Abstract
The main goal of the study was to determine the relationship between prenatal exposure to polycyclic aromatic hydrocarbons (PAHs) measured by PAH-DNA adducts in umbilical cord blood and early wheeze. The level of PAH-DNA adducts in the cord blood is assumed to reflect the cumulative dose of PAHs absorbed by the fetus over the prenatal period. The effect of prenatal PAH exposure on respiratory health measured by the incidence rate ratio (IRR) for the number of wheezing days in the subsequent four year follow-up was adjusted for potential confounding factors such as personal prenatal exposure to fine particulate matter (PM2.5), environmental tobacco smoke (ETS), gender of child, maternal characteristics (age, education and atopy), parity, and mold/dampness in the home. The study sample includes 339 newborns of non-smoking mothers 18-35 years of age and free from chronic diseases, who were recruited from ambulatory prenatal clinics in the first or second trimester of pregnancy. The number of wheezing days during the first two years of life was positively associated with prenatal level of PAH-DNA adducts (IRR = 1.69, 95%CI = 1.52 – 1.88), prenatal particulate matter (PM2.5) level dichotomized by the median (IRR = 1.38; 95%CI: 1.25 – 1.51), maternal atopy (IRR = 1.43; 95%CI: 1.29 – 1.58), moldy/damp house (IRR = 1.43; 95%CI: 1.27 – 1.61). The level of maternal education and maternal age at delivery were inversely associated with the IRRs for wheeze. The significant association between frequency of wheeze and the level of prenatal environmental hazards (PAHs and PM2.5) was not observed at ages 3 or 4 years. Although the frequency of wheezing at ages 3 or 4 years was no longer associated with prenatal exposure to PAHs and PM2.5, its occurrence depended on the presence of wheezing in the first two years of life, which nearly tripled the risk of wheezing in later life. In conclusion, the findings may suggest that driving force for early wheezing (<24 months of age) are different to those leading to later onset of wheeze. As we reported no synergistic effects between prenatal PAH (measured by PAH-DNA adducts) and PM2.5 exposures on early wheeze, this suggests the two exposures may exert independent effects via different biological mechanism on wheeze.
Keywords: prenatal exposure to polycyclic aromatic hydrocarbons, biomarkers of exposure, DNA adducts, early wheeze, 4-year olds, birth cohort study
Introduction
There is a compelling body of epidemiologic evidence that asthma and other respiratory diseases are a major health issue in childhood. They are the leading causes of visits to physicians for children and one of the main and increasing causes of hospitalization in young children and adolescents. The prevalence of asthma and wheezing symptoms in infants and children varies widely between populations and there is a debate concerning the nature and meaning of early wheezing for respiratory health in the course of later child and adult life (1 - 4). Wheeze originates in airways which may be narrowed by compression or by intrabronchial or intraluminal obstruction (inflammatory mucosal oedema, secretions or spasm), which cause an increase in velocity of gas through them with resultant oscillation. It is also suggested that wheezy lower respiratory illness in the first year of life is a consequence of anatomically small airway unrelated to the later development of atopic asthma (5).
The importance of early wheezing for the respiratory health of young children was emphasized by the findings that most cases of persistent wheezing beginning in early life are often associated with reduced infant lung function. For instance, the recent study in preschool children in the Manchester Asthma and Allergy Study (6) has shown that wheezers have reduced lung function compared with nonwheezing children, but the deficit was considerably greater in recurrent wheezers.
Many epidemiologic studies conducted so far have investigated the effects of particulate matter and environmental tobacco smoke (ETS) on the occurrence of respiratory symptoms in postnatal life (7 - 14), however, very scarce attempts have been made to measure prenatal environmental hazards. In the intrauterine period, during which the lung is developing and maturing, even very subtle influences on fetal airway development may have a lasting impact on the risks of respiratory disease later in life (15 - 18). As the relationship between prenatal exposure to environmental hazards and infant’s health is still poorly understood, the purpose of the study was to test the hypothesis that infants with higher levels of prenatal exposure to environmental pollutants may be at greater risk of developing respiratory symptoms. We focused our attention in particular on prenatal exposure to PAHs such as benzo(a)pyrene as they commonly contaminate outdoor environment as well as the indoor environment in which infants spend most of their time (19). The biological importance of PAH exposure stems from the fact that these compounds represent an important class of environmental immunotoxic contaminants, which may impair the immune function of the foetus and subsequently be responsible for increased susceptibility of newborns and young infants to respiratory infections.
The main goal of the study was to relate prenatal exposure to PAH compounds to the onset and frequency of wheezing in early childhood. Prenatal PAH exposure was measured by PAH-DNA adducts in the cord blood, which is specifically assumed to reflect the absorbed cumulative dose of fetal exposure over the course of gestation. The effect of prenatal PAH exposure on the frequency of wheeze in the first four years of life was adjusted for potential confounding factors such as personal exposure to prenatal PM2.5, ETS, gender of child, maternal characteristics (age, education and atopy), parity, and mouldy/damp home.
Material and Methods
This study uses data from an earlier established birth cohort of children in Krakow which is the product of a collaboration of Jagiellonian University with Columbia University in New York. The design of the study and the detailed selection of the population have been described previously (20). Pregnant women were recruited from ambulatory prenatal clinics in the first or second trimester of pregnancy. Only women 18-35 years of age, who claimed to be non-smokers, with singleton pregnancies, without illicit drug use and HIV infection, free from chronic diseases such as diabetes or hypertension, and resided in Krakow for at least one year prior to pregnancy were eligible for the study. Prior to participation, women read and signed an informed consent. The Ethical Committee of the Jagiellonian University approved the research.
Upon enrolment, a detailed questionnaire was administered to each woman to solicit information on demographic data, house characteristics, medical and reproductive history, occupational hazards, and smoking practices of others present in the home. A total of 505 pregnant women enrolled to the study born their children between January 2001 and February 2004, but the analysis has been based on 369 subjects with complete data over the whole follow-up period.
After delivery, newborns were followed-up every three months in their two years of life and every 6 months in their third and fourth years; trained interviewers carried out detailed face-to-face standardized interviews on infants’ health at each visit. All interviews were conducted with the mothers of infants present. Respiratory outcome variables included the number of wheezing days in the chest of children irrespective of respiratory infection as reported by mothers.
Prenatal exposure to fine particles was measured by the personal exposure monitors in the second trimester of pregnancy. ETS was assessed by interviewing mothers on their passive exposure to tobacco smoke over the course of their pregnancy, and was supplemented by the independent measurements of cotinine levels in the cord blood. Mould and dampness in the household was based on questions regarding noticeable moisture stains and visible mould growth on the walls within the household asked at the interviews given at each follow up time points. Maternal atopy was assumed in the case that the mother reported allergic skin disorders or allergy related respiratory diseases. Maternal education level (elementary, medium and higher) was an indicator of socio-economic status.
Dosimetry of personal prenatal exposure to fine particles
Monitoring of personal of fine particles (PM2.5) was carried out in all pregnant women over a 48-hour period during the second trimester of pregnancy. The women were instructed by the trained staff member as how to use personal monitor and asked to carry the monitoring device during the daytime hours for two consecutive days and place it by their bed at night. On the second day the air monitoring staff assistant and interviewer visited the woman’s home to change the battery-pack and to complete the questionnaire on the household characteristics.
