Abstract
Recently, a genome-wide association scan was completed in the IRAS (Insulin Resistance Atherosclerosis Study) Family Study (IRASFS) Hispanic-American cohort. Multiple single-nucleotide polymorphisms (SNPs) in the G-protein signaling 6 (RGS6) gene were found to be associated with adiposity phenotypes. RGS6 has shown downstream antagonistic interplay with opioid receptors, targets of fatty/sugary food agonists. The possibility that RGS6 promotes tolerance and tachyphylaxis among the opioid receptor is a plausible pathway for overconsuming fat/sugar-laden food. Therefore, we hypothesized that RGS6 variants are associated with intake of fatty/sugary foods. In 932 Hispanics from San Antonio and San Luis Valley, CO, the following dietary intake variables were assessed using the Block Brief 2000 food frequency questionnaire: total calories, total fat, % calories from fat, % calories from saturated fat, protein, % calories from protein, carbohydrates, % calories from carbohydrates, and daily frequency of servings of fats/oils/sweets. We tested for association between 23 SNPs in RGS6 and dietary intake using a variance components measured genotype approach. All models were adjusted for gender, recruitment site, admixture, BMI, and age. Using an additive genetic model, rs1402064 was associated with higher intake of fats/oils/sweets, total calories, total fat and saturated fat (P = 0.0007, 0.026, 0.023, and 0.024). SNPs rs847330 and rs847354 were associated with higher intake of fats/oils/sweets (P = 0.002 and 0.018), total fat (P = 0.040 and 0.048) and saturated fat (P = 0.044 and 0.041). Finally, rs769148 was associated with higher intake of fats/oils/sweets (P = 0.002). RGS6 is a new candidate gene for adiposity traits that may be associated with a behavioral tendency toward fat-laden food intake.
INTRODUCTION
Recently, a genome-wide association study (n = 229) and follow-up study in the IRAS (Insulin Resistance Atherosclerosis Study) Family Study (IRASFS) Hispanic cohort (n = 1,190) identified multiple single-nucleotide polymorphisms (SNPs) in the regulator of G-protein signaling 6 (RGS6) gene associated with BMI, visceral adipose tissue area and visceral-to-subcutaneous fat ratio, as measured by computed tomography scanning (associated at P < 1 × 10−3 (1)).
RGS6 is a regulator of G-protein signaling, and a member of the G7 superfamily (2), shown to have an interesting interplay with opioid receptors (G-protein coupled receptors). Under stress, cortisol levels go up and provoke inflammatory responses, but also promote negative feedback (3). Opioid receptors are normally part of this negative feedback, as they promote feelings of well-being, as well as inhibition of cortisol secretion. RGS6 acts downstream of opioid receptors to promote tachyphylaxis and tolerance. RGS6 binds active α-subunits to promote slow GTPase activity and sequesters them, slowing formation of ready G-protein coupled receptors. Thus, RGS6 modulates the opioid response to any stimulus. Fatty and sugary foods also provoke an opioid response (3), and increased preference for such foods are noted in mice, rats, and humans subject to chronic stress (4). Thus, we find it possible that RGS6 gene variation and/or polymorphisms associated with adiposity traits could also be associated with eating or perhaps addictive behaviors. Similar associations have been found between dietary behaviors and other obesity genes, such as FTO (5–8) and MC4R (9). We hypothesize that given known function and observed prior association, RGS6 will be associated with behavioral variables and adiposity in the IRASFS Hispanic population.
METHODS AND PROCEDURES
Subjects
The IRASFS was designed to explore genetic and epidemiologic contributions to abdominal adiposity and glucose homeostasis traits among Hispanic and African Americans using a family-based design (10). Large families were recruited from 2000 to 2002 at study centers in San Antonio, TX (Hispanics); San Luis Valley, CO (Hispanics); and Los Angeles, CA (African Americans); with probands identified from both the parent study IRAS (11) as well as the general population. Families were recruited based upon family size, not disease or phenotype status. A follow-up examination was conducted ~5 years after the baseline examination, during which dietary intake data were collected (described below). As the RGS6 genotyping was only done in Hispanics (1) the current study only includes the Hispanic cohort of the IRASFS. Individuals with diabetes remained in our dataset for analysis. The institutional review boards at each center approved the protocol; and informed consent was provided by each subject.
