Abstract
Physical exercise is the cornerstone of cardiovascular disease treatment. The present study investigated whether exercise training affects atherosclerotic plaque composition through the modification of inflammatoryrelated pathways in apolipoprotein E knockout (apoE−/−) mice with diabetic atherosclerosis. Forty-five male apoE−/− mice were randomized into three equivalent (n=15) groups: control (CO), sedentary (SED), and exercise (EX). Diabetes was induced by streptozotocin administration. High-fat diet was administered to all groups for 12 weeks. Afterwards, CO mice were euthanatized, while the sedentary and exercise groups continued high-fat diet for 6 additional weeks. Exercising mice followed an exercise program on motorizedtreadmill (5 times/week, 60 min/session). Then, blood samples and atherosclerotic plaques in the aortic root were examined. A considerable (P<0.001) regression of the atherosclerotic lesions was observed in the exercise group (180.339±75.613×103µm2) compared to the control (325.485±72.302×103 µm2) and sedentary (340.188±159.108×103µm2) groups. We found decreased macrophages, matrix metalloproteinase-2 (MMP-2), MMP-3, MMP-8 and interleukin-6 (IL-6) concentrations (P<0.05) in the atherosclerotic plaques of the exercise group. Compared to both control and sedentary groups, exercise training significantly increased collagen (P<0.05), elastin (P<0.001), and tissue inhibitor of matrix metalloproteinase-2 (TIMP-2) (P<0.001) content in the atherosclerotic plaques. Those effects paralleled with increased fibrous cap thickness and less internal elastic lamina ruptures after exercise training (P<0.05), while body-weight and lipid parameters did not significantly change. Plasma MMP-2 and MMP-3 concentrations in atherosclerotic tissues followed a similar trend. From our study we can conclude that exercise training reduces and stabilizes atherosclerotic lesions in apoE−/− mice with diabetic atherosclerosis. A favorable modification of the inflammatory regulators seems to explain those beneficial effects.
Key words: diabetes, atherosclerosis, exercise, matrix metalloproteinases, plaque stability.
Introduction
Diabetes mellitus (DM) is a multifaceted metabolic disease associated with precipitated atherogenesis and two- to four-fold increased cardiovascular risk.1 These pro-atherogenic properties of DM are usually ascribed to the presence of low-grade inflammation, which enhances the atherosclerotic plaque development and vulnerability.2 In the diabetic population, the use of properly prescribed exercise training can favourably affect atherosclerosis and its consequences (e.g., myocardial infarction, stroke) both in the primary and secondary prevention strategies.3,4 The modification of numerous cardiovascular risk factors (e.g., hypertension, hyperglycemia, hyperlipidemia, obesity, etc.) has long been proposed as the potential mechanism of the exercise-induced protection against diabetic atherosclerosis.5 Recently, clinical and experimental studies suggest that physical activity exerts multi-factorial, pleiotropic, actions which might retard atherosclerotic plaque development and enhance plaque stability.6,7 Although most of these pleiotropic mechanisms remain unclear, ever more data supports the exercise-induced suppression of the number and the activity of inflammatory cells.8
Inflammation predominantly contributes to all stages of atherosclerosis and influences the vascular extracellular matrix (ECM) homeostasis. 9 In particular, activated cells (e.g., macrophages, and endothelial cells) in inflamed atherosclerotic plaques produce matrix metalloproteinases (MMPs), which are zinc-proteolytic enzymes.10 MMPs degrade the ECM – the scaffold of the artery and the plaque – leading to plaque disruption and acute arterial thromobosis.11 There is a plethora of data supporting the involvement of MMPs and their tissue inhibitors of matrix metalloproteinases (TIMPs) in inflammatory pathways, atherogenesis and atherosclerotic plaque destabilization. 12,13 Consequently, pro-inflammatory cytokines and MMPs could serve both as systemic and local biomarkers of plaque stability. Unfortunately, there is limited data about the effects of exercise training on vascular MMPs.14,15 Thus, it would be of great interest to assess the impact of exercise training on inflammatory and connective tissue-associated mechanisms involved in the diabetic atherosclerotic plaque texture.
The aim of the present study was to investigate the impact of exercise training on morphological markers of plaque stability and systemic inflammation in apolipoprotein E knockout (apoE−/−) mice with diabetic atherosclerosis. As exercise training could develop antiatherosclerotic effects in the absence of systemic lipid-lowering and weight-loss, we further explored the underlying mechanisms by assessing the effects of exercise training on inflammatory mediators, such as MMPs, TIMP- 2 and interleukin-6 (IL-6).
Materials and Methods
Animals and experimental design
We used apoE−/− mice, backcrossed for 10 generations into the C57BL/6 background (Charles Rivers Laboratories, Milan, Italy). Housing in animal rooms was under specific pathogen free (SPF) conditions. Tap water and vacuum packed pelleted food were provided ad libitum. The population consisted of forty-five male ApoE−/− mice randomly assigned to three equally sized (n=15) groups: 1) control (CO), 2) sedentary (SED), and 3) exercise (EX). At baseline, the mice were 8 weeks old and weighed 20–24g. Diabetes was induced at the beginning of the study by peritoneal injections of streptozotocin (STZ) for 5 consecutive days (0.05 mg/g body weight in 0.05 mol/L citrate buffer, pH 4.5). Mice maintaining fasting glucose levels >200 mg/dL throughout the course of the study were considered diabetic and were included in analyses. A western-type diet (42% of total calories from milk fat and 0.15% from cholesterol, Harlan Teklad TD 88137) (Harlan Laboratories Inc., Madison, WI, USA) was administered to all groups for 12 weeks. This type of diet has been demonstrated to induce atherosclerotic lesions with characteristics similar to human plaques in the long-term.16 At the end of the 12th week of the study protocol (20th week of age), CO mice were euthanatized under deep anaesthesia with isoflurane. The remaining groups – SED and EX – maintained high-fat diet for 6 additional weeks, until they were euthanatized. SED mice were confined to their cages, while EX mice followed an exercise training protocol for the same time-period. At the end of this last period, both groups were euthanatized. Body weight measurements and blood samples were obtained at baseline (before diabetes induction and western-type diet administration) and at the end (just before euthanasia of all groups).
