Abstract
Background:
Asymptomatic solitary meningiomas are typically managed with clinical and radiographic follow-up. Multiple meningiomas represents a clinical entity distinct from solitary meningiomas and can be sporadic, radiation-induced, associated with neurofibromatosis, or exhibit other familial inheritance. The growth rate for multiple meningiomas is not known and therefore management of these complicated patients can be difficult.
Methods:
A retrospective chart review was performed on 12 patients with a total of 55 meningiomas. Patients with neurofibromatosis were not included. Serial enhanced magnetic resonance imaging was used to determine tumor growth rates. Treatment history was also reviewed and included for analysis.
Results:
Analysis of all 55 tumors demonstrated an average rate of growth of 0.46 cm3/year (range: −0.57-2.94 cm3/year). In the 23 tumors that received no treatment, the average rate of growth was 0.34 cm3/year (range: −0.03-1.8 cm3/year). Ten of the 23 tumors that received no treatment had no history of cranial irradiation. This group demonstrated a growth rate of 0.44 cm3/year (range: −0.01-1.8 cm3/year). Linear regression analysis did not yield any significant relationship between tumor burden and rates of growth.
Conclusion:
Tumor growth rates in patients with multiple meningiomas did not appear to be higher than reported rates for incidentally found solitary meningiomas. As such, asymptomatic multiple meningioma patients should be managed with clinical and radiographic follow-up.
Keywords: Meningioma, multiple meningioma, natural history
INTRODUCTION
Meningiomas are the most common brain tumors, representing approximately 35% of all brain tumors diagnosed in the United States.[3] Meningiomas are generally benign and slow growing, but growth rates can vary dramatically. Existing data suggests that meningiomas generally fall into three groups: Those that do not grow, those that exhibit linear growth, and those that exhibit exponential growth.[2,7] Greater use of diagnostic imaging has led to an increasing number of meningiomas being found while still asymptomatic or minimally symptomatic providing challenges in management. Most solitary incidental meningiomas, however, can be managed with close clinical and radiographic follow-up.[2,10]
Multiple meningiomas are found in only 1-10% of patients with meningiomas[5,6] [Figure 1]. It can occur sporadically or as part of a familial syndrome of either neurofibromatosis (NF) type 2 or familial multiple meningioma.[8] Development of multiple meningiomas in the setting of NF involves inactivation of the NF2 gene on chromosome 22, which affects the merlin tumor suppressor protein. Familial multiple meningioma demonstrates autosomal dominant inheritance, but does not typically involve the NF2 gene.[8] Many sporadic cases are also related to merlin inactivation and exhibit loss of one copy of chromosome 22.
Figure 1.
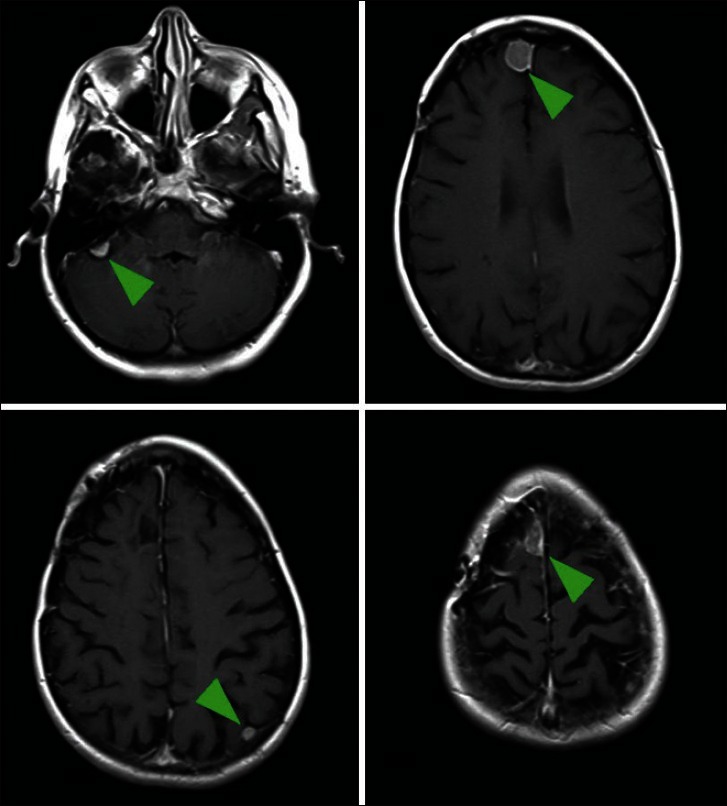
Axial images of a gadolinium-enhanced magnetic resonance imaging of the brain. The patient had a symptomatic right frontal meningioma treated with surgery, but had four additional separate meningiomas in discrete locations
Management of solitary meningiomas is often dictated by tumor size, location, symptoms, and patient preference. In carefully selected patients, a single surgical intervention can provide the opportunity for cure. Patients with multiple meningiomas are not typically able to achieve this result without multiple surgeries and additional risk. In fact, extensive surgical intervention in order to treat all foci may be unnecessary. Eucidating the natural history and growth rate of these tumors is therefore important to determine the best management strategy.
