarily to the postsynaptic muscle region of the NMJ (Fig. 4F,G; top panels). In addition, Par6 immunoreactivity was observed in a diffuse manner at presynaptic boutons (marked by the anti-HRP signal), being particularly prominent at presynaptic microtubule bundles (Ruiz-Canada et al., 2004) (Fig. 4F, top panels), and at low levels at the muscle cell cortex (Fig. 4F, top panels). MAGI immunoreactivity was also observed at presynaptic compartments, but without noticeable concentration at microtubule bundles (Fig. 4G, top panels). In torsin null mutants, postsynaptic localization of Par6 immunoreactivity as well as muscle cell cortex signal was severely reduced (Fig. 4F, bottom panels; Fig. 4H), while presynaptic localization of Par6 at microtubule bundles appeared normal (Fig. 4F, arrows). Similarly, postsynaptic MAGI protein localization was severely reduced in these mutants (Fig. 4G, bottom panels; Fig. 4I). Unlike Par6 and MAGI, DLG immunoreactivity was not changed in torsin mutants (Fig. 4J; Fig. SF4D), suggesting that the defect is not general but affects only a subset of postsynaptic proteins. Thus, disrupting Torsin function prevents normal localization of some synaptic mRNAs, and as a consequence, normal postsynaptic levels of their encoded proteins.
The functional consequence on NMJ structure of reduced Par6 and MAGI mRNA and protein levels at the postsynaptic compartment in torsin mutants was assessed by counting the number of normal and undifferentiated ghost boutons observable in the last (3rd instar) larval stage. Interfering with Torsin function resulted in a significantly reduced number of synaptic boutons (Fig. 4J,K), and a significantly increased number of undifferentiated ghost boutons (Fig. 4J, arrowheads, Fig. 4L).
Together, these results demonstrate that inhibiting Torsin function results in megaRNP accumulation at the NE, likely due to a defect in INM scission during primary envelopment. As a consequence, synaptic transcript-containing megaRNPs fail to efficiently exit the nucleus, limiting trafficking of the mRNAs contained within to postsynaptic sites where they are normally enriched. This reduced synaptic mRNA localization results in reduced levels of specific postsynaptic proteins during NMJ expansion, and thus in poorly developed NMJs containing fewer synaptic boutons and increased numbers of undifferentiated ghost boutons lacking postsynaptic proteins. These results provide the first mechanistic insight into the molecular machinery underlying nuclear egress of megaRNPs by NE budding. They also provide a novel mechanism by which Torsin influences synaptic development as well as important clues as to how torsinA dysfunction might lead to the alterations in synaptic plasticity observed in DYT1 mouse models and human patients.
EXPERIMENTAL PROCEDURES
Fly Strains
Flies were reared on standard Drosophila medium at 25°C. RNAi crosses and controls were performed at 29°C.
Molecular Biology
Torsin dsRNA was prepared by amplifying exon1 by PCR, and in-vitro transcribed using Ambion MEGAscript T7 kit.
S2 cell culture and dsRNA treatment
Drosophila SL2-NP2 cells were cultured and treated as in (Koles et al., 2012).
Immunocytochemistry
Third instar larval body wall muscles were dissected and fixed as in (Budnik et al., 1996).
Antibody Generation
Anti-MAGI was generated by immunizing rats with a bacterially produced Magi peptide (aa337-558).
Fluorescence In-situ Hybridization
Procedures for FISH were as in (Speese et al., 2012)
Transmission Electron Microscopy (TEM)
TEM was performed as in (Ashley et al., 2005).
Diaminobenzidine (DAB) conversion of Mini-SOG
DAB photoconversion was adapted from (Grabham and Goldberg, 1997).
Image Acquisition
Confocal images were acquired using a Zeiss LSM700 confocal microscope equipped with a Zeiss 63x Plan-Apochromat 1.4 NA DIC oil immersion objective.
