Abstract
Huntington’s disease (HD) is an inherited progressive neurodegenerative disorder resulting from CAG repeat expansion in the gene that encodes for the protein huntingtin. To identify neuroprotective compound (s) that can slow down disease progression and can be administered long term with few side effects in Huntington’s disease, we investigated the effect of sertraline, a selective serotonin reuptake inhibitor (SSRI) which has been shown to upregulate BDNF levels in rodent brains. We report here that in HD mice sertraline increased BDNF levels, preserved chaperone protein HSP70 and Bcl-2 levels in brains, attenuated the progression of brain atrophy and behavioral abnormalities and thereby increased survival. Sertraline also enhanced neurogenesis, which appeared to be responsible for mediating the beneficial effects of sertraline in HD mice. Additionally, the effective levels of sertraline are comparable to the safe levels achievable in humans. The findings suggest that sertraline is a potential candidate for treatment of HD patients.
Keywords: SSRI, serotonin, BDNF, Huntington’s disease, neurogenesis
Introduction
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disorder in which neurons in the striatum and the cerebral cortex degenerate, resulting in abnormal involuntary movements (chorea), and psychiatric and cognitive abnormalities (Reiner et al, 1998 Vonsattel et al., 1985; Myers et al., 1988). Death usually occurs within 10–20 years after onset of the first clinical symptoms. Although the mechanism of neuronal degeneration in HD is unclear, it is thought that disease onset and progression may be involved in transcription dysregulation (Cha 2000; Sugars and Rubinsztein, 2003) and deficits of neurotrophic factors (Zuccato et al., 2001; Zuccato et al., 2005; Zuccato and Cattaneo 2007; Ferrigno and Silver, 2000; Gauthier et al., 2004; Cowan and Raymond, 2006), with cumulative neuronal loss.
It has been demonstrated that levels of brain-derived neurotrophic factor (BDNF) are decreased in brain cells of HD patients and mutant huntingtin transgenic mice. Although the crucial mechanism(s) of neuronal death induced by mutant htt is still unclear, mutant huntingtin promotes neuronal degeneration, in part by suppressing BDNF transcription and/or blocking axonal transport of BDNF from cerebral cortex to striatum (Zuccato et al., 2001 and 2003; Gauthier et al., 2004). Other studies have shown that BDNF can protect neurons against insults relevant to the pathogenesis of HD (Bemelmans et al., 1999; Kells et al., 2004; Canals et al., 2004; Cepeda et al., 2004; Pineda et al., 2005; Zuccato et al., 2005; Lynch et al., 2007). In addition to promoting neuronal survival, BDNF also regulates neurogenesis (Duman, 2004; Cotman and Berchtold 2002). Altered neurogenesis occurred in mouse models of HD and in postmortem brains in humans with the disease (Phillips et al., 2006; Phillips et al., 2005; Grote et al., 2005; Gil et al., 2005; Tattersfield et al., 2005; Lazic et al., 2004; Curtis et al., 2003). Mutant huntingtin also interrupts other cell protective systems, such as chaperone protein, leading to dysfunction of the protein refolding system (Landles and Bates 2004).
There is currently no therapy to delay onset or prevent disease progression in HD patients. Promising approaches have been reported in preclinical trials, such as treating with FGF-2 (Jin et al., 2005) and GDNF (McBridge et al., 2006), which prevented neuronal death and dysfunction, or by replacing lost neurons by transplanting embryonic stem cells (Conti et al 2006). Nonetheless, most of these approaches are associated with ethical, technical, and immunological problems for application to human patients (Rosser and Dunnett, 2003). Searching for drugs that can delay onset and/or slow disease progression with few side effects after administration for long periods will be valuable for treating HD patients.
Selective serotonin reuptake inhibitors (SSRIs), a class of drugs that is widely used for the treatment of patients with depression and severe anxiety disorders, has been shown to increase BDNF expression in brain (Nibuya et al., 1995; Nibuya et al., 1996; Moltzen and Bang-Andersen, 2006). SSRIs can also stimulate neurogenesis and protect neurons against metabolic/oxidative insults and processes known to be responsive to BDNF (Malberg and Blendy, 2005). Moreover, SSRIs are very safe and well tolerated over long periods of administration. We previously reported that an SSRI, paroxetine, is beneficial in HD mice (Duan et al., 2004). In the present study we investigated the effect of another widely prescribed SSRI, sertraline, on disease progression and molecular mechanisms in N171-82Q HD mice. Sertraline has been reported to be effective as treatment for depression (Slaughter et al., 2001), obsessive compulsive disorders (Patzold and Brune., 2002), and severe aggressiveness (Ranen et al., 1996) in HD patients. In addition to its anti-psychiatric effect, however, whether sertraline is neuroprotective and whether it prevents neurodegeneration and disease progression of HD, to our knowledge, has not been studied.
We show here that sertraline increases BDNF levels, preserves chaperone protein HSP70 levels and anti-apoptotic protein Bcl-2 levels, restores depleted serotonin levels, retards motor behavioral impairment, enhances neurogenesis and increases survival in HD mice. The increased neurogenesis apparently mediates its beneficial effects in HD. The effective levels of sertraline in blood are comparable to the levels that are safe and achievable in humans. Taken together, we provide evidence that sertraline is neuroprotective in HD and the rationale for further clinical trials of SSRIs in HD patients.
Materials and Methods
Mice and drug administration
All animal experiments were performed according to procedures approved by the Institutional Animal Care and Use Committee. N171-82Q transgenic HD mice were mated to hybrid mice (C3H/HEJ × C57BL/6J F1, Taconic, NY). All HD mice were housed in cage conditions including an orange mouse igloo and a green nylabone setting in the cage, and wet mash was provided to all mice starting at weaning. We used male HD mice for all our studies since we found that there is a significant variability in all phenotypes between male and female HD mice (Duan et al 2003). Each group contained 15 mice at the beginning of experiments for survival and behavioral tests. Sertraline was freshly prepared by dissolving in vehicle 0.2% tween-80 and administered to 12-week-old mice by daily intraperitoneal injection until the end of studies.
Behavioral test
All mice were randomly divided into each group. Nontransgenic control mice are wild type littermates of HD mice. Each group contained 15 mice at the beginning of the rotarod test. We used the same set of animals for survival analyses and motor performance tests.
Motor behavioral performance was assessed using a rotarod apparatus with an accelerating speed (Columbus Instrument, OH). Each mouse was trained at speed of 4 rpm for 5 minutes, the training session was followed by a 1-hour rest period in the home cage. Mice were then placed back on the rotarod apparatus for three trials (maximal 3 min at accelerating speed 4–40 rpm) separated by a 30-minute rest period between trials. Mice were tested for 3 consecutive days, by which time a steady baseline level of performance was attained.
X-ray irradiation to eliminate neurogenesis
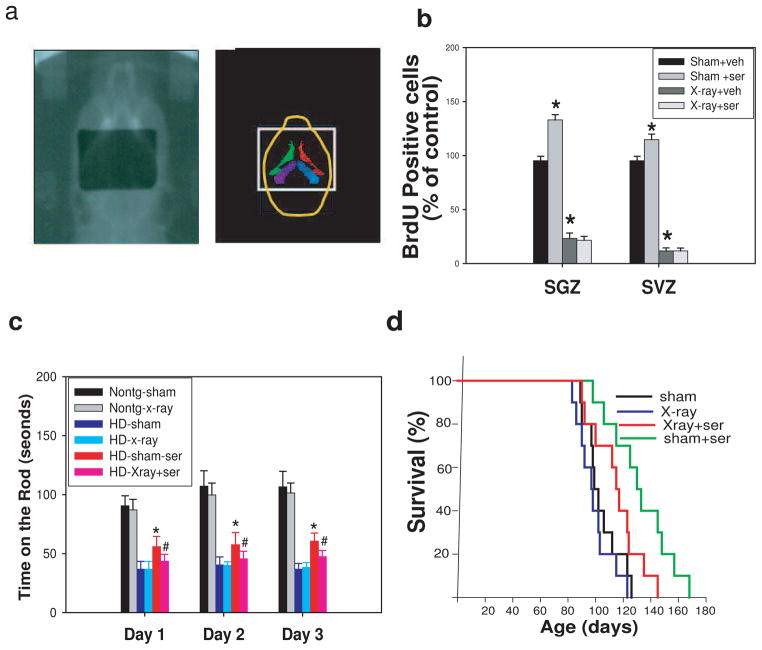
Radiation was delivered using the in-house small animal radiation research platform (SARRP) (Wong et al, 2007). The SARRP consists of an industrial X-ray tube with an adjustable anode voltage up to 225 kVp which delivers a beam through a collimator system. The animals were held on a robotic platform which permitted motion along the three principal axes as well as rotation. For these experiments, mice were positioned prone in a custom-designed stereotactic head holder system which was suitable for use of isoflurane anesthesia (2%). The beam was collimated using a custom lead cutout 8 × 10 mm at the isocenter (35 cm from the x-ray source). Using the robotic stages, the mice were positioned under the collimator. This set-up was intended to irradiate both the hippocampus and subventricular zone (SVZ) (see Figure 6a), regions that are known to harbor neuroprogenitor cells (Santarelli et al., 2003) and are known to be sensitive to radiation (Monje et al., 2002), while sparing the olfactory bulb and other brain parenchyma. Positioning was verified using radiographs taken at a source voltage of 100 kVp. After alignment, a single radiation dose of 10 Gy was delivered in 6.5 min. This radiation dose was measured using gafchromic EBT film and a water-equivalent solid water phantom arranged in a set-up to match the experimental conditions (For further details on film dosimetry see Deng et al. 2007). Sham radiations were performed by the same anesthesia procedure as above without X-ray delivery.
