Abstract
Endoscopic ultrasonography (EUS) has evolved into a useful therapeutic tool for treating a broad range of tumors since being introduced into clinical practice as a diagnostic modality nearly three decades ago. In particular, EUS-guided fine-needle injection has proven a successful minimally invasive approach for treating benign lesions such as pancreatic cysts, relieving pancreatic pain through celiac plexus neurolysis, and controlling local tumor growth of unresectable malignancies by direct delivery of anti-tumor agents. One such ablative agent, ethanol, is capable of safely ablating solid or cystic lesions in hepatic tissues via percutaneous injection. Recent research and clinical interest has focused on the promise of EUS-guided ethanol ablation as a safe and effective method for treating pancreatic tumor patients with small lesions or who are poor operative candidates. Although it is not likely to replace radical resection of localized lesions or systemic treatment of metastatic tumors in all patients, EUS-guided ablation is an ideal method for patients who refuse or are not eligible for surgery. Moreover, this treatment modality may play an active role in the development of future pancreatic tumor treatments. This article reviews the most recent clinical applications of EUS-guided ethanol ablation in humans for treating pancreatic cystic tumors, pancreatic neuroendocrine tumors, and metastatic lesions.
Keywords: Endoscopic ultrasonography, Ethanol, Tumor ablation, Pancreas cancer, Cystic tumor, Neuroendocrine tumors, Celiac plexus neurolysis
Core tip: Ethanol, a commonly used ablative agent, has been used to successfully and safely ablate solid and cystic hepatic lesions via percutaneous injection. Endoscopic ultrasonography (EUS)-guided ethanol ablation, a minimally invasive approach, was recently developed and has been successfully applied as treatment of pancreatic cysts, pancreatic neuroendocrine tumors, and abdominal metastatic lesions. Although it is not likely to replace radical resection for treating localized lesions or systemic therapy for managing metastatic tumors, EUS-guided ablation therapies represent an attractive alternative treatment modality for patients who refuse or are not eligible for surgery.
INTRODUCTION
Endoscopic ultrasonography (EUS) has been used in clinical practice for more than 30 years[1,2] since its development for the original purpose of diagnosing pancreatic disease and carrying out malignancy staging. In the early 1990s, the introduction of curvilinear array echoendoscope made it possible to perform fine-needle aspiration (FNA) biopsy under direct endosonographic visualization. The minimally invasive and safe nature of FNA led to a boon in use of therapeutic EUS techniques, including EUS-guided fine needle injection (EUS-FNI). More recently, EUS-FNI has been applied as a pancreatic cancer treatment aimed at controlling pain through nerve blockade, as a solid tumor therapy for introduction of brachytherapy seeds and viral vectors, and as a tool for ablation therapy[3-6].
Ethanol, a commonly used ablative agent, gained popularity according to its advantageous features of cost effectiveness, ready availability, and rapidly ablation function. The mechanism of ethanol ablation mainly involves cell death by causing cell membrane lysis, protein denaturation, and vascular occlusion[7]. Percutaneous ethanol injection has been used in the ablation of renal cysts, hepatic cysts, and solid tumors, such as liver or adrenal tumors[8-12]. EUS-guided ethanol injection is superior to the percutaneous application because it offers real-time image monitoring of the lesions located deep in the pancreas. In addition, EUS can provide precise measurement of lesions and identification of surrounding structures, and can readily deliver therapeutic agents to a target site, thereby minimizing damage to non-tumor tissue. Some EUS-guided ethanol ablation procedures for pancreatic tissue have been shown to be safe and feasible in animal models[13,14].
In this review, the recent applications, features, and outcomes of EUS-guided ethanol injection treatment for pancreatic cystic tumors, pancreatic neuroendocrine tumors (pNETs), celiac plexus, and metastatic lesions in humans are summarized.
EUS-GUIDED ETHANOL ABLATION THERAPY FOR PANCREATIC CYSTIC TUMORS
Pancreatic cysts are common, with the incidence of asymptomatic cysts being reported at about 2.5% in the general population[15]. The advances of various imaging modalities, such as computed tomography (CT) scanning, magnetic resonance imaging, and EUS, have been accompanied by an increase in detection of pancreatic cystic neoplasms (PCNs). Pancreatic cystic tumors are mainly classified as either mucinous cystic neoplasms (MCNs), serous cystic neoplasms (SCNs), or intraductal papillary mucinous neoplasms (IPMNs), according to histopathological features[16]. Some cystic tumors of the pancreas, such as MCNs and IPMNs, have the potential to become malignant and are often treated by surgical resection when malignancy is suspected. Although surgical resection is generally recommended, pancreatic cyst ablation is an increasingly popular treatment option, especially via injection of ethanol or other ablative agents into the cyst cavity under EUS guidance.