Dietary intake data
Dietary variables were derived from a Block Brief 2000 food frequency questionnaire (12) which was administered by interview at the follow-up visit. This questionnaire contains a 70-item food list and is designed to provide estimates of usual and customary dietary intake. The food list for this questionnaire was developed from the National Health and Nutrition Examination Survey III dietary recall data. The nutrient database was developed from the USDA Nutrient Database for Standard Reference. Individual portion size was asked, and pictures were provided in a standardized manner. Food frequency questionnaires were edited initially at the clinical sites and additional editing and quality control checks, including internal consistency and range, were conducted by NutritionQuest using the Block/DietSys edit check program. To address our hypothesis, we examined nutrient variables that marked fatty and sugary food choices, such as: total calories, total fat, % calories from fat, saturated fat, % calories from saturated fat, carbohydrates, % calories from carbohydrates, frequency of servings of fats/oils/sweets. We examined protein intake (total, and as % calories from protein) as a control variable.
To investigate other potential addictive behaviors, we examined percent of calories from alcohol that was derived from the Block Brief 2000 food frequency questionnaire. We also analyzed average daily alcohol consumption (i.e., drinks per day), and average daily cigarette use (i.e., packs per day), as collected via IRASFS questionnaires.
SNP selection and genotyping
SNP selection for the RGS6 gene region has been described previously (1). Briefly, the gene region was identified through a three step process. First, a genome-wide association study was conducted in a nondiabetic subset of the San Antonio sample (n = 229 from 34 families) from the IRASFS. SNPs that showed initial evidence of association with adiposity phenotypes at the P < 0.001, were selected for follow-up genotyping in the entire IRASFS Hispanic-American cohort (n = 1,190). Based on these results, four genic regions, including RGS6, were identified for additional SNP genotyping. SNP selection was restricted to the linkage disequilibrium (LD) block defined by the CEPH sample in the International HapMap project (Version 27, phase II and III) containing the associated SNP or SNPs. This resulted in a total of 23 SNPs in RGS6 region selected for analysis (1). Maximum likelihood estimates of allele frequencies were computed using the largest set of unrelated Hispanic-American individuals; the genotypes were also tested for departure from Hardy–Weinberg equilibrium expectations using a χ2 goodness-of-fit test and no evidence for departure was found (1). These 23 SNPs from the prior genome-wide association study and follow-up study (1) were tested for association with macronutrient intake variables in the 932 IRASFS Hispanic-American subjects who provided dietary intake information at the follow-up visit. Haploview version 4.1 was used to construct the SNP-based LD map (13).
Statistical analysis
A variance component measured genotype model as implemented in the software Simultaneous Oligogenic Linkage Analysis Routines was used to test for associations among the SNPs within RGS6 region and the macronutrient intake variables (14). The additive genetic model was assumed for all tests of SNP association as it is the most robust in the presence of either dominant or recessive transmission models. All models were adjusted for age, gender, admixture (described below), clinic site, and BMI. BMI was included as a covariate as a result of univariate association and prior assumptions that body mass would influence eating habits and/or their recall. Macronutrients (total fat, saturated fat, carbohydrates, protein) were adjusted for total energy intake using the residual method. In brief, the residuals from the regression model with total caloric intake as the independent variable and absolute nutrient intake as the dependent variable are added to a constant, which is the expected nutrient intake for the mean caloric intake of the study population, to calculate “calorie-adjusted” nutrient intakes (15). The macronutrient intake variables were transformed as follows to best approximate conditional normality and homogeneity of variance, conditional on the above covariates. Total calories, total fat, carbohydrates, and saturated fat were log transformed; frequency of fats/oils/sweets was transformed using a square root transformation. The alcohol consumption (drinks/day), % of calories from alcohol, and smoking (packs/day) variables were log transformed after adding one (i.e., log (y + 1)). To test for the potential modifying effects of gender, age, and BMI on the SNP associations, the corresponding interaction terms (i.e., SNP × gender, SNP × age, SNP × BMI were entered into the variance component measured genotype model.