The study complied with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. We obtained approval of the protocol by the Ethics Committee for Animal Experimentation of the Biomedical Research Foundation, Academy of Athens and the competent Veterinary Service.
Exercise training
On the basis of a previously published exercise protocol, we made apoE−/− mice perform exercise training on a motorized rodent treadmill with electrical shock-plate incentive (Exer- 6M Open Treadmill, Columbus Instruments, Columbus, OH, USA).17 Exercise training started after 12 weeks of western-type feeding (20th week of age), lasted 6 weeks and was performed 5 days/week. The duration of each session gradually increased within the first two weeks and approached 60min/session, with a 2-min rest interval. Similarly, the speed of the treadmill gradually increased from 8m/min to 15m/min. The latter parameters were kept constant from the 3rd to the end of the 6th week. The treadmill slope remained unchanged at 5° throughout the training period.
Biochemical measurements
We drawn all blood samples after an overnight fasting under isoflurane anaesthesia. At baseline, blood samples (~100 µL) were obtained from the retro-orbital vein system, while at the end of the study and before euthanasia they were procured through cardiac puncture. Total cholesterol, triglycerides and glucose were immediately assayed in fresh plasma samples (6 mice per group) using an enzymatic method (Chemwell 2910; Awareness Technology Inc., Palm City, FL, USA). Then, serum was separated by centrifugation at 3500 rpm for 5 min, and stored (−80°C) until analysis in the same assay. Commercially available ELISA kits (R&D Systems Inc, Minneapolis, MN, USA) determined serum levels of MMP-2 and MMP-3. As reported by the manufacturer, the inter-assay coefficients of variance (CVs) for MMP-2 and MMP-3 were 7.4%, and 7.8%, respectively, while the intra-assay CVs were 2% and 7.8%, respectively. Interleukin-6 (IL-6) serum levels were measured using ELISA kit (IBL, Minneapolis, MN, USA) with an intra- and inter-assay CV of 11.2% and 8.2%, respectively.
Glucose tolerance test
One week before euthanasia, 6–7 mice per group underwent an intraperitoneal glucose tolerance test (GTT). After an overnight fasting, anesthetized mice were intra-peritoneally injected glucose (D-Glucose 50% wt/vol solution) with a 27-gauge needle at a dose of 2 g/kg body weight. Blood samples were taken before glucose injection and after 30, 60, 90, and 120 min. Blood glucose was measured at each time point by Accu-Chek advantage glucose monitors (Roche Diagnostics, Indianapolis, IN, USA) and the area under the curve (AUC) was then determined using the trapezoid rule.18
Tissue preparation and quantification of atherosclerosis
At death, the heart and the aortic arch were excised and washed thoroughly with injection of phosphate-buffered saline through the left ventricle of the heart. After maintenance in 10% buffered formalin for 24 h, tissue specimens were embedded in paraffin. Thereafter, serial 7-µm-thick sections were cut from the apex towards the base of the heart until the aortic valve leaflets appeared. After the aortic arch transverse sectioning, 7-µm-thick slices were collected on poly-D-lysine-coated slides, according to standardized procedure.19 Precisely, six sections of the aortic root for each animal, representing every 7th serial section, over a distance of about 300 µm, were taken and stained with hematoxylin/eosin (H&E) for quantitative morphometric analysis. The total lumen area (in µm2) circumscribed by the internal elastic lamina (IEL) and the extent of the atherosclerotic plaques (in µm2) were calculated in each section using computerized image analysis software (Image Pro Plus Version 4.1; Media Cybernetics Inc., Rockville, MD, USA). Again, the IEL distinguished the plaque from the arterial wall. In each section, the proportion of total lumen area occupied by the atherosclerotic plaques expressed the percentage of luminal stenosis. For atherosclerotic lesion quantification, we averaged plaque and lumen area and the luminal stenosis in all H&E-stained sections per animal. The intimal surface area was calculated by subtracting the patent lumen area from the area circumscribed by the IEL.
Histochemical and immunohistochemical analysis
In order to assess the changes in the histological features of plaque vulnerability across groups, serial sections through the aortic arch were evaluated. Specimens were stained with sirius-red and orcein for collagen and elastin visualization, respectively. After sirius-red staining, we measured the fibrous cap thickness of each atherosclerotic plaque and we averaged the minimum values of all plaques per animal. In orcein-stained sections, the number of ruptures (i.e., discontinuities or fractures) of the IEL was determined. Serial paraffin sections of the aortic root after the necessary preparation were incubated overnight at 4°C either with anti-Mac-3 mouse macrophage antibody (BD Pharmigen, Franklin Lakes, NJ, USA) for macrophages, or with anti-smooth muscle actin antibody (Biocare Medical, Concord, CA, USA) for smooth muscle cells (SMCs). Consecutive sections were also stained with polyclonal antibodies against MMP-2 and MMP-3 (MBL International Corporation, Woburn, MA, USA), MMP-8 (Chemicon International Inc., Temecula, CA, USA), TIMP-2 (Acris Antibodies GmbH, Herford, Germany) or rat monoclonal anti-mouse IL-6 (Biolegends, San Diego, CA, USA). Immunohistochemical analyses were performed according to the manufacturers’ protocols. Each parameter was quantitatively assessed on 5–6 sections per animal (10 mice per group). Results were given as percentage of the positively stained tissue of the plaque. In each section, the segmental stained plaque area was expressed as the percentage of the whole atherosclerotic plaque area. Two independent investigators who were blinded to the study protocol participated in image analysis, using computer-assisted morphometry (Image Pro Plus Version 4.1; Media Cybernetics Inc., Rockville, MD, USA).
Statistical analysis
Results are expressed as mean±standard deviation. We used one-way ANOVA and posthoc Tuckey test for comparison among groups at baseline and at the end of the study. We further compared changes in body weight, glucose, lipids and serum levels of MMP-2, and MMP-3 among groups from the baseline to the end using one-way ANOVA test for repeated measures. For GTT we compared values of the three groups at each time-point using one-way ANOVA test. Corresponding areas from the trapezoid were compared among groups by one-way ANOVA test. We considered P<0.05 as statistically significant. The data was analyzed by using SPSS 16.0 (SPSS Inc., Chicago, IL, USA) software package.