A Personal Environmental Monitoring Sampler (PEMS) was used to measure particle mass. The single pump/two impactors sampling method has been developed at Harvard School of Public Health and is applicable for measuring particles and gases. Fine particles were collected on the PEMS Teflon membrane filter (37 mm Teflo™, Gelman Sciences).
In a subsample of 85 women measurements of PM2.5 were also assessed in the third trimester of pregnancy The concentration of PM2.5 (mean ±SD) in the second trimester was 42.3 ±30.8 μg/m3 and in the third trimester was 38.5 ± 29.9 μg/m3. This difference was not statistically significant (t = 1.015, p = 0.313).
Dosimetry of PAH-DNA adducts
Maternal blood (30-35 mL) was collected within 1 day postpartum, and umbilical cord blood (30-60 mL) was collected at delivery. Samples were transported to the laboratory immediately after collection. The buffy coat, packed red blood cells, and plasma were separated and stored at −70°C. BaP-DNA adducts in extracted WBC DNA were analyzed using the HPLC-fluorescence method of Alexandrov et al. (21), which detects BaP tetraols. The assay gives zero values when unexposed calf thymus DNA is tested (D. Tang, personal communication). The method has a coefficient of variation of 12% and a lower limit of detection of 0.25 adducts per 108 nucleotides. HPLC analysis of DNA samples for BaP-DNA adducts was performed in batches, with 18-paired maternal and newborn samples in the same batch.
Dosimetry of cord blood cotinine
Newborns at delivery provided cord blood specimens which before were stored at 70°C prior to laboratory analysis. The serum cotinine concentration was measured at CDC using the sensitive isotope-dilution high-performance liquid chromatographic/atmospheric pressure ionization tandem spectrometric (LC/MS/MS) procedure (22). The limit of detection (LOD) is below 0.050 ng/mL. About 25% of specimens had cotinine levels below the LOD. Maternal blood cotinine level below 15.0 ng/L was considered the borderline separating smokers from non-smokers (23 - 24).
Statistical Analysis
The purpose of the statistical analysis was to assess the impact of prenatal environmental hazards on the frequency of wheezing as monitored in cohort children’s first four years of life. To identify potential confounders, associations between population characteristics and outcome variables were investigated. The effect of environmental exposure on wheezing days in children under the follow-up was assessed by incidence rate ratios (IRRs) estimated by the Zero-inflated Poisson regression model (ZIP), which better fits the overdispersed count Poisson data with excess of observed zeros (null observations) than the traditional Poisson regression model (25 - 26). When there are far more 0 counts than allowed by the standard Poisson distribution, upon which the model is based, one can consider breaking up the model into a part indicating whether the individual has a given symptom and another part indicating the number of days with the symptom. For the zero-inflated model, the probability of observing a zero outcome equals the probability that the individual is in the always-zero group. The zero-inflated regression estimates two sets of parameters: one set for the “logistic portion” (the parameters of the regression models for the probability of extra zeros using the logistic function) and another set for the “Poisson portion” (the parameters of the Poisson regression model). For easy interpretation, the parameters of the first set are presented as odds ratio (OR) and those of the second set as incidence rate ratios (IRR): both come with 95% confidence intervals. The inverse ORs (1/OR) with their confidence intervals can be interpreted as approximations of relative risks of reporting the symptom during the given follow-up period. The estimates are mutually adjusted for all other variables of the regression models. The dependent variables are counts of total number of wheezing days reported in the follow-up period (0, 1, 2, 3, 4, etc). In the regression models a set of potential confounders or modifiers (gender of child, maternal education, parity, maternal atopy, prenatal exposure to ETS and fine particles and mould damp in household) were taken into consideration. In all statistical analyses prenatal PAH was classified as exposed (>0.250 adducts per 108 nucleotides) and non-exposed (PAH-DNA adducts below LOD level); and PM2.5 exposure was dichotomized by median values. Statistical analyses were performed with STATA 10 version software for Windows (27)
Results
Table 1 presents the characteristics of the study sample grouped by the prenatal PAH exposure level (PAH-DNA adducts). The group of children in the high PAH-DNA adduct group did not differ significantly with respect to important demographic characteristics from the group with nondetectable PAH-DNA adduct levels.
Table 1.
Characteristics of the study sample grouped by the level of the cord blood PAH-DNA adducts (N = 339)
| Total N=339 |
Low prenatal PAH exposure (PAH adducts ≤ 0.250 per 108 nucleotides) N=119 |
High prenatal PAH exposure (PAH adducts > 0.250 per 108 nucleotides) N=220 |
P-level for difference |
||
|---|---|---|---|---|---|
|
| |||||
