Abstract
Objective
To evaluate cord blood erythropoietin (EPO) and interleukin-6 (IL-6) levels to predict preterm infants at risk of developing intraventricular hemorrhage (IVH).
Methods
Levels of umbilical cord EPO, acid–base status and IL-6 were analyzed in 116 consecutive, preterm newborns (GA at delivery: 29 [23–34] weeks) born to mothers who had a clinically indicated amniocentesis to rule out infection. Early-onset neonatal sepsis (EONS) was diagnosed using symptoms, hematological criteria and blood cultures.
Results
IVH was diagnosed by cranial ultrasounds. The prevalence of IVH in our population was 25% (29/116). There was a direct relationship between cord blood EPO and cord blood IL-6 concentration (r = 0.225, p = 0.014), independent of GA at birth. Elevated cord blood EPO levels (r = 0.182, p = 0.016) and GA birth at birth (r = –0.236, p = 0.004) remained significant independent factors associated with the risk of IVH, when evaluated with stepwise logistic regression analyses. Cord blood IL-6, pH, and EONS were not associated with IVH. These relationships remained following correction for GA at birth (p = 0.027).
Conclusions
Our results suggest that elevation in cord blood EPO may predict newborns at risk for IVH, independent of fetal inflammatory status. Further studies are warranted to confirm this association.
Keywords: Erythropoietin, IL-6, premature, newborn, IVH
Introduction
In the premature neonate, intraventricular hemorrhage (IVH) is a major cause of morbidity and mortality. The relevance of IVH as a major clinical problem is emphasized by the observation that in the USA, 20–25% of all very low birth weight (VLBW) infants are diagnosed with IVH [1]. Among these infants, 10–15% have severe grades (grades 3–4) of IVH, and 75% of this sub-group develop mental retardation or cerebral palsy [1]. Annually, >3600 new cases of mental retardation are attributable to IVH, with lifetime care costs exceeding $3.6 billion [1].
The pathogenesis of IVH is multifactorial with a variety of genetic and environmental factors being implicated [1–3]. Among the latter, the antenatal factors include early gestational age (GA), absence of antenatal steroid exposure, maternal chorioamnionitis, and early-onset neonatal sepsis (EONS). Postnatal factors include respiratory distress syndrome (RDS), and hypotension requiring pressors [1–3].
An effective preventive approach to IVH could have significant clinical implications. Among antenatal interventions, use of steroids has proved to be beneficial [4–6]. A variety of postnatal preventive clinical and pharmacologic interventions have been tried [1]. Among them, indomethacin has been found to be most effective in decreasing the rate of IVH [7,8]. However, indomethacin appears to mostly impact on the more severe grades of IVH [7,9]. In addition, indomethacin does not appear to consistently improve long-term neurodevelopmental outcomes [9,10].
In a majority of cases, IVH occurs within the first 24 h of birth, and may progress over the next 48 h [1]. Biomarkers with the potential to identify the preterm neonate at high-risk for IVH, could allow for early and more effective targeted therapy to prevent this serious morbidity. For clinical relevance, such a biomarker would need to be detected at or soon after birth.
Maternal and/or intra-uterine infection has been suggested to possibly be associated with IVH, mostly in relation to histologic chorioamnionitis [11]. Among proinflammatory cytokines, cord blood interleukin-6 (IL-6) has not been consistently shown to be associated with IVH [12–14].
Erythropoietin (EPO) has been previously proposed as a potential marker for central nervous system (CNS) injury in neonates with asphyxia, meningitis, and IVH [15]. In this study, mean cerebrospinal fluid (CSF) EPO levels were higher in neonates with IVH, compared to controls [15]. Interestingly, in 11 patients with IVH, CSF EPO concentrations obtained closer to birth (and presumably to the time of hemorrhage) tended to be higher, as compared to those obtained later [15]. Plasma EPO levels of these patients with IVH were not significantly different from controls [15]. These results may have been affected by the small sample size, and variability in the timing of collection of samples that were obtained from neonates who had an IVH within 1 week before the spinal tap [15]. Several premature babies with culture-proven or clinically presumed meningitis were also studied in the aforementioned study; however, it was not reported whether these neonates were delivered in the setting of intra-amniotic infection/inflammation [15]. This distinction is important because as shown in humans and animal models of sepsis, intra-amniotic inflammation may sensitize the fetus to postpartum stressors or initiate early brain hemorrhage or IVH via cytokine effects on cardiovascular instability [16,17].
The above observation led us to hypothesize that cord blood IL-6 and/or EPO concentrations in preterm neonates exposed to intra-amniotic inflammation/infection are higher in infants who develop IVH. The objective of the present study was to evaluate, in a prospective fashion, the cord blood IL-6 and EPO concentrations of babies delivered in the setting of intra-amniotic inflammation-related preterm birth, and its’ relationship to IVH.