Recent studies have shown that EUS-guided ethanol injection into pancreatic cysts is safe and feasible, slows or inhibits growth, and avoids the risks associated with surgical resection. Gan et al[17] published the first report of safety and feasibility for the ethanol lavage and ablation of pancreatic cystic lesions using EUS-FNI. In that study, 25 patients with pancreatic cysts (MCNs, n = 13; IPMNs, n = 4; SCNs, n = 3; pseudocysts, n = 3; uncertain etiology, n = 2) were treated with escalating doses (5%-80%) of ethanol for 3-5 min under EUS guidance. The results demonstrated that this procedure effectively reduced tumors without inducing complications, such as pancreatitis, in the short- and long-term follow-up periods. Eight patients (35%) had complete resolution of the cysts, and five patients undergoing surgical resection showed histological evidence of epithelial ablation.
To further determine whether EUS-guided ethanol lavage can decrease pancreatic cyst size, a multicenter, randomized, prospective trial was performed in 2009[18]. The primary aim of that study was to compare the change of pancreatic cyst size after EUS-guided lavage with 80% ethanol or saline solution alone. Of the 42 patients enrolled, 25 patients were initially treated with 80% ethanol lavage and 17 patients were administered saline solution lavage. Three months after the first lavage, a second pancreatic cystic lavage was performed. Patients who initially received an ethanol lavage received a second ethanol lavage, and those who received an initial saline solution lavage were then given an 80% ethanol lavage. The results indicated that EUS-guided 80% ethanol injection resulted in a greater decrease in size of pancreatic cystic tumors compared with the saline solution injection, and that the overall resolution rate of pancreatic cystic tumors was 33.3%. Incidences of major complications, such as abdominal pain, intracystic bleeding during lavage, and acute pancreatitis, were similar in the two groups. Furthermore, nine of the study patients (75%) were followed-up for two years after initial cyst resolution, and no evidence of cyst recurrence was found in any patient[19].
To improve the effect of ethanol ablation therapy, Oh et al[20-22] performed EUS-guided ethanol lavage with a paclitaxel injection to treat cystic tumors of the pancreas. An initial study found that complete resolution of pancreatic cystic tumors was achieved in 11 of 14 patients after treatment with ethanol and paclitaxel injection; in addition, minor complications, including hyperamylasemia and abdominal pain, were observed in one patient[20], but no cases of acute pancreatitis occurred. The study’s collective findings demonstrated that ethanol lavage with paclitaxel injection is a safe, feasible, and effective method to treat pancreatic cystic tumors. However, only a small number of patients and only short-term outcomes were assessed in this study. A subsequent study by this group was carried out involving a larger population (n = 52) with a longer-term follow-up period (n = 47 for > 12 mo). All patients underwent 99% pure ethanol injection into the collapsed cyst for a 3-5 min lavage. After the injected ethanol was aspirated, paclitaxel solution was injected into the cyst cavity. Ultimately, complete resolution was achieved in 29 of the patients (62%). No treatment-related complications, including bleeding, bowel perforation, or severe pancreatitis, were observed[22].
DiMaio et al[23] retrospectively analyzed the effectiveness of multiple EUS-guided ethanol lavages for pancreatic cystic tumor treatment. The results showed that multiple ethanol lavage treatments resulted in a greater decrease in the size and surface area of PCNs compared with only one ethanol lavage treatment. Complete cyst resolution was not seen in any patient after the first EUS-ethanol lavage, but was achieved in 5 of the 13 patients (38%) who underwent two sequential EUS-ethanol lavage treatments. Again, no treatment-related complications were observed, with the exception of one patient complaining of minor abdominal pain.
Collectively, these preliminary studies suggest that ethanol ablation is relatively safe and feasible for clinical use in humans. Therefore, EUS-guided ablation of pancreatic cystic tumors via ethanol injection, a minimally invasive technique, may be an attractive choice to treat patients who refuse or are not eligible for surgery. However, there are a number of limitations in these studies. First, some studies only included a treatment group, and no control group was included. Second, there was not a definitive diagnosis made prior to application of the ethanol ablation procedure. Inclusive criteria were based on the imaging findings and some benign lesions might have been treated unnecessarily. Third, the patients were followed-up for a relatively short period of time for documentation of cyst resolution. Thus, EUS-guided cyst ablation is an experimental therapy modality that should be used with caution.