Results
Body weight, biochemical parameters and glucose tolerance test
Body weight and biochemical results are summarized in Table 1. At baseline (8th week of age), just before diabetes induction, study groups did not differ in body weight and blood levels of lipids, glucose, IL-6, MMP-2 and MMP-3. Throughout the study, all groups significantly (P<0.001) gain weight compared to baseline. At the end of the experiment, exercise group had slightly lower body weight than the other groups. However, that difference was not statistically significant (P>0.05). Likewise, the amount of change of total plasma cholesterol and triglycerides concentrations did not significantly differ among the groups (P>0.05), and the end values of these variables were similar (P>0.05). As for fasting plasma glucose (FPG) levels, they highly increased from baseline (P<0.001). Furthermore, the magnitude of increment in FPG was marginally lower in EX than in CO (P=0.047) and SED (P=0.046) groups. By the end of the study, serum concentrations of MMP-2, MMP-3 and IL-6 highly increased in all groups compared to baseline (P<0.001). Importantly, however, these serum concentrations were considerably downregulated in the EX group compared to the other groups since their magnitude of increment was remarkably lower in EX than in SED and CO groups (P<0.01).
Table 1. Body weight and biochemical parameters in ApoE−/− mice at baseline (8th week of age) and at the end (just before euthanasia). We comparatively evaluated the baseline, the final period and the amount of changes in body weight and biochemical parameters among groups. Results are expressed as mean±standard deviation.
| Groups | Time | CO (N=15) | SED (N=15) | EX (N=15) | P | P1 | P2 | P3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Body weight (g) | Baseline | 22.3±2.6 | 21.5±3.1 | 21.9±1.2 | 0.726 | 0.820 | 0.717 | 0.810 | ||||||||
| End | 35.3±8.1 | 36.2±3.9 | 33±3.5 | 0.224 | 0.692 | 0.332 | 0.166 | |||||||||
| FPG (mg/dL) | ||||||||||||||||
| Baseline | 123.2±39.3 | 122.8±65 | 133.8±24.2 | 0.271 | 0.910 | 0.506 | 0.867 | |||||||||
| End | 350±45.5 | 354.1±49.9 | 296±25.4*# | 0.147 | 0.961 | 0.145 | 0.137 | |||||||||
| MMP-2 (ng/mL) | ||||||||||||||||
| Baseline | 91.3±46.8 | 92.4±29.1 | 95.3±21.3 | 0.554 | 0.891 | 0.447 | 0.781 | |||||||||
| End | 146.31±48.1 | 158.65±23.1 | 119.33±33.5*# | 0.003 | 0.874 | 0.009 | <0.001 | |||||||||
| MMP-3 (ng/mL) | ||||||||||||||||
| Baseline | 25.3±12 | 22±17.3 | 22.2±10.9 | 0.870 | 0.646 | 0.941 | 0.951 | |||||||||
| End | 70.89±38 | 99.084±42.1 | 53.99±34.6*# | 0.029 | 0.302 | 0.122 | 0.013 | |||||||||
| IL-6 (pg/mL) | ||||||||||||||||
| Baseline | 21.2±6.4 | 19.42±4.2 | 23.79±3.1 | 0.833 | 0.893 | 0.888 | 0.791 | |||||||||
| End | 89±34.8 | 94.63±24.5 | 61.2±20.8*# | 0.031 | 0.766 | 0.041 | 0.025 | |||||||||
| TC (mg/dL) | ||||||||||||||||
| Baseline | 221.5±51.3 | 208±43.3 | 236.4±40.8 | 0.698 | 0.881 | 0.812 | 0.558 | |||||||||
| End | 679.5±212 | 638±203.2 | 556±110.7 | 0.593 | 0.857 | 0.608 | 0.901 | |||||||||
| TG (mg/dL) | ||||||||||||||||
| Baseline | 59.5±22.1 | 68.2±19 | 75.8±23.5 | 0.662 | 0.791 | 0.602 | 0.825 | |||||||||
| End | 66±32.1 | 70.1±41.1 | 89.2±35.5 | 0.478 | 0.860 | 0.447 | 0.871 | |||||||||
CO, control; SED, sedentary; EX, exercise. P, one-way ANOVA; P1, CO vs SED; P2, CO vs EX; P3, SED vs EX using post-hoc Tuckey test at baseline and at the end; FPG, fasting plasma glucose; MMP-2, matrix metalloproteinase-2; MMP-3, matrix metalloproteinase-3; IL-6, interleukin-6; TC, total cholesterol; TG, triglycerides. Comparison of the amount of changes of variables among groups using one-way ANOVA test for repeated measures and post-hoc Tuckey test:
P<0.05, EX vs CO group;
P<0.05, EX vs SED group.
Glucose disposal during the GTT was similar in CO and SED groups. Glucose tolerance was notably improved in EX group compared to the other two groups. The improved glucose disposal was observed despite the non-significant exercise effect on body weight during the 6-week treatment period (Figure 1).
Figure 1.
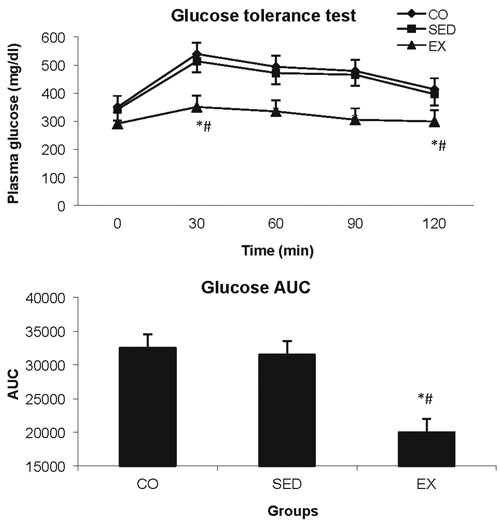
Glucose tolerance test results and the area under the curve (AUC) using the trapezoid rule. Comparisons were performed at each time point using one-way ANOVA, post-hoc Tuckey test. *P<0.05, exercise (EX) compared to control (CO) group; #P<0.05, EX compared to sedentary (SED) group.