Figure 6.
Neurogenesis induced by sertraline is required for its survival extension effect and improvement of motor performance in HD mice. (a) Radiographic alignment of the mouse brain with 8 ×10 mm radiation treatment port (left) and corresponding structures (right). The stereotactic head holder is visible in the radiograph as bars on either side of the head. Structures in the right panel are the right and left lateral ventricles (green, red) and dentate gyrus of hippocampus (purple, blue) from the atlas of Paxions and Franklin (2001). (b) There was significant reduction of cell proliferation in both SVZ and SGZ by X-ray irradiation, which was not rescued by sertraline treatment. The values are mean and SE from four HD mice in each group. *p<0.05 compared to the values of sham-vehicle group by standard student t-tests. (c) X-ray irradiation abolished the improved motor performance by sertraline in HD mice. Data are presented as mean and SE from 10 mice in each group. *p<0.05 compared to the values of HD-sham group, #p<0.05 compared to the values of HD-sham-sertraline group by standard student t-tests. (d) The survival extension effect of sertraline was partially eliminated by X-ray irradiation. Survival was analyzed by Kaplan-Meier analysis. The p value between sham group and sham+ser group is 0.00125; the p value between X-ray+ser group and sham+ser group is 0.0248.
BrdU administration, immunohistochemistry, and stereological quantification
For the neurogenesis study, all mice were received twice daily injections of 50 mg/kg BrdU (5-bromo-2-deoxyuridine; Sigma) in sterile 0.9% NaCl solution at 8 h apart for 5 consecutive days. Animals were perfused at 3 days after the last BrdU injection for assessing proliferation, and perfused at 3 weeks after the last injection for study of survival of newly generated cells. BrdU immunostaining was performed as we previously described (Lee et al., 2002). Briefly, coronal brain sections (40 μm) were made, endogenous peroxidases were quenched with 0.6% H2O2 for 30 minutes, the sections were incubated in 2 N HCl for 30 minutes at 37 °C and washed in 0.1 M borate buffer (pH 8.5) for 10 minutes. For light microscope quantification of BrdU-labeled cells, a series of every sixth 40-μm section was used. Sections were blocked in 1 x TBS containing 3% horse serum and 0.3% Triton X-100 for 1 h, followed by incubation in anti-BrdU antibody (1:200, BD Biosciences) or EM48 (1:200, rabbit polyclonal anti-polyglutamine antibody, Millipore) overnight at 4 °C. Sections were then incubated with biotinylated anti-mouse IgG for 1 h at room temperature. The immunoreactive product was detected using the vectastain ABC kit enhancing system (Vector Laboratory. CA) with diaminobenzadine (DAB) as substrate. Sections were counterstained with Nissl substrate for stereological quantification.
Stereology counting was performed in serial coronal sections on blind-coded slides. The numbers of BrdU-positive cells in the subgranule zone (SGZ) of the dentate gyrus (DG) were assessed by counting all positive cells in sections at the levels of the bregma −1.34 to −2.80- by using conventional light microscopy with 40 × objective, and distance between the sections of 240 μm. The “optical fractionator” was used to count BrdU-positive cells in the DG (Schmidt-Hieber et al., 2004). The optical fractionator technique estimates the number of cells by multiplying the sum of cells counted by the reciprocal of the fraction of the region sampled. For analysis of BrdU cells in the subventricular zone (SVZ), only the side adjacent to the striatum was quantified. On each section, the region of interest was outlined unilaterally under a 5 × objective and the enclosed area was calculated. The stereology investigator software automatically identified and measured profiles. All computer-identified cell profiles were manually verified as neurons and exported to Microsoft Excel. Cross-sectional areas were analyzed by using Sigmastat statistical program.
Measurement of volume of striatum and size of lateral ventricles
The volume of the striatum and lateral ventricle by histological analysis was evaluated according to the principle of Cavalieri (Cyr et al., 2005) (volume = s1d1 + s2d2…sndn, where s = surface area and d = distance between two sections). We used different sets of mice for the volumetric measurement study. Each group contained four mice. The Cavalieri approach requires an initial random cut through the reference space of interest, with subsequent cuts at consistent intervals. Provided that the sections through the reference space are systematic-random, and that all sections through the reference space had an equal probability of being sampled, the Cavalieri method gives an unbiased total volume estimate from reference areas on sections. By using the Cavalieri principle, the mean total volume of an arbitrary-shaped reference space can be estimated without bias from the areas on systematic-uniform-random sections. The Cavalieri principle was applied to histological data because of the limited number of sections selected. We chose the first section when the striatum was first detected, and chose every four series sections for volumetric analysis. We considered 11 anatomical levels of the striatum for the volume estimation of each brain by randomly selecting the first section. Images were captured by using a light microscope coupled to a color digital camera (Retina 2000R, QImaging). The surface area was calculated in each of the 11 Nissl-stained sections by contour drawing the striatum and lateral ventricle on each brain hemisphere with Aperio Imagescope software (Aperio Technologies). Data were expressed as the average Cavalieri volume (mm3) ± SEM of four mice per group.
Measurement of sertraline by HPLC/MS/MS
To measure the levels of sertraline in both blood and brain, both transgenic and nontransgenic littermates (6-week-old) were used. Four nontransgenic control mice and four HD mice were used in the study. Whole brain homogenates were mixed and centrifuged at 2500 × rpm for 10 minutes at 4 °C. A volume of 100 μl of the top organic layer was transferred to a disposable borosilicate glass culture tube (13×100 mm) and 100 μl of deionized water were added to this tube, mixed vigorously for 10 s, and centrifuged at 13,000 × rpm for 5 minutes. Volumes of 20 μl were injected onto the HPLC instrument for quantitative analysis. Serum samples were prepared as previously described (Zhao et al., 2005). Chromatographic analysis was performed with a Waters X-Terra ™ MS (C18 3.5 μm, 50 × 2.1 mm i.d). Separation of the analyses from potential interfering material was achieved at ambient temperature by using Waters X-Terra ™ MS (C18 3.5 μm, 50 × 2.1 mm i.d) packed with a 3.5-μM ODS stationary phase, protected by a guard column packed with Waters Xterra TM (RP18, 3 μm). The mobile phase used for chromatographic separation was composed of 70% formic acid (0.1%) in CH3CN, 30% ammonium acetate (10 mM), and was isocratic at a flow rate of 0.2 ml/min. Method validation runs for serum and brain tissue calibration standard and quality controls were performed on 3 consecutive days and include a calibration curve processed in duplicate, and quality control samples at four different concentrations in triplicate. The accuracy and precision of the assay was assessed by the mean relative percentage deviation from the nominal concentrations and the within- and between-run, respectively.
