Abstract
Existing methods of cardiac gene delivery can be classified by the site of injection, interventional approach and type of cardiac circulation at the time of transfer. General criteria to assess the efficacy of a given delivery method include: global versus regional myocardial transduction, technical complexity and the pathophysiological effects associated with its use, delivery-related collateral expression and the delivery-associated inflammatory and immune response. Direct gene delivery (intramyocardial, endocardial, epicardial) may be useful for therapeutic angiogenesis and for focal arrhythmia therapy but with gene expression which is primarily limited to regions in close proximity to the injection site. An often unappreciated limitation of these techniques is that they are frequently associated with substantial systemic vector delivery. Percutaneous infusion of vector into the coronary arteries is minimally invasive and allows for transgene delivery to the whole myocardium. Unfortunately, efficiency of intracoronary delivery is highly variable and the short residence time of vector within the coronary circulation and significant collateral organ expression limit its clinical potential. Surgical techniques, including the incorporation of cardiopulmonary bypass with isolated cardiac recirculation, represent novel delivery strategies that may potentially overcome these limitations; yet, these techniques are complex with inherent morbidity that must be thoroughly evaluated before safe translation into clinical practice. Characteristics of the optimal technique for gene delivery include low morbidity, increased myocardial transcapillary gradient, extended vector residence time in the coronary circulation and exclusion of residual vector from the systemic circulation after delivery to minimize extracardiac expression and to mitigate a cellular immune response. This article is part of a Special Section entitled “Special Section: Cardiovascular Gene Therapy”.
Keywords: Cardiac gene therapy, Gene delivery, Cardiopulmonary bypass, Ex vivo perfusion
1. Introduction
Our knowledge of the molecular mechanisms in heart disease has increased dramatically in recent years. Therefore, new forms of treatment designed to target the underlying molecular processes affecting failing cardiomyocytes have significant potential. However, an important prerequisite for introducing cardiac gene therapy into clinical practice is the development of simple and efficient gene delivery techniques.
During the last two decades, we have witnessed the development of several experimental gene delivery strategies with potential therapeutic value for the transition from the preclinical phase into clinical trials. Yet, efforts at gene transfer will require solutions to several problems including those related to delivery of the vector to the target tissue, improved safety and efficacy, prevention of complications, creation of new delivery devices and techniques, and improved geographical specificity of gene delivery to areas of therapeutic interest while simultaneously minimizing systemic spillover. Although cardiac tissue-specific promoters may mitigate collateral organ gene expression, only truly cardiac-specific gene delivery methods can diminish the biodistribution of vector capsids to extracardiac organs by limiting antigen presenting cell exposure, providing another mechanism to limit the potential for a T-cell mediated immune response to the vector capsid. The most optimal gene-delivery system should be combined with an appropriate vector. Recombinant AAV vectors have rapidly evolved as tools for cardiac gene therapy. For numerous target diseases, they offer advantages over other viral vector systems. Multiple AAV serotypes have been isolated in recent years. Among all AAV serotypes, AAV2 vectors have been successfully used in several experimental approaches such as protection from ischemia/reperfusion injury, inotropic therapy, beneficial effects on neoangiogenesis. From the most recent reports it appears that serotype 6 and 9 facilitate relatively stable cardiac gene expression and are superior to others in the heart, probably due to enhanced cellular internalization and nuclear uncoating in cardiomyocytes. Successful solutions to these and other challenges will undoubtedly help to achieve transmural, homogeneous, high-density cardiac gene transfer [1,2].
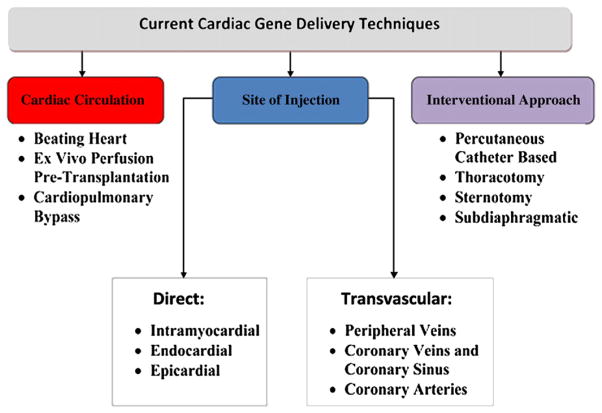
There are many published methods to transduce myocardium; yet, most of these approaches have led to limited transduction efficiency and have had limited efficacy in demonstrating adequacy for effective therapy of diseases involving the whole heart [3]. Existing methods of gene delivery can be classified by the site of injection, interventional approach and the physiology of the cardiac circulation (Fig. 1).
Fig. 1.
Cardiac gene delivery techniques.
In this review, we have tried to describe both the most common and innovative gene delivery methods and have further attempted to outline future developments in this exciting and dynamic field of cardiac gene therapy.
2. Direct gene delivery
Direct gene delivery methods have been utilized for at least two decades and some authors continue to reference and use them in their preclinical cardiac gene therapy studies. Many have also successfully been translated for use in clinical trials. The most relevant of them are usually classified as either an open technique, that is to include the surgical opening of the chest or a closed technique, i.e., transcutaneous or minimally invasive. Less invasiveness is desirable and can also be achieved via a subdiaphragmatic or thoracoscopic approach.
2.1. Intrapericardial injection
The rationale underlying intrapericardial gene delivery is related to the advantage of the anatomical connection between the pericardium and the myocardium, and the accessibility of the pericardial sac for percutaneous vector delivery [4] (Fig. 2a). Zhang et al. made a percutaneous puncture of the pericardium at the left costoxiphoid angle of the anterior chest with injection of Ad.CMV.lacZ. After three days, significant lacZ expression was observed in the epicardium, myocardium and endocardium of neonatal mice. The authors concluded that intrapericardial injection is an efficient technique to achieve transmural gene expression. Unfortunately, these results appear to be age-specific and are far less efficient in adult (rather than neonatal) animals. Furthermore, the group later found at two months that expression only persisted in atrial tissue and not in ventricles, the presumed target area to address the majority of cardiomyopathic diseases. Additionally, this method was associated with high levels of hepatic transduction [5].
Fig. 2.
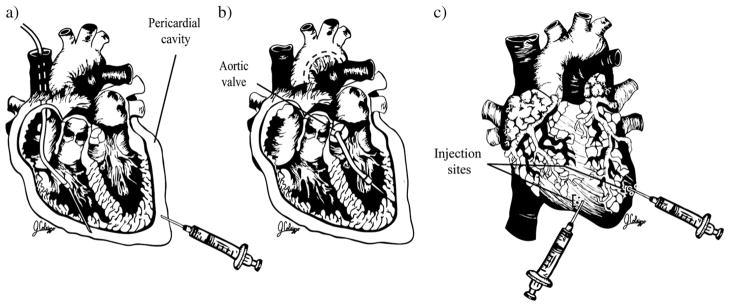
Direct methods of gene delivery. a. Epicardial. b. Endocardial. c. Intramyocardial.
Fromes et al. performed intrapericardial injections with a transdiaphragmatic approach, using adenovirus encoding β-galactosidase in rats. The staining observed was exclusively restricted to the pericardial cell layers; however, injecting a mixture of proteolytic enzymes with the virus led to an increase of transgene expression to 40% of the myocardium at day seven. Not surprisingly, using an adenoviral vector, the expression decreased to 0.5% at day 28. The authors also found positive β-galactosidase activity in distant organs [6]. March et al. used a hollow, helical-tipped penetrating catheter for vector delivery in the canine myocardium. The catheter was introduced percutaneously and advanced into the pericardial space through the apex of the right ventricle. All the animals tolerated the procedure without incident, demonstrating the feasibility of localized cardiac gene delivery via a catheter-based pericardial approach [7]. The administration of Ad2.CMV.LacZ into the pericardial sac produced extensive transfection of the visceral and parietal pericardium and doxycycline pretreatment increased this effect [8].