As part of the IRASFS genome-wide association study publication (1), a principal component analysis was computed using a set of 80 ancestry informative markers and the principal component that explained genetic variation along the Hispanic ancestry axis was including as a covariate in the above variance component models.
In this paper, a total of 23 SNPs in the RGS6 gene region are being tested for association with the macronutrient intake variables and some adjustment for multiple comparisons is necessary. However, these 23 SNPs are not independent as they exhibit modest, yet varying degrees of LD (Figure 1, R2 statistics shown). Thus, to more accurately account for the number of independent tests, a principal component analysis was computed on these 23 SNPs. Because of the correlations among these SNPs (i.e., LD), 13 principal components explained >99% of the genetic variation in these SNPs. Thus, for each phenotype 13 independent dimensions were assumed and a conservative Bonferroni threshold for significance can be defined as P value = 0.0038 (0.05/13).
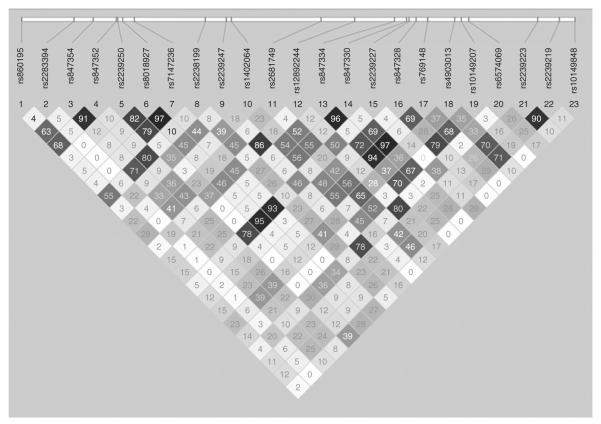
Figure 1.
Relative single-nucleotide polymorphism (SNP) position map and linkage disequilibrium plot of the all 23 genotyped SNPs in RGS6 which represents a ~100 kb region surrounding the original two SNPs from the genome-wide association study analysis, rs2239247 and rs2239227 (1) (above). The pairwise R2 statistics are given in the Gabriel plot (below).
RESULTS
The demographic and dietary intake characteristics of the IRASFS Hispanic cohort are summarized in Table 1. This sample was 61.9% female, with a mean age of 47.8 ± 14.1 years, and mean BMI of 29.8 ± 6.8 kg/m2 (Table 1). Eighty eight pedigrees were analyzed with a mean of 11.3 ± 6.8 individuals in each family. The pairwise R2 statistics are given in the Gabriel plot (Figure 1) for the ~100 kb gene region.
Table 1.
Demographic and dietary intake description of the Hispanic cohort in the IRAS (Insulin Resistance Atherosclerosis Study) Family Study
| Characteristic | Mean ± Standard Deviation or % |
|---|---|
| Total (n) | 932 |
| Gender (% female) | 61.9% |
| Age (years) | 47.8 ± 14.1 |
| BMI (kg/m2) | 29.8 ± 6.2 |
| Calories (kcal/day) | 1,816.6 ± 943.4 |
| Total fat (g/day)a | 75.2 ± 42.6 |
| Carbohydrate (g/day)a | 210.7 ± 34.6 |
| Saturated fat (g/day)a | 27.5 ± 5.0 |
| Protein (g/day)a | 75.2 ± 16.3 |
| Percent of calories from fat | 36.9 ± 5.8 |
| Percent of calories from carbohydrates | 46.5 ± 7.4 |
| Percent of calories from protein | 16.6 ± 3.4 |
| Percent of calories from sweets | 11.8 ± 9.4 |
| Servings of fats/oils/sweets (per day) | 2.1 ± 1.2 |
Adjusted for total energy intake using the residual method (15).