ELISA analysis of BDNF protein levels
Brain tissues were homogenized in lysis buffer (137 mM NaCl, 20 mM Tris, 1% NP-40 detergent, 10% glycerol, 1 mM phenylmethylsulfonylfluoride, 10 mg/ml aprotinin, 1 mg/ml leupeptin, and 0.5 mM sodium orthovanadate; pH 7.2) at 4°C. Homogenates were centrifuged at 2000 × g for 20 minutes at 4°C, and supernatants were used for ELISA analysis. BDNF protein levels were quantified by the BDNF Emax Immunoassay (Promega) as described previously (Duan et al., 2003). The intra- and inter-assay variability for the BDNF ELISA was 0.55 pg and 1.24 pg, respectively. The concentrations of BDNF in each sample were determined in duplicate, and the average of the two values was used as the value for that mouse.
Measurement of serotonin levels
Brain regions obtained from each animal were weighed and ultrasonicated in 0.1 M perchloric acid. Tissues were weighed and stored at −80°C until extraction. The tissues obtained from each animal were homogenized in 0.1 M perchloric acid and centrifuged at 25,000 × g for 12 minutes. Serotonin contents in supernatants of the brain homogenates were measured by HPLC with electrochemical detection (Krasnova et al., 2000). The analytical column was a Symmetry C18 3.5 μm (4.6×150.0 mm) from Waters (Milford, MA). The mobile phase consisted of 0.01 M sodium dihydrogen phosphate, 0.01 M citric acid, 2 mM sodium EDTA, 1 mM sodium octylsulfate, 10% methanol, pH 3.5, and was used at flow rate 0.9 ml/min and temperature of 25°C. The installation consisted of a Waters 717 Plus automated injection system, a Waters 1525 Binary pump, and Coulochem III detector (ESA, Chelmsford, MA). The Waters Breeze system was used for data collection and analysis.
Western blot analysis
Briefly, 50 μg of soluble proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was blocked in presence of 5% nonfat milk, and then incubated overnight with primary antibodies at 4 °C: mouse monoclonal anti-HSP70, (1:5000, Sigma), mouse monoclonal anti-β actin, (1:5000, Sigma), mouse anti-MnSOD (1:1000, R&D system, Inc), mouse anti-Bcl-2 (1:1000, cell signaling). The membrane was then exposed for 1 h to HRP-conjugated secondary antibody (1:3000; Jackson ImmunoResearch Labs) and immunoreactive proteins were visualized using a chemiluminescence-based detection kit according to the manufacturer’s protocol (ECL kit; Amersham Corp.).
Double immunofluoresent staining
Sections were quenched and blocked as described above, then incubated in a mixture of antibodies (rat anti-BrdU, 1:200, Serotec; mouse anti-NeuN, 1:200, or rabbit anti-GFAP, 1:1000, Chemicon Inc) overnight at 4 °C. A cocktail of secondary antibodies (FITC-anti-rat, Serotec; Cy3-anti-mouse or Cy3 anti-rabbit, Jackson Immuno Inc) was applied for 1 h at room temperature. Images were captured and analyzed in Zeiss LSM 510 confocal microscope.
Statistics
The data are expressed as the mean ± SEM or SE. Statistical comparisons between two groups were compared by student t-test. Survival data were analyzed by Kaplan-Meier analysis.
Results
Sertraline extends survival and ameliorates impaired motor performance of HD mice when administered after the onset of motor dysfunction
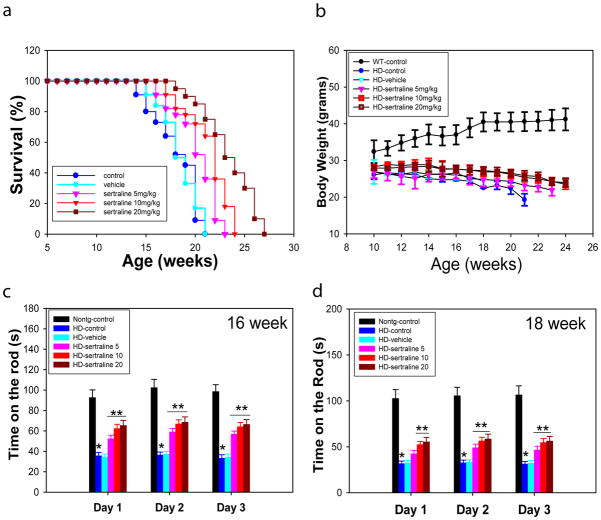
In order to determine whether sertraline might modify the course of the symptomatic phase of the disease, beginning at 12 weeks of age (age of onset of motor deficit), HD mice were injected with sertraline (5, 10, or 20 mg/kg daily injection) or vehicle 0.2% tween-80 (0.1 ml/10 g body weight). The survival of sertraline-treated HD mice was significantly and dose-dependently increased compared to the survival of vehicle -treated HD mice (Fig. 1a). Sertraline increased the median lifespan by approximately 10% at dose of 5 mg/kg group, 15% at dose of 10 mg/kg group, and 20% in the 20 mg/kg sertraline-treated group (Fig. 1a). HD mice exhibit a progressive weight loss, but sertraline administration only slightly attenuated the body weight loss (Fig. 1b)
Figure 1.
Sertraline increases survival and improves motor behavioral performance, but does not affect body weight loss in HD mice. Twelve-week-old male HD mice were administered sertraline daily at doses of 5, 10, and 20 mg/kg or vehicle (0.2% tween -80) until the end of study. (a) Survival of HD mice was significantly extended by sertraline treatment in a dose-dependent manner. The p values are 0.041 between vehicle and sertraline 5 mg/kg groups, 0.039 between vehicle and sertraline 10 mg/kg groups, and 0.012 between vehicle and sertraline 20 mg/kg groups by Kaplan-Meier analysis. (b) There was no significant difference in body weight loss by sertraline treatment. (c and d) Motor behavioral performance was evaluated by an accelerating rotarod apparatus in 16-(c) and 18-(d) week-old mice, n=8–15. The values are the mean and SE. *p<0.05, compared to the value of nontransgenic-control group; **p<0.05 compared to the values of HD-vehicle group by Standard student t-tests.
We next evaluated motor function in HD mice at indicated ages. There was significant impairment in performance of HD mice on the rotarod apparatus, indicated by reduced time spent on the rotarod compared to time spent by the nontransgenic mice. HD mice treated with sertraline exhibited superior motor performance compared to vehicle-treated or control HD mice (Fig. 1c, d).
Sertraline reduces brain atrophy not huntingtin inclusions
To determine whether the improved motor function and increased survival of HD mice treated with sertraline resulted from a slowed progression of the neurodegenerative process and/or prevention of pathogenesis in the brain, we performed histological analyses of the brains of HD mice that had been treated with sertraline or vehicle. As the disease progressed, brain atrophy occurred in the HD mice as indicated by an increase in the size of the lateral ventricles and decrease in volume of striatum compared to those in nontransgenic mice (Fig 2a–c). The magnitude of lateral ventricular enlargement and striatal atrophy was significantly decreased in HD mice that had been treated with sertraline compared to that of HD mice treated with vehicle (Fig. 2b &c). Intranuclear inclusions of aggregated huntingtin protein in striatal and cortical neurons were present in HD mice, as well as in HD patients (Ho et al., 2001). Whether the aggregation is pathogenic or a protective response is still controversial. Nevertheless, huntingtin aggregation acts as a phenotypic readout that can reflect pathologically relevant processes such as huntingtin cleavage, misfolding, proteolysis, transglutaminase activity, oxidative stress, and protein sequestration. The mutant huntingtin inclusion is a marker to evaluate effect of drugs and to determine a potential mechanism for the drugs. We used immunostaining by EM48 antibody to visualize the mutant huntingtin aggregates in HD mouse brains. Sertraline treatment did not affect huntingtin inclusions (Fig 2d).
Figure 2.