2.2. Endocardial injection
The feasibility of fluoroscopy-guided, percutaneous endocardial vector injection was demonstrated by Gwon et al. in the porcine heart [9] (Fig. 2b). Sanborn et al. used the same approach with a coaxial catheter for endocardial delivery of adenovirus encoding VEGF in the porcine myocardium. Regional VEGF expression was found to be significantly greater in targeted zones as compared with non-targeted zones [10]. Several authors believe that the electromechanical mapping-guided approach allows for better deployment of the tip of the catheter around areas of ischemia [11–13]. However, this system and the equipment needed to execute the procedure are quite complex and expensive.
In a porcine model, Grossman et al. compared endomyocardial and epicardial microsphere injection, finding that endomyocardial injection performed with the Stiletto system was associated with 43% microsphere retention, compared with 15% after epicardial injection. Reduction of injectate volume (10 μl) resulted in significantly improved retention compared to 100 μl injection, a typical volume used in clinical trials. These authors also found evidence of significant viral transfection in the liver and spleen after injection of adenovirus encoding β-galactosidase [14]. The study of Naimark et al. highlights the importance of enhancing the biocompatibility of the catheter for endocardial and epicardial gene delivery [15]. Despite the simplicity and safety of catheter-based endocardial and epicardial injection, there is an increased potential for complications associated with ventricular perforation, cardiac tamponade, endocardial thrombosis and intramyocardial hematoma, especially in patients with chronic ischemia who have thinned and scarred myocardium [9].
Along with demonstration of the feasibility of percutaneous endocardial gene transfer and gene expression, many questions still exist regarding this approach [10]. In patients with ischemic cardiomyopathy, should vector/transgene administration be targeted to ischemic areas alone or to “border zone” to stimulate collateral flow? What quantity represents the ideal dose and how many injections are necessary, especially given that the patients have multiple areas of ischemia?
2.3. Intramyocardial injection
The majority of successful preclinical studies have involved direct administration of vector. This technique allows for the application of a high concentration of vector directly at the target site. Several groups have demonstrated the feasibility of delivering transgenes to the heart via direct intramyocardial injection of plasmid DNA [16–19] (Fig. 2c). Although these studies have been encouraging because plasmid DNA may be expressed for up to six months by cardiomyocytes adjacent to the area of injection, estimates of the number of myocytes that can be transfected in vivo have been as low as 60 to 100 cells per injection [16]. This low efficiency has made it difficult to measure the physiological effects of gene expression in myocytes, making it unlikely that clinically significant effects will result [20]. The low transduction efficiency of plasmid DNA vectors lead to the search for improved gene transfer efficiency with direct injection of an adenovirus vector. Hearts transfected with an adenovirus vector containing the β-galactosidase gene showed significantly increased β-galactosidase enzymatic activity compared with hearts injected with β-galactosidase plasmid. Unfortunately, the gene expression persisted only one week after injection and it was noted to be coupled with an acute inflammatory response, which the authors considered to be related to the injury produced by direct injection as well as a cellular immune response against the adenovirus itself [20].
The studies of French et al. [21] demonstrated for the first time in a porcine model a number of important points relevant to this technique: 1) direct intramyocardial injection of replication-deficient adenovirus is 140,000 times more efficient than injection of an equal number of genome copies of recombinant plasmid DNA and can program recombinant gene expression in the cardiomyocytes of a large animal species; 2) the impact of this procedure on cardiac function appears to be negligible; 3) the amount of recombinant protein produced increases with the amount of virus; 4) the expression of recombinant genes following intramyocardial injection is similar in the left and right ventricles; and 5) the percentage of cardiomyocytes expressing β-galactosidase in the needle track adjacent to the injection site may be quite high but rarely are lacZ positive cells detected farther than 5 mm from the injection site [21]. In a study in dogs using adenovirus encoding chloramphenicol acetyl transferase, peak gene expression was noted at two days and decreased by an order of magnitude 14 days after direct single myocardial administration. In this study, there was no significant transduction of distant organs and no documented changes in global or regional LV function [22]. However, the feasibility of adenovirus-mediated gene transfer has been limited by the cellular immune response which causes myocardial inflammation and results in transient recombinant gene expression [23]. Svensson et al. showed that stable β-galactosidase expression can be achieved without evidence of myocardial inflammation or myocyte necrosis after substituting rAAV for adenovirus vector-mediated gene transfer [24]. Further, in an interesting study, Tomiyasu et al., succeeded in augmenting cardiac function in cardiomyopathic hamsters with heart failure by transfecting cardiac muscle with the β2-AR gene after direct intramuscular injection. Echocardiographic examination revealed that stroke volume and cardiac output were significantly elevated at two to four days after β2-AR gene transfer [25].
Rengo et al. studied myocardial gene transfer to post-myocardial infarct rats with intramyocardial direct injection. In order to stop beating of the heart (for 2.5 min), adenosine was injected and both ascending aorta and pulmonary artery were clamped. A volume of 4×1011 total particles of rAAV6-βARKct was injected in the LV free wall. The investigators found robust transgene expression in the LV at 12 weeks after delivery. An interesting finding was that βARKct (gene of interest) significantly improved cardiac contractility and reversed LV remodeling in this heart failure model [26].
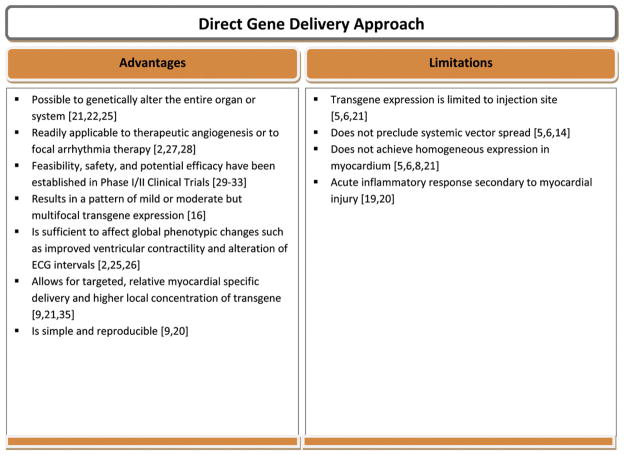
In summary, the direct gene delivery approach was the first among others that helped establish the therapeutic efficacy of cardiac gene therapy. Furthermore, the use of this method resulted in successful therapeutic myocardial angiogenesis [27], and focal treatment of cardiac arrhythmias through effects on cellular electrophysiology [28]; thus, making this platform very relevant and still widely used. Finally, this approach has been successfully utilized in some experimental models and Phase I/II clinical trials demonstrating its potential therapeutic relevance [29–35] (Fig. 3).
Fig. 3.
Advantages and limitations of direct gene delivery approach.
3. Transvascular gene delivery
There are many potential therapeutic targets that are inaccessible directly in vivo and, hence, require the transvascular administration of gene delivery vectors. A few candidate therapeutic applications include essential hypertension and pulmonary hypertension. Effective therapy in these diseases will likely require a gene delivery method capable of globally transducing the myocardium and selected other tissues and organs [36–38]. This paradigm is particularly valid in heart failure gene therapy where most authors agree that gene transfer should be as diffuse and homogeneous as possible [39,40].