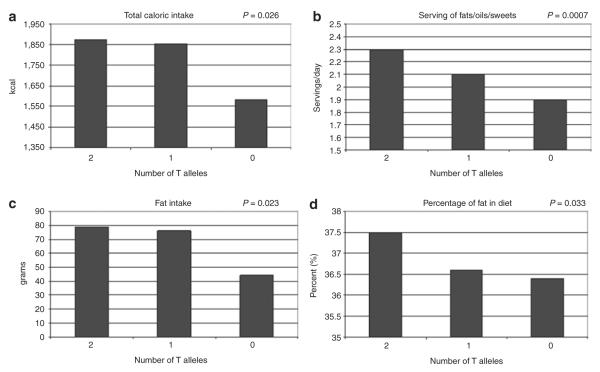
Adjusting for age, gender, clinic site, admixture, and BMI, RGS6 SNPs rs1402064 (P = 0.0007), rs847330 (P = 0.002), and rs769148 (P = 0.002) were all associated with the frequency of servings of fats/oils/sweets under an additive model (Table 2), with P values less than the Bonferroni adjusted threshold of P = 0.0038. SNP rs1402064 demonstrated the most consistent association trends with the dietary intake and drinking behaviors. In addition to frequency of servings of fats/oils/sweets, rs1402064 was associated with total caloric intake (P = 0.026), total fat intake (P = 0.023), saturated fat intake (P = 0.024), carbohydrate intake (P = 0.018), percent of calories from fat (P = 0.033) (Table 2), number of drinks per day (P = 0.034), and percentage of total calories from alcohol (P = 0.027) (Table 3). Covariate unadjusted means by genotype in rs1402064 for total caloric intake were 1874.7 ± 980 for individuals with two T alleles, 1855.75 ± 982 for one T allele, and 1583.85 for no T alleles; for frequency of servings of fats/oils/sweets means by genotypes were 2.3 ± 1.1, 2.1 ± 1.2, 1.89 ± 1.2; for dietary fat means by genotype were 78.9 ± 45.1, 76.2 ± 44.1, and 64.5 ± 36.4; and for percentage of calories from fat means by genotype were 37.5 ± 5.7, 36.6 ± 5.8, and 36.3 ± 5.4 (Figure 2).
Table 2.
Association between regulator of G-protein signaling 6 (RGS6) single-nucleotide polymorphisms (SNPs) and dietary intake
| Nutrient densities |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Minor allele frequency |
Total calories (kcal) |
Frequency of fats/oils/ sweets (servings/day) |
Total fat (g)a,b |
Carbohydrates (g)a,b |
Protein (g)a,b |
Saturated fat (g)a,b |
Fat (% of calories) |
Protein (% of calories) |
Carbohydrates (% of calories) |
| rs860195 | 0.39 | 0.136 | 0.332 | 0.145 | 0.132 | 0.238 | 0.164 | 0.806 | 0.121 | 0.204 |
| rs2283394 | 0.08 | 0.597 | 0.923 | 0.440 | 0.748 | 0.492 | 0.475 | 0.605 | 0.860 | 0.781 |
| rs847354 | 0.42 | 0.061 | 0.018 | 0.040 | 0.068 | 0.068 | 0.041 | 0.160 | 0.554 | 0.879 |
| rs847352 | 0.42 | 0.137 | 0.082 | 0.112 | 0.128 | 0.180 | 0.123 | 0.371 | 0.664 | 0.636 |
| rs2239250 | 0.14 | 0.137 | 0.082 | 0.946 | 0.978 | 0.767 | 0.978 | 0.365 | 0.881 | 0.636 |
| rs8018927 | 0.12 | 0.980 | 0.689 | 0.463 | 0.482 | 0.736 | 0.403 | 0.090 | 0.758 | 0.356 |
| rs7147236 | 0.11 | 0.516 | 0.312 | 0.416 | 0.426 | 0.679 | 0.363 | 0.078 | 0.772 | 0.155 |
| rs2238199 | 0.47 | 0.473 | 0.368 | 0.050 | 0.066 | 0.074 | 0.067 | 0.441 | 0.630 | 0.754 |
| rs2239247c | 0.23 | 0.064 | 0.037 | 0.432 | 0.245 | 0.493 | 0.359 | 0.145 | 0.911 | 0.696 |
| rs1402064 | 0.37 | 0.025 | 0.0007 d | 0.023 | 0.018 | 0.062 | 0.024 | 0.033 | 0.512 | 0.108 |
| rs2681749 | 0.42 | 0.257 | 0.030 | 0.266 | 0.260 | 0.326 | 0.293 | 0.984 | 0.716 | 0.532 |