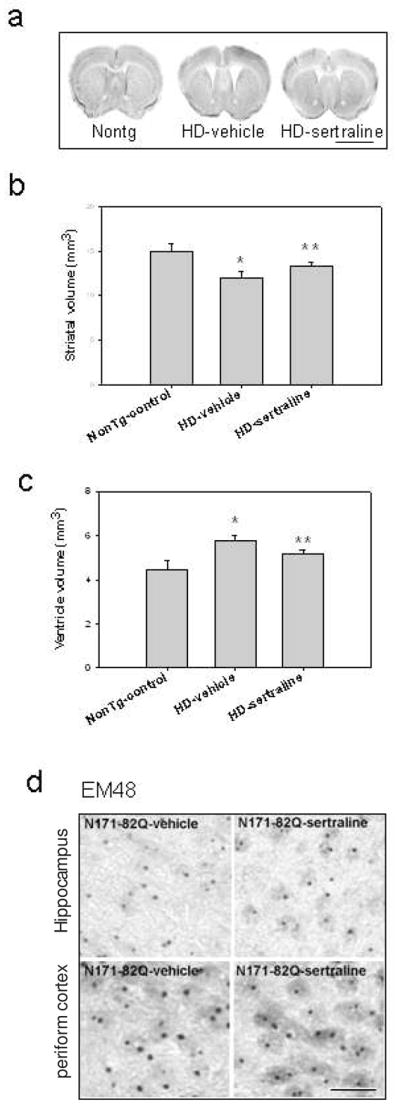
Sertraline reduces brain atrophy but not huntingtin inclusions in N171-82Q HD mice. (a) Representative photomicrographs of brain sections of HD mice and nontransgenic mice (nontg), scale bar=120 μm. (b) Quantification of striatal volume and (c) cerebral lateral ventricle volume. Data are presented as mean and SE, n= 4 mice in each group. *p<0.05, compared to the value of nontg control group; **p<0.05 compared to the value of HD-vehicle group by Standard Student t-tests. (d) Representative micrographs of htt inclusions immunostained by EM48 antibody in HD mice treated with sertraline or vehicle. Scale bar =20 μm.
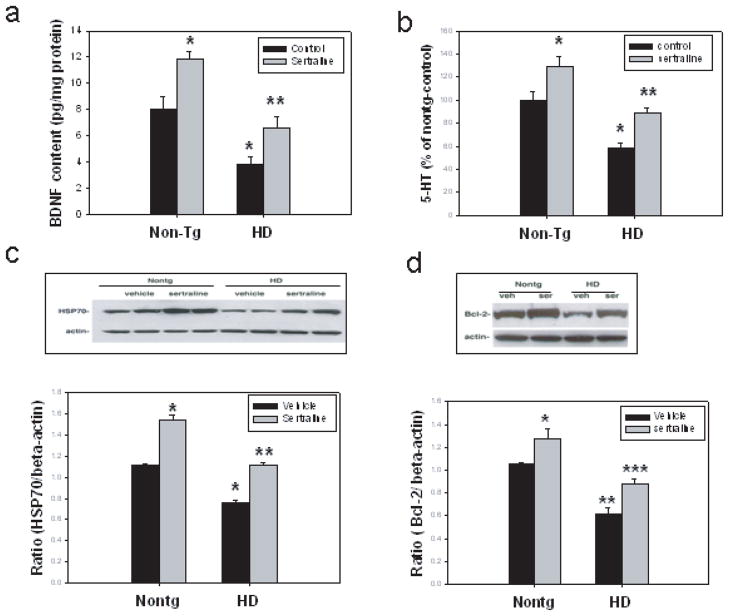
Sertraline attenuates depletion of BDNF and serotonin, and increases levels of HSP70 and Bcl-2 in HD mice
BDNF protein levels were significantly decreased, by more than 50%, in the cortex of HD mice compared to the cortex of nontransgenic mice. Sertraline administration significantly increased BDNF protein levels in both HD and NonTg mice (Fig. 3a). We also measured the content of serotonin (5-HT) in striatal tissues of mice. There was significant depletion of 5-HT levels in striatal tissues of N171-82Q HD mice. These results are consistent with disrupted serotonergic system demonstrated in other HD mice (Yohrling IV et al., 2002; Reynolds et al., 1999). Sertraline treatment significantly increased serotonin levels both in HD and nontransgenic mice (Fig. 3b).
Figure 3.
Sertraline normalizes BDNF protein levels and serotonin (5-HT) levels, and upregulates HSP70 and Bcl-2 levels. Twelve-week-old mice were administered sertraline at 10 mg/kg. Mice were euthanized 4 weeks after sertraline administration, (a) BDNF protein levels were measured in cortical tissue by ELISA. (b) Serotonin levels were measured by HPLC method in striatum. The values are the mean and SE, n=6. *p<0.05, compared to the values of nontg control group, **p<0.05 compared to the values of HD-control group by Standard Student t-tests. (c and d). HSP70 protein levels and Bcl-2 levels were measured in striatal tissues by Western blot analysis, the top panels are representative blots, and the bottom panel is the quantification data (mean and SE) from three mice in each group, *p<0.05 compared to the values of nontg-vehicle group; **p<0.05 compared the values of HD-vehicle group by Standard Student t-tests.
Mutant huntingtin interacts with chaperone proteins, and decreases their ability to refold abnormal structured proteins including huntingtin (Landles and Bates, 2004). We found that HSP70 levels were decreased in N171-82Q HD mice (Duan et al., 2003). We therefore investigated whether sertraline modulates HSP70 levels. The levels of HSP-70 were reduced in striatum of HD mice, consistent with our previous findings. Sertraline administration restored HSP-70 levels in HD mice (Fig. 3c).
Neuroprotective effects of BDNF may result from induction of the expression of cytoprotective molecules such as Bcl-2 (Schabitz et al., 2000) and MnSOD (Mattson et al., 1995). In order to determine whether these cytoprotective proteins are altered by mutant huntingtin and sertraline, we performed Western blots on mouse striatal tissues. There were decreased levels of Bcl-2 protein in HD mice and sertraline treatment increased Bcl-2 levels in HD mice and nontransgenic mice as well (Fig 3d). There was no change of MnSOD levels in HD mice and mice treated with sertraline (data not shown).
Sertraline increases basal neurogenesis and ameliorates impaired neurogenesis in HD mice
In the hippocampus of both rodents and primates, adult-generated neuronal cells arise from progenitor cells in the SGZ and migrate into granule cell layer, where they differentiate into granular neurons. These neurons have been shown to be capable of functional integration into hippocampal circuitry. The newly generated cells in SVZ can migrate into affected striatum and differentiated to medium spiny neurons in HD (Jin et al., 2005) and under ischemic conditions (Yamashita et al., 2006).
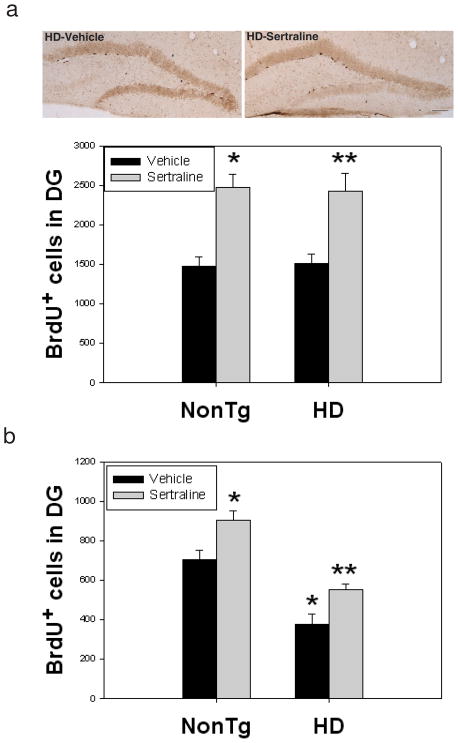
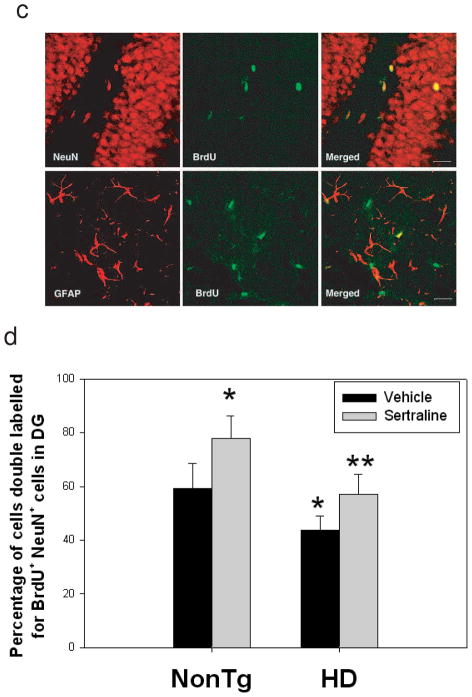
There are some controversial reports on neurogenesis between HD human brains and mouse models (Phillips et al., 2006; Phillips et al., 2005; Grote et al., 2005; Gil et al., 2005; Tattersfield et al., 2005; Lazic et al., 2004; Curtis et al., 2003). It has been reported that altered neurogenesis appeared in other HD mice such as R6/2 mice (Phillips et al., 2006; Phillips et al., 2005; Gil et al., 2005) and R6/1 mice (Lazic et al., 2004). There is no report on the status of neurogenesis in N171-82Q HD mice. In the present study, we asked whether there is an alteration of neurogenesis affected by mutant huntingtin and sertraline. There was no difference in numbers of proliferating cells in the SGZ of hippocampus between HD mice and nontransgenic mice. Sertraline increased proliferation of progenitor cells as evidenced by increased numbers of cells that incorporated BrdU in both genotypes (Fig 4a). There was a significant decrease of survival of newly generated cells (BrdU-positive cells) in HD mice compared to that in nontransgenic control mice. Sertraline treatment significantly enhanced survival of newly generated cells (Fig. 4b). Analysis of BrdU-positive cells showed that over 60% BrdU-positive cells expressed NeuN in nontransgenic control mice (Fig 4 c&d). The percentages of NeuN-positive BrdU-positive cells were decreased in HD mice. Sertraline administration increased the percentage population of cells co-labeled with BrdU and NeuN, indicating that sertraline promotes cells to differentiate to neurons (Fig 4d).