EUS-guided pancreatic cyst ablation is mainly used currently for the following indications: (1) patients who refuse surgery or are high-risk surgical candidates; (2) cysts that appear to be benign and are morphologically indeterminate; and (3) asymptomatic cysts that increase in size during the follow-up period. Before ethanol ablation for pancreatic cysts can be widely accepted, a prospective, randomized, controlled trial with a long follow-up period is required to determine the clinical efficacy of ethanol ablation therapy compared with surgical resection.
EUS-GUIDED ETHANOL ABLATION THERAPY FOR PANCREATIC NEUROENDOCRINE TUMORS
pNET account for a small percentage of all pancreatic tumors (1.3%), but their incidence is increasing[24]. Although surgical enucleation or resection is considered as the treatment of choice for pNET, a few patients are not suitable candidates for surgery because of old age or comorbidities. Recently, successful application of EUS-guided ablation therapy using ethanol has been reported for the treatment of pNET. Jürgensen et al[25] reported successful resolution of a 13 mm insulinoma by EUS-guided ethanol ablation therapy in a 78-year-old woman (diagnosed by laboratory findings and EUS-FNA). Because the patient was in poor general condition and refused surgical resection, EUS-guided ethanol injection was performed. Although mild procedure-associated pancreatitis was experienced after the ethanol ablation procedure, complete resolution was achieved in the patient. The patient had durable glycemic control throughout the recovery and follow-up and no indications of insulinoma were observed by follow-up EUS.
Muscatiello et al[26,27] described another case of successful ethanol ablation involving a patient with a pancreatic endocrine tumor. A female patient with multiple endocrine neoplasia type 1 (11 and 7 mm, respectively) underwent 40% ethanol ablation therapy. Two months after the ethanol ablation, EUS with contrast enhancement revealed areas of fibrosis. However, subsequent resolution of the accompanying perturbed levels of vasoactive intestinal peptide and chromogranin A was observed. The main complication in this case was a small pancreatic necrotic lesion, which was presumably caused by minimal ethanol effusion and managed by laparoscopic necrosectomy.
Deprez et al[28] reported treatment of a patient with insulinoma of the pancreas head involving a 98% ethanol injection under EUS guidance. The patient achieved complete resolution (evidenced by imaging) at 3 mo after the procedure and remained asymptomatic and normoglycemic for more than two years later. Vleggaar et al[29] reported an ethanol ablation operation for an 82-year-old patient with a pNET, for which 96% ethanol was delivered to under EUS guidance. Two months after the ablation therapy, EUS examination indicated that a significant reduction in the diameter of the tumor had been achieved. Finally, Levy et al[30] retrospectively reviewed eight patients with insulinomas who received US-guided ethanol ablation. Five of those patients underwent EUS-guided ethanol injection and the remaining three underwent intra-operative ultrasound (IOUS)-guided ethanol injection. No complications occurred during or after the EUS-guided procedure. The IOUS-guided ethanol injection, however, was associated with minor peritumoral bleeding (n = 1), pseudocyst (n = 1), and pancreatitis with peri-pancreatic fluid (n = 1). Hypoglycemia-related symptoms completely disappeared in five patients who underwent EUS-guided ethanol injection, and significantly improved in the three patients who underwent IOUS-guided ethanol injection.
Based on these case reports, EUS-guided ethanol ablation seems to be an alternative treatment option for pancreatic solid tumors. However, some issues remain that require further study, such as the choice of target area and adequate ethanol dose to achieve successful ablation without causing serious complications. In addition, EUS-guided ethanol ablation therapy for pNETs appears to harbor the possibility of late relapse requiring re-intervention, as well as incomplete ablation and risk of metastasis. While currently the strongest indication for intratumoral therapy is patients who refuse surgery or are poor surgical candidates, a clinical trial enrolling more patients with longer follow-up is required to more definitively determine the particular physiological indications.
EUS-GUIDED ETHANOL ABLATION THERAPY FOR CELIAC PLEXUS ABLATION
Pain is one of the major complications of advanced pancreatic cancer. It is estimated that pain is present in 80%-85% of pancreatic cancer patients at the time of diagnosis, and it can be difficult to control, even with high doses of analgesics[31,32]. Celiac plexus neurolysis (CPN), a chemical splanchnicectomy of the celiac plexus, has been well established as an effective method for controlling pain and decreasing morphine consumption in patients with locally advanced or unresectable pancreatic cancer[32,33]. Before the advent of EUS, CPN was performed by radiological guidance or intraoperative method, both of which are associated with a risk of the serious complications of paraesthesia, paraplegia, and pneumothorax[34,35]. With the development of the EUS technique, EUS-guided CPN (EUS-CPN) has become a novel option to treat cancer-related pain in patients with inoperable pancreatic cancer.