Material and methods
Study population and research design
We prospectively studied 116 consecutive preterm neonates born to mothers who had amniocenteses to rule out intra-amniotic infection. Women were recruited following admission to the Labor and Birth or to the Maternal Special Care antepartum units at Yale New Haven Hospital (YNHH). The decision to perform amniocenteses was made by clinicians responsible for the care of the patient, independent of our study protocol. The Human Investigation Committee of Yale University approved our study.
Amniotic fluid (AF) was collected by ultrasound-guided amniocentesis for all the participants and each woman was followed prospectively up to the point of delivery. Inclusion criteria included: GA ≥ 23.1 weeks, symptoms of preterm labor, preterm premature rupture of membranes (PPROM), advanced cervical dilatation (≥3 cm), and/or uterine contractions intractable to tocolysis. Women with anhydramnios, human immunodeficiency or hepatitis viral infections were not enrolled. GA was determined based on last menstrual period and ultrasound information obtained prior to 20 weeks of gestation [18]. Preterm labor was defined as the presence of regular uterine contractions and documented cervical effacement and/or dilatation in patients <37 weeks GA. The diagnosis of PPROM was confirmed by vaginal AF “pooling”, “nitrazine”, “ferning” or by an amniocentesis-dye positive test. No digital exams were permitted, and in the absence of infection, PPROM was managed expectantly. Patients received corticosteroids for fetal lung maturity and antibiotic therapy in accordance to the American Congress of Obstetrics and Gynecology (ACOG) recommendations [19]. Delivery was performed for routine obstetrical indications and the decision to deliver was made by the clinical team responsible for the care of the patient. The neonatology resuscitation team was present at the time of delivery for all cases.
AF was cultured for aerobic and anaerobic bacteria, Ureaplasma urealyticum and Mycoplasma hominis. Additional clinical laboratory tests performed for the purpose of diagnosing infection/inflammation included: glucose, lactate dehydrogenase (LDH), Gram stain, and white blood cell (WBC) count. For clinical management, an AF glucose cut-off of ≤15 mg/dl and LDH levels ≥419 U/l were considered suggestive of intra-amniotic infection [20,21]. AF not used for clinical purposes was spun at 3000g at 4°C for 20 min, aliquoted and immediately stored at –80°C until IL-6 levels were measured by a specific and sensitive immunoassay.
Umbilical cord blood
Umbilical cord blood was obtained by aseptic puncture of the clamped umbilical vein at the time of delivery. Acid–base status was determined within 10 min of delivery. Immediately following collection, the cord blood was centrifuged at 1000g for 15 min. Serum was aliquoted in sterile polypropylene tubes and stored at –80°C until IL-6 and EPO levels were examined.
Immunoassays for IL-6 and EPO
Enzyme-linked immunosorbent assays for human IL-6 (Pierce-Endogen, Rockford, IL) and EPO (R&D Systems, Minneapolis, MN) were performed in duplicate according to manufacturers’ instructions by investigators unaware of the AF and umbilical cord blood sample origin. The minimal detectable concentration for the umbilical cord and AF IL-6 was 1 pg/ml. The minimal detectable concentration for EPO was 0.6 mIU/ml. The inter- and intra-assay coefficients of variation were <10%.
Evaluation of early-onset neonatal sepsis
Neonatal hematological indices and sepsis categorization were assessed from blood specimens and cultures obtained within 2 h from the time of birth by an investigator (VB) unaware of the results of the umbilical cord IL-6 levels. All 116 neonates were admitted to the Yale Newborn Special Care Unit (NBSCU). EONS was defined as the presence of confirmed or suspected sepsis at ≤72 h after birth. A diagnosis of EONS was based on clinical symptoms corroborated with hematological laboratory results [22,23]. Any two or more of the following hematological criteria were used as indicators of EONS [23]: (1) absolute neutrophil count of <7500 or >14,500 cells /mm3; (2) absolute band count >1500 cells/mm3; (3) immature/total neutrophil (I:T) ratio >0.16; (4) platelet count <150,000 cells/mm3. EONS was dichotomized into present (when sepsis was either confirmed or suspected) or absent. All neonates with confirmed or suspected sepsis received antibiotic therapy, per institutional protocol.
Evaluation of IVH
This was done using cranial ultrasounds, as per nursery protocol, and read by experienced pediatric radiologists. Routinely, the first scans are done by day of life (DOL) 3, and repeated at DOL 7–10 and DOL 30. Additional scans are done if clinically indicated. We used the worst grade of IVH for our analyses.
Statistical analysis
Statistical analyses were performed with Sigma Stat, version 2.03 (SPSS Inc, Chicago, IL) and MedCalc (Broekstraat, Belgium) statistical software. Normality testing was performed using the Kolmogorov–Smirnov test. Data were compared with one-way ANOVA followed by Dunnet's tests (parametric) or Mann–Whitney Rank Sum Test, or Kruskal–Wallis on ranks followed by Dunn's tests (non-parametric), to adjust for multiple comparisons as appropriate. The IL-6 concentrations were presented as arithmetic means with inter-quartile range. Statistical analysis was completed before (Kruskal–Wallis ANOVA) or after (one-way ANOVA) logarithmic transformation of data. Pearson correlations were used to measure co-linearity between the selected independent variables as well as other relevant relationships between dependent and independent variables. Comparisons between proportions were done with Chi-square tests. Stepwise multivariable regression analysis was used to determine concurrent relationships between variables and to correct for possible influences of GA and BW. A p value of <0.05 was considered significant throughout the analysis.