| rs12892244 | 0.23 | 0.337 | 0.186 | 0.678 | 0.523 | 0.778 | 0.589 | 0.239 | 0.925 | 0.399 |
| rs847334 | 0.28 | 0.097 | 0.004 | 0.078 | 0.112 | 0.206 | 0.072 | 0.153 | 0.397 | 0.468 |
| rs847330 | 0.29 | 0.059 | 0.002 d | 0.048 | 0.064 | 0.138 | 0.044 | 0.198 | 0.339 | 0.818 |
| rs2239227c | 0.11 | 0.581 | 0.311 | 0.264 | 0.312 | 0.529 | 0.225 | 0.038 | 0.671 | 0.385 |
| rs847328 | 0.23 | 0.239 | 0.022 | 0.273 | 0.176 | 0.424 | 0.307 | 0.352 | 0.790 | 0.621 |
| rs769148 | 0.30 | 0.069 | 0.002 d | 0.058 | 0.072 | 0.161 | 0.053 | 0.188 | 0.356 | 0.786 |
| rs4903013 | 0.49 | 0.318 | 0.229 | 0.170 | 0.339 | 0.300 | 0.192 | 0.631 | 0.480 | 0.106 |
| rs10149207 | 0.27 | 0.541 | 0.069 | 0.550 | 0.489 | 0.755 | 0.558 | 0.764 | 0.619 | 0.870 |
| rs6574069 | 0.26 | 0.892 | 0.689 | 0.700 | 0.844 | 0.661 | 0.720 | 0.296 | 0.457 | 0.316 |
| rs2239223 | 0.48 | 0.844 | 0.593 | 0.833 | 0.964 | 0.934 | 0.987 | 0.293 | 0.121 | 0.182 |
| rs2239219 | 0.49 | 0.144 | 0.079 | 0.091 | 0.238 | 0.156 | 0.092 | 0.823 | 0.511 | 0.753 |
| rs10149848 | 0.12 | 0.619 | 0.517 | 0.739 | 0.449 | 0.912 | 0.758 | 0.892 | 0.049 | 0.781 |
All P values are generated under an additive genetic model, adjusting for age, gender, clinic site, admixture, and BMI. Boldface values are statistically significant at 0.05 level.
Total calories, total fat, carbohydrates, and saturated fat were log transformed; Frequency of fats/oils/sweets was transformed using square root.
Adjusted for total energy intake using the residual method.
Both SNPs were found through the initial GWAS of adiposity traits (1).
P values meet the conservative Bonferroni multiple correction threshold of P = 0.0038.
Table 3.
Association between regulator of G-protein signaling 6 (RGS6) single-nucleotide polymorphisms (SNPs) and alcohol and smoking behaviors
| Alcohol intake |
|||
|---|---|---|---|
| SNPs | Alcohol consumption (drinks/dayc) |
% of calories from alcoholb,c |
Current smoking (packs/dayc) |
| rs860195 | 0.255 | 0.041 | 0.635 |
| rs2283394 | 0.520 | 0.621 | 0.775 |
| rs847354 | 0.464 | 0.093 | 0.799 |
| rs847352 | 0.428 | 0.148 | 0.799 |
| rs2239250 | 0.690 | 0.634 | 0.535 |
| rs8018927 | 0.408 | 0.898 | 0.866 |
| rs7147236 | 0.430 | 0.967 | 0.691 |
| rs2238199 | 0.923 | 0.267 | 0.781 |
| rs2239247 | 0.025 | 0.058 | 0.464 |
| rs1402064 | 0.034 | 0.027 | 0.903 |
| rs2681749 | 0.781 | 0.640 | 0.781 |
| rs12892244 | 0.093 | 0.337 | 0.611 |
| rs847334 | 0.667 | 0.972 | 0.317 |
| rs847330 | 0.417 | 0.467 | 0.295 |
| rs2239227 | 0.339 | 0.638 | 0.753 |
| rs847328 | 0.649 | 0.802 | 0.528 |
| rs769148 | 0.328 | 0.416 | 0.489 |
| rs4903013 | 0.464 | 0.640 | 0.153 |
| rs10149207 | 0.953 | 0.576 | 0.717 |
| rs6574069 | 0.062 | 0.239 | 0.081 |
| rs2239223 | 0.278 | 0.212 | 0.040 |
| rs2239219 | 0.630 | 0.577 | 0.139 |
| rs10149848 | 0.370 | 0.215 | 0.399 |
P values lower than P = 0.05 are presented in boldface.