EUS-CPN consists of an injection of absolute alcohol, a neurolytic agent, into the celiac ganglia to permanently destroy neural tissue of celiac ganglia. The first applications of EUS-CPN were reported by Wiersema et al[36]. Thirty patients with pain due to intra-abdominal malignancies were injected with 98% dehydrated absolute alcohol under EUS guidance into the celiac plexus via the transgastric route. The majority of patients (79%-88%) experienced a significant decrease in pain scores at the 10 wk post-treatment (median follow-up time).
Subsequent studies have demonstrated that EUS-CPN is a safe and effective method to relieve severe pain from advanced pancreatic cancer[3,37-39]. In a meta-analysis of these EUS-CPN studies, pain relief was observed in about 80% of 289 patients with pain due to pancreatic cancer[40]. While EUS-CPN can be delivered on either or both sides of the aorta, a recent study showed that bilateral injection was more effective than a central single injection[41]. The common complications of EUS-CPN, transient diarrhea and hypotension, were observed in 9% and 10%-15% of cases, respectively, and both complications were self-limiting in most cases[37].
EUS-GUIDED ETHANOL ABLATION THERAPY FOR OTHER TUMORS
EUS-guided injection of alcohol is also used for ablation of other abdominal tumors, such as gastrointestinal stromal tumor (GIST) and intra-abdominal metastatic lesions located in the liver, adrenal glands, and pelvic lymph nodes. Günter et al[42] reported a case of EUS-guided ethanol ablation of a GIST involving a 59-year-old man with a 4 cm lesion in the muscularis propria of the stomach that was diagnosed by EUS and EUS-guided FNA. Severe comorbidity precluded surgery and the patient was treated with an injection of 1.5 mL of 95% ethanol under EUS guidance. Seven weeks after the injection, no endosonographic evidence of residual tumor was seen, but a 1.5-cm ulcer at the treatment site was present, which was resolved by acid suppression therapy. Two years after the ethanol ablation treatment, a follow-up examination showed complete remission of the tumor. No severe complications were observed, except for self-limiting pain in the upper abdomen.
Recently, two cases of EUS-guided ethanol ablation of a solid hepatic metastasis carcinoma have been reported[43,44]. In the first, a 65-year-old man with a 3.3-cm lesion in the left liver and a markedly elevated carcinoembryonic antigen level was considered as having metastatic cancer from either a pancreatic adenocarcinoma or prior colorectal cancer. Multiple EUS-guided injections of absolute alcohol were administered over several years. The lesion size decreased over time, as well as the levels of corresponding tumor markers, indicating that this approach was effective in controlling tumor growth. Furthermore, the multiple EUS-guided hepatic tumor injections did not result in major complications, except for a small subcapsular hematoma that resolved spontaneously[43].
Our group also reported a case of a 47-year-old man with a hepatic metastatic carcinoma from a pancreatic adenocarcinoma treated by injection of anhydrous ethanol under EUS guidance (Figure 1). One month later, an abdominal CT follow-up scan revealed a decrease in size of the lesion (Figure 2). No significant procedure-related complications were observed, except for a transient low fever[44].
Figure 1.
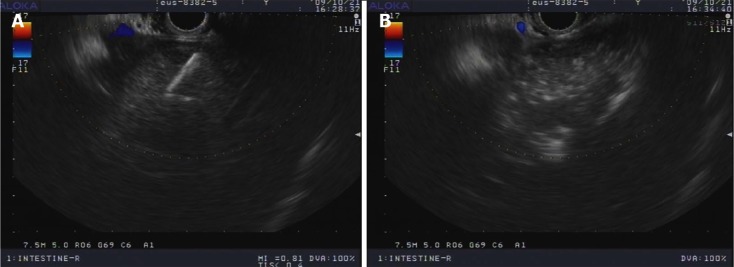
Endoscopic ultrasonography image of hepatic tumor. A: During ethanol injection; B: Hyperechoic appearance after ethanol injection.
Figure 2.