Figure 4.
Sertraline increases neurogenesis in subgranular zone (SGZ) of dentate gyrus in HD mice and control mice. (a) Sertraline (10 mg/kg) was administered to mice at 12 weeks of age for 4 weeks. Mice then received 5-bromo-2-deoxyuridine (BrdU) and were perfused at 3 days after the last BrdU injection for cell proliferation, Scale bar = 100 μm. (b) mice were perfused 3 weeks after the last BrdU injection for quantifying survival of newly generated cells. (c) representative sections were double labeled with anti-BrdU and anti-NeuN antibody (neuronal marker) or anti-GFAP antibody (astrocyte marker) to identify the phenotypes of newly generated cells. Representative images from confocal microscope, green shows BrdU-positive staining and red shows NeuN- or GFAP-positive staining. Scale bar = 20 μm. (d) Quantification of newly generated neurons in dentate gyrus. All values are the mean and SE. n=4 mice per group. *p<0.05, compared to the values of nontg-vehicle group, **p<0.05 compared to the values of HD vehicle group by standard student t-tests.
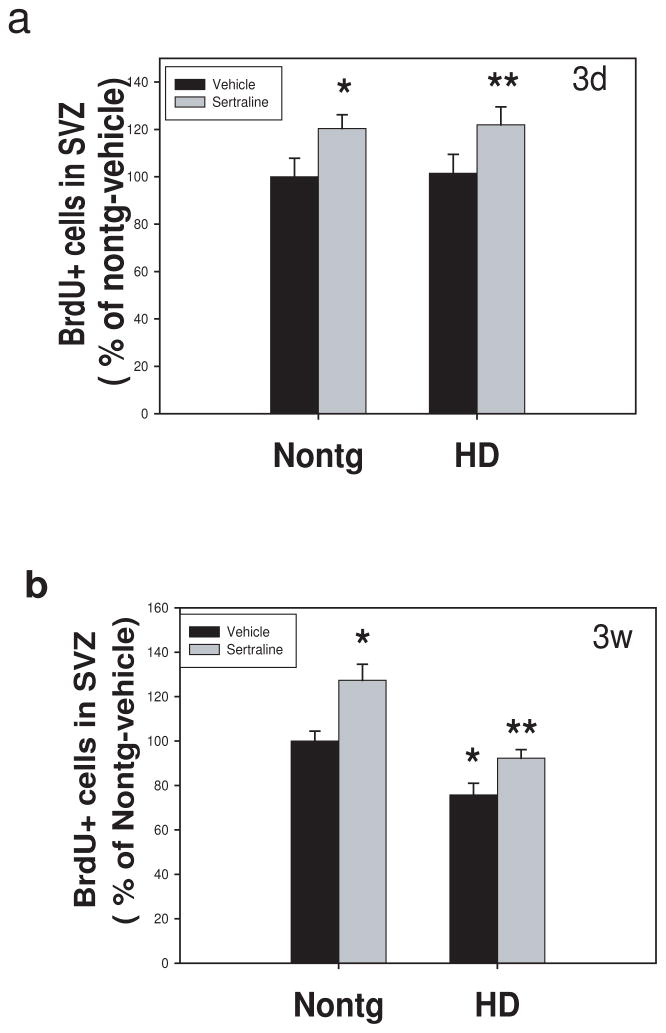
There was no difference in cell proliferation in SVZ between HD and nontransgenic mice (Fig. 5a). In contrast, there was a significant decrease in survival of BrdU cells in SVZ (Fig. 5b). Sertraline administration significantly increased both cell proliferation and survival of newly generated cells in SVZ. These results indicate that sertraline increases neurogenesis by increasing neural stem cell proliferation, enhancing survival, and promoting differentiation into neurons.
Figure 5.
Sertraline increases neurogenesis in subventricular zone (SVZ) of mice. Mice were administered sertraline at 10 mg/kg from 12 weeks of age for 4 weeks. Mice were perfused at 3 days after the last BrdU injection for cell proliferation study indicating BrdU-positive cells in SVZ (a) and 3 weeks after the last injection for quantifying survival of newly generated cells indicated by remaining BrdU-positive cells (b). All values are the mean and SE. n=4 mice per group. *p<0.05, compared to the values of nontg vehicle group, **p<0.05 compared to the values of HD vehicle group by standard student t-tests.
Increased neurogenesis mediates the beneficial effects of sertraline in HD mice
In order to determine whether increased neurogenesis by sertraline is required for its protection in HD mice, we employed X-ray irradiation (10 Gy) to eliminate the neurogenesis in both SGZ and SVZ regions (Fig 6a), as this dose X-ray has been shown to cause long periods of decreased neurogenesis (Mizumatsu et al., 2003; Tada et al., 2000). We treated mice with either X-ray or sham operation at 12 weeks of age; mice then received sertraline or vehicle daily injection until the end of study, and BrdU was injected at 4 weeks after sertraline administration. Some of the mice were euthanized at 3 days after BrdU injection to analyze neurogenesis by BrdU incorporation. There was a significant decrease of the numbers of cells incorporating BrdU in both SVZ and SGZ of hippocampus (Fig 6b), suggesting that neurogenesis was inhibited by X-ray irradiation. Blocking the neurogenesis by X-ray abolished the protection of motor function by sertraline in HD mice (Fig 6c), and significantly eliminated the extended survival by sertraline (Fig 6d). These results suggest that neurogenesis is a mediator of neuroprotection provided by sertraline in HD mice.
Effective levels of sertraline in mice are comparable to achievable levels in humans
In order to study whether effective doses in mice are comparable to doses achievable in humans, we established the LC/MS/MS method to measure the levels of sertraline in blood and brain tissues. Sertraline was injected daily to mice for a week, and blood samples and brain tissues were collected for measuring drug concentrations. The blood concentrations are 25.1 ± 4.9 ng/ml (mean ± SD) at 5 mg/kg group and 43.4 ± 4.3 ng/ml (Mean ± SD) at 10 mg/kg group, these levels are comparable to the effective levels in human patients with depression (oral administration of 100–200 mg/day, the blood concentration ranges around 40–60 ng/ml). Drug concentrations in brain tissues are 920 ± 230 ng/g wet weight at 5 mg/kg group and 2090 ± 380 ng/g wet weight at 10 mg/kg group, the brain/blood ratio of sertraline is over 35, indicating higher penetration of sertraline through the blood-brain barrier.
Discussion
Sertraline has been widely used to treat depressed patients, including those with HD (Slaughter et al 2001). Whether sertraline has a neuroprotective effect, however, is not known. In the current study, we provide evidence that sertraline suppressed brain atrophy, improved motor performance, and increased the survival in HD mice, and most importantly, sertraline increased neurogenesis in HD mice, which is required for its beneficial effect of in HD mice. This is also the first report that impaired neurogenesis occurs in N171-82Q HD mice.