3.1. Selective coronary catheterization with antegrade intracoronary delivery
To date, one of the most attractive and most common of gene delivery methods involves catheter-based, percutaneous infusion of vector into the coronary arteries (Fig. 4a). The benefits of this technique include its minimal invasiveness, the possibility of transgene delivery to all four myocardial chambers and the delivery of vector genomes using endovascular coronary catheterization—a procedure for which there is established clinical experience. Early reports using simple antegrade intracoronary delivery achieved very limited myocardial transduction efficiency [22,39,41,42]. The variability in transduction was due to a number of factors that include differences between animal species, biocompatibility of catheter and vector, different pharmacological agents used to permeabilize the vasculature, and vector-related variables such as vector serotype and titer [4]. Using intracoronary perfusion in explanted hearts, Donahue et al. reported highly effective gene transfer to the heart and identified critical parameters influencing the efficiency of intracoronary gene transfer. These included exposure time, high coronary flow rate and perfusion pressure, the use of crystalloid solution as opposed to whole blood, virus concentration, and temperature [37]. Several authors believe that aside from these factors, a major deficiency in intracoronary gene transfer is the short residence time of vector within the coronary circulation [39,43,44]. Attempts to resolve this shortcoming have resulted in a number of strategies. Logeart et al. showed that brief interruption of coronary flow is required to obtain significant myocyte transduction during single-pass delivery in the isolated rat heart model [42]. In other experiments, this laboratory obtained similar results in vivo, when adenoviruses were delivered downstream of an occluded artery and when occlusion was maintained for 30 s following adenovirus injection [42]. This procedure raises questions as to the potential role of ischemia in enhancing gene transfer by increasing microvascular permeability. The authors also demonstrated that selective catheterization of the coronary venous sinus, which was transiently occluded and retroperfused with saline during their procedures, increased the pressure inside post-capillary venules, which in turn improved gene transfer by increasing vector residence time in coronary vessels [39].
Fig. 4.
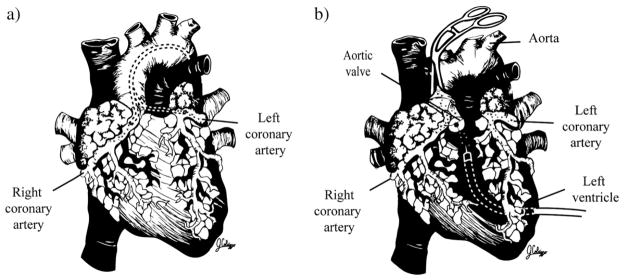
Transvascular gene delivery. a. Selective coronary catheterization with antegrade delivery. b. Nonselective intracoronary delivery.
Hayase et al. used the technique of antegrade intracoronary gene delivery with concomitant coronary venous blockade. The myocardium was preconditioned with 1 min of occlusion of the left anterior descending and circumflex arteries. Quantitative β-galactosidase analysis showed that gene expression was improved after selective coronary venous blockade [40]. Donahue et al. found that decreasing perfusate Ca2+ concentration or pre-treating with serotonin or bradykinin significantly decreased the exposure time necessary to achieve widespread transfection [45]. To prolong viral exposure time, Ding et al. pharmacologically induced transient cardiac arrest, simultaneously, as they occluded the aorta and obstructed venous return to the heart. Cardiac arrest of 2 min allowed for transfection of 18% of cells, whereas an extended time to 5 min resulted in a cardiac transfection of about 43% of cells [43].
Increasing perfusion pressure and flow augments myocardial expression perhaps by increasing the fenestration width between capillary endothelial cells, permitting better viral transendothelial transfer and enhancing virus–myocyte interaction [46,47]. Emani et al. used an apparatus consisting of a constant flow infusion pump with pressure transducer and examined the effects of altering intracoronary flow rate, while obtaining a seal between the catheter and the coronary lumen. The results indicate that efficient cardiac transgene expression is dependent upon the infusion flow rate and requires an intraluminal seal. Excessive flow rate is associated with myocardial injury [48].
3.2. Nonselective (indirect) intracoronary delivery
Hajjar et al. observed that adenovirus infusion into the left ventricle during brief aortic clamping results in efficient adenoviral gene transfer, perhaps due to the resulting high perfusion pressure inside the coronary vessels (Fig. 4b). Later, to achieve diffuse cardiac gene transfer in vivo, the authors developed a catheter-based technique in rodents. In this approach, a catheter was inserted in the LV apex and advanced beyond the aortic valve. A high concentration of an adenoviral preparation was then injected through the catheter while the aorta and pulmonary artery were cross-clamped, distal to the catheter tip for a period of 10 to 40 s. This method achieves grossly homogeneous transduction of cardiac myocytes throughout the left and right ventricles. By cross-clamping both the pulmonary artery and the aorta, the left ventricular end-diastolic pressure does not increase because blood return to the left ventricle is minimal. This method relies on the creation of a transcoronary myocardial perfusion gradient for vector delivery. This allows perfusion of the virus at relatively low downstream pressure, and the endocardium can be efficiently transfected. It is noteworthy, according to the authors, that aortic occlusion during aortic valvuloplasty is well tolerated in generally ill patients for periods of time comparable to those required for gene transfer in animal models [36,49]. Maurice et al. used a similar technique in the leporine model, whereby a catheter was placed into the LV chamber through the apex of the heart. The adenovirus solution was injected while the aorta was cross-clamped for 40 s. After six days, the authors found global myocardial β-galactosidase expression in both ventricles. However, three weeks later, β2-AR over-expression was minimal [50]. This method shunts the virus down the coronary arteries, and global transgene expression is possible; yet, there is a risk of systemic ischemia and acute LV overload during the aortic cross-clamping, and time must be limited [51]. This data was supported by Parsa et al., who showed in a rabbit model with cross-clamping of aorta, a significant decrease in dP/dtmax, which they explained, indicates a negative effect on cardiac contractility after aortic occlusion with elevation of afterload [46]. Kaspar et al. also used aortic and pulmonary artery cross-clamping for indirect coronary delivery of AAV encoding GFP. Gene expression was evaluated at four time points up to one year after vector delivery, revealing 20–32% transmural gene expression in the left ventricle [52]. Eckhart et al. showed that in vivo myocardial gene delivery in rabbits using this LV/cross-clamp delivery method of either the β2AR transgene or a gene encoding βARKct can enhance cardiac function in normal hearts as well as failing hearts [53]. Variations of this method may include clamping of the aorta without pulmonary artery occlusion, occluding the distal rather than ascending aorta, and the use of hypothermia to prolong cross-clamp times [4].
3.3. Selective coronary sinus or coronary venous catheterization with retrograde delivery
The feasibility and efficacy of percutaneous retrograde gene delivery by selective pressure-regulated retroinfusion of the coronary veins has been demonstrated by Boekstegers et al. Using their constructed apparatus, consisting of a pump unit, extracorporeal circuit, retroinfusion catheter and suction device, the authors demonstrated advantages of retrograde delivery compared to ante-grade and confirmed the results from several groups that blocking the venous outflow and coronary ischemia can significantly increase viral transfection of the myocardium [44,54]. These authors believe that selective coronary retroinfusion prolongs adhesion time of the vector and increases endothelial permeability. This finding has led to an important advance in this field of delivery, although this method does not reduce transduction of extracardiac organs like liver and lung [55]. Also, Hou et al. showed that a single retrograde coronary venous administration resulted in efficient regional myocyte transfection of human Del-1 and GFP. The authors believe that the coronary venous approach offers minimal washout and allows for controlled dwell times for longer exposure [56]. Kaye at al. developed the V-Focus delivery system for a minimally invasive percutaneous procedure, which was designed to isolate the coronary circulation from the systemic. This system includes percutaneous intervention catheters, extracorporeal pumpoxygenator circuit, infusion pump and monitors. According to the authors, this system achieves superior myocardial gene expression in contrast to intracoronary delivery and is associated with lower systemic expression [57,58]. Although the authors represent this method as a “closed-loop” recirculation, careful analysis of the authors’ reported quantitative PCR results indicates that the vector genome concentration was 26 times higher in the liver than in the heart and thus isolation of the heart was not achieved as claimed [59].