| Maternal age: ≤ 25years | n (%) | 78 (23.0) | 25 (21.0) | 53 (24.1) | 0.380 |
| 26–30 years | n (%) | 191 (56.3) | 73 (61.3) | 118 (53.6) | |
| > 30 years | n (%) | 70 (20.6) | 21 (17.6) | 49 (22.3) | |
|
| |||||
| Maternal education: | |||||
| Elementary | n (%) | 32 (9.4) | 8 (6.7) | 24 (10.9) | 0.163 |
| Secondary | n (%) | 78 (23.0) | 23 (19.3) | 55 (25.0) | |
| Higher | n (%) | 229 (67.6) | 88 (73.9) | 141 (64.1) | |
|
| |||||
| Gender: Boys | n (%) | 170 (50.1) | 64 (53.8) | 106 (48.2) | 0.384 |
| Girls | n (%) | 169 (49.9) | 55 (46.2) | 114 (51.8) | |
|
| |||||
| Parity: 1 | n (%) | 217 (64.0) | 79 (66.4) | 138 (62.7) | 0.581 |
| ≥ 2 | n (%) | 122 (36.0) | 40 (33.6) | 82 (37.3) | |
|
| |||||
| Breastfeeding: ≤ 6 months | n (%) | 105 (31.0) | 33 (27.7) | 72 (32.7) | 0.4085 |
| > 6 months | n (%) | 234 (69.0) | 86 (72.3) | 148 (67.3) | |
|
| |||||
| Maternal atopy (+): | n (%) | 85 (25.1) | 32 (26.9) | 53 (24.1) | 0.663 |
|
| |||||
| Prenatal ETS (+): | n (%) | 81 (23.9) | 29 (24.4) | 52 (23.6) | 0.986 |
|
| |||||
| Postnatal ETS (+): | n (%) | 63 (18.6) | 25 (21.0) | 38 (17.3) | 0.485 |
|
| |||||
| Mould (+) or dampness: | n (%) | 104 (30.7) | 32 (26.9) | 72 (32.7) | 0.3227 |
|
| |||||
| Prenatal exposure to fine particulate matter (PM2.5 μg/m3): Median |
33.4 | 31.8 | 35.1 | 0.087 | |
| IQ range | 22.3–50.7 | 22.3–44.8 | 22.2–53.8 | ||
| Missing data | 3 | 1 | 2 | ||
|
| |||||
| Cord blood cotinine (ng/mL): Median | 0.08 | 0.08 | 0.08 | 0.201 | |
| IQ range | 0.05–0.15 | 0.05–0.16 | 0.05–0.14 | ||
| Missing data | 15 | 4 | 11 | ||
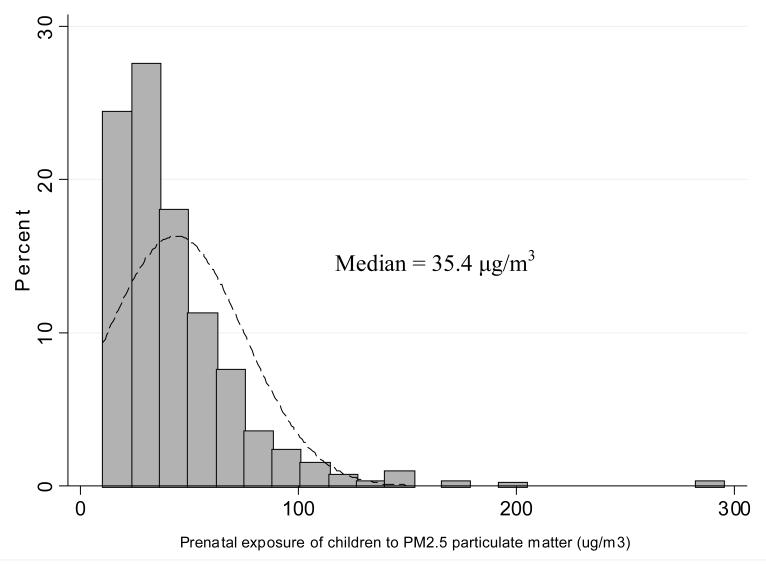
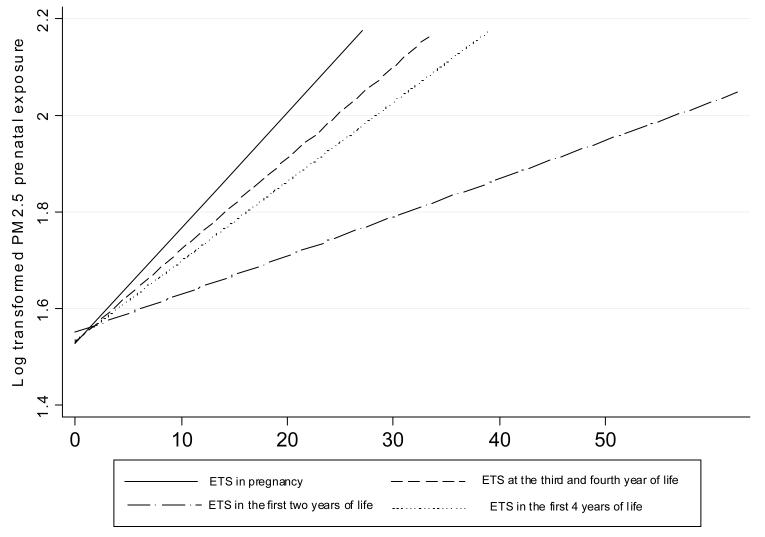
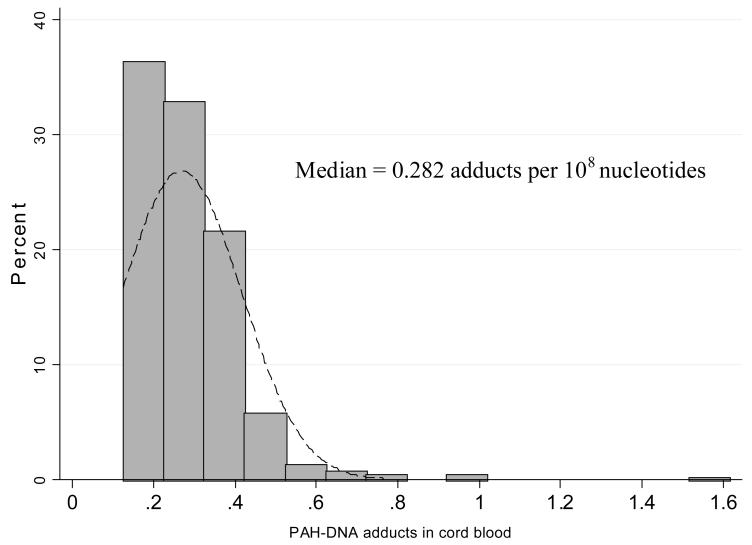
Personal measurements of prenatal daily exposure to PM2.5 particles were within a wide range of 10.3 μg/m3 – 294.9 μg/m3 with the median of 35.4 μg/m3 (Figure 1). PM2.5 was modestly, but significantly, correlated not only with the average number of cigarettes smoked daily in the presence of the mother over the course of pregnancy (r = 0.21, p<0.0001) but also with the reported average number of cigarettes smoked at home in the children’s first four years of life (rs = 0.21, p = 0.0001) (Figure 2). Cord blood cotinine was also significantly associated with PM2.5 (rs = 0.315, p <0.0001) and the reported number of cigarettes smoked at the presence of mother in pregnancy (rs = 0.572, p<0.0001). In 119 (35.1%) newborns, PAH DNA-adducts were below the detectable level (0.250 adducts per 108 nucleotides) and this group of children was treated as unexposed to PAHs in the prenatal period. Those who were above the detectable level had PAH DNA adducts within the range of 0.250 – 1.615 with a median of 0.318 adducts per 108 nucleotides (Figure 3). The association between cord blood PAH – DNA adducts and prenatal PM2.5 level was not significant (rs = 0.083, p = 0.078); and cord blood PAH-DNA adducts were neither significantly correlated with reported prenatal ETS (rs = 0.06, p = 0.226), nor with cord blood cotinine concentrations (rs = 0.044, p = 0.355), nor with postnatal ETS over the first 4 years of life (r = −0.0004, p = 0.994).
Figure 1.
Prenatal exposure of children to PM2.5 particulate matter (μg/m3)
Figure 2.
Fitted linear regression lines for PM2.5 (log transformed μg/m3) and the reported number of cigarettes smoked in various periods of the follow-up
Figure 3.
Distribution of PAH-DNA adducts in the total sample of children
In the sample of children followed over 4 years, 139 children (41.0%) were reported by their mothers to experience at least one day of wheezing. The onset of wheezing in the first two years of life was recorded in 97 infants (28.6%) and incidence of wheezing beyond the first two years of age was observed in 42 children (12.4%). The number of wheezing days was significantly higher in children with higher PAH exposure, but only in the first two years of life (table 2).
Table 2.
Number of wheezing days occurring in the follow-up periods according to the level of prenatal exposure to PAHs (PAH-DNA adducts)
| Follow-up periods |
N | Mean | SE | 95%CI interval exact Poisson |
|---|---|---|---|---|
| PAH-DNA adducts <=0.250 |
||||
| 1 – 2 years | 150 | 3.35 | 0.15 | 3.06 – 3.65 |
| 3 – 4 years | 119 | 3.53 | 1.17 | 3.21 – 3.89 |
| PAH-DNA adducts>0.250 |
||||
| 1 – 2 years | 267 | 5.55 | 0.14 | 5.28 – 5.84 |
| 3 –4 years | 219 | 2.65 | 0.11 | 2.44 – 2.87 |
Tables 3 - 4 present the relationship between the frequency of wheeze in the subsequent years of follow-up and the prenatal PAH exposure (PAH-DNA adducts) and the prenatal PM2.5 level assessed by the ZIP statistical models. Estimates of IRRs were adjusted for maternal characteristics (age, education, atopy), the number of older siblings, gender of child and dampness/mould in the home. Due to the colinearity between both prenatal/postnatal ETS variables and PM2.5, ETS variables were dropped from the regression models. The tables in the upper part contain the Poisson portion on IRR of number of wheezing days through age 4 years; and the lower parts contain the logistic portion which is based on zero days for wheeze and reports the inverse odds ratios (1/OR), which can be interpreted as an approximation of relative risks of reporting the symptom during the given follow-up period.