MATERIALS AND METHODS
A retrospective chart review was performed on patients treated at NorthShore Neurologic Institute between 1990 and 2010 to identify patients with more than one meningioma. Thirteen patients were identified with a total of 69 meningiomas. One patient had a history of NF type 2 and was excluded for the purposes of our study. The remaining 12 patients had 55 tumors. Eleven patients underwent treatment, while one had no treatment during the period of follow-up. Seven of the eleven had surgical treatment of one or more tumors. Three of eleven had stereotactic radiosurgery (SRS). One patient had whole brain radiation therapy (WBRT) and one patient had a combination of SRS, surgery, and WBRT. Of the 12 patients, 6 had a history of cranial irradiation suspected to be the cause of the multiple meningiomas.
Serial gadolinium-enhanced magnetic resonance imaging (MRI) was analyzed to determine tumor burden in each patient as well as growth over time. Growth was assessed using volumetric estimations determined by the "abc/2" method.[1] In order to ascertain a better estimation of the natural history of multiple meningiomas, two subgroup analyses were performed: The first utilized growth data on tumors that have not been treated by any of the above modalities (surgery, SRS, or WBRT). The second analysis was performed on the portion of untreated tumors that were not believed to be the result of previous cranial radiation exposure.
Statistical analysis was performed using Microsoft Excel with StatPro. Simple linear regression analysis was used to determine whether there was any correlation between tumor burden and rate of growth. Square of the sample correlation coefficients were also calculated and used for analysis.
RESULTS
Twelve patients with a total of 55 meningiomas were included for analysis [Table 1]. Eleven patients (92%) were female. The average number of tumors per patient was 4.6 (range: 2-10). Average age at diagnosis was 37 years (range: 19-61 years). Average tumor size at time of diagnosis was 0.84 cm3(range: 0.04-4.51 cm3). The average length of follow-up was 61 months (range: 24-101 months).
Table 1.
Patient characteristics
The overall average growth rate for all 55 meningiomas was 0.46 cm3/year (range: −0.57-2.94cm3/year). Of the 55 tumors, 23 were untreated. The average tumor size at time of diagnosis for untreated tumors was 0.92 cm3 (range: 0.04-4.51cm3). The average rate of growth was 0.34 cm3/year (range: −0.03-1.8 cm3/year). Of the 23 untreated tumors, 13 occurred in patients believed to have radiation-induced multiple meningiomas. The remaining 10 tumors were distributed among four patients with an average growth rate of 0.44 cm3/year (range: −0.01-1.8 cm3/year). The average tumor size at time of diagnosis was 1.47 cm3(range: 0.04-4.51cm3).
The relationship between tumor burden and rate of growth was analyzed using linear regression analysis [Figure 2a–c]. In the total group data, no correlation was found between tumor burden and rate of tumor growth. In subgroup analysis of untreated and untreated without history of cranial irradiation, there was little, if any, correlation.
Figure 2.