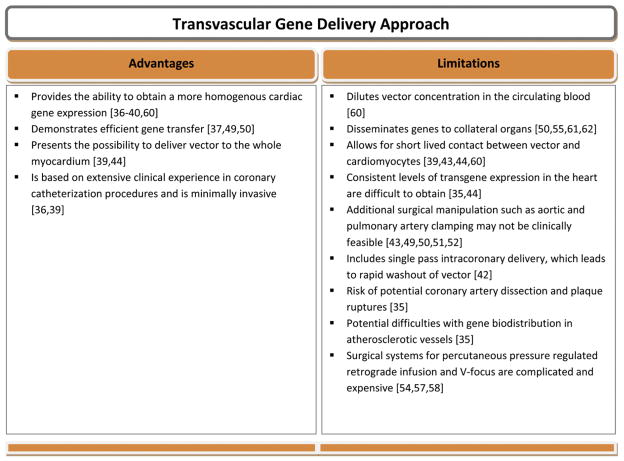
In summary transvascular gene delivery provides the ability to obtain homogenous [60] and efficient cardiac gene expression accomplished with rapid dilution of vector in circulating blood with significant extracardiac expression [61–62]. Surgical manipulations included temporary aortic/pulmonary clamping or coronary arterial/venous occlusion but both have significant clinical limitations and would require more translational research, preferably in large animals, prior to considering clinical application (Fig. 5).
Fig. 5.
Advantages and limitations of transvascular gene delivery approach.
4. Ex vivo gene delivery
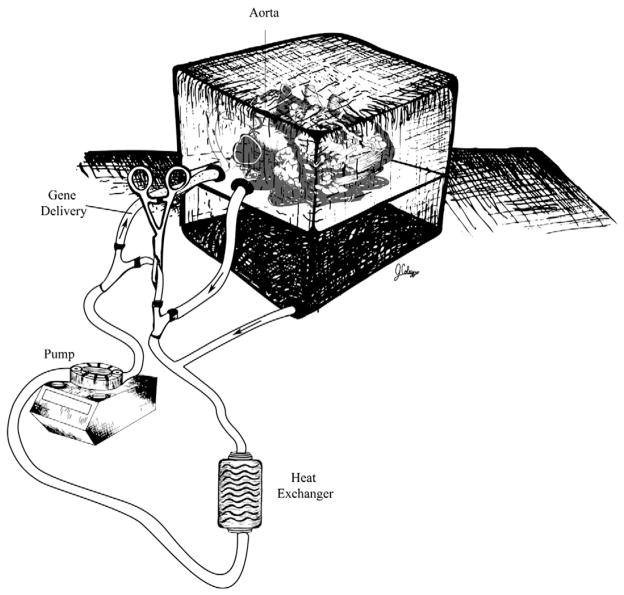
Even though there is a shortage of donor organs, over 2000 heart transplants are performed annually in the United States. Ex vivo, myocardial gene delivery to the donor heart before reimplantation is an active area of investigation [63] (Fig. 6). Such an approach is clinically relevant in the setting of clinical heart transplantation and in surgical treatment of heart failure. Strategies to improve cardiac allograft function could potentially increase the longevity of current allografts, modulate the host immune response for prevention of allograft rejection, treat ischemia–reperfusion injury and make available additional marginal organs for transplantation that are currently not utilized [64]. Multiple studies have demonstrated the ability to transfect cardiac allografts with intracoronary, intramuscular and transported delivery of vectors prior to transplantation [60,65–67]. Because gene delivery is carried out in the donor heart, total body virus exposure is limited in the recipient, thus making it clinically attractive [68]. Using intracoronary perfusion in explanted hearts, Donahue et al. reported highly effective gene transfer to the heart [37].
Fig. 6.
Ex vivo gene delivery.
In the study of Griscelli et al., recombinant adenoviruses encoding β-galactosidase were injected into the coronary vessels of the harvested non-beating hearts of piglets at a dose 1010–1011 pfu. The hearts were maintained in contact with the vector-containing solution for 1 h at 4 °C and the coronary vascular bed was then washed out by injecting 100 ml of cardioplegia solution and the last virus titer in the coronary sinus effluent was three logs below that of the infused viral solution. Gene transfer to allografts was evaluated four days after heterotopic reimplantation. In four out of 11 animals transgene expression was detected in all cardiac areas, PCR analysis revealed minimal collateral organ transfection [60], and gene expression was not enhanced by the exclusion of blood [47]. Shah et al. performed ex vivo perfusion of cardiac allografts with adenovirus encoding transgenes that enhance β-AR signaling [69]. The group demonstrated that five days after heterotopic transplantation, left ventricular systolic and diastolic performance was significantly increased in transfected grafts compared with controls. Further, Svensson et al. showed that 15 min of perfusion with AAV.CMV.lacZ was sufficient to result in transduction of 40% of cardiomyocytes after four weeks [24].
5. CPB and cardioplegic arrest
Another possible application of gene therapy is its use during operations with extracorporeal circulation or cardiopulmonary bypass (CPB), which has become a routine cardiac procedure. In fact, in 2009 more than one million cardiac procedures that depend upon CPB were performed worldwide. Potentially widespread clinical application would involve intracoronary (antegrade or retrograde) gene transfer in the setting of CPB and cardioplegic arrest. The ability to augment myocardial performance with gene transfer could potentially reduce the need for long-term inotropic or mechanical support in the post-bypass setting, avoiding the complications associated with these interventions [64]. High-risk patients undergoing revascularization or valve replacement with coexisting severely reduced ventricular function and inherited forms of cardiomyopathy might particularly benefit from concomitant gene therapy. Bridges et al. and Davidson et al. first hypothesized that cardiopulmonary bypass may facilitate cardiac-selective gene transfer using recombinant replication-deficient adenovirus [70,71]. The absence of a significant influence of cold temperatures on transgene expression in an in vivo model with CPB was described by Jones et al. [72]. They also demonstrated that, the presence of crystalloid cardioplegia compared with blood cardioplegia within the coronary circulation had no effect on transgene expression and hypothesized that endothelial contact with cardioplegia and the associated relative ischemia likely increased endothelial permeability [73]. Ikeda et al. evaluated the feasibility of restoring δ-sarcoglycan deficiency in cardiomyopathic hamsters after injection of a cardioplegia solution containing an adenoviral vector encoding δ-sarcoglycan into the aortic root [74]. At one and three weeks after transfection, immunostaining showed extensive restoration of deficient membrane proteins with significantly less progression of LV dysfunction compared with controls. Davidson et al. demonstrated the feasibility of myocardial gene delivery during CPB with cold, hyperkalemic cardioplegic arrest in the porcine model [71] (Fig. 7a). Unlike previous studies that utilized a single-pass perfusion technique, Bridges et al. were the first to create an isolated “closed-loop” recirculating model of vector-mediated cardiac gene delivery in the large animal heart using cardiopulmonary bypass with an antegrade delivery approach, allowing for vector recirculation for 20 min [70]. Later, they used CPB with high-pressure retrograde coronary sinus infusion with multiple-pass recirculation of vector through the heart and washed out of the cardiac circuit prior to weaning from CPB, which limited extracardiac gene expression (Fig. 7b). They were able to show an increase of several orders of magnitude in cardiac marker gene activities compared with controls. Furthermore, there was minimal gene expression in the liver and other collateral organs [75]. These results validate this surgical technique as a potentially clinically translatable approach for cardiac gene therapy in carefully selected patients.