Morphometric analysis of the atherosclerotic lesions
Figure 2A depicts histomorphometrical data. At the end of the study, EX group had significantly lower percentage of luminal stenosis (32.65±6.63%) than SED (51.36±7.98%; P<0.001) and CO (48.09±6.07%; P<0.001) groups. Further analysis revealed that there was no change in luminal stenosis or lesion surface area between SED and CO group (P>0.05). As for the plaque cross-sectional area, EX group developed less extensive lesions at the time of tissue harvest compared to SED and CO groups (P<0.001) (Table 2).
Figure 2.
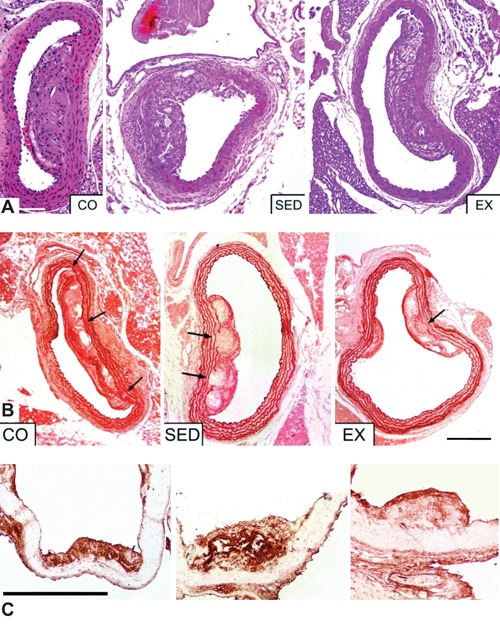
A) Hematoxylin/eosin staining: representative examples of the effects of exercise on plaque size across the aortic arch, after; CO, control group; SED, sedentary group; EX, exercise group; the average plaque size in the aortic arch was significantly reduced in the exercise training group; scale bar: 400 µm. B) Orcein staining: exercise training significantly increased the elastin content of the atherosclerotic plaques and protected the integrity of internal elastic lamina (IEL); arrows point to ruptures of IEL; CO, control group; SED, sedentary group; EX, exercise group; scale bar: 400 µm. C) Exercise training significantly decreased MMP-8 content of the atherosclerotic plaques. CO, control group; SED, sedentary group; EX, exercise group; scale bar: 400 µm.
Table 2. Morphometric characteristics of the atherosclerotic lesions and the percentage of positively-stained tissue in the plaques at the end of the study. Results are expressed as mean±standard deviation.
| CO | SED | EX | |
|---|---|---|---|
| Plaque area (×103 µm2) | 325.485±72.302 | 340.188±159.108 | 180.339±75.613*# |
| Lumen area (×103 µm2) | 986.365±292.914 | 927.482±370.340 | 1038.346±717.256*# |
| Elastic lamina ruptures per mm of arterial girth | 5.2±1.1 | 5.4±0.6 | 2.1±0.2*# |
| Fibrous cap thickness (µm) | 20±0.7 | 20.6±1.6 | 31.3±1.7*# |
| Elastin (%) plaque | 12.6±4.2 | 13.9±2.9 | 24±2.1*# |
| Collagen (%) plaque | 19±3.9 | 18.5±6.3 | 30.1±5.9*# |
| α-actin (SMCs) (%) plaque | 7.8±0.8 | 6.9±2.8 | 10.7±5.7 |
| Mac-3 (macrophages) (%) plaque | 33.9±4.1 | 36.4±5 | 16.7±4.3*# |
CO, control; SED, sedentary; EX, exercise. SMCs, smooth muscle cells; (%), positive staining areas in relation to total plaque area.
P<0.05 EX vs CO group;
P<0.05 EX vs SED group at post-hoc analysis of one-way ANOVA test. Analysis of data regards 8 to 10 mice per group.
Histological analysis of plaque Composition
As shown in Table 2, aortic specimens from the EX group showed a noteworthy area stained for collagen (Sirius-red staining) in the atherosclerotic plaque compared to SED (P<0.001) and CO (P<0.001) mice. Moreover, exercise-treated mice rather than -untreated mice showed considerably enhanced atherosclerotic plaque fibrous cap thickness (P<0.05). Quantitative analysis showed greater elastin-stained (orcein) lesion areas in the EX than in SED (P<0.001) and CO (P<0.001) groups. Besides, atherosclerosis progression resulted in multiple IEL breaks which were either under a plaque or independent of an overlying plaque. In our study, exercising mice presented less multiple frank breaks in IEL than CO and SED groups (P<0.001) (Figure 2B). No significant differences in the histochemical parameters mentioned above were observed between SED and CO groups (P>0.05).
Immunohistochemical analysis of plaque composition
Exercise training was associated with more than 50% reduction in macrophages infiltration in the atherosclerotic lesions (P<0.001). Exercise training had no effect on SMCs abundance in the atherosclerotic lesions (P>0.05) (Table 2). A significant reduction in the MMP-2, MMP-3, MMP-8 and IL-6 positive immunestained area was observed in EX mice vs SED and CO mice (P<0.05) (Figure 2C). In sequential sections, MMP-3 was mostly co-localized with macrophages in the shoulder region of the plaques and in areas surrounding the lipid core, thus indicating macrophages and their derivative foam cells as the predominant source of MMP-3 (Figure 3). On the contrary, a greater extent of positive immunoreactivity for TIMP-2 was found in the atherosclerotic plaques of EX group than SED and CO groups (P<0.001). Notably, no significant difference in these parameters was detected between SED and CO groups (P>0.05). All immunohistochemical results are given in Figure 4.
Figure 3.