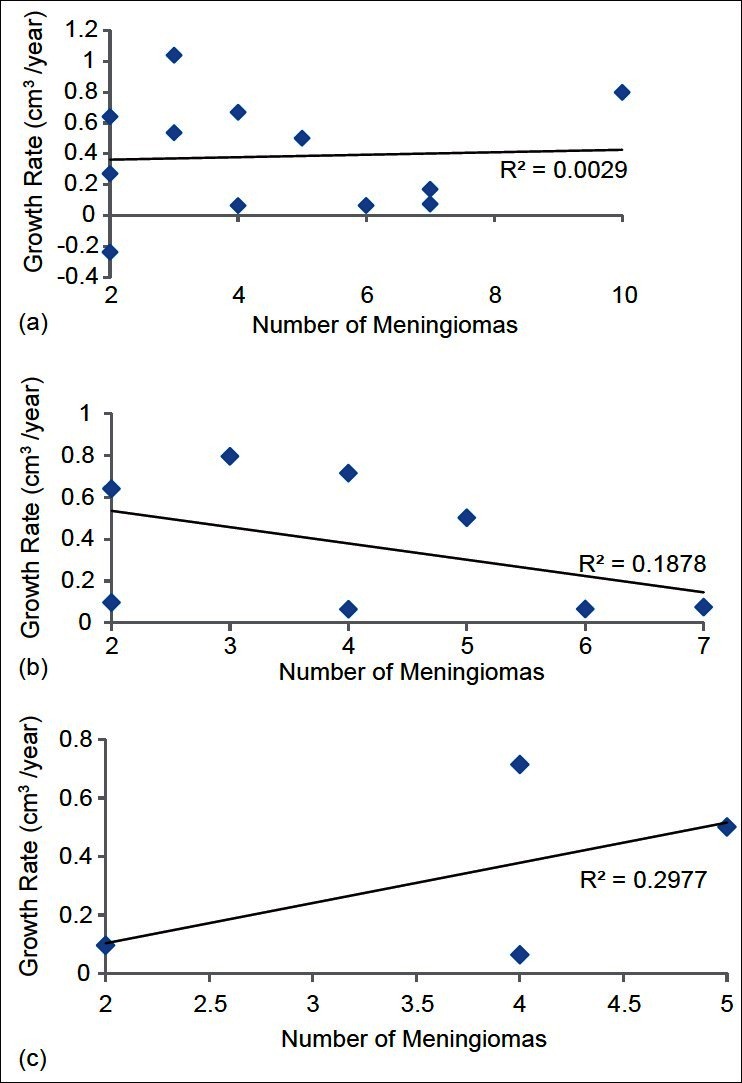
Simple linear regression analysis of tumor burden and growth rate in three groups: (a) all tumors, (b) all untreated tumors, (c) untreated tumors without a history of cranial irradiation. Square of the sample correlation coefficients are included (r2)
DISCUSSION
To the best of our knowledge, this represents the first attempt at determining the growth rate for nonsyndromic multiple meningiomas. In our analysis of 55 tumors distributed over 12 patients and subgroup analysis of untreated tumors and untreated tumors without any history of cranial radiation, we found average growth rates of 0.34-0.46 cm3/year with an overall range of −0.57-2.94 cm3/year. Nakasu et al., reported average growth rates for solitary meningiomas to be 0.796 cm3/year (range: 0.03-2.62 cm3/year).[7] While our average result was lower, it may not represent a statistically significant difference.
We further analyzed the relationship between tumor multiplicity and growth rates through several linear regression analyses. In the overall group, as well as the subgroups, we were unable to find any significant relationship between tumor burden, expressed as number of meningiomas per patient, and rates of growth. This suggests that over the range of this study, patients with 10 meningiomas do not significantly differ in their course when compared with patients with 2 meningiomas with respect to tumor growth rates.