Fig. 7.
Cardiopulmonary bypass-mediated gene delivery. a. Gene delivery via conventional cardiopulmonary bypass. b. Gene transfer via cardiopulmonary bypass with complete cardiac isolation and recirculating delivery (MCARD).
In summary, despite the attractiveness of ex vivo and CPB-mediated gene delivery approaches, it should be noted that one cannot exclude possible attendant morbidity. Morbidity may be related to technique-associated complications as well as the additional CPB time required. Finally, these methods have not been translated to clinical trials, and the only evidence we have that they are effective is founded on the basis of data derived from experimental studies. Therefore, it is difficult to judge objectively about the likely clinical efficacy of these gene delivery platforms (Fig. 8).
Fig. 8.
Advantages and limitations of ex vivo and cardiopulmonary bypass-mediated gene delivery approach.
6. Cardiac gene delivery in clinical trials
A significant proportion of patients with myocardial ischemia and congestive heart failure remain refractory to pharmacological therapies and unsuitable for percutaneous or surgical interventions [76]. Trials involving gene therapy in patients with ischemic heart disease have been undertaken to stimulate angiogenesis. Rosengart et al. reported a Phase I clinical trial involving 21 patients with clinically significant coronary artery disease that utilized adenovirus encoding human VEGF 121 cDNA with a goal to induce therapeutic angiogenesis. The vector construct was administered by direct myocardial injection into an area of reversible ischemia either as an adjunct to coronary artery bypass grafting or as sole therapy via a minithoracotomy. In both groups, coronary angiography and stress sestamibi scans suggested improvement in angina class patients after therapy [33,77]. Percutaneous catheter-based myocardial gene transfer of naked plasmid DNA encoding phVEGF-2 was tested in human subjects by Vale et al. [78]. After the completion of electromechanical mapping, the injection catheter was introduced percutaneously via a femoral arteriotomy across the aortic valve into the LV and the needle was advanced 4 to 6 mm intramuscular to administer six injections of plasmid DNA into the ischemic myocardium. All patients experienced reduced angina, reduced nitroglycerin usage and improved myocardial perfusion by SPECT-sestamibi scanning. Also, results of single intracoronary administration of Ad5-FGF4 show evidence of favorable anti-ischemic effects in patients with stable angina pectoris [79]. In this study the transgene was infused over a period of 90 s through subselective catheters into all major patent coronary arteries that could be engaged. Nevertheless, it should be noted that these Phase I studies have not measured the amount of recombinant protein produced; hence, it has not been possible to make accurate, quantitative determinations of the relative efficiency of gene transfer, and a placebo-induced therapeutic effect cannot be excluded.
Hajjar et al. and Jaski et al. performed the first-in-human Phase I/II clinical trial with single intracoronary infusion of AAV1.SERCA2a in advanced heart failure. Standard percutaneous catheter engagement technique with the coronary arteries was performed. This usually involves delivering two-thirds of the dose to the anterolateral and one-third to the posterolateral myocardium, based on the coronary anatomy. Of the nine patients, several demonstrated symptomatic, functional and biomarker improvements from baseline to month six. The Phase II results of this trial are not yet available [80,81].
7. Future directions
Although this review has outlined progressive improvement in efficiency and safety of delivery systems, many hurdles still have to be overcome. Direct delivery approaches should: (1) lead to homogeneous delivery to the target tissue and appropriate regional distribution of the vector; (2) be accompanied by techniques such as electromechanical mapping to determine areas of ischemic, border and viable myocardium; (3) be used in additional research studies and clinical trials confirming evidence of enhanced collateral flow after myocardial injections of angiogenic factors and demonstrating appropriate atrio-ventricular node genetic modification. Future developments in systemic delivery approaches have to improve safety by minimizing the possibility of extracardiac expression and its potentially toxic effects. Additionally, the known catheter-based platforms need further modification to create more reliable “closed-loop” coronary recirculation systems. Gene transfer using CPB should be less complex, ideally through the development of minimally invasive approaches that achieve equivalent, highly efficient and cardiac-specific delivery results.
8. Conclusions
On the basis of the reviewed methods, characteristics of optimal technique for cardiac gene delivery must be safe, clinically translatable and (ideally) incorporate the following: (1) route of delivery: retrograde transvenous through the coronary sinus/coronary veins or antegrade intracoronary through coronary arteries; (2) washout of vector after gene transfer to minimize collateral expression; (3) increased myocardial transcapillary gradient and/or enhanced transendothelial transport of viral particles from the vasculature into the interstitium, using physical or pharmacological methods; (4) creation of a “closed-loop” for extended transgene residence time in the coronary circulation; and (5) myocardial preconditioning with temporary local ischemia or concomitant coronary venous blockade.
Acknowledgments
This work was sponsored in part by the National Heart Lung and Blood Institute (1-R01-HL083078-01A2).
References
- 1.Melo LG, Pachori AS, Gnecchi M, Dzau VJ. Genetic therapies for cardiovascular diseases. Trends Mol Med. 2005;11:240–50. doi: 10.1016/j.molmed.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Donahue JK. Gene therapy for cardiac arrhythmias. Ann NY Acad Sci. 2004;1015:332–7. doi: 10.1196/annals.1302.029. [DOI] [PubMed] [Google Scholar]
- 3.Bernecker OY, del Monte F, Hajjar RJ. Gene therapy for the treatment of heart failure-calcium signaling. Semin Thorac Cardiovasc Surg. 2003;15:268–76. doi: 10.1016/s1043-0679(03)70006-9. [DOI] [PubMed] [Google Scholar]
- 4.Ly H, Kawase Y, Yoneyama R, Hajjar RJ. Gene therapy in the treatment of heart failure. Physiology. 2007;22:81–96. doi: 10.1152/physiol.00037.2006. [DOI] [PubMed] [Google Scholar]