All P values were generated under an additive genetic model, adjusting for age, gender, clinic site, admixture, and BMI.
Derived from the Block Brief food frequency questionnaire.
Phenotypes were log (y + 1) transformed to normalize the distributions.
Figure 2.
Unadjusted, genotypic means are shown graphically in each of the four dietary intake graphs above for single-nucleotide polymorphism rs1402064: (a) Total caloric intake/day (kcal); (b) Frequency serving of fats, oils, or sweets/day; (c) Total fat intake residual-adjusted for total energy intake; and (d) Percent of calories from fat. The P values represent the association between the number of T alleles of rs1402064 and each of the four dietary intake variables under the additive model adjusted for age, gender, clinic site, admixture, and BMI.
Additionally, rs847354 and rs847330 were more modestly associated with the dietary behaviors: frequency fats/oils/sweets (P = 0.018 and P = 0.002, respectively), total fat (P = 0.04 and 0.048, respectively), and saturated fat (P = 0.041 and 0.044, respectively) (Table 2). SNPs rs769148, rs2239247, and rs847238 were associated with frequency of fats/oils/sweets (servings/day), rs22382199 was associated with total fat, and rs2239227 was associated with percentage of fat in the diet. One association was seen between percent of calories from protein and rs1014948 (P = 0.049), but no other associations were observed between the protein variables and RGS6 SNPs; and rs1014948 was not associated with any other dietary intake behavior. SNPs rs860195 and rs2239247 were associated with alcohol consumption as a percentage of total calories and number of drinks/day (P = 0.041 and 0.025, respectively). Rs2239223 was associated with number of cigarette packs/day (P = 0.040). No statistically significant interactions were seen.
DISCUSSION
In a Hispanic-American sample, we found that dietary intake of fat and fatty/sugary foods is associated with variation in RGS6, a novel candidate gene for adiposity (1). These results support the hypothesis that RGS6 may be involved in obesity development by potentially affecting the intake of palatable energy-dense foods. This predisposition toward high caloric intake, fat intake, and frequency of consumption of fatty/sweet food reveals a possible interesting genetic influence on behavior that should be examined in other populations.
RGS6 is widely expressed in a range of tissue and cell types, among them cardiac, bladder, ovarian, neuronal (including spinal column and retina), platelet, and brain (16–21). In the rodent brain, RGS6 is expressed in areas including the hypothalamus and those involved in reward, smell, taste, and impulsivity, such as the pons-medulla, striatum, medial habenula, and olfactory bulb (22). Both the aforementioned FTO and MC4R are likewise expressed in the hypothalamus and appear consistent with an important role for central nervous system processes in weight regulation (22), satiety, and energy homeostasis (6,23,24). The RGS6 gene is one of the R7 subfamily of regulators of G-protein signaling, which has slow-acting GTPase activity (3,23). RGS6 may bind to the active α-subunits of G-proteins to increase their sequestration, slowing reformation of ready G-proteins at G-protein coupled receptors (2), demonstrating a mechanism for tolerance.
Food preference, like addiction behavior, is complex. There are multiple oral and postoral signals involved in the biochemistry of behavior; opioid receptors appear to be a major one (24–26) with multiple possible mechanisms. First, there is a strong connection between stress and diet-induced obesity. Rodents and humans show increased secretion of cortisol and obesogenic change in feeding and eating behavior when subjected to chronic or social stress, prior caloric restriction, or fatty food choice (3,27–29). Cortisol causes opioid release to protect the organism from the negative effects of extreme or prolonged stress, which (in addition to the psychological effects) inhibits further cortisol secretion; palatable foods (such as those with a high sugar or fat content) likewise cause opioid release. Second, drug manipulation of opioid receptors has effects on food palatability. Pharmacologic manipulation with naloxene (an opioid antagonist) alters fat palatability in mammal models through dopamine activation (26). It is plausible that RGS6 polymorphism/s alter the expression or function of the gene, favoring increased consumption of fatty foods (and also obesity) due to promotion of opioid receptor tolerance.