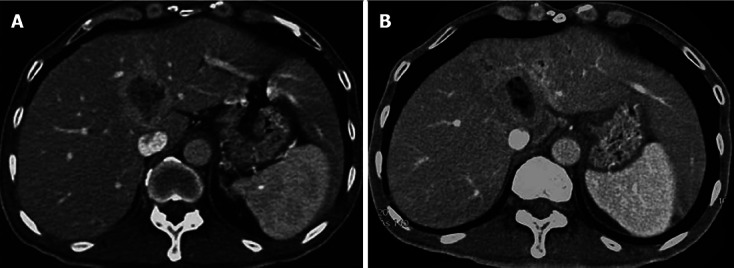
Computer tomography scans of hepatic metastatic carcinoma. A: Before ethanol injection; B: After ethanol injection.
Artifon et al[45] described a case of EUS-guided alcohol ablation of a 5 cm left adrenal metastatic carcinoma in a 52-year-old man. For this patient, the main presenting complaint was abdominal pain and the diagnosis of left adrenal metastasis from non-small-cell lung carcinoma was made by EUS-FNA. Three days after the alcohol ablation therapy treatment (15 mL of 98% absolute alcohol), the symptom of abdominal pain disappeared. At the one-month follow-up, EUS revealed a hyperechoic nodule between the pancreas and the left kidney, which was presumed to represent alcohol ablation-induced fibrosis.
More recently, DeWitt et al[46] reported the successful EUS-guided alcohol ablation of metastatic pelvic lymph nodes in a patient with rectal cancer. The two injected metastatic pelvic lymph nodes received 4 and 2 mL of ethanol, respectively, which achieved local complete resolution at 10 mo post-treatment. No procedure-related complications were reported.
The basic characteristics and outcomes of the studies described herein of EUS-guided ethanol ablation therapy for pancreatic cystic tumors, pNETs, and other tumors are summarized in Table 1, Table 2 and Table 3, respectively.
Table 1.
Summary of endoscopic ultrasonography-guided ethanol ablation for pancreatic cystic tumor
| Ref. | n | Median size, mm (range) | Ablative agents | Complete resolution | Complications |
| Gan et al[17] | 25 | 19.4 (6-30) | Ethanol | 35% | None |
| Oh et al[20] | 14 | 25.5 (17-52) | Ethanol and paclitaxel | 79% | Acute pancreatitis (n = 1) |
| Hyperamylasemia (n = 6) | |||||
| Vague abdominal pain (n = 1) | |||||
| Oh et al[21] | 10 | 29.5 (20-68) | Ethanol and paclitaxel | 60% | Mild pancreatitis (n = 1) |
| DeWitt et al[18] | 42 | 22.4 (10-58) | Saline vs ethanol | 33% | Abdominal pain at 2 h (n = 2) |
| Abdominal pain at 7 d (n = 5) | |||||
| Pancreatitis (n = 1) | |||||
| Acystic bleeding (n = 1) | |||||
| Oh et al[22] | 52 | 31.8 (17-68) | Ethanol and paclitaxel | 62% | Fever (n = 1) |
| Vague abdominal discomfort (n = 1) | |||||
| Mild pancreatitis (n = 1) | |||||
| Splenic vein obliteration (n = 1) |
Table 2.
Summary of endoscopic ultrasound-guided ethanol ablation for pancreatic neuroendocrine tumor
| Ref. | n | Maximum diameter (mm) | Ethanol | Volume (mL) | Complications |
| Jurgensen et al[25] | 1 | 13 | 95% | 8.0 | Pain in the upper abdomen |
| A mild increase of serum lipase activity | |||||
| Muscatiello et al[26] | 1 | 11 and 7 | 40% | 2.0 | A small pancreatic necrotic lesion |
| Deprez et al[28] | 98% | 3.5 | A mild elevation of pancreatic enzymes | ||
| Hematoma ulceration of the duodenal wall | |||||
| Vleggaar et al[29] | 1 | 10 | 96% | 0.3 | None |
| Levy et al[30] | 5 | 18 | 95% | 0.1 | None |
| 0.4 | |||||
| 0.1 | |||||
| 20 | 98% | 0.1 | |||
| 0.3 | |||||
| 21 | 98% | 1.0 | |||
| 0.3 | |||||
| 8 | 98% | 3.0 | |||
| 1.5 | |||||
| 16 | 99% | 0.7 | |||
| 1.0 |
Table 3.