The mechanism of how mutant huntingtin leads to the devastating course of events that culminates in cell death in HD remains elusive. It has been reported that levels of serotonin are decreased in the striatum of HD mice (Reynolds et al., 1999) and the serotonergic system is disrupted in earlier stages of disease in other HD mice (Yohrling IV et al., 2002). Our previous study (Duan et al., 2004) showed reduced 5-HT levels in N171-82Q mice and we had consistent results in the current study. These reports are in contrast to those reported in human postmortem HD brains, however. Specifically, several groups have found that 5-HT levels are elevated in blood and brains of HD patients (Reynolds and Pearson 1987; Kish et al., 1987; Beal MF et al., 1990). One possibility is that striatal atrophy that occurs in HD may account for these findings. There is a reciprocal interaction between BDNF and serotonergic signaling, in which BDNF enhances serotonin production and release, while serotonin stimulates BDNF production. Serotonin might also have direct neuroprotective effects on striatal and cortical neurons, because other signals that activate cyclic AMP and CREB have been shown to protect neurons against excitotoxic, oxidative, and metabolic insults relevant to HD (Mattson et al., 2004). The decreased levels of BDNF in the HD brains also suggest reduced ability of neurons to cope with stress in HD. SSRIs increase extracellular serotonin levels and serotonin synthesis (Goggi et al., 2002), which consequently could enhance serotonergic signaling and increase BDNF expression. We confirmed these effects of sertraline in the current studies.
The protein folding system has been shown to be compromised in HD. We and others found that chaperone proteins are decreased in brains of HD mice (Landles and Bates, 2004; Duan et al., 2003). The decreased levels of HSP-70 in the brains of HD mice suggest a reduced ability of neurons to cope with stress in HD. Sertraline increased HSP70 levels in brain cells, suggesting a plausible mechanism whereby sertraline reduced pathological changes in the HD mice. Bcl-2 is a target gene of cyclic AMP-CREB signaling, which is known to be a cell-protective protein. We indeed found that Bcl-2 levels are decreased in N171-82Q HD brains. Reduced Bcl-2 protein levels in HD mice indicate that neurons in HD mice have reduced capacity to cope with further insults and stress. Sertraline-treated mice have levels of Bcl-2 similar to those in normal mice, suggesting that sertraline treatment improved cellular protective ability in neurons of HD, which may contribute its beneficial effects.
Adult neurogenesis might be part of the physiological regenerative response and might thereby alter or alleviate symptoms, but it might also become impaired by the disease mechanism and thereby contribute to the symptoms of neurodegeneration. The increased neurogenesis in human HD indicates that neurogenesis is stimulated in the disease, possibly representing an adaptive response directed toward neuronal replacement (Curtis et al., 2003; Curtis et al., 2007). There is a significant decrease of neurogenesis in HD mice, however, which suggests that neurogenesis is compromised in HD mice. Two brain regions have been identified by the active adult neurogenesis: subventricular zone (SVZ) and subgranular zone (SGZ) of the hippocampus. In response to the degeneration that occurs in the caudate nucleus in Huntington’s disease, the SVZ increases production of progenitor cells that migrate towards the site of the damage where they can differentiate into mature neurons and glial cells (Cutis et al., 2007). The SVZ comprises heterogeneous cell types that are maintained in an environment that is permissive to neurogenesis and gliogenesis; and this zone responds to neurodegenerative changes in adjacent brain regions by increasing progenitor cell proliferation and neurogenesis in an attempt to replace the cells that die as a result of neurodegeneration. At the earlier stage of disease, when neurons self-regenerate, neurodegeneration can be overcome, cells are still functional, and no severe symptoms appear. As the disease progresses, the regeneration ability may not be sufficient to cope with the neurodegenerative process, and symptoms will start to appear. It was reported that HD patients had increased cell proliferation as estimated by cell cycle marker proliferating cell nuclear antigen (PCNA) immunostaining (Curtis et al. 2003). We did not find differences in the number of proliferating cells by labeling with BrdU in the SVZ of N171-82Q mice compared to that in nontransgenic control mice. It is possible that there is a difference in markers for labeling proliferating cells. PCNA is expressed in cells undergoing DNA repair (Tomasevic et al., 1998). The different stage of disease observed in HD patients and HD mice may also contribute to the discrepancy. Further analysis of HD brains by using combinations of several markers of proliferation is therefore warranted.
Previous studies reported that neurogenesis is required for behavioral effects of SSRIs in normal mice (Santarelli et al., 2003). We found that neurogenesis was decreased in N171-82Q mice and that neurogenesis was enhanced by sertraline administration. To further address the question whether increased neurogenesis is required for the beneficial effects of sertraline in HD mice, we used X-ray to block the neurogenesis in both SVZ and SGZ. We confirmed that blocking neurogenesis abolished the improvement of motor function and significantly reduced the extension of survival by sertraline treatment in HD mice, suggesting that the neurogenesis induced by sertraline is required for its beneficial effects in HD. These findings also indicate that drugs that promote neurogenesis might be beneficial in HD.
Misfolding and aggregation of abnormal proteins are believed to be on the pathogenic pathway in HD; however, several lines of evidence indicate that inclusions are not the main cause of toxicity, and may represent a cellular protective response (DiFiglia., 1997; Bates 2003). Developing therapies for the misfolding diseases requires finding chemical compounds that can either clear toxic misfolded protein, or can protect neurons from their impact (Ross and Poirier 2004 & 2005; Bodner et al., 2006;). Mutant huntingtin engages multiple pathogenic pathways leading to neuronal death, and mutant huntingtin aggregation may not be a predictor of cell loss (Aronin et al., 1999; Kummerle et al., 1999); thus aggregation of mutant huntingtin can be dissociated from the extent of cell death. This is the case in the effect of sertraline. There was no effect of sertraline on neuronal inclusions in our study, suggesting that beneficial effects of sertraline were not mediated by inhibiting htt aggregation.
Finally, we emphasize that the effective levels of sertraline in HD mice are comparable to the achievable levels in human, which is very safe for human. Since sertraline is an FDA-approved drug with few side effects after long term administration, the preclinical findings obtained in the present study provide a rationale for moving directly to clinical trials of SSRI in humans with HD.