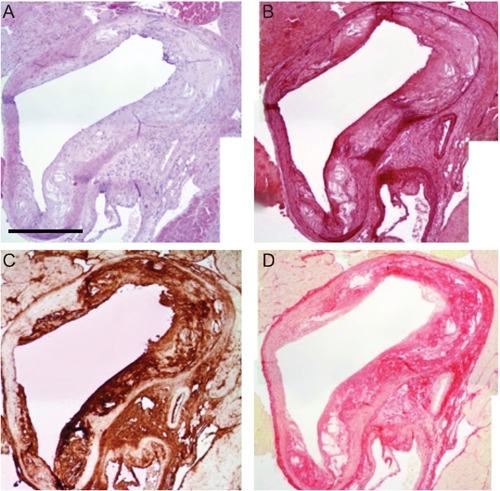
Representative examples of four serial sections of the aortic arch after staining with hematoxylin-eosin (A), orcein (B), MMP-3 (C), Sirius red (D), in a control mice. Scale bar: 400 µm.
Figure 4.
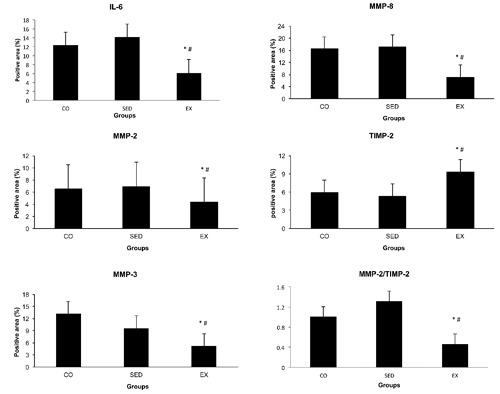
Effects of exercise training on IL-6, MMP-2, MMP-3, MMP-8 and TIMP-2 content of the atherosclerotic lesions. CO, control group; SED, sedentary group; EX, exercise group. Results are expressed as mean ± standard deviation.*P<0.05, EX compared to CO group; #P<0.05, EX compared to SED group.
Discussion
In the present study, exercise training effectively decreased the area of advanced atherosclerotic lesions in the aortic arch of diabetic apoE−/− mice. Most importantly, it enhanced morphological markers of atherosclerotic plaque stability by increasing fibrous cap thickness, elastin, and collagen component and decreasing macrophages content and average quantity of IEL ruptures. Those effects were associated with favorable alterations of novel cardiovascular risk factors, like MMP-2, MMP-3, MMP-8, TIMP-2 and IL-6, thus providing additional benefit on the inflammatory status. This benefit was accrued regardless of the systemic effects on lipids or body weight.
Most previous studies, carried out in animal models of atherosclerosis, reported attenuated progression20 or even regression of atherosclerosis after exercise training.7,21 Accordingly, we demonstrated a considerable regression of plaque area after 6 weeks of treadmill training in diabetic apoE−/− mice despite the minimally altered body weight and lipid profile. Human studies have focused on the anti-atherogenic mechanisms – mainly lipid lowering and weight loss – of including exercise.22 The mechanisms that mediated the beneficial effects on plaque composition and plaque size may involve inflammation and MMPs homeostasis. Therefore, the above findings support the notion of the pleiotropic effects of exercise training on plaque burden and composition regardless of the modification of traditional cardiovascular risk factors.
In our study, one of the most striking findings was the beneficial alteration of plaque composition after exercise training. It is widely accepted that plaque composition rather than plaque size determines its vulnerability leading to acute cardiovascular events.23 Rupture-prone or vulnerable lesions are indirectly recognized by a number of histological characteristics, including thinning of the fibrous cap, decreased amount of collagen and elastin, large necrotic core and excessive macrophages accumulation.24,25 To this day, the influence of exercise on plaque composition has yielded only limited data17 since the majority of trials have focused only on the exerciserelated changes in the atherosclerotic burden.4 In the present study, exercise training was significantly associated with restrained macrophage infiltration, and increased collagen and elastin content of the plaques. Those findings were further supported by additional signs of atherosclerotic lesion stabilization, such as low number of IEL ruptures and thicker fibrous cap,26 despite the generally low incidence of spontaneous plaque rupture in apoE−/− mice.27 The negative impact of exercise training on macrophages number – the principal cellular source of inflammatory cytokines and MMPs – provides a plausible explanation for the atherosclerosis regression and lowers the possibility of fibrous cap disruption.28
Both inflammation and MMPs play an important role in the progress of vulnerable atherosclerotic plaques.29 Modulation of inflammatory process and MMPs may be a potential molecular basis of the exercise-mediated plaque regression and plaque-stabilizing activity of exercise training. Growing evidence supports the key role of MMP-3 in the occurrence of acute coronary syndromes and the severity of coronary atherosclerosis.30,31 In animal studies, fat-fed, apoE−/−/MMP-3−/− double knockout mice appeared with smaller crosssectional plaque area and enhanced stability compared to apoE−/− mice.32 In our study, lesion concentrations of MMP-3 were more than 50% higher in CO and SED mice compared to EX mice. These same values were also observed in circulating levels of MMP-3 in the atherosclerotic lesions. Moreover, the exercise-induced MMP-3 reduction was associated with significant improvement in histological markers of atherosclerotic plaque stability, such as fewer IEL ruptures, and larger collagen and elastin content. No such effects have been previously reported on the pro-atherogenic function of MMP-3 and its key role in the activation of other members of MMPs (e.g., MMP-8, MMP-9, etc.),33 thus proving their scientific importance. While looking for the underlying mechanisms, we did not detect any relationship between MMP-3 and inflammatory factors in both atherosclerotic tissue and circulation. On the basis of previous studies, the influence of inflammation on MMP-3 may be divergent.34,35 On the other hand, MMP-3 staining was associated with Mac-3 macrophages staining across groups indicating their preponderance in MMP-3 production.34 A vicious cycle of less macrophages producing less MMP-3, which further reduces macrophages infiltration, provides a possible explanation for the atherosclerotic plaque regression and stabilization in EX mice.