Cases of endoscopic ultrasound-guided ethanol ablation for other tumor
| Target tumor | Maximum diameter (mm) | Ethanol | Volume (mL) | Complications |
| Gastrointestinal stromal tumor[42] | 40 | 95% | 1.5 | None |
| Hepatic metastasis carcinoma from colorectal carcinoma[43] | 33 | 98% | 6.0 | A tiny subcapsular hematoma |
| Hepatocellular carcinoma from pancreatic adenocarcinoma[44] | 35 | 100% | 10.0 | Low-grade fever |
| Left adrenal metastasis carcinoma from non-small-cell lung carcinoma[45] | 50 | 98% | 15.0 | None |
| Pelvic metastatic lymph nodes from rectal cancer[46] | 11 and 10 | Not stated | 4.0 and 2.0 | None |
CONCLUSION
With the advent of curvilinear EUS, therapeutic EUS has emerged as an important approach to manage malignancies. While prominent advances have been made in applications of EUS-FNI for specific antitumor therapy, the field of EUS-guided ethanol ablation therapy for malignancies continues to evolve. Currently, our understanding of the safety and efficacy of EUS-guided ethanol ablation as a tumor therapy is primarily limited by the small sample sizes and short-term follow-up periods of related case studies. Prospective, large trials should be performed to better evaluate this technique: its indications and complications before it is recommended for widespread use in clinical practice. For example, particular patient populations may receive more benefit than others from the EUS-guided ethanol ablation with or without systemic therapies, and prospective clinical trials will help to define these patients.
ACKNOWLEDGMENTS
We thank Medjaden Bioscience Limited for assisting in the preparation of this manuscript.
Footnotes
Supported by A grant from the Shanghai Science and Technology Committee Foundation, No. 11D21921605
P- Reviewers Nakajima N, Oh HC, ReshetnyakVI, Tanno S S- Editor Zhai HH L- Editor A E- Editor Xiong L
References
- 1.DiMagno EP, Buxton JL, Regan PT, Hattery RR, Wilson DA, Suarez JR, Green PS. Ultrasonic endoscope. Lancet. 1980;1:629–631. doi: 10.1016/s0140-6736(80)91122-8. [DOI] [PubMed] [Google Scholar]
- 2.Strohm WD, Phillip J, Hagenmüller F, Classen M. Ultrasonic tomography by means of an ultrasonic fiberendoscope. Endoscopy. 1980;12:241–244. doi: 10.1055/s-2007-1021752. [DOI] [PubMed] [Google Scholar]
- 3.Wiechowska-Kozłowska A, Boer K, Wójcicki M, Milkiewicz P. The efficacy and safety of endoscopic ultrasound-guided celiac plexus neurolysis for treatment of pain in patients with pancreatic cancer. Gastroenterol Res Pract. 2012;2012:503098. doi: 10.1155/2012/503098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin Z, Du Y, Li Z, Jiang Y, Chen J, Liu Y. Endoscopic ultrasonography-guided interstitial implantation of iodine 125-seeds combined with chemotherapy in the treatment of unresectable pancreatic carcinoma: a prospective pilot study. Endoscopy. 2008;40:314–320. doi: 10.1055/s-2007-995476. [DOI] [PubMed] [Google Scholar]
- 5.Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, Soetikno RM, Kirn DH, Freeman SM. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9:555–561. [PubMed] [Google Scholar]
- 6.Yoon WJ, Brugge WR. Endoscopic ultrasonography-guided tumor ablation. Gastrointest Endosc Clin N Am. 2012;22:359–69, xi. doi: 10.1016/j.giec.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Gelczer RK, Charboneau JW, Hussain S, Brown DL. Complications of percutaneous ethanol ablation. J Ultrasound Med. 1998;17:531–533. doi: 10.7863/jum.1998.17.8.531. [DOI] [PubMed] [Google Scholar]
- 8.Bean WJ. Renal cysts: treatment with alcohol. Radiology. 1981;138:329–331. doi: 10.1148/radiology.138.2.7455112. [DOI] [PubMed] [Google Scholar]
- 9.Omerović S, Zerem E. Alcohol sclerotherapy in the treatment of symptomatic simple renal cysts. Bosn J Basic Med Sci. 2008;8:337–340. doi: 10.17305/bjbms.2008.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larssen TB, Jensen DK, Viste A, Horn A. Single-session alcohol sclerotherapy in symptomatic benign hepatic cysts. Long-term results. Acta Radiol. 1999;40:636–638. doi: 10.3109/02841859909175601. [DOI] [PubMed] [Google Scholar]