Table 3.
Zero-inflated Poisson model for number of wheezing events in the first two-years of life associated with prenatal exposure to PAHs, fine particulate matter and other potential risk factors
| IRR | P>z | [95% Conf. | Interval] | |
|---|---|---|---|---|
| Poisson portion | ||||
| Maternal age | ||||
| <=20 years | Reference | |||
| 20 – 30 years | 0.441 | 0.000 | 0.370 | 0.525 |
| >30 years | 0.435 | 0.000 | 0.340 | 0.557 |
| Maternal education | ||||
| Elementary | Reference | |||
| Secondary | 0.747 | 0.000 | 0.647 | 0.863 |
| Higher | 0.893 | 0.155 | 0.764 | 1.044 |
| Maternal atopy | 1.428 | 0.000 | 1.292 | 1.578 |
| Number of older siblings | 1.043 | 0.215 | 0.976 | 1.115 |
| Gender of child | 1.073 | 0.161 | 0.972 | 1.184 |
| Damp/mould | 1.429 | 0.000 | 1.265 | 1.614 |
| Cord blood PAH-adducts | 1.686 | 0.000 | 1.517 | 1.875 |
| Prenatal exposure to fine | 1.377 | 0.000 | 1.252 | 1.514 |
| particulate matter | ||||
| Logistic portion | 1/OR | |||
| Maternal age | 0.377 | 0.001 | 0.202 | 0.681 |
| Maternal education | 1,037 | 0.842 | 0.731 | 1.471 |
| Maternal atopy | 1.346 | 0.255 | 0.807 | 2.246 |
| Number of older siblings | 2.121 | 0.000 | 1.468 | 3.062 |
| Gender of child | 0.662 | 0.072 | 0.422 | 0.963 |
| Damp/mould | 1.441 | 0.294 | 0.728 | 2.852 |
| Cord blood PAH-adducts | 0.772 | 0.278 | 0.482 | 1.234 |
| Prenatal exposure to fine particulate matter |
1.324 | 0.225 | 0.841 | 2.083 |
| Constant | 0.779 | 0.744 | 0.174 | 3.483 |
Table 4.
Zero-inflated Poisson model for number of wheezing events in the third and fourth years of life due to prenatal exposure to PAH compounds, fine particulate matter and other potential risk factors
| IRR | P>z | [95% Conf. |
Interval] | |
|---|---|---|---|---|
| Poisson portion | ||||
| Maternal age | ||||
| <=20 years | ||||
| 20 – 30 years | 0.961 | 0.820 | 0.682 | 1.353 |
| >30 years | 0.491 | 0.001 | 0.317 | 0.761 |
| Maternal education | ||||
| Elementary | ||||
| Secondary | 0.888 | 0.376 | 0.683 | 1.155 |
| Higher | 1.095 | 0.502 | 0.840 | 1.426 |
| Maternal atopy | 1.351 | 0.000 | 1.176 | 1.552 |
| Number of older siblings | 1.130 | 0.021 | 1.018 | 1.254 |
| Gender of child | 0.873 | 0.045 | 0.765 | 0.997 |
| Damp/mould | 1.669 | 0.000 | 1.390 | 2.005 |
| Cord blood PAH-adducts | 0.956 | 0.506 | 0.836 | 1.093 |
| Prenatal exposure to fine | 1.063 | 0.397 | 0.923 | 1.223 |
| particulate matter | ||||
| Presence of wheeze in the first two years of life |
1.190 | 0.013 | 1.037 | 1.365 |
| Logistic portion | 1/OR | |||
| Maternal age | 0.412 | 0.017 | 0.198 | 0.856 |
| Maternal education | 1.560 | 0.054 | 0.993 | 2.452 |
| Maternal atopy | 0.995 | 0.987 | 0.544 | 1.859 |
| Number of older siblings | 1.142 | 0.564 | 0.727 | 1.765 |
| Gender of child | 1.124 | 0.671 | 0.656 | 1.925 |
| Damp/mould | 2.102 | 0.083 | 0.908 | 4.865 |
| Cord blood PAH-DNA adducts |
0.845 | 0.551 | 0.488 | 1.467 |
| Prenatal exposure to fine particulate matter |
1.027 | 0.922 | 0.596 | 1.772 |
| Presence of wheeze in the first two years of life |
2.601 | 0.001 | 1.486 | 4.549 |
| Constant | 0.361 | 0.288 | 0.055 | 2.368 |
Table 3 shows that the frequency of wheeze (overall number of wheezing days during the first two years of postnatal life) was positively associated with prenatal PAH-DNA adducts (IRR = 1.69, 95%CI = 1.52 – 1.88), prenatal PM2.5 level dichotomized by median (IRR = 1.38; 95%CI: 1.25 – 1.51), maternal atopy (IRR = 1.43; 95%CI: 1.29 – 1.58), mould/damp in the home (IRR = 1.43; 95%CI: 1.27 – 1.61). The level of maternal education and maternal age at delivery were inversely associated with the IRRs for wheeze. The relative risk for the onset of wheeze across the follow-up period increased significantly with the number of older siblings (1/OR = 2.12, 95%CI: 1.47 – 3.06) and was lower in girls (1/OR = 0.66, 95%CI: 0.42 – 0.96).
In the subsequent analysis carried out for the 3 and 4 year-olds (Table 4) we included an additional potential confounding variable (presence of wheeze in the preceding two year period). The results of the analysis revealed that both the effects of prenatal PAH and PM2.5 exposures became insignificant, although the association between frequency of wheeze and maternal atopy, presence of mould/damp in the home and the number of older siblings remained significant as in the preceding follow-up period. It has to be added that the presence of wheeze in the first two years of life almost tripled the relative risk of reporting wheeze at 3 – 4 years of age (1/OR = 2.60, 95% CI: 1.49 – 4.55).
DISCUSSION
Nearly half of the children (41 %) in our study sample experienced wheezing in the first four years of life; and in about two third of these cases, the symptoms developed in the first two years of life. The data showed a strong association between wheezing in the first two years of life and prenatal exposure to air pollutants (PAHs and PM2.5). Although the wheezing at ages 3 or 4 years was not significantly associated with prenatal exposure to PAHs and PM2.5, its frequency depended on the presence of wheezing in the first two years of life. The occurrence of wheeze in the first two years almost tripled the risk of onset of wheezing in later postnatal life. The findings may indicate that driving force for early transient wheezing are different to those leading to later wheeze. As we could not confirm the synergistic effects of PAH and PM2.5 exposures on early wheeze, this suggests that the effects of the exposures are independent and may exert different biological mechanisms. This study also suggests that the wheezing attributable to prenatal exposure is more strongly associated with the total absorbed dose of PAH in pregnancy (PAH-DNA adducts) than the exposure to fine particulate matter.