Results
The demographics and clinical characteristics of the maternal and neonatal study population are presented in Table I. The prevalence of IVH in our population was 25% (29/116). Mothers of infants who developed IVH were more likely to have a lower GA at enrollment (p = 0.006) and evidence of intra-amniotic infection (positive AF cultures) (p = 0.04) and inflammation as reflected by the AF IL-6 (p = 0.0007). Infants without IVH were more likely to be delivered via cesearean section, have significantly higher GA, BW, and Apgar scores at 1 and 5 min (all p ≤ 0.02).
Table I.
Demographic and clinical characteristics of women and their newborns.
| IVH |
|||
|---|---|---|---|
| Variable | No, n = 87 | Yes, n = 29 | p-value |
| Characteristics at enrollment | |||
| Maternal age, years★ | 29 [23–35] | 26 [20–32] | 0.182 |
| Maternal parity★ | 1 [0–2] | 0 [0–1] | 0.063 |
| Non-Caucasian race, n (%)† | 48 (55) | 22 (76) | 0.080 |
| Product of twin gestation, n (%)† | 12 (14) | 6 (21) | 0.554 |
| Gestational age at enrollment, weeks★ | 28 [25–30] | 26 [24–28] | 0.006 |
| PPROM, n (%)† | 52 (60) | 13 (45) | 0.235 |
| Clinical chorioamnionitis, n (%)† | 6 (7) | 7 (24) | 0.027 |
| Steroid exposure during pregnancy, n (%)† | 79 (91) | 29 (100) | 0.204 |
| Intra-amniotic infection (positive cultures), n (%)† | 34 (39) | 18 (62) | 0.044 |
| Intra-amniotic inflammation IL-6, ng/ml★ | 6.8 [1.4–23.7] | 24.6 [6.5–98.8] | 0.007 |
| Outcome characteristics | |||
| Gestational age at delivery, weeks★ | 29 [26–30] | 26 [25–29] | 0.011 |
| Amniocentesis-to-delivery, days★ | 0.5 [0.2–2.2] | 0.4 [0.2–0.8] | 0.747 |
| Birth weight, grams★ | 1,190 [849–1,503] | 955 [730–1,225] | 0.018 |
| Cesarean delivery, n (%)† | 51 (59) | 9 (31) | 0.018 |
| Apgar score 1 min,★ | 7 [5–8] | 4 [2–6] | 0.001 |
| Apgar score 5 min,★ | 8 [8–9] | 6 [6–8] | < 0.001 |
| Male sex, n (%)† | 43 (50) | 16 (55) | 0.748 |
| Histological chorioamnionitis stages II & III, n (%)† | 49 (56) | 21 (72) | 0.189 |
| Histological funisitis grades 1–4, n (%)† | 37 (43) | 16 (55) | 0.333 |
| Early-onset neonatal sepsis (EONS), n (%)† | 21 (24) | 13 (45) | 0.060 |
| EONS confirmed by blood cultures, n (%)† | 3 (3) | 3 (10) | 0.333 |
PPROM, preterm premature rupture of membranes; AF, amniotic fluid.
Data presented as median [interquartile range – IQR] and analyzed by Mann–Whitney tests.
Data presented as n (%) and analyzed by Chi-square tests.
Table II shows the results of umbilical vein IL-6 and EPO levels. While there were no differences in the EPO levels, the cord blood IL-6 values were significantly elevated in infants with IVH (p = 0.004). Since intra-amniotic infection/inflammation increases the risk of a fetal inflammatory status [24], we analyzed the hematological indices from the infants’ blood, done as part of their sepsis evaluation. Infants with IVH had significantly lower hematocrits (p = 0.003), but elevated absolute band count (p = 0.035) and I:T ratios (p = 0.007). EPO levels were not different based on the route of delivery (cesarean section versus vaginal delivery, p = 0.454), and did not significantly correlate with Apgars at 1 (r = –0.101, p = 0.282) or 5 min (r = –0.049, p = 0.607).
Table II.