- 11.Livraghi T, Bolondi L, Lazzaroni S, Marin G, Morabito A, Rapaccini GL, Salmi A, Torzilli G. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis. A study on 207 patients. Cancer. 1992;69:925–929. doi: 10.1002/1097-0142(19920215)69:4<925::aid-cncr2820690415>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 12.Xiao YY, Tian JL, Li JK, Yang L, Zhang JS. CT-guided percutaneous chemical ablation of adrenal neoplasms. AJR Am J Roentgenol. 2008;190:105–110. doi: 10.2214/AJR.07.2145. [DOI] [PubMed] [Google Scholar]
- 13.Aslanian H, Salem RR, Marginean C, Robert M, Lee JH, Topazian M. EUS-guided ethanol injection of normal porcine pancreas: a pilot study. Gastrointest Endosc. 2005;62:723–727. doi: 10.1016/j.gie.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 14.Matthes K, Mino-Kenudson M, Sahani DV, Holalkere N, Brugge WR. Concentration-dependent ablation of pancreatic tissue by EUS-guided ethanol injection. Gastrointest Endosc. 2007;65:272–277. doi: 10.1016/j.gie.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 15.Laffan TA, Horton KM, Klein AP, Berlanstein B, Siegelman SS, Kawamoto S, Johnson PT, Fishman EK, Hruban RH. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802–807. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salvia R, Festa L, Butturini G, Tonsi A, Sartori N, Biasutti C, Capelli P, Pederzoli P. Pancreatic cystic tumors. Minerva Chir. 2004;59:185–207. [PubMed] [Google Scholar]
- 17.Gan SI, Thompson CC, Lauwers GY, Bounds BC, Brugge WR. Ethanol lavage of pancreatic cystic lesions: initial pilot study. Gastrointest Endosc. 2005;61:746–752. doi: 10.1016/s0016-5107(05)00320-2. [DOI] [PubMed] [Google Scholar]
- 18.DeWitt J, McGreevy K, Schmidt CM, Brugge WR. EUS-guided ethanol versus saline solution lavage for pancreatic cysts: a randomized, double-blind study. Gastrointest Endosc. 2009;70:710–723. doi: 10.1016/j.gie.2009.03.1173. [DOI] [PubMed] [Google Scholar]
- 19.DeWitt J, DiMaio CJ, Brugge WR. Long-term follow-up of pancreatic cysts that resolve radiologically after EUS-guided ethanol ablation. Gastrointest Endosc. 2010;72:862–866. doi: 10.1016/j.gie.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 20.Oh HC, Seo DW, Lee TY, Kim JY, Lee SS, Lee SK, Kim MH. New treatment for cystic tumors of the pancreas: EUS-guided ethanol lavage with paclitaxel injection. Gastrointest Endosc. 2008;67:636–642. doi: 10.1016/j.gie.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 21.Oh HC, Seo DW, Kim SC, Yu E, Kim K, Moon SH, Park do H, Lee SS, Lee SK, Kim MH. Septated cystic tumors of the pancreas: is it possible to treat them by endoscopic ultrasonography-guided intervention? Scand J Gastroenterol. 2009;44:242–247. doi: 10.1080/00365520802495537. [DOI] [PubMed] [Google Scholar]
- 22.Oh HC, Seo DW, Song TJ, Moon SH, Park do H, Soo Lee S, Lee SK, Kim MH, Kim J. Endoscopic ultrasonography-guided ethanol lavage with paclitaxel injection treats patients with pancreatic cysts. Gastroenterology. 2011;140:172–179. doi: 10.1053/j.gastro.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 23.DiMaio CJ, DeWitt JM, Brugge WR. Ablation of pancreatic cystic lesions: the use of multiple endoscopic ultrasound-guided ethanol lavage sessions. Pancreas. 2011;40:664–668. doi: 10.1097/MPA.0b013e3182128d06. [DOI] [PubMed] [Google Scholar]
- 24.Yao JC, Eisner MP, Leary C, Dagohoy C, Phan A, Rashid A, Hassan M, Evans DB. Population-based study of islet cell carcinoma. Ann Surg Oncol. 2007;14:3492–3500. doi: 10.1245/s10434-007-9566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jürgensen C, Schuppan D, Neser F, Ernstberger J, Junghans U, Stölzel U. EUS-guided alcohol ablation of an insulinoma. Gastrointest Endosc. 2006;63:1059–1062. doi: 10.1016/j.gie.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 26.Muscatiello N, Salcuni A, Macarini L, Cignarelli M, Prencipe S, di Maso M, Castriota M, D’Agnessa V, Ierardi E. Treatment of a pancreatic endocrine tumor by ethanol injection guided by endoscopic ultrasound. Endoscopy. 2008;40 Suppl 2:E258–E259. doi: 10.1055/s-2007-966962. [DOI] [PubMed] [Google Scholar]