Many treatment modalities are used in the management of meningiomas. This includes surgery, whole brain radiation, and SRS. Selection of treatment should include consideration for both the benefits and the risks. Radiation effects are conventionally categorized into acute, subacute, and delayed effects.[4] Acute effects typically occur within several weeks of treatment and include symptoms of headache, nausea, and somnolence. These effects are believed to be mediated by radation-induced cerebral edema. Subacute effects occur between several weeks and several months of treatment. This is thought to be a result of injury to oligodendrocytes leading to demyelination and neurologic dysfunction. These effects are generally believed to be transient. Delayed effects occur after several months, but within the first 3 years after treatment. The most devastating manifestation of this is a progressive, space-occupying process of radiation necrosis thought to be the result of vascular endothelial damage. The risk of radiation necrosis is dependent on fractionation dose, total dose, treatment duration, treatment volume, patient age, and comorbidities, though reported risks range from 3% to 24%.[9]
Overall, in both rates of growth and relationship of disease burden to growth rates, we did not find any significant differences compared with solitary meningiomas. Given the inherent risks in treatment, the unlikely possibility of complete cure, and the slow growth rate, we recommend that management of these patients be dictated by clinical exam. Asymptomatic patients with multiple meningiomas can be managed through close clinical and radiographic follow-up.
Limitations
The present study is limited by its retrospective design and small sample size. In addition, the patient group had some important heterogeneous characteristics, such as history of radiation exposure and differing treatment strategies. We attempted to address these by performing subgroup analyses to obtain more representative data, but obviously those results are limited by even smaller sample sizes. Larger, prospective studies are required to obtain this information.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2013/4/1/71/112617
Disclaimer: The authors of this article have no conflicts of interest to disclose, and have adhered to SNI's policies regarding human/animal rights, and informed consent. Advertisers in SNI did not ask for, nor did they receive access to this article prior to publication
Contributor Information
Ricky H. Wong, Email: wong.ricky@gmail.com.
Andrew K. Wong, Email: andrew.wong8@gmail.com.
Nicholas Vick, Email: nvick@northshore.org.
Hamad I. Farhat, Email: hfarhat@northshore.org.
REFERENCES
- 1.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–93. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 2.Chamoun R, Krisht KM, Couldwell WT. Incidental meningiomas. Neurosurg Focus. 2011;31:E19. doi: 10.3171/2011.9.FOCUS11220. [DOI] [PubMed] [Google Scholar]
- 3.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro Oncol. 2012;14(Suppl 5):v1–49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fink J, Born D, Chamberlain MC. Radiation necrosis: Relevance with respect to treatment of primary and secondary brain tumors. Curr Neurol Neurosci Rep. 2012;12:276–85. doi: 10.1007/s11910-012-0258-7. [DOI] [PubMed] [Google Scholar]
- 5.Huang H, Buhl R, Hugo HH, Mehdorn HM. Clinical and histological features of multiple meningiomas compared with solitary meningiomas. Neurol Res. 2005;27:324–32. doi: 10.1179/016164105X39932. [DOI] [PubMed] [Google Scholar]
- 6.Mocker K, Holland H, Ahnert P, Schober R, Bauer M, Kirsten H, et al. Multiple meningioma with different grades of malignancy: Case report with genetic analysis applying single-nucleotide polymorphism array and classical cytogenetics. Pathol Res Pract. 2011;207:67–72. doi: 10.1016/j.prp.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Nakasu S, Fukami T, Nakajima M, Watanabe K, Ichikawa M, Matsuda M. Growth pattern changes of meningiomas: Long-term analysis. Neurosurgery. 2005;56:946–955. [PubMed] [Google Scholar]
- 8.Shen Y, Nunes F, Stemmer-Rachamimov A, James M, Mohapatra G, Plotkin S, et al. Genomic profiling distinguishes familial multiple and sporadic multiple meningiomas. BMC Med Genomics. 2009;2:42. doi: 10.1186/1755-8794-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siu A, Wind JJ, Iorgulescu JB, Chan TA, Yamada Y, Sherman JH. Radiation necrosis following treatment of high grade glioma-a review of the literature and current understanding. Acta Neurochir. 2012;154:191–201. doi: 10.1007/s00701-011-1228-6. [DOI] [PubMed] [Google Scholar]
- 10.Sughrue ME, Rutkowski MJ, Aranda D, Barani IJ, McDermott MW, Parsa AT. Treatment decision making based on the published natural history and growth rate of small meningiomas. J Neurosurg. 2010;113:1036–42. doi: 10.3171/2010.3.JNS091966. [DOI] [PubMed] [Google Scholar]