Quantification
Categorization of DFz2C/LamC foci at the light microscopy level. To categorize nuclear DFz2C/LamC foci, larval body wall muscle preparations were labeled with antibodies to DFz2C and LamC and the number of nuclei at muscle 6 (segment A2-4) with foci of the following categories were counted and divided by the total number of nuclei. A focus was considered “normal” if it contained a DFz2C spot localized within a thickening of the lamina (Fig. 1A), “with small DFz2C puncta” if small DFz2C puncta localized at the lamina but there was no observable thickening of the lamina at this site (Fig. 1B), or “containing depleted foci” if a thickening of the lamina devoid of DFz2C signal was observed (Fig. 1C). Ultrastructural categorization of megaRNPs. Micrographs of foci at 78,000–110,000X total magnification were examined and megaRNPs were categorized as “within INM invaginations” if they appeared as a granule or granule cluster surrounded by an INM invagination, as “with a collared neck” if the granule was located within an enlarged perinuclear space and tethered to the INM through an electron dense neck, or as “amorphous” if the granule was larger than 250nm. Categorization of NE associated FISH signal. To determine the % of signal outside the lamina, the number of lamina-associated FISH puncta was subdivided into those that were present either on the nucleoplasmic or cytoplasmic side of the LamC-immunoreactive lamina. Measurements of postsynaptic protein levels. Normalized postsynaptic protein levels were determined as in (Ramachandran et al., 2009). Quantification of bouton and ghost bouton number. Bouton and ghost bouton numbers were assessed as in (Speese et al., 2012).
Statistical Analysis
(See additional methods for complete Statistical Analysis)
Supplementary Material
HIGHLIGTS.
Large RNPs (megaRNPs) can exit the nucleus via nuclear envelope budding.
The dystonia-linked AAA-ATPase Torsin is required for nuclear exit of megaRNPs.
Torsin is involved in scission of the inner nuclear membrane during megaRNP egress.
torsin mutants have decreased postsynaptic mRNAs and proteins, and abnormal synapses.
Acknowledgments
We thank Drs. Xandra Breakefield, Mary Munson, Reid Gilmore, Emiliano Ricci, and members of the Budnik lab for helpful comments on the manuscript and discussions. We would like to thank the UMassMed Electron Microscopy Facility for support in our ultrastructural studies, and the Vienna Drosophila RNAi facility for providing the Torsin-RNAi line. We also thank Drs. Pamela Gayer, Yosef Gruenbaum, Georg Krohne, and Gertrud Schupbach for generous gift of antibodies. Supported by NIH grant R01 NS063228 to VB. MJM is an HHMI investigator.
Footnotes
Author contributions:
VJ carried out most of the experiments and contributed intellectually to the project. JA carried out some of the ultrastructural studies and contributed intellectually to the project and manuscript writing. JN carried out most the ultrastructural experiments. AN performed supporting FISH in wild type and mutant larvae. NI and NW-I provided the unpublished TorsinΔE fly strain and contributed to discussions. MJM provided critical intellectual input, and helped in manuscript writing. VB directed the project and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashley J, Packard M, Ataman B, Budnik V. Fasciclin II signals new synapse formation through amyloid precursor protein and the scaffolding protein dX11/Mint. J Neurosci. 2005;25:5943–5955. doi: 10.1523/JNEUROSCI.1144-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataman B, Ashley J, Gorczyca D, Gorczyca M, Mathew D, Wichmann C, Sigrist SJ, Budnik V. Nuclear trafficking of Drosophila Frizzled-2 during synapse development requires the PDZ protein dGRIP. Proc Natl Acad Sci U S A. 2006;103:7841–7846. doi: 10.1073/pnas.0600387103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataman B, Ashley J, Gorczyca M, Ramachandran P, Fouquet W, Sigrist SJ, Budnik V. Rapid activity-dependent modifications in synaptic structure and function require bidirectional wnt signaling. Neuron. 2008;57:705–718. doi: 10.1016/j.neuron.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barco A, Lopez de Armentia M, Alarcon JM. Synapse-specific stabilization of plasticity processes: the synaptic tagging and capture hypothesis revisited 10 years later. Neurosci Biobehav Rev. 2008;32:831–851. doi: 10.1016/j.neubiorev.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9:222–234. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- Budnik V, Koh YH, Guan B, Hartmann B, Hough C, Woods D, Gorczyca M. Regulation of synapse structure and function by the Drosophila tumor suppressor gene dlg. Neuron. 1996;17:627–640. doi: 10.1016/s0896-6273(00)80196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild RE, Kim CE, Dauer WT. Loss of the dystonia-associated protein torsinA selectively disrupts the neuronal nuclear envelope. Neuron. 2005;48:923–932. doi: 10.1016/j.neuron.