Since the last decade, MMP-8 – known also as neutrophil elastase – has been associated with collagen cleavage which leads to atherosclerotic plaque vulnerability and progression. 36,37 To this day, little attention has been paid to pharmaceutical and non-pharmaceutical modification of MMP-8. To our knowledge, this is the first study of clinical importance demonstrating an exercise-related reduction in MMP-8 content of the atherosclerotic lesions. The regulatory mechanisms of MMP-8 expression and activity seem to correlate with inflammation. Dollery et al. recently established that among vascular cells, macrophages constituted the predominant source of MMP-8 within plaques.38 In our study we observed the co-localization of MMP-8 with macrophages, which indicated the pivotal role of macrophage infiltration in MMP-8 production. Thus, the exercise-induced reduction in MMP-8 could be attributed to the parallel decrease of macrophages in our diabetic apoE−/− mice. Another plausible explanation derived from the correlation of pro-inflammatory cytokines (e.g., IL-1 , TNF-α) with MMP-8 expression.39
Indeed, we observed that MMP-8 content correlated with IL-6 levels in the atherosclerotic plaque. A recently published study has also demonstrated a positive correlation between MMP-8 and IL-6 concentrations in human carotid plaques.40 All in all, exercise training possibly reduced atherosclerotic plaque MMP-8 content via the favourable modulation of the inflammatory pathway.
Clinical and experimental studies correlated MMP-2 (gelatinase A) with atherosclerosis development since it degrades basement membrane and facilitates SMCs migration and proliferation. 41,42 Still, the compensatory atheroprotective or bystander role of TIMPs remains a point of debate. Most, but not all, studies have shown an inverse relationship between atherosclerosis and TIMP-2.43,44 The equilibrium between MMPs and TIMPs determines the net proteolytic activity of the degradating enzymes and their consequences.12 We must take into consideration that hyperglycemia exerts opposite effects on MMP-2 and TIMP-2 activities leading to further downregulation of MMP-2/TIMP-2 ratio.45 To our knowledge this is the first study investigating the influence of exercise on MMP-2/TIMP-2 homeostasis. In particular, our study showed how exercise training considerably decreases in both circulating and atherosclerotic plaque levels of MMP-2, while it significantly increased TIMP-2. A possible reason for this is that suppressed MMP-2 concentration in EX group mirrored the improved function of its cellular sources, the SMCs. Indeed, exercise training has been recently proved to alter beneficially the function of SMCs in atherosclerotic plaques.46 Moreover, conditioned medium collected from endothelial NO synthase (eNOS) transfected SMCs showed increased MMP-2 and decreased TIMP-2 activity, leading to the inhibition of vascular smooth muscle cells (VSMCs) migration.47 Although we did not examine it, eNOS – the key factor of the anti-atherogenic effects of exercise – might have mediated exercise effects on the SMCs and MMP-2/TIMP-2 ratio.7 As a whole, we assumed that exercise training in a valid animal model of diabetic atherosclerosis, switched the MMP/TIMP ratio to lower proteolytic activity, which might have promoted atherosclerotic lesion regression and stabilization.
Several shortcomings should be considered in our results interpretation. First, fibrous cap rupture or erosion is rarely observed in the atherosclerotic plaques of apoE−/− mice. The transfer of our experimental data to humans should be done with caution. Human atherosclerosis progression lasts decades, with numerous histological stages and concomitant precipitating risk factors (e.g., diabetes, smoking, hereditary etc.). Thus, the matching of time-frame and conditions between humans and animal model is quite difficult. However, such an animal model compresses the observational time and provides valuable histological data, which is usually obtained post-mortem in humans. Moreover, the great similarities in atherosclerotic plaques morphology between our animal model of atherosclerosis and a human one allow us to evaluate the effects of atherosclerotic modulators. Second, further studies are needed to evaluate whether exercise training reduces the activity of macrophages and MMPs rather than the actual number of inflammatory cells and MMPs concentrations in advanced atherosclerotic lesions.
To conclude, our study showed that exercise training can stabilize vulnerable atherosclerotic plaques through the modulation of inflammatory pathways and MMPs in diabetic atherosclerosis-prone apoE−/− mice. Our findings may provide an alternative mechanism of exercise which contributes to primary and secondary prevention of cardiovascular diseases beyond the modification of traditional cardiovascular risk factors.
Acknowledgements:
part of the present study was presented as an oral announcement and awarded the first prize at the 11th National Congress of Diabetes - 2009, organized by the Hellenic Diabetological Society.
References
- 1.Averill MM, Bornfeldt KE. Lipids versus glucose in inflammation and the pathogenesis of macrovascular disease in diabetes. Curr Diabetes Rep. 2009;9:18–25. doi: 10.1007/s11892-009-0005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nanni S, Melandri G, Hanemaaijer R, Cervi V, Tomasi L, Altimari A, et al. Matrix metalloproteinases in premature coronary atherosclerosis: influence of inhibitors, inflammation, and genetic polymorphisms. Transl Res. 2007;149:137–44. doi: 10.1016/j.trsl.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2007;115:114–26. doi: 10.1161/CIRCULATIONAHA.106.179294. [DOI] [PubMed] [Google Scholar]