Cord blood IL-6 and EPO levels with hematological indices for evaluation of sepsis in infants.
| IVH |
|||
|---|---|---|---|
| Variable | No, n = 87 | Yes, n = 29 | p-value |
| Umbilical vein research analytes | |||
| Interleukin-6, pg/ml★ | 12.4 [6.6–69.2] | 89.3 [14.9–724.5] | 0.004 |
| Erythropoietin, mIU/ml★ | 8.0 [4.0–15.1] | 8.2 [5.2–21.2] | 0.287 |
| Hematological indices | |||
| Hematocrit, %★ | 46 [43–50] | 41 [39–48] | 0.003 |
| Hemoglobin, g/dl★ | 15 [14–16] | 13 [12–15] | 0.002 |
| WBC, cells × 1000/mm3★ | 10 [8–14] | 8 [6–17] | 0.386 |
| Platelets, cells × 1000/mm3★ | 242 [212–308] | 233 [198–288] | 0.241 |
| Segmented, %★ | 33 [24–43] | 28 [21–34] | 0.048 |
| Lymphocytes, %★ | 41 [29–57] | 42 [26–56] | 0.962 |
| ANC, cells/mm3★ | 3,529 [2,091–5,875] | 2,323 [1,206–5,600] | 0.154 |
| ABC, cells/mm3★ | 336 [146–1,177] | 910 [409–2,455] | 0.035 |
| I:T ratio, %★ | 4 [1–12] | 12 [4–20] | 0.007 |
| NRBC, cells/mm3★ | 18 [13–33] | 20 [13–33] | 0.382 |
ANC, absolute neutrophil count; ABC, absolute band count; I:T, immature/total neutrophil count; NRBC, nucleated red blood cells.
Data presented as median [interquartile range – IQR] and analyzed by Mann–Whitney tests.
The cord blood gases were available for 85 neonates (Table III). Our analysis demonstrated that the pO2 and O2 saturation values were significantly higher, while pCO2 and HCO3 values significantly lower, in the umbilical vein of infants who developed IVH. At birth, none of the infants had either acidemia or hypoxia as defined by the ACOG criteria [25]. IL-6 levels were significantly higher in the infants who developed IVH, with no difference in EPO values, in this subset, similar to the entire study cohort.
Table III.
Umbilical cord blood analysis.
| IVH |
|||
|---|---|---|---|
| Variable | No, n = 69 | Yes, n = 16 | p-value |
| Umbilical artery clinical gas analysis | |||
| pH★ | 7.3 [7.2–7.4] | 7.3 [7.3–7.3] | 0.926 |
| pO2, mmols/l★ | 22 [18–33] | 28 [25–35] | 0.048 |
| O2 sat, %★ | 47 [27–63] | 59 [405–755] | 0.133 |
| pCO2, mmols/l★ | 42 [37–48] | 44 [40–48] | 0.854 |
| HCO3, mmols/l★ | 21 [19–22] | 22 [20–23] | 0.344 |
| Base deficit, mmols/l★ | 4.9 [6.2–3.6] | 4.7 [5.3–3.7] | 0.493 |
| Umbilical vein clinical gas analysis | |||
| pH★ | 7.4 [7.3–7.4] | 7.4 [7.3–7.4] | 0.971 |
| pO2, mmols/l★ | 31 [24–40] | 39 [33–54] | 0.004 |
| O2 sat, %★ | 65 [40–80] | 83 [65–93] | 0.013 |
| pCO2, mmols/l★ | 38 [36–41] | 33 [29–38] | 0.030 |
| HCO3, mmols/l★ | 21 [19–22] | 19 [17–21] | 0.005 |
| Base deficit, mmols/l★ | 4.0 [4.9–2.0] | 4.3 [6.7–3.7] | 0.051 |
| Umbilical vein research analytes | |||
| Interleukin-6, pg/ml★ | 12.4 [6.8–56.7] | 201.2 [25.3–1,651] | < 0.001 |
| Erythropoietin, mIU/ml★ | 7.2 [4.1–15.6] | 12.3 [5.7–39.9] | 0.139 |
Data presented as median [interquartile range – IQR] and analyzed by Mann–Whitney tests.
When examining the relationships with GA at birth we found that cord blood IL-6 levels were dependent and inversely correlated (r = –0.298, p= 0.001) with GA at birth. In contrast, cord blood EPO concentration appeared to vary independently of GA at birth (r = 0.131, p = 0.162).
Relationship between severity of IVH and IL-6 and EPO values
The distribution of the severity of IVH in our study population, and the IL-6 as well as EPO levels in the respective categories have been shown in Table IV. There was a significant difference between no IVH (Grade 0) and severe (Grades 3–4) IVH, for both analytes (Table IV).
Table IV.
IL-6 and EPO levels and severity of IVH.
| IVH groups | n | IL-6, pg/ml‡ | EPO, mIU/ml‡ |
|---|---|---|---|
| Grade 0 | 87 | 12.4 [6.6–69.2] | 8.0 [4.1–15.2] |
| Grade 1 | 7 | 21.4 [8.6–30.0] | 6.0 [4.3–7.5] |
| Grade 2 | 9 | 89.3 [13.8–109.4] | 8.6 [4.3–43.8) |
| Grades 3–4 | 13 | 563.0 [53.2–3026.6]★ | 17.0 [7.6–39.3)★★ |
IVH, intraventricular hemorrhage; EPO, erythropoietin; IL-6, interleukin-6.
Data presented as median [interquartile range – IQR] and analyzed by Mann–Whitney tests.
p < 0.001, vs. Grade 0
p = 0.037, vs. Grade 0.