- 5.Zhang JCL, Woo YJ, Chen JA, Swain JL, Sweeney HL. Efficient transmural cardiac gene transfer by intrapericardial injection in neonatal mice. J Mol Cell Cardiol. 1999;31:721–32. doi: 10.1006/jmcc.1998.0905. [DOI] [PubMed] [Google Scholar]
- 6.Fromes Y, Salmon A, Wang X, Collin H, Rouche A, Hagege A, et al. Gene delivery to the myocardium by intrapericardial injection. Gene Ther. 1999;6:683–8. doi: 10.1038/sj.gt.3300853. [DOI] [PubMed] [Google Scholar]
- 7.March KL, Woody M, Mehdi K, Zipes DP, Brantly M, Trapnell BC. Efficient in vivo catheter-based pericardial gene transfer mediated by adenoviral vectors. Clin Cardiol. 1999;22(1):123–9. doi: 10.1002/clc.4960221308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamping KG, Rios CD, Chun JA, Ooboshi H, Davidson BL, Heistad DD. Intrapericardial administration of adenovirus for gene transfer. Am J Physiol. 1997;272:H310–7. doi: 10.1152/ajpheart.1997.272.1.H310. [DOI] [PubMed] [Google Scholar]
- 9.Gwon HC, Jeong JO, Kim HJ, Park SW, Lee SH, Park SJ, et al. The feasibility and safety of fluoroscopy-guided percutaneous intramyocardial gene injection in porcine heart. Int J Cardiol. 2001;79:77–88. doi: 10.1016/s0167-5273(01)00410-7. [DOI] [PubMed] [Google Scholar]
- 10.Sanborn TA, Hackett NR, Lee LY, El-Sawy T, Blanco I, Tarazona N, et al. Percutaneous endocardial transfer and expression of genes to the myocardium utilizing fluoroscopic guidance. Cathet Cardiovasc Intervent. 2001;52:260–6. doi: 10.1002/1522-726x(200102)52:2<260::aid-ccd1061>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 11.Vale PR, Losordo DW, Tkebuchava T, Chen D, Milliken CE, Isner JM. Catheter-based myocardial gene transfer utilizing nonfluoroscopic electromechanical left ventricular mapping. J Am Coll Cardiol. 1999;34:246–54. doi: 10.1016/s0735-1097(99)00143-6. [DOI] [PubMed] [Google Scholar]
- 12.Kornowski R, Leon MB, Fuchs S, Vodovotz Y, Flynn MA, Gordon DA, et al. Electromagnetic guidance for catheter-based transendocardial injection: a platform for intramyocardial angiogenesis therapy.Results in normal and ischemic porcine models. J Am Coll Cardiol. 2000;35:1031–9. doi: 10.1016/s0735-1097(99)00642-7. [DOI] [PubMed] [Google Scholar]
- 13.Sylven C, Sarkar N, Unsulander P, Kennebäck G, Blomberg P, Islam K, et al. Catheter-based transendocardial myocardial gene transfer. J Interv Cardiol. 2002;15:7–13. doi: 10.1111/j.1540-8183.2002.tb01026.x. [DOI] [PubMed] [Google Scholar]
- 14.Grossman PM, Han Z, Palasis M, Barry JJ, Lederman RJ. Incomplete retention after direct myocardial injection. Cathet Cardiovasc Intervent. 2002;55:392–7. doi: 10.1002/ccd.10136. [DOI] [PubMed] [Google Scholar]
- 15.Naimark WA, Lepore JJ, Klugherz BD, Wang Z, Guy TS, Osman H, et al. Adenovirus–catheter compatibility increases gene expression after delivery to porcine myocardium. Hum Gene Ther. 2003;14:161–6. doi: 10.1089/104303403321070856. [DOI] [PubMed] [Google Scholar]
- 16.Acsadi G, Jiao S, Jani A, Duke D, Williams P, Chong W, et al. Direct gene transfer and expression into rat heart in vivo. New Biol. 1991;3:71–81. [PubMed] [Google Scholar]
- 17.Lin H, Parmacek MS, Morle G, Bolling S, Leiden JM. Expression of recombinant genes in myocardium in vivo after direct injection of DNA. Circulation. 1990;82:2217–21. doi: 10.1161/01.cir.82.6.2217. [DOI] [PubMed] [Google Scholar]
- 18.Buttrick PM, Kass A, Kitsis RN, Kaplan ML, Leinwand LA. Behavior of genes directly injected into the rat heart in vivo. Circ Res. 1992;70:193–8. doi: 10.1161/01.res.70.1.193. [DOI] [PubMed] [Google Scholar]
- 19.von Harsdorf R, Schott RJ, Shen YT, Vatner SF, Mahdavi V, Nadal-Ginard B. Gene injection into canine myocardium as a useful model for studying gene expression in the heart of large mammals. Circ Res. 1993;72:688–95. doi: 10.1161/01.res.72.3.688. [DOI] [PubMed] [Google Scholar]
- 20.Guzman RJ, Lemarchand P, Crystal RG, Epstein SE, Finkel T. Efficient gene transfer into myocardium by direct injection of adenovirus vectors. Circ Res. 1993;73:1202–7. doi: 10.1161/01.res.73.6.1202. [DOI] [PubMed] [Google Scholar]
- 21.French BA, Mazur W, Geske RS, Bolli R. Direct in vivo gene transfer into porcine myocardium using replication-deficient adenoviral vectors. Circulation. 1994;90:2414–24. doi: 10.1161/01.cir.90.5.2414. [DOI] [PubMed] [Google Scholar]
- 22.Magovern CJ, Mack CA, Zhang J, Hahn RT, Ko W, Isom OW, et al. Direct in vivo gene transfer to canine myocardium using a replication-deficient adenovirus vector. Ann Thorac Surg. 1996;62:425–33. [PubMed] [Google Scholar]
- 23.Barr E, Carroll J, Kalynych AM, Tripathy SK, Kozarsky K, Wilson JM, et al. Efficient catheter-mediated gene transfer into the heart using replication-defective adenovirus. Gene Ther. 1994;1:51–8. [PubMed] [Google Scholar]
- 24.Svensson EC, Marshall DJ, Woodard K, Lin H, Jiang F, Chu L, et al. Efficient and stable transduction of cardiomyocytes after intramyocardial injection or intracoronary perfusion with recombinant adeno-associated virus vectors. Circulation. 1999;99:201–5. doi: 10.1161/01.cir.99.2.201. [DOI] [PubMed] [Google Scholar]
- 25.Tomiyasu K, Oda Y, Nomura M, Satoh E, Fushiki S, Imanishi J, et al. Direct intra-cardiomuscular transfer of β2-adrenergic receptor gene augments cardiac output in cardiomyopathic hamsters. Gene Ther. 2000;7:2087–93. doi: 10.1038/sj.gt.3301329. [DOI] [PubMed] [Google Scholar]
- 26.Rengo G, Lymperopoulos A, Zincarelli C, Donniacuo M, Soltys S, Rabinowitz JE, et al. Myocardial adeno-associated virus serotype 6-βARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation. 2009;119:89–98. doi: 10.1161/CIRCULATIONAHA.108.803999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koransky ML, Robbins RC, Blau HM. VEGF gene delivery for treatment of ischemic cardiovascular disease. Trends Cardiovasc Med. 2002;12:108–14. doi: 10.1016/s1050-1738(01)00158-x. [DOI] [PubMed] [Google Scholar]
- 28.Edelberg JM, Huang DT, Josephson ME, Rosenberg RD. Molecular enhancement of porcine cardiac chronotropy. Heart. 2001;86:559–62. doi: 10.1136/heart.86.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isner JM, Vale PR, Symes JF, Losordo DW. Assessment of risks associated with cardiovascular gene therapy in human subjects. Circ Res. 2001;89:389–400. doi: 10.1161/hh1701.096259. [DOI] [PubMed] [Google Scholar]
- 30.Lathi KG, Vale PR, Losordo DW, Cespedes RM, Symes JF, Esakof DD, et al. Gene therapy with vascular endothelial growth factor for inoperable coronary artery disease: anesthetic management and results. Anesth Analg. 2001;92:19–25. doi: 10.1097/00000539-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Vale PR, Losordo DW, Milliken CE, McDonald MC, Gravelin LM, Curry CM, et al. Randomized, single-blind, placebo-controlled pilot study of catheter-based myocardial gene transfer for therapeutic angiogenesis using left ventricular electromechanical mapping in patients with chronic myocardial ischemia. Circulation. 2001;103:2138–43. doi: 10.1161/01.cir.103.17.2138. [DOI] [PubMed] [Google Scholar]