Acknowledgments
We thank Gay Rudow in Neuropathology at Johns Hopkins University for expert technical support with the stereology analysis, Laragen Inc for genotyping service, and Dr. Pamela Talalay for her dedicated editorial assistance. This research was supported by the High Q foundation (to W. Duan) and NS NINDS 16375 (to CAR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aronin N, Kim M, Laforet G, DiFiglia M. Are there multiple pathways in the pathogenesis of Huntington’s disease? Philos Trans R Soc Lond B Biol Sci. 1999;354(1386):995–1003. doi: 10.1098/rstb.1999.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates G. Huntingtin aggregation and toxicity in Huntington’s disease. Lancet. 2003;361(9369):1642–4. doi: 10.1016/S0140-6736(03)13304-1. [DOI] [PubMed] [Google Scholar]
- Beal MF, Walker LC, Storey E, Segar L, Price DL, Cork LC. Kynurenine pathway measurements in Huntington’s disease striatum: evidence for reduced formation of kynurenic acid. J Neurochem. 1990;55(4):1327–39. doi: 10.1111/j.1471-4159.1990.tb03143.x. [DOI] [PubMed] [Google Scholar]
- Bemelmans AP, Horellou P, Pradier L, Brunet I, Colin P, Mallet J. Brain-derived neurotrophic factor-mediated protection of striatal neurons in an excitotoxic rat model of Huntington’s disease, as demonstrated by adenoviral gene transfer. Hum Gene Ther. 1999;10(18):2987–97. doi: 10.1089/10430349950016393. [DOI] [PubMed] [Google Scholar]
- Bodner RA, Housman DE, Kazantsev AG. New directions for neurodegenerative disease therapy: using chemical compounds to boost the formation of mutant protein inclusions. Cell Cycle. 2006;5(14):1477–80. doi: 10.4161/cc.5.14.2929. [DOI] [PubMed] [Google Scholar]
- Canals JM, Pineda JR, Torres-Peraza JF, Bosch M, Martin-Ibanez R, Munoz MT, Mengod G, Ernfors P, Alberch J. Brain-derived neurotrophic factor regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington’s disease. J Neurosci. 2004;24(35):7727–39. doi: 10.1523/JNEUROSCI.1197-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Starling AJ, Wu N, Nguyen OK, Uzgil B, Soda T, Andre VM, Ariano MA, Levine MS. Increased GABAergic function in mouse models of Huntington’s disease: reversal by BDNF. J Neurosci Res. 2004;78(6):855–67. doi: 10.1002/jnr.20344. [DOI] [PubMed] [Google Scholar]
- Cha JH. Transcriptional dysregulation in Huntington’s disease. Trends Neurosci. 2003;23:387–392. doi: 10.1016/s0166-2236(00)01609-x. [DOI] [PubMed] [Google Scholar]
- Conti L, Reitano E, Cattaneo E. Neural stem cell systems: diversities and properties after transplantation in animal models of diseases. Brain Pathol. 2006;16:143–154. doi: 10.1111/j.1750-3639.2006.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Cowan CM, Raymond LA. Selective neuronal degeneration in Huntington’s disease. Curr Top Dev Biol. 2006;75:25–71. doi: 10.1016/S0070-2153(06)75002-5. [DOI] [PubMed] [Google Scholar]
- Curtis MA, Penney EB, Pearson AG, van Roon-Mom WM, Butterworth NJ, Dragunow M, Connor B, Faull RL. Increased cell proliferation and neurogenesis in the adult human Huntington’s disease brain. Proc Natl Acad Sci U S A. 2003;100:9023–9027. doi: 10.1073/pnas.1532244100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Eriksson PS, Faull RL. Progenitor cells and adult neurogenesis in neurodegenerative diseases and injuries of the basal ganglia. Clin Exp Pharmacol Physiol. 2007;34:528–532. doi: 10.1111/j.1440-1681.2007.04609.x. [DOI] [PubMed] [Google Scholar]
- Cyr M, Caron MG, Johnson GA, Laakso A. Magnetic resonance imaging at microscopic resolution reveals subtle morphological changes in a mouse model of dopaminergic hyperfunction. NeuroImage. 2005;26:83–90. doi: 10.1016/j.neuroimage.2005.01.039. [DOI] [PubMed] [Google Scholar]
- Deng H, Kennedy CW, Armour E, Tryggestad E, Ford E, McNutt T, et al. The small-animal radiation research platform (SARRP): Dosimetry of a focused lens system. Phys Med Biol. 2007;52(10):2729–40. doi: 10.1088/0031-9155/52/10/007. [DOI] [PubMed] [Google Scholar]
- DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel JP, Aronin N. Aggregation of huntingtin in neuronal intracellular inclusions and dystrophic neuritis in brain. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- Duan W, Guo Z, Jiang H, Ware M, Li XJ, Mattson MP. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc Natl Acad Sci U S A. 2003;100:2911–2916. doi: 10.1073/pnas.0536856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W, Guo Z, Jiang H, Ladenheim B, Xu X, Cadet JL, Mattson MP. Paroxetine retards disease onset and progression in Huntingtin mutant mice. Ann Neurol. 2004;55:590–4. doi: 10.1002/ana.20075. [DOI] [PubMed] [Google Scholar]
- Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med. 2004;5:11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- Ferrigno P, Silver PA. Polyglutamine expansions: proteolysis, chaperones, and the dangers of promiscuity. Neuron. 2000;26:9–12. doi: 10.1016/s0896-6273(00)81132-0. [DOI] [PubMed] [Google Scholar]
- Gauthier LR, Charrin BC, Borrell-Pages M, Dompierre JP, Rangone H, Cordelieres FP, De Mey J, MacDonald ME, Lessmann V, Humbert S, Saudou F. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118:127–138. doi: 10.1016/j.cell.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Gil JM, Mohapel P, Araujo IM, Popovic N, Li JY, Brundin P, Petersen A. Reduced hippocampal neurogenesis in R6/2 transgenic Huntington’s disease mice. Neurobiol Dis. 2005;20:744–751. doi: 10.1016/j.nbd.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Goggi J, Pullar IA, Carney SL, Bradford HF. Modulation of neurotransmitter release induced by brain-derived neurotrophic factor in rat brain striatal slices in vitro. Brain Res. 2002;941:34–42. doi: 10.1016/s0006-8993(02)02505-2. [DOI] [PubMed] [Google Scholar]
- Grote HE, Bull ND, Howard ML, van Dellen A, Blakemore C, Bartlett PF, Hannan AJ. Cognitive disorders and neurogenesis deficits in Huntington’s disease mice are rescued by fluoxetine. Eur J Neurosci. 2005;22:2081–2088. doi: 10.1111/j.1460-9568.2005.04365.x. [DOI] [PubMed] [Google Scholar]
- Ho LW, Carmichael J, Swartz J, Wyttenbach A, Rankin J, Rubinsztein DC. Differential loss of striatal projection neurons in Huntington disease. Proc Natl Acad Sci USA. 1998;85:5733–5737. doi: 10.1073/pnas.85.15.5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, LaFevre-Bernt M, Sun Y, Chen S, Gafni J, Crippen D, Logvinova A, Ross CA, Greenberg DA, Ellerby LM. FGF-2 promotes neurogenesis and neuroprotection and prolongs survival in a transgenic mouse model of Huntington’s disease. Proc Natl Acad Sci U S A. 2005;102:18189–94. doi: 10.1073/pnas.0506375102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kells AP, Fong DM, Dragunow M, During MJ, Young D, Connor B. AAV-mediated gene delivery of BDNF or GDNF is neuroprotective in a model of Huntington disease. Mol Ther 2004. 2004 May;9(5):682–8. doi: 10.1016/j.ymthe.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, Hornykiewicz O. Elevated serotonin and reduced dopamine in subregionally divided Huntington’s disease striatum. Ann Neurol. 1987;22(3):386–9. doi: 10.1002/ana.410220318. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Bychkov ER, Lioudyno VI, Zubareva OE, Dambinova SA. Intracerebroventricular administration of substance P increases dopamine content in the brain of 6-hydroxydopamine-lesioned rats. Neuroscience. 2000;95:113–117. doi: 10.1016/s0306-4522(99)00400-5. [DOI] [PubMed] [Google Scholar]
- Kuemmerle S, Gutekunst CA, Klein AM, Li XJ, Li SH, Beal MF, Hersch SM, Ferrante RJ. Huntington aggregates may not predict neuronal death in Huntington’s disease. Ann Neurol. 1999;46(6):842–9. [PubMed] [Google Scholar]