Relationships between IVH, EPO, EONS, and IL-6
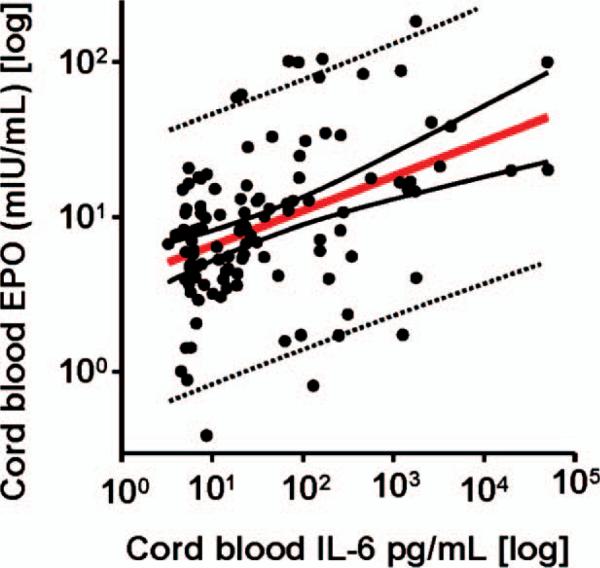
There was a direct relationship between cord blood EPO and cord blood IL-6 concentration (r = 0.225, p = 0.014) (Figure 1), independent of GA. However, in multivariate regression analysis, elevated cord blood EPO levels, but not cord blood IL-6, pH and EONS status, was the strongest predictor of IVH. These relationships remained following correction for GA (p = 0.027). Table V shows the results of stepwise logistic regression analysis. The model included IVH (dichotomized) as dependent variable and GA at delivery along with BW, IL-6, and EPO as independent variables. When our outcome data were evaluated by using stepwise logistic regression analyses, elevated cord blood EPO levels (r = 0.182, p = 0.016) and GA at birth (r = –0.236, p = 0.004) remained significant independent risk factors associated with the risk of IVH. Cord blood IL-6 and BW were excluded from the model (p > 0.1 for both).
Figure 1.
Relationship between the umbilical cord blood interleukin-6 (IL-6) and erythropoietin (EPO) levels. Distribution of the cord blood IL-6 (y-axis) in relationship to the EPO on the x-axis. Data presented in logarithmic format. (pg, picograms; mIU, milli international units; ml, milliliters). The regression line (solid red) and 95% confidence (solid black lines) and prediction (dotted black lines) intervals are also shown (n = 116).
Table V.
Logistic regression analyses for predicting IVH.
| Variables | R | p value |
|---|---|---|
| GA at delivery | –0.236 | 0.004 |
| CB EPO | 0.182 | 0.016 |
| CB IL-6 | 0.135 | >0.1 |
| Birth weight | –0.219 | >0.1 |
GA, gestation age; CB, cord blood; EPO, erythropoietin; IL-6, interleukin-6.
Discussion
The prevalence of IVH in our population mirrors what has been reported nationally [1]. While a variety of factors [1–3,26,27] have been associated with the risk of developing IVH, a majority of them (VLBW, RDS, severity of illness) are related to the GA at delivery. In our study, however, the association of IVH with cord blood EPO levels remained significant after correcting for GA. Cesarean delivery has not been shown to impact on IVH [28], and our study noted a higher incidence of Cesarean-section delivery in infants without IVH. As noted in Table I, we also did not find differences in antenatal steroid exposure or gender [29] in the infants with or without IVH in our study cohort.
The cord blood EPO levels reported in our study in the infants with IVH ranged from 1.6 to 184.7 mIU/ml, similar to the plasma levels (4.7 to 164.2 mU/ml) reported in the earlier study [15]. In that study, no differences were found in patients with IVH versus controls; however, the sample size was small and the timing of collection of samples was quite variable [15]. Interestingly, we did find that severe IVH (Grades 3–4) had higher levels of cord blood IL-6 and EPO, compared to infants with no IVH (Table IV).
While we did not have detailed information about hypotension [30] or postnatal blood gases collected in our study cohort, these and other commonly cited clinically-relevant factors were not found to be significant risk factors for severe IVH [3]. It is also important to point out that our NBSCU utilized the indomethacin protocol for prophylaxis against IVH [7,8] during the study period.
With regards to IL-6 levels and IVH, elevated umbilical vein levels (median: 87; 25–75 centile: 30–310 pg/ml) in VLBW infants (n = 69) with IVH, versus those without (median: 0; 25–75 centile: 0–4 pg/ml) have been reported [31]. Increased post-natal serum IL-6 values have also been suggested as a marker for IVH [32]. Our results are in accord with these studies. However, following correction for GA we found that IL-6, pH and EONS were not associated with IVH.
While one study has shown an association of IVH with severe acidemia (pH < 7) at birth [33], the majority of studies have reported that cord blood gas analyses are not helpful in predicting IVH in VLBW infants [3,16,34,35]. Our blood gas data results support that latter contention.