The consistency of associations across a number of dietary behaviors after adjustment for total caloric intake is consistent with our hypothesis of a genetically determined predisposition toward fat and sugar-laden food. Additional associations with carbohydrates were observed, but these observations were not as consistently associated across the residual-adjusted and nutrient density models as the fat intake variables. This lack of consistency with the total carbohydrates variable supports our conclusion that fat/simple carbohydrates are the true association. Our models were additionally adjusted for BMI, suggesting that the associations are independent of overall body mass, which may, on a cross-sectional basis, appear to influence reported or actual macronutrient intake. Associations between RGS6 variants and alcohol intake were also seen, adding further weight to the proposed mechanistic involvement of this obesity candidate gene in addiction-related behavior. Given the overall association pattern generated by testing multiple macronutrient/behavioral components, our results are suggestive that variation in the RGS6 gene predisposes to a preference of fatty foods.
Potential pitfalls to this analysis include three possible areas. First, previous biological information suggested that RGS6 operates in response to stress (3). Unfortunately, markers of stress are unavailable in the IRASFS. Second, it is important to note the SNPs presented here are all intronic, and non-coding (1); thus likely to be in LD with the relevant locus. Third, only three SNPs demonstrate association at the Bonferroni adjusted threshold with a single phenotype, and P values presented in the paper are unadjusted for multiple comparisons; whereas many other observations, while consistent, were only nominally significant at the P < 0.05 level. Thus, we believe the RGS6 SNPs that we typed are likely a regional marker of the functional/important variation not captured completely in this study.
In summary, RGS6 represents a candidate gene region for adiposity that appears to act through increased dietary preference of fat and sugar-laden food. Continued follow-up work on the variation could reveal the function by which our marked variation is operating.
ACKNOWLEDGMENTS
This research was supported in part by: National Institutes of Health grants HL060894, HL060931, HL060944, HL061019, and HL061210; the General Clinical Research Centers Program, National Center for Research Resources grant M01RR00069 and the Diabetes Endocrinology Research Center Grant DDK063491, and the Wake Forest University Health Sciences Center for Public Health Genomics.
Footnotes
DISCLOSURE The authors declared no conflict of interest.
REFERENCES
- 1.Norris JM, Langefeld CD, Talbert ME, et al. Genome-wide association study and follow-up analysis of adiposity traits in Hispanic Americans: the IRAS Family Study. Obesity (Silver Spring) 2009;17:1932–1941. doi: 10.1038/oby.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garzón J, López-Fando A, Sánchez-Blázquez P. The R7 subfamily of RGS proteins assists tachyphylaxis and acute tolerance at mu-opioid receptors. Neuropsychopharmacology. 2003;28:1983–1990. doi: 10.1038/sj.npp.1300263. [DOI] [PubMed] [Google Scholar]
- 3.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Warne JP. Shaping the stress response: interplay of palatable food choices, glucocorticoids, insulin and abdominal obesity. Mol Cell Endocrinol. 2009;300:137–146. doi: 10.1016/j.mce.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 5.Cecil JE, Tavendale R, Watt P, Hetherington MM, Palmer CN. An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med. 2008;359:2558–2566. doi: 10.1056/NEJMoa0803839. [DOI] [PubMed] [Google Scholar]
- 6.Speakman JR, Rance KA, Johnstone AM. Polymorphisms of the FTO gene are associated with variation in energy intake, but not energy expenditure. Obesity (Silver Spring) 2008;16:1961–1965. doi: 10.1038/oby.2008.318. [DOI] [PubMed] [Google Scholar]