- 4.Kadoglou NP, Iliadis F, Liapis CD. Exercise and carotid atherosclerosis. Eur J Vasc Endovasc. 2008;35:264–72. doi: 10.1016/j.ejvs.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 5.Kadoglou NP, Iliadis F, Angelopoulou N, Sailer N, Fotiadis G, Voliotis K, et al. Cardiorespiratory capacity is associated with favourable cardiovascular risk profile in patients with Type 2 diabetes. J Diabetes Complicat. 2009;23:160–6. doi: 10.1016/j.jdiacomp.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Kozakova M, Palombo C, Mhamdi L, Konrad T, Nilsson P, Staehr PB, et al. Habitual physical activity and vascular aging in a young to middle-age population at low cardiovascular risk. Stroke. 2007;38:2549–55. doi: 10.1161/STROKEAHA.107.484949. [DOI] [PubMed] [Google Scholar]
- 7.Okabe TA, Shimada K, Hattori M, Murayama T, Yokode M, Kita T, et al. Swimming reduces the severity of atherosclerosis in apolipoprotein E deficient mice by antioxidant effects. Cardiovasc Res. 2007;74:537–45. doi: 10.1016/j.cardiores.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 8.Kadoglou NP, Iliadis F, Angelopoulou N, Perrea D, Ampatzidis G, Liapis CD, et al. The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur J Cardiov Prev R. 2007;14:837–43. doi: 10.1097/HJR.0b013e3282efaf50. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura K, Sasaki T, Cheng XW, Iguchi A, Sato K, Kuzuya M. Statin prevents plaque disruption in apoE-knockout mouse model through pleiotropic effect on acute inflammation. Atherosclerosis. 2009;206:355–61. doi: 10.1016/j.atherosclerosis.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Morancho A, Rosell A, García-Bonilla L, Montaner J. Metalloproteinase and stroke infarct size: role for anti-inflammatory treatment? Ann NY Acad Sci. 2010;1207:123–33. doi: 10.1111/j.1749-6632.2010.05734.x. [DOI] [PubMed] [Google Scholar]
- 11.Visse R, Negase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function and biochemistry. Circ Res. 2003;92:827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 12.Kadoglou NP, Daskalopoulou SS, Perrea D, Liapis CD. Matrix metalloproteinases and diabetic vascular complications. Angiology. 2005;56:173–89. doi: 10.1177/000331970505600208. [DOI] [PubMed] [Google Scholar]
- 13.Bisoendial RJ, Birjmohun RS, Akdim F, van't Veer C, Spek CA, Hartman D, et al. Creactive protein elicits white blood cell activation in humans. Am J Med. 2009;122:582–89. doi: 10.1016/j.amjmed.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 14.Kadoglou NPE, Kostomitsopoulos N, Kapelouzou A, Moustardas P, Katsimpoulas M, Giagini A, et al. Effects of exercise training on the severity and composition of atherosclerotic plaque in apoE-deficient mice. J Vasc Res. 2011;48:347–56. doi: 10.1159/000321174. [DOI] [PubMed] [Google Scholar]
- 15.Xu X, Wan W, Ji L, Lao S, Powers AS, Zhao W, et al. Exercise training combined with angiotensin II receptor blockade limits post-infarct ventricular remodelling in rats. Cardiovasc Res. 2008;78:523–32. doi: 10.1093/cvr/cvn028. [DOI] [PubMed] [Google Scholar]
- 16.Li G, Sanders JM, Bevard MH, Sun Z, Chumley JW, Galkina EV, et al. CD40 ligand promotes Mac-1 expression, leukocyte recruitment, and neointima formation after vascular injury. Am J Pathol. 2008;172:1141–52. doi: 10.2353/ajpath.2008.070633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pynn M, Schäfer K, Konstantinides S, Halle M. Exercise training reduces neointimal growth and stabilizes vascular lesions developing after injury in apolipoprotein e-deficient mice. Circulation. 2004;109:386–92. doi: 10.1161/01.CIR.0000109500.03050.7C. [DOI] [PubMed] [Google Scholar]
- 18.Hofmann SM, Perez-Tilve D, Greer TM, Coburn BA, Grant E, Basford JE, et al. Defective lipid delivery modulates glucose tolerance and metabolic response to diet in apolipoprotein E-deficient mice. Diabetes. 2008;57:5–12. doi: 10.2337/db07-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tangirala RK, Rubin EM, Palinski W. Quantitation of atherosclerosis in murine models: correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. J Lipid Res. 1995;36:2320–8. [PubMed] [Google Scholar]
- 20.Napoli C, Williams-Ignarro S, De Nigris F, Lerman LO, Rossi L, Guarino C, et al. Longterm combined beneficial effects of physical training and metabolic treatment on atherosclerosis in hypercholesterolemic mice. P Natl Acad Sci USA. 2004;101:8797–802. doi: 10.1073/pnas.0402734101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimada K, Kishimoto C, Okabe TA, Hattori M, Murayama T, Yokode M, et al. Exercise training reduces severity of atherosclerosis in apolipoprotein E knockout mice via nitric oxide. Circ J. 2007;71:1147–51. doi: 10.1253/circj.71.1147. [DOI] [PubMed] [Google Scholar]
- 22.Kadoglou NP, Perrea D, Iliadis F, Angelopoulou N, Liapis C, Alevizos M. Exercise reduces resistin and inflammatory cytokines in patients with type 2 diabetes. Diabetes Care. 2007;30:719–21. doi: 10.2337/dc06-1149. [DOI] [PubMed] [Google Scholar]
- 23.Verhoeven B, Hellings WE, Moll FL, de Vries JP, de Kleijn DP, de Bruin P, et al. Carotid atherosclerotic plaques in patients with transient ischemic attacks and stroke have unstable characteristics compared with plaques in asymptomatic and amaurosis fugax patients. J Vasc Surg. 2005;42:1075–81. doi: 10.1016/j.jvs.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Gonçalves I, Ares MP, Moberg A, Moses J, To F, Montan J, et al. Elastin- and collagenrich human carotid plaques have increased levels of the cysteine protease inhibitor cystatin C. J Vasc Res. 2008;45:395–401. doi: 10.1159/000121474. [DOI] [PubMed] [Google Scholar]
- 25.Fishbein MC. The vulnerable and unstable atherosclerotic plaque. Cardiovasc Pathol. 2010;19:6–11. doi: 10.1016/j.carpath.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Moreno PR, Purushothaman KR, Fuster V, O'Connor WN. Intimomedial interface damage and adventitial inflammation is increased beneath disrupted atherosclerosis in the aorta: implications for plaque vulnerability. Circulation. 2002;105:2504–11. doi: 10.1161/01.cir.0000017265.52501.37. [DOI] [PubMed] [Google Scholar]