Hypoxia could be implicated as a potential mechanism, since EPO is well known to increase in the CNS following hypoxia [15]. Elevated circulating nucleated erythrocytes (NRBCs) in the newborn period can be a marker of chronic fetal hypoxia and levels measured in the first 6 days of postnatal life have been associated with IVH [36]. In the absence of hypoxia, our earlier work has noted that elevations in NRBCs in the early neonatal period may be a direct response of exposure to inflammatory mediators in utero [37]. In our cohort, however, there was no difference in NRBC counts between newborns with and without IVH (Table II). In addition, the data on cord blood gases also do not support an acute hypoxic perinatal event in our study cohort.
The lack of association of EONS with IVH in our study are in accord with a recent study in 125 premature newborns, where funisitis (OR 1.6, p = 0.06) and fetal inflammation (OR 2.6, p = 0.06) were not associated with early hemorrhage [12]. In that study, fetal inflammation was defined as cord blood IL-6 concentrations >7.6 pg/ml [12]. The data on the association of histological chorioamnionitis with IVH is controversial [38–40].
In VLBW infants, most of the literature regarding the CNS has focused on the use of recombinant EPO as a neuroprotective strategy, specifically, against hypoxic-ischemic encephaolopathy [41] or IVH and periventricular leukomalacia [42]. We are unaware of any previous study evaluating cord blood EPO levels in relationship to IVH in VLBW infants. Our data, however, are supported by the earlier report of the levels and timing of collection in CSF in infants with IVH [15]. Most importantly, our results remained true even after adjusting for GA.
In conclusion, our results suggest that the level of cord blood EPO, in association with GA at birth, may predict newborns at risk for IVH, independent of fetal inflamma-tory status. Further studies are warranted to confirm this association.
Acknowledgements
CSB was supported by NICHD RO3 RO3HD50249-01 and K12 HD047018-O1 WRHR. IAB was supported by NICHD RO1 HD 047321.
References
- 1.McCrea HJ, Ment LR. The diagnosis, management, and postnatal prevention of intraventricular hemorrhage in the preterm neonate. Clin Perinatol. 2008;35:777–792. vii. doi: 10.1016/j.clp.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. 2010;67:1–8. doi: 10.1203/PDR.0b013e3181c1b176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarkar S, Bhagat I, Dechert R, Schumacher RE, Donn SM. Severe intraventricular hemorrhage in preterm infants: comparison of risk factors and short-term neonatal morbidities between grade 3 and grade 4 intraventricular hemorrhage. Am J Perinatol. 2009;26:419–424. doi: 10.1055/s-0029-1214237. [DOI] [PubMed] [Google Scholar]
- 4.Eriksson L, Haglund B, Ewald U, Odlind V, Kieler H. Short and long-term effects of antenatal corticosteroids assessed in a cohort of 7,827 children born preterm. Acta Obstet Gynecol Scand. 2009;88:933–938. doi: 10.1080/00016340903111542. [DOI] [PubMed] [Google Scholar]
- 5.Blickstein I, Reichman B, Lusky A, Shinwell ES. Plurality-dependent risk of severe intraventricular hemorrhage among very low birth weight infants and antepartum corticosteroid treatment. Am J Obstet Gynecol. 2006;194:1329–1333. doi: 10.1016/j.ajog.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 6.Crane J, Armson A, Brunner M, De La Ronde S, Farine D, Keenan-Lindsay L, Leduc L, Schneider C, Van Aerde J. Antenatal corticosteroid therapy for fetal maturation. J Obstet Gynaecol Can. 2003;25:45–52. doi: 10.1016/s1701-2163(16)31081-7. [DOI] [PubMed] [Google Scholar]
- 7.Ment LR, Oh W, Ehrenkranz RA, Philip AG, Vohr B, Allan W, Duncan CC, Scott DT, Taylor KJ, Katz KH, et al. Low-dose indomethacin and prevention of intraventricular hemorrhage: a multicenter randomized trial. Pediatrics. 1994;93:543–550. [PubMed] [Google Scholar]
- 8.Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts RS, Saigal S, Solimano A, Vincer M, Wright LL. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med. 2001;344:1966–1972. doi: 10.1056/NEJM200106283442602. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, Solimano A, Tin W. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357:1893–1902. doi: 10.1056/NEJMoa073679. [DOI] [PubMed] [Google Scholar]