- 32.Losordo DW, Vale PR, Symes J, Dunnington C, Esakof D, Maysky M, et al. Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation. 1998;98:2800–4. doi: 10.1161/01.cir.98.25.2800. [DOI] [PubMed] [Google Scholar]
- 33.Rosengart TK, Lee LY, Patel SR, Kligfield PD, Okin PM, Hackett NR, et al. Six-month assessment of a phase I trial of angiogenic gene therapy for the treatment of coronary artery disease using direct intramyocardial administration an adenovirus vector expressing the VEGF121 cDNA. Ann Surg. 1999;230:466–72. doi: 10.1097/00000658-199910000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muhlhauser J, Jones M, Yamada I, Cirielli C, Lemarchand P, Gloe TR, et al. Safety and efficacy of in vivo gene transfer into the porcine heart with replication-deficient, recombinant adenovirus vectors. Gene Ther. 1996;3:145–53. [PubMed] [Google Scholar]
- 35.Kornowski R, Fuchs S, Leon MB, Epstein SE. Delivery strategies to achieve therapeutic myocardial angiogenesis. Circulation. 2000;101:454–8. doi: 10.1161/01.cir.101.4.454. [DOI] [PubMed] [Google Scholar]
- 36.Hajjar RJ, del Monte F, Matsui T, Rosenzweig A. Prospects for gene therapy for heart failure. Circ Res. 2000;86:616–21. doi: 10.1161/01.res.86.6.616. [DOI] [PubMed] [Google Scholar]
- 37.Donahue JK, Kikkawa K, Johns DC, Marban E, Lawrence JH. Ultrarapid, highly efficient viral gene transfer to the heart. Proc Natl Acad Sci USA. 1997;94:4664–8. doi: 10.1073/pnas.94.9.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker AH. Development and use of gene transfer for treatment of cardiovascular disease. J Card Surg. 2002;17:543–8. doi: 10.1046/j.1540-8191.2002.01011.x. [DOI] [PubMed] [Google Scholar]
- 39.Logeart D, Hatem SN, Heimburger M, Roux AL, Michel JB, Mercadier JJ. How to optimize in vivo gene transfer to cardiac myocytes: mechanical or pharmacological procedures? Hum Gene Ther. 2001;12:1601–10. doi: 10.1089/10430340152528101. [DOI] [PubMed] [Google Scholar]
- 40.Hayase M, del Monte F, Kawase Y, MacNeill BD, McGregor J, Yoneyama R, et al. Catheter-based antegrade intracoronary viral gene delivery with coronary venous blockade. Am J Physiol Heart Circ Physiol. 2005;288:H2995–3000. doi: 10.1152/ajpheart.00703.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaplitt MG, Xiao X, Samulski RJ, Li J, Ojamaa K, Klein IL, et al. Long-term gene transfer in porcine myocardium after coronary infusion of an adeno-associated virus vector. Ann Thorac Surg. 1996;62:1669–76. doi: 10.1016/s0003-4975(96)00946-0. [DOI] [PubMed] [Google Scholar]
- 42.Logeart D, Hatem SN, Rucker-Martin C, Chossat N, Nevo N, Haddada H, et al. Highly efficient adenovirus-mediated gene transfer to cardiac myocytes after single-pass coronary delivery. Hum Gene Ther. 2000;11:1015–22. doi: 10.1089/10430340050015329. [DOI] [PubMed] [Google Scholar]
- 43.Ding Z, Fach C, Sasse A, Gődecke A, Schrader J. A minimally invasive approach for efficient gene delivery to rodent hearts. Gene Ther. 2004;11:260–5. doi: 10.1038/sj.gt.3302167. [DOI] [PubMed] [Google Scholar]
- 44.Boekstegers P, Kupatt C. Current concepts and applications of coronary venous retroinfusions. Basic Res Cardiol. 2004;99:373–81. doi: 10.1007/s00395-004-0486-3. [DOI] [PubMed] [Google Scholar]
- 45.Donahue JK, Kikkawa K, Thomas AD, Marban E, Lawrence JH. Acceleration of widespread adenoviral gene transfer to intact rabbit hearts by coronary perfusion with low calcium and serotonin. Gene Ther. 1998;5:630–4. doi: 10.1038/sj.gt.3300649. [DOI] [PubMed] [Google Scholar]
- 46.Parsa CJ, Reed RC, Walton GB, Pascal LS, Thompson RB, Petrofski JA, et al. Catheter-mediated subselective intracoronary gene delivery to the rabbit heart: introduction of a novel method. J Gene Med. 2005;7:595–603. doi: 10.1002/jgm.704. [DOI] [PubMed] [Google Scholar]
- 47.Wright MJ, Wightman LML, Latchman DS, Marber MS. In vivo myocardial gene transfer: optimization and evaluation of intracoronary gene delivery in vivo. Gene Ther. 2001;8:1833–9. doi: 10.1038/sj.gt.3301614. [DOI] [PubMed] [Google Scholar]
- 48.Emani SM, Shah AS, Bowman MK, Emani S, Wilson K, Glower DD, et al. Catheter-based intracoronary myocardial adenoviral gene delivery: importance of intraluminal seal and infusion flow rate. Mol Ther. 2003;8:306–13. doi: 10.1016/s1525-0016(03)00149-7. [DOI] [PubMed] [Google Scholar]
- 49.Hajjar RJ, Schmidt U, Matsui T, Guerrero JL, Lee KH, Gwathmey JK, et al. Modulation of ventricular function through gene transfer in vivo. Proc Natl Acad Sci USA. 1998;95:5251–5156. doi: 10.1073/pnas.95.9.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maurice JP, Hata JA, Shah AS, White DC, McDonald PH, Dolber PC, et al. Enhancement of cardiac function after adenoviral-mediated in vivo intracoronary β2-adrenergic receptor gene delivery. J Clin Invest. 1999;104:21–9. doi: 10.1172/JCI6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parsa CJ, Koch WJ. Gene interventions in the beta-adrenergic system for treating heart failure. Semin Thorac Cardiovasc Surg. 2003;15:259–67. doi: 10.1016/s1043-0679(03)70005-7. [DOI] [PubMed] [Google Scholar]
- 52.Kaspar BK, Roth DM, Lai NC, Drumm JD, Erickson DA, McKirnan MD, et al. Myocardial gene transfer and long-term expression following intracoronary delivery of adeno-associated virus. J Gene Med. 2005;7:316–24. doi: 10.1002/jgm.665. [DOI] [PubMed] [Google Scholar]
- 53.Eckhart AD, Koch WJ. Beta-Adrenergic gene therapy for cardiovascular disease. Curr Control Trials Cardiovasc Med. 2000;1:131–4. doi: 10.1186/cvm-1-3-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boekstegers P, von Degenfeld G, Giehrl W, Heinrich D, Hullin R, Kupatt C, et al. Myocardial gene transfer by selective pressure-regulated retroinfusion of coronary veins. Gene Ther. 2000;7:232–40. doi: 10.1038/sj.gt.3301079. [DOI] [PubMed] [Google Scholar]
- 55.Raake PW, Hinkel R, Muller S, Delker S, Kreuzupointer R, Kupatt C, et al. Cardio-specific long-term gene expression in a porcine model after selective pressure-regulated retroinfusion of adeno-associated viral (AAV) vectors. Gene Ther. 2008;15:12–7. doi: 10.1038/sj.gt.3303035. [DOI] [PubMed] [Google Scholar]
- 56.Hou D, Maclaughlin F, Thiesse M, Panchal V, Bekkers BC, Wilson EA, et al. Widespread regional myocardial transfection by plasmid encoding Del-1 following retrograde coronary venous delivery. Cathet Cardiovasc Intervent. 2003;58:207–11. doi: 10.1002/ccd.10417. [DOI] [PubMed] [Google Scholar]