The biological mechanisms whereby PAH and PM2.5 exposure might cause adverse health outcomes in children are yet unclear. Both variables are a proxy measure of a complex of toxic agents present in the environment that could adversely affect fetal growth and maturation of the lung in utero and in early childhood. The developing fetal lung, as well as the infant lung, is more susceptible to injury by lung toxicants that included air pollutants at doses below the no-effect levels for adults. Animal studies indicate that intrauterine as well as postnatal exposure to air pollutants can lead to impaired lung growth (28, 29). In our prior studies we showed that prenatal exposure to PAHs and fine particulate matter was associated with significant deficits of weight, length and head circumference of newborns (30 - 34). The inhibitory effect of fine particles on fetal growth may affect the airway caliber of infants, making the airways more susceptible to ambient and its hazards (35 - 39). In this case, early wheeze could have been brought about by smaller lung size. In our understanding these observations would be in a good agreement with the original publication of Martinez et al. (40) who documented that infants with early wheeze had a reduced lung function possibly attributable to smaller airways.
However, fine particles may act as carriers of allergens, in addition to PAH compounds (41), which may easily penetrate deep into respiratory system. Moreover, transplacental exposure of newborns to PAHs may result in production of an “allergic response” typified by proliferation of Th2 type T lymphocytes that secrete proinflammatory cytokines such as interleukin (IL)-4, IL-5, and IL-13. The very recent birth cohort study carried out by Tadaki et al (42) has found that out of the 17 cytokines and chemokines investigated in serum cord blood serum there was a positive relationship between high IL-8 concentration and wheezing in infants at 1 yr of age. The Th2 cytokines promote allergen-specific IgE antibody and induce eosinophil-dominated inflammatory tissue responses (43 - 48). The association of high cord blood IgE and sensitization to aeroallergens and recurrent wheezing illness also in later childhood was confirmed by Ferguson et al. (49). Earlier findings that living in high-traffic areas and being exposed to diesel exhaust particles, have been associated with increased respiratory symptoms and a greater risk of allergization (50 - 54) would go along with our study results.
The first epidemiologic observation of an adverse effect of prenatal personal PAH exposure, in combination with postnatal ETS, on respiratory outcomes by age 1 to 2 years was made by Miller et al. in the birth cohort recruited from northern Manhattan (55). These findings were also confirmed in our initial birth cohort followed over the first year of life for whom data from prenatal personal air monitoring of mothers in the second trimester of pregnancy were available (56). In the latter study an increased risk related to prenatal PAH exposure was observed for various respiratory symptoms such as wheezing, sore throat, ear infection, cough irrespective of respiratory infections, and cough without cold. The importance of maternal use of domestic chemicals during pregnancy on wheezing and lung function in children aged <8.5 years was also investigated in the Avon longitudinal study of parents and children (57). The authors have shown that an increased household chemical exposure score was associated with early and late onset of wheeze and decrements in FEV1 and FEF25-75%.
To our knowledge, our study provided the first epidemiologic evidence on the association between cord blood PAH-DNA adducts and the respiratory health of children. Earlier epidemiologic studies were mostly concerned with the traditional measurements of airborne PAHs exposure. DNA adducts have been found in various human tissues and there is already a sufficiently large scientific basis to justify the application of DNA adduct measurements as biomarkers in exposure assessment although their use in risk-assessment requires further investigation (58). In epidemiological studies, correlations between the level of PAH exposure and the number of PAH-DNA adducts have been found, including that between coke oven exposure and PAH-DNA adducts in blood cells (59, 60) and that between cigarette smoking and PAH-DNA adducts also in blood cells (61). PAH-DNA adduct measurements have several advantages over traditional exposure assessment. First, higher PAH-DNA adduct levels integrate exposure over a longer period of time and account for all exposure routes. Second, PAH-DNA adducts may better account for inter-individual differences in uptake, elimination, distribution, metabolism and repair amongst exposed individuals.
In our study we were not able to separate effects of ETS and PM2.5 on the severity of symptoms because there was a significant correlation between PM2.5 and both prenatal and postnatal ETS. This interrelationship creates colinearity in regression models and difficulties in separating the effect of ETS on the health outcomes from that attributed to fine particles. Stepwise regression indicated that adding both ETS variables into the models did not further explain the variability in severity of wheeze, compared to PM2.5 alone. The harmful impact of ETS confirmed in some previous studies may result from its correlation with fine particles.
Our observations on the importance of older siblings for wheezing episodes in children are in good agreement with the Tucson Children’s Respiratory Study (62), which has shown that children with more exposure at home or at day care were more likely to have frequent wheezing at 2 years of age than children with little or no exposure. In the birth cohort COAST study (63) day care attendance and/or the presence of siblings significantly increased the likelihood of contracting viral infections (1.5 to 2.1 fold increase) during infancy. The higher risk of respiratory infections in children having older siblings is assumed to be related to the fact that older siblings introduce bacterial or viral infections into the family circle.
A number of earlier studies have also found significant associations between respiratory infections and family history of asthma or atopy. For example, Gurwitz and coworkers found that children hospitalized with respiratory syncytial virus (RSV) had a higher proportion of first-degree relatives with bronchial hyperactivity (64). Similarly, Trefny et al. (65) found that infants hospitalized with RSV bronchiolitis were more likely to have a family history of asthma. The role of family history of atopy in the occurrence of respiratory infections is not fully understood as yet. It may be a proxy of intrinsic genetic susceptibility, cytokine deregulation, lung development, altered antiviral immunity or increased inflammatory response. The influence of family history is likely to be clarified by ongoing genetic studies taking into consideration gene-environment interactions.
An inverse association between maternal age and wheezing observed in our study still requires explanation. Maternal age may be a proxy for some unknown social factors not considered in the analysis. Maybe younger mothers are not as responsive as older mothers to their infants’ needs or present some less favorable behavior during early infancy of children. The way in which mothering skills may affect the young child and respiratory health problems is also unknown. Interestingly, the effects of maternal education showed a similar impact as that found for maternal age and this again might indicate that some important mothering skills in caring for newborns and infants related to maternal education may be important for respiratory health of babies. The educational level of mothers is not only a proxy for the socioeconomic status of the family, but it may be related to other relevant factors such as maternal lifestyle, dietary habits before and during pregnancy, or feeding practices of infants and young children. In this respect the results of our study calls for more research efforts aiming to explain the other factors hidden behind proxy measures of quality of maternal care of babies.
A limitation of our study results from the fact that we could not clearly distinguish the effect of prenatal PAH and PM2.5 exposure from that of postnatal exposure. However, the postnatal level of exposure to PAHs based on measurement of 1-hydroxypyrene (1-HP) in urine, which is commonly used as an overall marker of PAH exposure (66 - 68), was measured in the subsample of 220 children at age of three years. Since the measurements have shown no significant difference in concentrations of 1-HP between the groups of children with low and high PAH exposure level (444.6 pg/mL vs. 398.3 pg/mL, p = 0.184), we believe that postnatal exposure to PAHs could not significantly confound the main study results. The important potential confounders of the relationship between prenatal ambient risk factors and the respiratory outcomes of infants such as chronic diseases or active tobacco smoking by mothers have been removed through entry criteria. Other risk factors that are thought to affect the probability of respiratory diseases in infants such as maternal atopy, prenatal exposure to fine particles representing also air pollution attributed to passive smoking. and presence of dampness/moulds in the households have been taken into consideration in the analysis. A significant feature of our study is the prenatal personal monitoring of PM2.5 exposure together with measurements of PAH-DNA adducts in cord blood, which are highly relevant measures of individual exposure to environment toxicants in question. Previous studies have attempted to assign exposure values to individual study subjects based on the concentration of pollutants measured in the area of residence. Another strong point of our study is very careful monitoring data of respiratory health in children performed by face-to-face interviews taken by trained interviewers over 12 time points in the follow-up. Potential bias in reporting wheezing episodes by interviews with mothers could have some impact of the study results since the maternal reports were not verified by physician assessment of wheezing phenomena. However, a recently published international study has shown high internal consistency and validity of questionnaire-based wheezing data for children below 36 months of age (69).