- 7.Timpson NJ, Emmett PM, Frayling TM, et al. The fat mass- and obesity-associated locus and dietary intake in children. Am J Clin Nutr. 2008;88:971–978. doi: 10.1093/ajcn/88.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wardle J, Carnell S, Haworth CM, et al. Obesity associated genetic variation in FTO is associated with diminished satiety. J Clin Endocrinol Metab. 2008;93:3640–3643. doi: 10.1210/jc.2008-0472. [DOI] [PubMed] [Google Scholar]
- 9.Pichler M, Kollerits B, Heid IM, et al. Association of the melanocortin-4 receptor V103I polymorphism with dietary intake in severely obese persons. Am J Clin Nutr. 2008;88:797–800. doi: 10.1093/ajcn/88.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henkin L, Bergman RN, Bowden DW, et al. Genetic epidemiology of insulin resistance and visceral adiposity. The IRAS Family Study design and methods. Ann Epidemiol. 2003;13:211–217. doi: 10.1016/s1047-2797(02)00412-x. [DOI] [PubMed] [Google Scholar]
- 11.Wagenknecht LE, Mayer EJ, Rewers M, et al. The insulin resistance atherosclerosis study (IRAS) objectives, design, and recruitment results. Ann Epidemiol. 1995;5:464–472. doi: 10.1016/1047-2797(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 12.Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology. 1990;1:58–64. doi: 10.1097/00001648-199001000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Barrett JC. Haploview: Visualization and analysis of SNP genotype data. Cold Spring Harb Protoc. 2009;2009 doi: 10.1101/pdb.ip71. pdb.ip71. [DOI] [PubMed] [Google Scholar]
- 14.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 Suppl):1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. [DOI] [PubMed] [Google Scholar]
- 16.Berman DM, Wang Y, Liu Z, et al. A functional polymorphism in RGS6 modulates the risk of bladder cancer. Cancer Res. 2004;64:6820–6826. doi: 10.1158/0008-5472.CAN-04-1916. [DOI] [PubMed] [Google Scholar]
- 17.Chen FS, Shim H, Morhardt D, et al. Functional redundancy of R7 RGS proteins in ON-bipolar cell dendrites. Invest Ophthalmol Vis Sci. 2010;51:686–693. doi: 10.1167/iovs.09-4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doupnik CA, Xu T, Shinaman JM. Profile of RGS expression in single rat atrial myocytes. Biochim Biophys Acta. 2001;1522:97–107. doi: 10.1016/s0167-4781(01)00342-6. [DOI] [PubMed] [Google Scholar]
- 19.Hurst JH, Mendpara N, Hooks SB. Regulator of G-protein signalling expression and function in ovarian cancer cell lines. Cell Mol Biol Lett. 2009;14:153–174. doi: 10.2478/s11658-008-0040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Z, Chatterjee TK, Fisher RA. RGS6 interacts with SCG10 and promotes neuronal differentiation. Role of the G gamma subunit-like (GGL) domain of RGS6. J Biol Chem. 2002;277:37832–37839. doi: 10.1074/jbc.M205908200. [DOI] [PubMed] [Google Scholar]
- 21.Zhang JH, Simonds WF. Copurification of brain G-protein beta5 with RGS6 and RGS7. J Neurosci. 2000;20:RC59. doi: 10.1523/JNEUROSCI.20-03-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gold SJ, Ni YG, Dohlman HG, Nestler EJ. Regulators of G-protein signaling (RGS) proteins: region-specific expression of nine subtypes in rat brain. J Neurosci. 1997;17:8024–8037. doi: 10.1523/JNEUROSCI.17-20-08024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerken T, Girard CA, Tung YC, et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318:1469–1472. doi: 10.1126/science.1151710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lam TK, Schwartz GJ, Rossetti L. Hypothalamic sensing of fatty acids. Nat Neurosci. 2005;8:579–584. doi: 10.1038/nn1456. [DOI] [PubMed] [Google Scholar]
- 25.Sclafani A. Psychobiology of food preferences. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S13–S16. doi: 10.1038/sj.ijo.0801905. [DOI] [PubMed] [Google Scholar]
- 26.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 27.Dallman MF, Akana SF, Laugero KD, et al. A spoonful of sugar: feedback signals of energy stores and corticosterone regulate responses to chronic stress. Physiol Behav. 2003;79:3–12. doi: 10.1016/s0031-9384(03)00100-8. [DOI] [PubMed] [Google Scholar]
- 28.Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun. 2005;19:275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Greeno CG, Wing RR. Stress-induced eating. Psychol Bull. 1994;115:444–464. doi: 10.1037/0033-2909.115.3.444. [DOI] [PubMed] [Google Scholar]