- 10.Ment LR, Vohr BR, Makuch RW, Westerveld M, Katz KH, Schneider KC, Duncan CC, Ehrenkranz R, Oh W, Philip AG, et al. Prevention of intraventricular hemorrhage by indo-methacin in male preterm infants. J Pediatr. 2004;145:832–834. doi: 10.1016/j.jpeds.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Kaukola T, Herva R, Perhomaa M, Paakko E, Kingsmore S, Vainionpaa L, Hallman M. Population cohort associating chorioamnionitis, cord inflammatory cytokines and neurologic outcome in very preterm, extremely low birth weight infants. Pediatr Res. 2006;59:478–483. doi: 10.1203/01.pdr.0000182596.66175.ee. [DOI] [PubMed] [Google Scholar]
- 12.Babnik J, Stucin-Gantar I, Kornhauser-Cerar L, Sinkovec J, Wraber B, Derganc M. Intrauterine inflammation and the onset of peri-intraventricular hemorrhage in premature infants. Biol Neonate. 2006;90:113–121. doi: 10.1159/000092070. [DOI] [PubMed] [Google Scholar]
- 13.Goldenberg RL, Andrews WW, Faye-Petersen OM, Cliver SP, Goepfert AR, Hauth JC. The Alabama preterm birth study: corticosteroids and neonatal outcomes in 23- to 32-week newborns with various markers of intrauterine infection. Am J Obstet Gynecol. 2006;195:1020–1024. doi: 10.1016/j.ajog.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 14.Satar M, Turhan E, Yapicioglu H, Narli N, Ozgunen FT, Cetiner S. Cord blood cytokine levels in neonates born to mothers with prolonged premature rupture of membranes and its relationship with morbidity and mortality. Eur Cytokine Netw. 2008;19:37–41. doi: 10.1684/ecn.2008.0118. [DOI] [PubMed] [Google Scholar]
- 15.Juul SE, Stallings SA, Christensen RD. Erythropoietin in the cerebrospinal fluid of neonates who sustained CNS injury. Pediatr Res. 1999;46:543–547. doi: 10.1203/00006450-199911000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Salafia CM, Minior VK, Rosenkrantz TS, Pezzullo JC, Popek EJ, Cusick W, Vintzileos AM. Maternal, placental, and neonatal associations with early germinal matrix/intraventricular hemorrhage in infants born before 32 weeks’ gestation. Am J Perinatol. 1995;12:429–436. doi: 10.1055/s-2007-994514. [DOI] [PubMed] [Google Scholar]
- 17.Buhimschi CS, Baumbusch MA, Dulay AT, Oliver EA, Lee S, Zhao G, Bhandari V, Ehrenkranz RA, Weiner CP, Madri JA, et al. Characterization of RAGE, HMGB1, and S100beta in inflammation-induced preterm birth and fetal tissue injury. Am J Pathol. 2009;175:958–975. doi: 10.2353/ajpath.2009.090156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hadlock FP, Deter RL, Harrist RB, Park SK. Computer assisted analysis of fetal age in the third trimester using multiple fetal growth parameters. J Clin Ultrasound. 1983;11:313–316. doi: 10.1002/jcu.1870110605. [DOI] [PubMed] [Google Scholar]
- 19.ACOG Practice Bulletin No. 80: premature rupture of membranes. Clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2007;109:1007–1019. doi: 10.1097/01.AOG.0000263888.69178.1f. [DOI] [PubMed] [Google Scholar]
- 20.Edwards RK, Clark P, Locksmith Gregory J, Duff P. Performance characteristics of putative tests for subclinical chorioamnionitis. Infect Dis Obstet Gynecol. 2001;9:209–214. doi: 10.1155/S1064744901000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garry D, Figueroa R, Aguero-Rosenfeld M, Martinez E, Visintainer P, Tejani N. A comparison of rapid amniotic fluid markers in the prediction of microbial invasion of the uterine cavity and preterm delivery. Am J Obstet Gynecol. 1996;175:1336–1341. doi: 10.1016/s0002-9378(96)70051-0. [DOI] [PubMed] [Google Scholar]
- 22.Buhimschi CS, Buhimschi IA, Abdel-Razeq S, Rosenberg VA, Thung SF, Zhao G, Wang E, Bhandari V. Proteomic biomarkers of intra-amniotic inflammation: relationship with funisitis and early-onset sepsis in the premature neonate. Pediatr Res. 2007;61:318–324. doi: 10.1203/01.pdr.0000252439.48564.37. [DOI] [PubMed] [Google Scholar]
- 23.Bhandari V, Wang C, Rinder C, Rinder H. Hematologic profile of sepsis in neonates: neutrophil CD64 as a diagnostic marker. Pediatrics. 2008;121:129–134. doi: 10.1542/peds.2007-1308. [DOI] [PubMed] [Google Scholar]
- 24.Buhimschi CS, Dulay AT, Abdel-Razeq S, Zhao G, Lee S, Hodgson EJ, Bhandari V, Buhimschi IA. Fetal inflammatory response in women with proteomic biomarkers characteristic of intra-amniotic inflammation and preterm birth. BJOG. 2009;116:257–267. doi: 10.1111/j.1471-0528.2008.01925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ACOG Committee Opinion No. 348, November 2006: Umbilical cord blood gas and acid-base analysis. Obstet Gynecol. 2006;108:1319–1322. doi: 10.1097/00006250-200611000-00058. [DOI] [PubMed] [Google Scholar]