In conclusion, the results of our study indicate that the likelihood of wheezing increased with prenatal exposure to PAHs and PM2.5, maternal atopy, presence of dampness/moulds in the house, but was inversely correlated with maternal age and education. The findings may suggest that driving force for early wheezing (<24 months of age) are different to those leading to the later onset of wheeze. As we could not confirm the synergistic effects of PAH and PM2.5 exposures on early wheeze, this suggests that the effects of the exposures are independent and may exert different biological mechanisms. The data support the hypothesis that the risk of respiratory symptoms in early childhood and possibly in later life may be programmed by environment hazards during the prenatal period when the respiratory system is completing its growth and maturation.
Acknowledgements
This is part of an ongoing comparative longitudinal investigation on the health impact of prenatal exposure to outdoor/indoor air pollution in infants and children being conducted in New York City and Krakow. The study received funding from an RO1 grant entitled, “Vulnerability of the Fetus/Infant to PAH, PM2.5 and ETS” (5 RO1 ES10165 NIEHS; 02/01/00 - 01/31/04) and from the NIEHS (RO1 ES010165-0451) the Lundin Foundation and the Gladys T. and Roland Harriman Foundation. Principal investigator: Prof. FP Perera
The authors thank Dr Rachel L.Miller, for her comments and suggestions on the first draft of the manuscript.
References
- 1.Burr ML, Butland BK, King S, Vaughan-Williams E. Changes in asthma prevalence: two surveys 15 years apart. Arch Dis Child. 1989;64:1452–56. doi: 10.1136/adc.64.10.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yunginger J, Reed CE, O’Connel EJ, Melton LJ, O’Fallon WM, Silverstaein MD. A community-based study of the epidemiology of asthma. Incidence rates, 1964-1983. Am Rev Respir Dis. 1992;146:888–94. doi: 10.1164/ajrccm/146.4.888. [DOI] [PubMed] [Google Scholar]
- 3.Steering committee of the International Study on Asthma and Allergies in Childhood (ISAAC) Worldwide variations in the prevalence of asthma symptoms: the international study of asthma and allergies in childhood (ISAAC) Eur Respir J. 1998;12:315–335. doi: 10.1183/09031936.98.12020315. [DOI] [PubMed] [Google Scholar]
- 4.Koopman LP, Brunekreef B, de Jongste JC, Neijens HJ. Definition of respiratory symptoms and disease in early childhood in large prospective birth cohort studies that predict the development of asthma. Pediatr Allergy Immunol. 2001;12:118–124. doi: 10.1034/j.1399-3038.2001.012003118.x. [DOI] [PubMed] [Google Scholar]
- 5.Sly PD, Willet K. Developmental physiology. In: Silverman M, editor. Childhood Asthma and Other Wheezing Disorders. Chapman & Hall; London: 1995. pp. 55–66. [Google Scholar]
- 6.Lowe LA, Simpson A, Woodcock A, Morris J, Murray C, Custovic A. Wheeze phenotypes and lung function in preschool children. Am J Respir Crit Care Med. 2005;171:231–237. doi: 10.1164/rccm.200406-695OC. [DOI] [PubMed] [Google Scholar]
- 7.Fergusson DM, Horwood LJ, Shannon FT, Taylor B. Parental smoking and lower respiratory illness in the first three years of life. J. Epidemiol. Comm Health. 1981;35:180–184. doi: 10.1136/jech.35.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gortmaker SL, Walker DK, Jacobs FH, Ruch-Ross H. Parental smoking and the risk of childhood asthma. Am J Publ Health. 1982;72:574–579. doi: 10.2105/ajph.72.6.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedreira FA, Guandolo VL, Feroli EJ, Mella GW, Weiss IP. Involuntary smoking and incidence of respiratory illness during the first year of life. Pediatrics. 1985;75:594–597. [PubMed] [Google Scholar]
- 10.Neuspiel DR, Rush D, Butler NR, Golding J, Bijur PE, Kurzon M. Parental smoking and post-infancy wheezing in children: a prospective study. Am J Publ Health. 1989;79:168–171. doi: 10.2105/ajph.79.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forastiere F, Corbo GM, Michelozzi P, Pistelli R, Agabiti N, Brancato G, Ciappi G, Perucci CA. Effects of environment and passive smoking on the respiratory health of children. Int J Epidemiol. 1992;21:66–73. doi: 10.1093/ije/21.1.66. [DOI] [PubMed] [Google Scholar]
- 12.Martinez FD, Cline M, Burrows B. Increased incidence of asthma in children of smoking mothers. Pediatrics. 1992;89:21–26. [PubMed] [Google Scholar]
- 13.Ronchetti R, Bonci E, Cutrera R, de Castro G, Indinnimeo L, Midulla F, Tancredi G, Martinez FD. Enhanced allergic sensitization related to parental smoking. Arch Dis Child. 1992;67:496–500. doi: 10.1136/adc.67.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mannino DM, Moorman JE, Kingsley B, Rose D, Repace J. Health effects related to environmental tobacco smoke exposure in children in the United States: data from the third National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 2001;155:36–41. doi: 10.1001/archpedi.155.1.36. [DOI] [PubMed] [Google Scholar]
- 15.Hislop AA, Wigglesworth JS, Desai R. Alveolar development in the human fetus and infant. Early Human Develop. 1986;13:1–11. doi: 10.1016/0378-3782(86)90092-7. [DOI] [PubMed] [Google Scholar]
- 16.Zeltmer TB, Burri PH. The postnatal development and growth of the human lung. II Morphology. Respiration Physiology. 1986;67:269–282. doi: 10.1016/0034-5687(87)90058-2. [DOI] [PubMed] [Google Scholar]
- 17.Pinkerton KE, Joad JP. The mammalian respiratory system and critical windows of exposure for children’s health. Environ Health Perspect. 2000;108(suppl 3):457–462. doi: 10.1289/ehp.00108s3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dockery DW, Skerret PJ, Walters D, Gilliland F. In momograph Effects of Air Pollution on Children’Health and develoment. WHO Regional Office for Europe; 2005. Development of lung function; pp. 108–134. Review of evidence. [Google Scholar]
- 19.Spengler JD, Samet JM, McCarthy JF. Indoor Air Quality Handbook. McGraw-Hill; 2001. Chapter 9: Air cleaning-particles, pp 9.1-9,28; Chapter 26: Multiple chemical intolerance and indoor air quality pp 26.1-26.27. Chapter 70: Risk analysis framework pp 70.3-70.38. [Google Scholar]