- Landles C, Bates GP. Huntingtin and the molecular pathogenesis of Huntington’s disease. Fourth in molecular medicine review series. EMBO Rep. 2004;5:958–963. doi: 10.1038/sj.embor.7400250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazic SE, Grote H, Armstrong RJ, Blakemore C, Hannan AJ, van Dellen A, Barker RA. Decreased hippocampal cell proliferation in R6/1 Huntington’s mice. Neuroreport. 2004;15:811–813. doi: 10.1097/00001756-200404090-00014. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Lynch G, Kramar EA, Rex CS, Jia Y, Chappas D, Gall CM, Simmons DA. Brain-derived neurotrophic factor restores synaptic plasticity in a knock-in mouse model of Huntington’s disease. J Neurosci. 2007;27(16):4424–34. doi: 10.1523/JNEUROSCI.5113-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Blendy JA. Antidepressant action: to the nucleus and beyond. Trends Pharmacol Sci. 2005;26:631–8. doi: 10.1016/j.tips.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Lovell MA, Furukawa K, Markesbery WR. Neurotrophic factors attenuate glutamate-induced accumulation of peroxides, elevation of intracellular Ca2+ concentration, and neurotoxicity and increase antioxidant enzyme activities in hippocampal neurons. J Neurochem. 1995;65:1740–1751. doi: 10.1046/j.1471-4159.1995.65041740.x. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Maudesley S, Martin B. A neural signaling triumvirate that influences ageing and age-related disease: insulin/IGF-1, BDNF and serotonin. Ageing Res Rev. 2004;3(4):445–64. doi: 10.1016/j.arr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- McBride JL, Ramaswamy S, Gasmi M, Bartus RT, Herzog CD, Brandon EP, Zhou L, Pitzer MR, Berry-Kravis EM, Kordower JH. Viral delivery of glial cell line-derived neurotrophic factor improves behavior and protects striatal neurons in a mouse model of Huntington’s disease. Proc Natl Acad Sci U S A. 2006;103:9345–9350. doi: 10.1073/pnas.0508875103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumatsu S, Monje ML, Morhardt DR, Rola R, Pamler TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of x-irradiation. Cancer Res. 2003;63:4021–4027. [PubMed] [Google Scholar]
- Moltzen EK, Bang-Andersen B. Serotonin reuptake inhibitors: the corner stone in treatment of depression for half a century--a medicinal chemistry survey. Curr Top Med Chem. 2006;6:1801–1823. doi: 10.2174/156802606778249810. [DOI] [PubMed] [Google Scholar]
- Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8(9):955–62. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- Myers RH, Vonsattel JP, Stevens TJ, Cupples LA, Richardson EP, Martin JB, Bird ED. Clinical and neuropathologic assessment of severity in Huntington’s disease. Neurology. 1988;38(3):341–7. doi: 10.1212/wnl.38.3.341. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15(11):7539–47. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16(7):2365–72. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. Tht mouse brain in stereotaxic coordinates. 3. 2001. [Google Scholar]
- Patzold T, Brune M. Obsessive compulsive disorder in Huntington disease: a case of isolated obsessions successfully treated with sertraline. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15(3):216–219. [PubMed] [Google Scholar]
- Pineda JR, Canals JM, Bosch M, Adell A, Mengod G, Artigas F, Ernfors P, Alberch J. Brain-derived neurotrophic factor modulates dopaminergic deficits in a transgenic mouse model of Huntington’s disease. J Neurochem. 2005;93(5):1057–68. doi: 10.1111/j.1471-4159.2005.03047.x. [DOI] [PubMed] [Google Scholar]
- Phillips W, Jennifer Morton A, Barker RA. Limbic neurogenesis/plasticity in the R6/2 mouse model of Huntington’s disease. Neuroreport. 2006;17:1623–1627. doi: 10.1097/01.wnr.0000236855.85962.f6. [DOI] [PubMed] [Google Scholar]
- Phillips W, Morton AJ, Barker RA. Abnormalities of neurogenesis in the R6/2 mouse model of Huntington’s disease are attributable to the in vivo microenvironment. J Neurosci. 2005;25:11564–11576. doi: 10.1523/JNEUROSCI.3796-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranen NG, Lipsey JR, Treisman G, Ross CA. Sertraline in the treatment of severe aggressiveness in Huntington’s disease. J Neuropsychiatry Clin Neurosci. 1996;8:338–340. doi: 10.1176/jnp.8.3.338. [DOI] [PubMed] [Google Scholar]
- Reiner A, Albin RL, Anderson KD, D’Amato CJ, Penney JB, Young AB. Differential loss of striatal projection neurons in Huntington disease. Proc Natl Acad Sci USA. 1998;85:5733–5737. doi: 10.1073/pnas.85.15.5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GP, Dalton CF, Tillery CL, Mangiarini L, Davies SW, Bates GP. Brain neurotransmitter deficits in mice transgenic for the Huntington’s disease mutation. J Neurochem. 1999;72:1773–1776. doi: 10.1046/j.1471-4159.1999.721773.x. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Pearson SJ. Decreased glutamic acid and increased 5-hydroxytryptamine in Huntington’s disease brain. Neurosci Lett. 1987;22;78(2):233–8. doi: 10.1016/0304-3940(87)90639-2. [DOI] [PubMed] [Google Scholar]
- Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10 (Suppl):S10–7. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- Ross CA, Poirier MA. Opinion: What is the role of protein aggregation in neurodegeneration? Nat Rev Mol Cell Bio. 2005;6:891–898. doi: 10.1038/nrm1742. [DOI] [PubMed] [Google Scholar]
- Rosser AE, Dunnett SB. Neural transplantation in patients with Huntington’s disease. CNS Drugs. 2003;17:853–867. doi: 10.2165/00023210-200317120-00001. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Schabitz WR, Sommer C, Zoder W, Kiessling M, Schwaninger M, Schwab S. Intravenous brain-derived neurotrophic factor reduces infarct size and counterregulates Bax and Bcl-2 expression after temporary focal cerebral ischemia. Stroke. 2000;31:2212–2217. doi: 10.1161/01.str.31.9.2212. [DOI] [PubMed] [Google Scholar]
- Schechter LE, Ring RH, Beyer CE, Hughes ZA, Khawaja X, Malberg JE. Rosenzweig-Lipson S. Innovative approaches for the development of antidepressant drugs: current and future strategies. NeuroRx. 2005;2:590–611. doi: 10.1602/neurorx.2.4.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter JR, Martens MP, Slaughter KA. Depression and Huntington’s disease: prevelance, clinical manifestations, etiology, and treatment. CNS Spectr. 2001;6:306–326. doi: 10.1017/s109285290002201x. [DOI] [PubMed] [Google Scholar]
- Sugars KL, Rubinsztein DC. Transcriptional abnormalities in Huntington disease. Trends Genet. 2003;19:233–238. doi: 10.1016/S0168-9525(03)00074-X. [DOI] [PubMed] [Google Scholar]
- Tada E, Parent JM, Lowenstein DH, Fike JR. X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neurosci. 2000;99(1):33–41. doi: 10.1016/s0306-4522(00)00151-2. [DOI] [PubMed] [Google Scholar]
- Tattersfield AS, Croon RJ, Liu YW, Kells AP, Faull RL, Connor B. Neurogenesis in the striatum of the quinolinic acid lesion model of Huntington’s disease. Neuroscience. 2004;127:319–332. doi: 10.1016/j.neuroscience.2004.04.061. [DOI] [PubMed] [Google Scholar]
- Tomasevic G, Kamme F, Wieloch T. Changes in proliferating cell nuclear antigen, a protein involved in DNA repair, in vulnerable hippocampal neurons following global cerebral ischemia. Brain Res Mol Brain Res. 1998;60(2):168–76. doi: 10.1016/s0169-328x(98)00173-9. [DOI] [PubMed] [Google Scholar]
- Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP., Jr Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol. 1985;44(6):559–77. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- Wong JW, Armour E, Kazanzedis P, Iordachita I, Tryggestad E, Deng H, et al. A high-resolution small animal radiation research platform (SARRP) with x-ray CT guidance. Int J Radiat Oncol Biol Phys. 2007 doi: 10.1016/j.ijrobp.2008.04.025. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, Araki N, Abe K, Okano H, Sawamoto K. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohrling GJ, IV, Jiang GC, DeJohn MM, Robertson DJ, Vrana KE, Cha JH. Inhibition of tryptophan hydroxylase activity and decreased 5-HT1A receptor binding in a mouse model of Huntington’s disease. J Neurochem. 2002;82:1416–1423. doi: 10.1046/j.1471-4159.2002.01084.x. [DOI] [PubMed] [Google Scholar]
- Zhao M, Hartke C, Jimeno A, Li J, He P, Zabelina Y, Hidalgo M, Baker SD. Specific method for determination of gefitinib in human plasma, mouse plasma and tissues using high performance liquid chromatography coupled to tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;819:73–80. doi: 10.1016/j.jchromb.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Tartari M, Crotti A, Goffredo D, Valenza M, Conti L, Cataudella T, Leavitt BR, Hayden MR, Timmusk T, Rigamonti D, Cattaneo E. Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nat Genet. 2003;35:76–83. doi: 10.1038/ng1219. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Role of brain-derived neurotrophic factor in Huntington’s disease. Prog Neurobiol. 2007;81(5–6):294–330. doi: 10.1016/j.pneurobio.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Ciammola A, Rigamonti D, Leavitt BR, Goffredo D, Conti L, MacDonald ME, Friedlander RM, Silani V, Hayden MR, Timmusk T, Sipione S, Cattaneo E. Loss of huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science. 2001;293(5529):493–8. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Liber D, Ramos C, Tarditi A, Rigamonti D, Tartari M, Valenza M, Cattaneo E. Progressive loss of BDNF in a mouse model of Huntington’s disease and rescue by BDNF delivery. Pharmacol Res 2005. 2005 Aug;52(2):133–9. doi: 10.1016/j.phrs.2005.01.001. [DOI] [PubMed] [Google Scholar]