- 26.Synnes AR, Macnab YC, Qiu Z, Ohlsson A, Gustafson P, Dean CB, Lee SK. Neonatal intensive care unit characteristics affect the incidence of severe intraventricular hemorrhage. Med Care. 2006;44:754–759. doi: 10.1097/01.mlr.0000218780.16064.df. [DOI] [PubMed] [Google Scholar]
- 27.Heuchan AM, Evans N, Henderson Smart DJ, Simpson JM. Perinatal risk factors for major intraventricular haemorrhage in the Australian and New Zealand Neonatal Network, 1995–97. Arch Dis Child Fetal Neonatal Ed. 2002;86:F86–90. doi: 10.1136/fn.86.2.F86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paul DA, Sciscione A, Leef KH, Stefano JL. Caesarean delivery and outcome in very low birthweight infants. Aust N Z J Obstet Gynaecol. 2002;42:41–45. doi: 10.1111/j.0004-8666.2002.00047.x. [DOI] [PubMed] [Google Scholar]
- 29.Cuestas E, Bas J, Pautasso J. Sex differences in intraventricular hemorrhage rates among very low birth weight newborns. Gend Med. 2009;6:376–382. doi: 10.1016/j.genm.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Perlman JM. The relationship between systemic hemodynamic perturbations and periventricular-intraventricular hemorrhage – a historical perspective. Semin Pediatr Neurol. 2009;16:191–199. doi: 10.1016/j.spen.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Kassal R, Anwar M, Kashlan F, Smulian J, Hiatt M, Hegyi T. Umbilical vein interleukin-6 levels in very low birth weight infants developing intraventricular hemorrhage. Brain Dev. 2005;27:483–487. doi: 10.1016/j.braindev.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Heep A, Behrendt D, Nitsch P, Fimmers R, Bartmann P, Dembinski J. Increased serum levels of interleukin 6 are associated with severe intraventricular haemorrhage in extremely premature infants. Arch Dis Child Fetal Neonatal Ed. 2003;88:F501–504. doi: 10.1136/fn.88.6.F501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lavrijsen SW, Uiterwaal CS, Stigter RH, de Vries LS, Visser GH, Groenendaal F. Severe umbilical cord acidemia and neurological outcome in preterm and full-term neonates. Biol Neonate. 2005;88:27–34. doi: 10.1159/000084096. [DOI] [PubMed] [Google Scholar]
- 34.Beverley DW, Chance GW, Coates CF. Intraventricular haemorrhage – timing of occurrence and relationship to perinatal events. Br J Obstet Gynaecol. 1984;91:1007–1013. doi: 10.1111/j.1471-0528.1984.tb03679.x. [DOI] [PubMed] [Google Scholar]
- 35.Vergani P, Patane L, Doria P, Borroni C, Cappellini A, Pezzullo JC, Ghidini A. Risk factors for neonatal intraventricular haemorrhage in spontaneous prematurity at 32 weeks gestation or less. Placenta. 2000;21:402–407. doi: 10.1053/plac.1999.0499. [DOI] [PubMed] [Google Scholar]
- 36.Green DW, Hendon B, Mimouni FB. Nucleated erythrocytes and intraventricular hemorrhage in preterm neonates. Pediatrics. 1995;96:475–478. [PubMed] [Google Scholar]
- 37.Dulay AT, Buhimschi IA, Zhao G, Luo G, Abdel-Razeq S, Cackovic M, Rosenberg VA, Pettker CM, Thung SF, Bahtiyar MO, et al. Nucleated red blood cells are a direct response to mediators of inflammation in newborns with early-onset neonatal sepsis. Am J Obstet Gynecol. 2008;198:426, e421–429. doi: 10.1016/j.ajog.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarkar S, Kaplan C, Wiswell TE, Spitzer AR. Histological chorioamnionitis and the risk of early intraventricular hemorrhage in infants born < or = 28 weeks gestation. J Perinatol. 2005;25:749–752. doi: 10.1038/sj.jp.7211399. [DOI] [PubMed] [Google Scholar]
- 39.Furukawa S, Sameshima H, Ikenoue T. Circulatory disturbances during the first postnatal 24 hours in extremely premature infants 25 weeks or less of gestation with histological fetal inflammation. J Obstet Gynaecol Res. 2008;34:27–33. doi: 10.1111/j.1447-0756.2007.00678.x. [DOI] [PubMed] [Google Scholar]
- 40.Mehta R, Nanjundaswamy S, Shen-Schwarz S, Petrova A. Neonatal morbidity and placental pathology. Indian J Pediatr. 2006;73:25–28. doi: 10.1007/BF02758255. [DOI] [PubMed] [Google Scholar]
- 41.McPherson RJ, Juul SE. Erythropoietin for infants with hypoxic-ischemic encephalopathy. Curr Opin Pediatr. 2010;22:139–145. doi: 10.1097/MOP.0b013e328336eb57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fauchere JC, Dame C, Vonthein R, Koller B, Arri S, Wolf M, Bucher HU. An approach to using recombinant erythropoietin for neuroprotection in very preterm infants. Pediatrics. 2008;122:375–382. doi: 10.1542/peds.2007-2591. [DOI] [PubMed] [Google Scholar]