- 20.Jedrychowski W, Whyatt R, Camann D, Bawle U, Peiki K, Spengler J, Dumyahn T, Perera F. Effect of prenatal PAH exposure on birth outcomes and neurocognitive development among a cohort of Polish mothers and newborns. Study design and preliminary ambient data. Int J Occup Med Environ Hlth. 2003;16:21–29. [PubMed] [Google Scholar]
- 21.Alexandrov K, Rojas M, Geneste O, Castegnaro M, Camus A-M, Petruzzelli S, Giuntini C, Bartsch H. An improved fluorimetric assay for dosimetry of benzo[a]pyrene diolepoxide-DNA adducts in smokers’ lung: comparisons with total bulky adducts and aryl hydrocarbon hydroxylase activity. Cancer Res. 1992;51:6248–6253. [PubMed] [Google Scholar]
- 22.Bernert JT, McGuffey JE, Morrison MA, et al. Comparison of serum and salivary cotinine measurements by a sensitive high-performance liquid chromatography-tandem mass spectrometry method as an indicator of exposure to tobacco smoke among smokers and nonsmokers. J Anal Toxicol. 2000;24:333–339. doi: 10.1093/jat/24.5.333. [DOI] [PubMed] [Google Scholar]
- 23.Jarvis MJ, Tunstall-Pedoe H, Feyerabend C, Vesey C, Saloojee Y. Comparison of tests used to distinguish smokers from nonsmokers. American Journal of Public Health. 1987;77:435–438. doi: 10.2105/ajph.77.11.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peacock J, Cook DG, Carey JM, Jarvis MJ, Bryant AE, Anderson HR. Maternal cotinine level during pregnancy and birthweight for gestational age. Int J Epidemiol. 1998;27:647–656. doi: 10.1093/ije/27.4.647. [DOI] [PubMed] [Google Scholar]
- 25.Kohler U, Kreuter F. Data analysis using STATA. Stata Press Publication; College Station, Texas: 2005. [Google Scholar]
- 26.Hardin JW, Hilbe JM. Generalized linear models and extensions. 2nd ed. STATA Press Publication; Stata Corp LP, Texas: 2007. [Google Scholar]
- 27.STATA software for windows, release 10. StaCorp; Texas: 2007. [Google Scholar]
- 28.Lieberman E, Torday J, Barbieri R, et al. Association of intrauterine cigarette smoke exposure with indices of fetal lung maturation. Obstet Gynecol. 1992;79:564–70. [PubMed] [Google Scholar]
- 29.Dezateux C, Stocks J, Wade AM, Dundas I, Fletcher ME. Airway function at one year: association with premorbid airway function, wheezing and maternal smoking. Thorax. 2001;56:680–686. doi: 10.1136/thorax.56.9.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perera F, Whyatt R, Jedrychowski W, Manchester D, Santella R, Ottman R. A study of the effects of environmental polycyclic aromatic hydrocarbons on birth outcomes in Poland. Am. J. Epidemiol. 1998;147:309–314. doi: 10.1093/oxfordjournals.aje.a009451. [DOI] [PubMed] [Google Scholar]
- 31.Perera FP, Rauh V, Tsai WY, Kinney P, Camann D, Barr D, Bernert T, garfinkel R, Tu YH, Diaz D, Dietrich J, Whyatt RM. Effects of transplacental exposure to environmental pollutants on birth outcomes in multiethnic population. Environ Health Perspect. 2003;2003;111:201–205. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perera FP, Rauh V, Whyatt RM, Tsai WY, Bernert JT, Tu YH, et al. Molecular evidence of an interaction between prenatal environmental exposures and birth outcomes in a multiethnic population. Environ Hlth Perspect. 2004;112:626–630. doi: 10.1289/ehp.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jedrychowski W, Bendkowska I, Flak E, Penar A, Jacek R, Kaim I, Spengler JD, Camann D, Perera FP. Estimated risk for altered fetal growth resulting from exposure to fine particles during pregnancy: an epidemiologic prospective cohort study in Poland. Environ Health Perspect. 2004;112:1398–402. doi: 10.1289/ehp.7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi H, Jedrychowski W, Spengler J, Camman DE, Whyatt RM, Rauh V, Tsai WY, Perera FP. International studies of prenatal exposure to polycyclic aromatic hydrocarbons and fetal growth. Environ Health Perspect. 2006;114:1744–1750. doi: 10.1289/ehp.8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan KN, Noble-Jamieson CM, Elliman A, Bryan EM, Silverman M. Lung function in children of low birth weight. Arch Dis Child. 1989;64:1284–93. doi: 10.1136/adc.64.9.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rona RJ, Gulliford MC, Chinn S. Effects of prematurity and intrauterine growth on respiratory health and lung function in childhood. BMJ. 1993;306:817–20. doi: 10.1136/bmj.306.6881.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaheen SO, Sterne JA, Tucker JS, Florey V du C. Birth weight, childhood lower respiratory tract infection, and adult lung function. Thorax. 1998;53:549–53. doi: 10.1136/thx.53.7.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edwards CA, Osman LM, Godden DJ, Campbell DM, Douglas JG. Relationship between birth weight and adult lung function: controlling for maternal factors. Thorax. 2003;58:1061–1065. doi: 10.1136/thorax.58.12.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lawlor DA, Ebrahim S, Smith GD. Association of birth weight with adult lung function: findings from the British women’s heart and health study and meta-analysis. Thorax. 2005;60:851–858. doi: 10.1136/thx.2005.042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ, et al. Asthma and wheezing in the first six years of life. NEJM. 1996;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 41.Ormstad H. Suspended particulate matter in indoor air: adjuvants and allergen carriers. Toxicology. 2000;152:53–68. doi: 10.1016/s0300-483x(00)00292-4. [DOI] [PubMed] [Google Scholar]
- 42.Tadaki H, Arakawa H, Sugiyama M, Ozawa K, Mizuno T, Mochizuki H, Tokuyama K, Morikawa A. Association of cord blood cytokine production with wheezy infants in the first year of life. Pediatr Allergy Immunol. 2009;20:227–233. doi: 10.1111/j.1399-3038.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- 43.Takano H, Yoshikawa T, Ichinose T, et al. Diesel exhaust particles enhance antigen-induced airway inflammation and local cytokine expression. Am J Respir Crit Care Med. 1997;156:36–42. doi: 10.1164/ajrccm.156.1.9610054. [DOI] [PubMed] [Google Scholar]
- 44.Fujieda S, Diaz-Sanchez D, Saxon A. Combined nasal challenge with diesel exhaust particles and allergen induces invivo IgE isotype switching. Am J Respir Cell Mol Biol. 1998;19:507–512. doi: 10.1165/ajrcmb.19.3.3143. [DOI] [PubMed] [Google Scholar]
- 45.Ohta K, Yamashita N, Tajima M, et al. Diesel exhaust particulate induces airway hyperresponsiveness in a murine model: essential role of GM-CSF. J Allergy Clin Immunol. 1999;104:1024–1030. doi: 10.1016/s0091-6749(99)70084-9. [DOI] [PubMed] [Google Scholar]