- 27.Von der Thüsen JH, van Berkel TJ, Biessen EA. Induction of rapid atherogenesis by perivascular carotid collar placement in apolipoprotein E-deficient and low-density lipoprotein receptor-deficient mice. Circulation. 2001;103:1164–70. doi: 10.1161/01.cir.103.8.1164. [DOI] [PubMed] [Google Scholar]
- 28.DeGraba TJ. Immunogenetic susceptibility of atherosclerotic stroke: implications on current and future treatment of vascular inflammation. Stroke. 2004;35:2712–9. doi: 10.1161/01.STR.0000143788.87054.85. [DOI] [PubMed] [Google Scholar]
- 29.Ding S, Zhang M, Zhao Y, Chen W, Yao G, Zhang C, et al. The role of carotid plaque vulnerability and inflammation in the pathogenesis of acute ischemic stroke. Am J Med Sci. 2008;336:27–31. doi: 10.1097/MAJ.0b013e31815b60a1. [DOI] [PubMed] [Google Scholar]
- 30.Chen QJ, Lu L, Peng WH, Hu J, Yan XX, Wang LJ, et al. Polymorphisms of MMP-3 and TIMP-4 genes affect angiographic coronary plaque progression in non-diabetic and type 2 diabetic patients. Clin Chim Acta. 2009;405:97–103. doi: 10.1016/j.cca.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 31.White AJ, Duffy SJ, Walton AS, Ng JF, Rice GE, Mukherjee S, et al. Matrix metalloproteinase- 3 and coronary remodelling: implications for unstable coronary disease. Cardiovasc Res. 2007;75:813–20. doi: 10.1016/j.cardiores.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Silence J, Lupu F, Collen D, Lijnen HR. Persistence of atherosclerotic plaque but reduced aneurysm formation in mice with stromelysin-1 (MMP-3) gene inactivation. Arterioscler Thromb Vasc Biol. 2001;21:1440–5. doi: 10.1161/hq0901.097004. [DOI] [PubMed] [Google Scholar]
- 33.Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253:269–85. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- 34.Becker JW, Marcy AI, Rokosz LL, Axel MG, Burbaum JJ, Fitzgerald PM, et al. Stromelysin-1: three-dimensional structure of the inhibited catalytic domain and of the C-truncated proenzyme. Protein Sci. 1995;4:1966–76. doi: 10.1002/pro.5560041002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samnegård A, Hulthe J, Silveira A, Ericsson CG, Hamsten A, Eriksson P. Gender specific associations between matrix metalloproteinases and inflammatory markers in post myocardial infarction patients. Atherosclerosis. 2009;202:550–6. doi: 10.1016/j.atherosclerosis.2008.05.050. [DOI] [PubMed] [Google Scholar]
- 36.Tuomainen AM, Nyyssönen K, Laukkanen JA, Tervahartiala T, Tuomainen TP, Salonen JT, et al. Serum matrix metalloproteinase- 8 concentrations are associated with cardiovascular outcome in men. Arterioscler Thromb Vasc Biol. 2007;27:2722–8. doi: 10.1161/ATVBAHA.107.154831. [DOI] [PubMed] [Google Scholar]
- 37.Turu MM, Krupinski J, Catena E, Rosell A, Montaner J, Rubio F, et al. Intraplaque MMP-8 levels are increased in asymptomatic patients with carotid plaque progression on ultrasound. Atherosclerosis. 2005;187:161–9. doi: 10.1016/j.atherosclerosis.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 38.Dollery CM, Owen CA, Sukhova GK, Krettek A, Shapiro SD, Libby P. Neutrophil elastase in human atherosclerotic plaques: production by macrophages. Circulation. 2003;107:2829–36. doi: 10.1161/01.CIR.0000072792.65250.4A. [DOI] [PubMed] [Google Scholar]
- 39.Pauschinger M, Rutschow S, Chandrasekharan K, Westermann D, Weitz A, Schwimmbeck LP, et al. Carvedilol improves left ventricular function in murine coxsackievirus-induced acute myocarditis association with reduced myocardial interleukin-1beta and MMP-8 expression and a modulated immune response. Eur J Heart Fail. 2005;7:444–52. doi: 10.1016/j.ejheart.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Ionita MG, Vink A, Dijke IE, Laman JD, Peeters W, van der Kraak PH, et al. High levels of myeloid-related protein 14 in human atherosclerotic plaques correlate with the characteristics of rupture-prone lesions. Arterioscler Thromb Vasc Biol. 2009;29:1220–7. doi: 10.1161/ATVBAHA.109.190314. [DOI] [PubMed] [Google Scholar]
- 41.Newby AC. Matrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc Res. 2006;69:614–24. doi: 10.1016/j.cardiores.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Pasterkamp G, Schoneveld AH, Hijnen DJ, de Kleijn DP, Teepen H, van der Wal AC, et al. Atherosclerotic arterial remodeling and the localization of macrophages and matrix metalloproteases 1, 2 and 9 in the human coronary artery. Atherosclerosis. 2000;150:245–53. doi: 10.1016/s0021-9150(99)00371-8. [DOI] [PubMed] [Google Scholar]
- 43.Tayebjee MH, Lip GY, Tan KT, Patel JV, Hughes EA, MacFadyen RJ. Plasma matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-2 and CD40 ligand levels in patients with stable coronary artery disease. Am J Cardiol. 2005;96:339–45. doi: 10.1016/j.amjcard.2005.03.072. [DOI] [PubMed] [Google Scholar]
- 44.Kruk M, Kalińczuk L, Pregowski J, Przyluski J, Janas J, Chmielak Z, et al. Serum tissue inhibitor of metalloproteinases- 1 and higher risk features of coronary plaque: a volumetric multivessel intravascular ultrasound study. Athero - sclerosis. 2007;194:57–63. doi: 10.1016/j.atherosclerosis.2006.08.045. [DOI] [PubMed] [Google Scholar]
- 45.Ho FM, Liu SH, Lin WW, Liau CS. Opposite effects of high glucose on MMP-2 and TIMP-2 in human endothelial cells. J Cell Biochem. 2007;101:442–50. doi: 10.1002/jcb.21192. [DOI] [PubMed] [Google Scholar]
- 46.Pellegrin M, Berthelot A, Houdayer C, Gaume V, Deckert V, Laurant P. New insights into the vascular mechanisms underlying the beneficial effect of swimming training on the endothelial vasodilator function in apolipoprotein E-deficient mice. Atherosclerosis. 2007;190:35–42. doi: 10.1016/j.atherosclerosis.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Gurjar MV, Sharma RV, Bhalla RC. eNOS gene transfer inhibits smooth muscle cell migration and MMP-2 and MMP-9 activity. Arterioscler Thromb Vasc Biol. 1999;19:2871–7. doi: 10.1161/01.atv.19.12.2871. [DOI] [PubMed] [Google Scholar]