- 57.Kaye DM, Preovolos A, Marshall T, Burne M, Hoshijima M, Hajjar R, et al. Percutaneous cardiac recirculation-mediated gene transfer of an inhibitory phospholamban peptide reverses advanced heart failure in large animals. J Am Coll Cardiol. 2007;50:253–60. doi: 10.1016/j.jacc.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 58.Byrne MJ, Power JM, Preovolos A, Mariani JA, Hajjar RJ, Kaye DM. Recirculating cardiac delivery of AAV2/1SERCA2a improves myocardial function in an experimental model of heart failure in large animals. Gene Ther. 2008;15:1550–7. doi: 10.1038/gt.2008.120. [DOI] [PubMed] [Google Scholar]
- 59.Bridges CR. Recirculating method of cardiac gene delivery should be called “non-recirculating” method. Gene Ther. 2009;16:939–40. doi: 10.1038/gt.2009.35. [DOI] [PubMed] [Google Scholar]
- 60.Griscelli F, Belli E, Opolon P, Musset K, Connault E, Perricaudet M, et al. Adenovirus-mediated gene transfer to the transplanted piglet heart after intracoronary injection. J Gene Med. 2003;5:109–19. doi: 10.1002/jgm.322. [DOI] [PubMed] [Google Scholar]
- 61.Shah AS, Lilly E, Kypson AP, Tai O, Hata JA, Pippen A, et al. Intracoronary adenovirus-mediated delivery and overexpression of the β2-adrenergic receptor in the heart. Prospects for molecular ventricular assistance. Circulation. 2000;101:408–14. doi: 10.1161/01.cir.101.4.408. [DOI] [PubMed] [Google Scholar]
- 62.Barr E, Carroll J, Kalynych AM, Tripathy SK, Kozarsky K, Wilson JM, et al. Efficient catheter-mediated gene transfer into the heart using replication-defective adenovirus. Gene Ther. 1994;1:51–8. [PubMed] [Google Scholar]
- 63.Donahue JK, Heldman AW, Fraser H, McDonald AD, Miller JM, Rade JJ, et al. Focal modifications of electrical conduction in the heart by viral gene transfer. Nat Med. 2000;6:1395–8. doi: 10.1038/82214. [DOI] [PubMed] [Google Scholar]
- 64.White DC, Milano CA. Gene therapy in cardiovascular disease: state of the art. Ann Surg. 2003;238:S90–9. doi: 10.1097/01.sla.0000097772.28752.38. [DOI] [PubMed] [Google Scholar]
- 65.Lee J, Laks H, Drinkwater DC, Blitz A, Lam L, Shiraishi Y, et al. Cardiac gene transfer by intracoronary infusion of adenovirus vector-mediated reporter gene in the transplanted mouse heart. J Thorac Cardiovasc Surg. 1996;111:246–52. doi: 10.1016/S0022-5223(96)70422-1. [DOI] [PubMed] [Google Scholar]
- 66.Kypson AP, Peppel K, Akhter SA, Lilly RE, Glower DD, Lefkowitz RJ, et al. Ex vivo adenovirus-mediated gene transfer to the adult rat heart. J Thorac Cardiovasc Surg. 1998;115:623–30. doi: 10.1016/S0022-5223(98)70327-7. [DOI] [PubMed] [Google Scholar]
- 67.Wang JYM, Knechtle SJ. Adenovirus-mediated gene transfer into rat cardiac allografts: comparison of direct injection and perfusion. Transplantation. 1996;61:1726–9. doi: 10.1097/00007890-199606270-00011. [DOI] [PubMed] [Google Scholar]
- 68.Gojo S, Niwaya K, Taniguchi S, Nishizaki K, Kitamura S. Gene transfer into the donor heart during cold preservation for heart transplantation. Ann Thorac Surg. 1998;65:647–52. doi: 10.1016/s0003-4975(97)01295-2. [DOI] [PubMed] [Google Scholar]
- 69.Shah AS, White DC, Tai O, Hata JA, Wilson KH, Pippen A, et al. Adenovirus-mediated genetic manipulation of the myocardial β-adrenergic signaling system in transplanted hearts. J Thorac Cardiovasc Surg. 2000;120:581–8. doi: 10.1067/mtc.2000.107519. [DOI] [PubMed] [Google Scholar]
- 70.Bridges CR, Burkman JM, Malekan R, Konig SM, Chen H, Yarnall CB, et al. Global cardiac-specific transgene expression using cardiopulmonary bypass with cardiac isolation. Ann Thorac Surg. 2002;73:1939–46. doi: 10.1016/s0003-4975(02)03509-9. [DOI] [PubMed] [Google Scholar]
- 71.Davidson MJ, Jones JM, Emani SM, Wilson KH, Jaggers J, Koch WJ, et al. Cardiac gene delivery with cardiopulmonary bypass. Circulation. 2001;104:131–3. doi: 10.1161/01.cir.104.2.131. [DOI] [PubMed] [Google Scholar]
- 72.Jones JM, Wilson KH, Koch WJ, Milano CA. Adenoviral gene transfer to the heart during cardiopulmonary bypass: effect of myocardial protection technique on transgene expression. Eur J Cardiothorac Surg. 2002;21:847–52. doi: 10.1016/s1010-7940(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 73.Jones JM, Koch WJ. Gene therapy approaches to cardiovascular disease. Methods Mol Med. 2005;112:15–35. doi: 10.1385/1-59259-879-x:015. [DOI] [PubMed] [Google Scholar]
- 74.Ikeda Y, Gu Y, Iwanada Y, Hoshijima M, Oh SS, Giordano FJ, et al. Restoration of deficient membrane proteins in the cardiomyopathic hamster by in vivo cardiac gene transfer. Circulation. 2002;105:502–8. doi: 10.1161/hc0402.102953. [DOI] [PubMed] [Google Scholar]
- 75.Bridges CR, Gopal K, Holt DE, Yarnall C, Cole S, Anderson RB, et al. Efficient myocyte gene delivery with complete cardiac surgical isolation in situ. J Thorac Cardiovasc Surg. 2005;130:1364–70. doi: 10.1016/j.jtcvs.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 76.Vinge LE, Raake PW, Koch WJ. Gene therapy in heart failure. Circ Res. 2008;102:1458–70. doi: 10.1161/CIRCRESAHA.108.173195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rosengart TK, Lee LY, Patel SR, Sanborn TA, Parikh M, Bergman GW, et al. Angiogenesis gene therapy: phase I assessment of direct intramyocardial administration of an adenovirus vector expressing VEFG 121 cDNA to individuals with clinically significant severe coronary artery disease. Circulation. 1999;100:468–74. doi: 10.1161/01.cir.100.5.468. [DOI] [PubMed] [Google Scholar]
- 78.Vale PR, Losordo DW, Milliken CE, McDonald MC, Gravelin LM, Curry CM, et al. Randomized, single-blind, placebo-controlled pilot study of catheter-based myocardial gene transfer for therapeutic angiogenesis using left ventricular electromechanical mapping in patients with chronic myocardial ischemia. Circulation. 2001;103:2138–43. doi: 10.1161/01.cir.103.17.2138. [DOI] [PubMed] [Google Scholar]
- 79.Grines CL, Watkins MW, Helmer G, Penny W, Brinker J, Marmur JD, et al. Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris. Circulation. 2002;105:1291–7. doi: 10.1161/hc1102.105595. [DOI] [PubMed] [Google Scholar]
- 80.Hajjar RJ, Zsebo K, Deckelbaum L, Thompson C, Rudy J, Yaroshinsky A, et al. Design of a phase 1/2 trial of intracoronary administration of AAV1/SERCA2a in patients with heart failure. J Card Fail. 2008;14:355–67. doi: 10.1016/j.cardfail.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 81.Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail. 2009;15:171–81. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]