- 46.Bommel H, Li-Weber M, Serfling E, et al. The environmental pollutant pyrene induces the production of IL-4. J Allergy Clin Immunol. 2000;105:796–802. doi: 10.1067/mai.2000.105124. [DOI] [PubMed] [Google Scholar]
- 47.Salvi SS, Nordenhall C, Blomberg A, et al. Acute exposure to diesel exhaust increases Il-8 and GRO-a production in healthy human airways. Am J Respir Crit Care Med. 2000;161:550–557. doi: 10.1164/ajrccm.161.2.9905052. [DOI] [PubMed] [Google Scholar]
- 48.Heo Y, Saxon A, Hankinson O. Effect of diesel exhaust particles and their components on the allergen-specific IgE and IgG1 response in mice. Toxicology. 2001;159:143–158. doi: 10.1016/s0300-483x(00)00418-2. [DOI] [PubMed] [Google Scholar]
- 49.Ferguson A, Dimich-Ward H, Becker A, Watson W, DyBuncio A, Carlsten C, Chan-Yeung M. Elevated cord blood IgE is associated with recurrent wheeze and atopy at 7 yrs in a high risk cohort. Pediatr Allergy Immunol. 2009 doi: 10.1111/j.1399-3038.2009.00869.x. DOI: 10.1111/j.1399-3038.2009.00869.x. [DOI] [PubMed] [Google Scholar]
- 50.Diaz-Sanchez D. The role of diesel exhaust particles and their associated polycyclic aromatic hydrocarbons in the induction of allergic airway disease. Allergy. 1997;52:52–56. doi: 10.1111/j.1398-9995.1997.tb04871.x. [DOI] [PubMed] [Google Scholar]
- 51.Wyler C, Braun-Fahrlander C, Kunzli N, et al. Exposure to motor vehicle traffic and allergic sensitization. Epidemiology. 2000;11:450–456. doi: 10.1097/00001648-200007000-00015. [DOI] [PubMed] [Google Scholar]
- 52.Gehring U, Cyrys J, Sedlimeir G, et al. Traffic-related air pollution and respiratory health during the first 2 years of life. Eur Respir J. 2002;19:690–698. doi: 10.1183/09031936.02.01182001. [DOI] [PubMed] [Google Scholar]
- 53.Pandya RJ, Solomon G, Kinner A, et al. Diesel exhaust and asthma: hypotheses and molecular mechanisms of action. Environ Health Perspect. 2002;101:103–112. doi: 10.1289/ehp.02110s1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Behrens T, Taeger D, Maziak W, Duhme H, Rzehak P, Weiland SK, Keil U. Self-reported traffic density and atopic disease in children. Results of the ISAAC Phase III survey in Muenster, Germany. PediatrAllergy Immunol. 2004;15:331–339. doi: 10.1111/j.1399-3038.2004.00157.x. [DOI] [PubMed] [Google Scholar]
- 55.Miller RI, Garfinkel R, Horton M, Camann, Perera FP, Whyatt R, Kinney PL. Polycyclic aromatic hydrocarbons, environmental tobacco smoke, and respiratory symptoms in an inner-city birth cohort. Chest. 2004;126:1071–1078. doi: 10.1378/chest.126.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jedrychowski W, Galas A, Flak E, Jacek R, Penar A, Spengler J, Perera FP. Increased burden of respiratory disease in the first six months of life due to prenatal environmental tobacco smoke: Krakow birth cohort study. Early Child Development and Care. 2007;177:369–381. [Google Scholar]
- 57.Henderson J, Sherriff A, Farrow A, Ayres JG. Household chemicals,persistent wgeezing and lung function: effect modification by atopy? Eur Respir J. 2008;31:547–554. doi: 10.1183/09031936.00086807. [DOI] [PubMed] [Google Scholar]
- 58.Hemminki K, Koskinen M, Rajaniemi H, Zhao C. DNA adducts, mutation, and cancer 2000. Regul Toxicol Pharmacol. 2000;32:264–275. doi: 10.1006/rtph.2000.1431. [DOI] [PubMed] [Google Scholar]
- 59.Harris CC, Vähäkangas K, Newman MJ, Trivers GE, Shamsuddin A, Sinopoli N, Mann DL, Wright WE. Detection of benzo[a]pyrene diol epoxide-DNA adducts in peripheral blood lymphocytes and antibodies to the adducts in serum from coke oven workers. Proc Natl Acad Sci USA. 1985;82:6672–6676. doi: 10.1073/pnas.82.19.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haugen A, Becher G, Benestad C, Vähäkangas K, Trivers GE, Newman MJ, Harris CC. Determination of polycyclic aromatic hydrocarbons in the urine, benzo(a)pyrene diol epoxide-DNA adducts in lymphocyte DNA, and antibodies to the adducts in sera from coke oven workers exposed to measured amounts of polycyclic aromatic hydrocarbons in the work atmosphere. Cancer Res. 1986;46:4178–4183. [PubMed] [Google Scholar]
- 61.Poirier MC, Santella R, Weston A. Carcinogen macromolecular adducts and their measurement. Carcinogenesis. 2000;21:353–359. doi: 10.1093/carcin/21.3.353. [DOI] [PubMed] [Google Scholar]
- 62.Ball TM, Catro-Rodrigez JA, Griffith KA, Holberg CJ, Martinez ED, Wright AL. Siblings day-care attendance, and risk of asthma and wheezing during childhood. NEJM. 2000;343:538–543. doi: 10.1056/NEJM200008243430803. [DOI] [PubMed] [Google Scholar]
- 63.Lemanske RF., jr. The child origins of ASTma (COAST) study. Pediatr Allergy Immunol. 2002;13:38–43. doi: 10.1034/j.1399-3038.13.s.15.8.x. [DOI] [PubMed] [Google Scholar]
- 64.Gurwitz D, Mindorff, Levison H. Increased incidence of bronchial reactivity in children with a history of bronchiolitis. J Pediatr. 1981;98:551–555. doi: 10.1016/s0022-3476(81)80758-5. [DOI] [PubMed] [Google Scholar]
- 65.Trefny P, Stricker T, Baerlocher C, Sennhauser FH. Family history of atopy and clinical course of RSV infection in ambulatory and hospitalized infants. Pediatr Pulmonol. 2000;30:302–306. doi: 10.1002/1099-0496(200010)30:4<302::aid-ppul5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 66.Keimig SD, Kirby KW, Morgan DP, Keiser JE, Hubert TD. Identification of 1-hydroxypyrene as`a major mtabolite of pyrene in pig urine. Xenobiotica. 1993;13:415–420. doi: 10.3109/00498258309052279. [DOI] [PubMed] [Google Scholar]
- 67.Jongeneelen FJ. Methods for routine biological monitoring of carcinogenic PAH-mixture. Sci Total Environ. 1997;199:141–149. doi: 10.1016/s0048-9697(97)00064-8. [DOI] [PubMed] [Google Scholar]
- 68.Dor F, Dab W, Empereur-Bisonnet P, Zmirou D. Validity of biomarkers in environmental studies; the case of PAHs and benzene. Crit Rev Toxicol. 1999;29:129–168. doi: 10.1080/10408449991349195. [DOI] [PubMed] [Google Scholar]
- 69.Bianca AC, Wandalsen GF, Miyagi K, Camargo L, Cezarin D, mallol J, Sole D. International study of wheezing in infants (EISL): validation of written questionnaire for children aged below 3 years. Invest Allergol Clin Immunol. 2009;19:35–42. [PubMed] [Google Scholar]