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Grabham PW, Goldberg DJ. Nerve growth factor stimulates theaccumulation of beta1 integrin at the tips of filopodia in the growth cones of sympathetic neurons. J Neurosci. 1997;17:5455–5465. doi: 10.1523/JNEUROSCI.17-14-05455.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald D, Singer RH, Rout M. Nuclear export dynamics of RNA-protein complexes. Nature. 2011;475:333–341. doi: 10.1038/nature10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer LM, Leipe DD, Koonin EV, Aravind L. Evolutionary history and higher order classification of AAA+ ATPases. J Struct Biol. 2004;146:11–31. doi: 10.1016/j.jsb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Kim CE, Perez A, Perkins G, Ellisman MH, Dauer WT. A molecular mechanism underlying the neural-specific defect in torsinA mutant mice. Proc Natl Acad Sci U S A. 2010;107:9861–9866. doi: 10.1073/pnas.0912877107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler A, Hurt E. Exporting RNA from the nucleus to the cytoplasm. Nat Rev Mol Cell Biol. 2007;8:761–773. doi: 10.1038/nrm2255. [DOI] [PubMed] [Google Scholar]
- Koles K, Nunnari J, Korkut C, Barria R, Brewer C, Li Y, Leszyk J, Zhang B, Budnik V. Mechanism of evenness interrupted (evi)-exosome release at synaptic boutons. J Biol Chem. 2012;287:16820–16834. doi: 10.1074/jbc.M112.342667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maric M, Shao J, Ryan RJ, Wong CS, Gonzalez-Alegre P, Roller RJ. A functional role for TorsinA in herpes simplex virus 1 nuclear egress. J Virol. 2011;85:9667–9679. doi: 10.1128/JVI.05314-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew D, Ataman B, Chen J, Zhang Y, Cumberledge S, Budnik V. Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2. Science. 2005;310:1344–1347. doi: 10.1126/science.1117051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter TC, Klupp BG, Granzow H. Herpesvirus assembly: a tale of two membranes. Curr Opin Microbiol. 2006;9:423–429. doi: 10.1016/j.mib.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Naismith TV, Heuser JE, Breakefield XO, Hanson PI. TorsinA in the nuclear envelope. Proc Natl Acad Sci U S A. 2004;101:7612–7617. doi: 10.1073/pnas.0308760101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwald AF, Aravind L, Spouge JL, Koonin EV. AAA+: A class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 1999;9:27–43. [PubMed] [Google Scholar]
- Ozelius LJ, Hewett JW, Page CE, Bressman SB, Kramer PL, Shalish C, de Leon D, Brin MF, Raymond D, Corey DP, et al. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat Genet. 1997;17:40–48. doi: 10.1038/ng0997-40. [DOI] [PubMed] [Google Scholar]
- Packard M, Koo ES, Gorczyca M, Sharpe J, Cumberledge S, Budnik V. The Drosophila wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell. 2002;111:319–330. doi: 10.1016/s0092-8674(02)01047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran P, Barria R, Ashley J, Budnik V. A critical step for postsynaptic F-actin organization: regulation of Baz/Par-3 localization by aPKC and PTEN. Dev Neurobiol. 2009;69:583–602. doi: 10.1002/dneu.20728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter JD. Think globally, translate locally: what mitotic spindles and neuronal synapses have in common. Proc Natl Acad Sci U S A. 2001;98:7069–7071. doi: 10.1073/pnas.111146498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Canada C, Ashley J, Moeckel-Cole S, Drier E, Yin J, Budnik V. New Synaptic Bouton Formation Is Disrupted by Misregulation of Microtubule Stability in aPKC Mutants. Neuron. 2004;42:567–580. doi: 10.1016/s0896-6273(04)00255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu X, Lev-Ram V, Deerinck TJ, Qi Y, Ramko EB, Davidson MW, Jin Y, Ellisman MH, Tsien RY. A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS Biol. 2011;9:e1001041. doi: 10.1371/journal.pbio.1001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speese SD, Ashley J, Jokhi V, Nunnari J, Barria R, Li Y, Ataman B, Koon A, Chang YT, Li Q, et al. Nuclear Envelope Budding Enables Large Ribonucleoprotein Particle Export during Synaptic Wnt Signaling. Cell. 2012;149:832–846. doi: 10.1016/j.cell.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe LM, Kim CE, Alagem N, Dauer WT. Primary dystonia: molecules and mechanisms. Nat Rev Neurol. 2009;5:598–609. doi: 10.1038/nrneurol.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi-Ito N, Doherty OM, Moriyama H, Breakefield XO, Gusella JF, O’Donnell JM, Ito N. Dtorsin, the Drosophila ortholog of the early-onset dystonia TOR1A (DYT1), plays a novel role in dopamine metabolism. PLoS ONE. 2011;6:e26183. doi: 10.1371/journal.pone.0026183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JE, Saraste M, Runswick MJ, Gay NJ. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. Embo J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersma-Meems R, Van Minnen J, Syed NI. Synapse formation and plasticity: the roles of local protein synthesis. Neuroscientist. 2005;11:228–237. doi: 10.1177/1073858404274110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.