- 27.Muscatiello N, Nacchiero M, Della Valle N, Di Terlizzi F, Verderosa G, Salcuni A, Macarini L, Cignarelli M, Castriota M, D’Agnessa V, et al. Treatment of a pancreatic endocrine tumor by ethanol injection (PEI) guided by endoscopic ultrasound. Endoscopy. 2008;40 Suppl 2:E83. doi: 10.1055/s-2007-995540. [DOI] [PubMed] [Google Scholar]
- 28.Deprez PH, Claessens A, Borbath I, Gigot JF, Maiter D. Successful endoscopic ultrasound-guided ethanol ablation of a sporadic insulinoma. Acta Gastroenterol Belg. 2008;71:333–337. [PubMed] [Google Scholar]
- 29.Vleggaar FP, Bij de Vaate EA, Valk GD, Leguit RJ, Siersema PD. Endoscopic ultrasound-guided ethanol ablation of a symptomatic sporadic insulinoma. Endoscopy. 2011;43 Suppl 2 UCTN:E328–E329. doi: 10.1055/s-0030-1256775. [DOI] [PubMed] [Google Scholar]
- 30.Levy MJ, Thompson GB, Topazian MD, Callstrom MR, Grant CS, Vella A. US-guided ethanol ablation of insulinomas: a new treatment option. Gastrointest Endosc. 2012;75:200–206. doi: 10.1016/j.gie.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 31.Moore JC, Adler DG. Celiac plexus neurolysis for pain relief in pancreatic cancer. J Support Oncol. 2009;7:83–87, 90. [PubMed] [Google Scholar]
- 32.Yan BM, Myers RP. Neurolytic celiac plexus block for pain control in unresectable pancreatic cancer. Am J Gastroenterol. 2007;102:430–438. doi: 10.1111/j.1572-0241.2006.00967.x. [DOI] [PubMed] [Google Scholar]
- 33.Wyse JM, Carone M, Paquin SC, Usatii M, Sahai AV. Randomized, double-blind, controlled trial of early endoscopic ultrasound-guided celiac plexus neurolysis to prevent pain progression in patients with newly diagnosed, painful, inoperable pancreatic cancer. J Clin Oncol. 2011;29:3541–3546. doi: 10.1200/JCO.2010.32.2750. [DOI] [PubMed] [Google Scholar]
- 34.O’Toole TM, Schmulewitz N. Complication rates of EUS-guided celiac plexus blockade and neurolysis: results of a large case series. Endoscopy. 2009;41:593–597. doi: 10.1055/s-0029-1214868. [DOI] [PubMed] [Google Scholar]
- 35.Eisenberg E, Carr DB, Chalmers TC. Neurolytic celiac plexus block for treatment of cancer pain: a meta-analysis. Anesth Analg. 1995;80:290–295. doi: 10.1097/00000539-199502000-00015. [DOI] [PubMed] [Google Scholar]
- 36.Wiersema MJ, Wiersema LM. Endosonography-guided celiac plexus neurolysis. Gastrointest Endosc. 1996;44:656–662. doi: 10.1016/s0016-5107(96)70047-0. [DOI] [PubMed] [Google Scholar]
- 37.Gunaratnam NT, Sarma AV, Norton ID, Wiersema MJ. A prospective study of EUS-guided celiac plexus neurolysis for pancreatic cancer pain. Gastrointest Endosc. 2001;54:316–324. doi: 10.1067/mge.2001.117515. [DOI] [PubMed] [Google Scholar]
- 38.Kaufman M, Singh G, Das S, Concha-Parra R, Erber J, Micames C, Gress F. Efficacy of endoscopic ultrasound-guided celiac plexus block and celiac plexus neurolysis for managing abdominal pain associated with chronic pancreatitis and pancreatic cancer. J Clin Gastroenterol. 2010;44:127–134. doi: 10.1097/MCG.0b013e3181bb854d. [DOI] [PubMed] [Google Scholar]
- 39.Levy MJ, Topazian MD, Wiersema MJ, Clain JE, Rajan E, Wang KK, de la Mora JG, Gleeson FC, Pearson RK, Pelaez MC, et al. Initial evaluation of the efficacy and safety of endoscopic ultrasound-guided direct Ganglia neurolysis and block. Am J Gastroenterol. 2008;103:98–103. doi: 10.1111/j.1572-0241.2007.01607.x. [DOI] [PubMed] [Google Scholar]
- 40.Puli SR, Reddy JB, Bechtold ML, Antillon MR, Brugge WR. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: a meta-analysis and systematic review. Dig Dis Sci. 2009;54:2330. doi: 10.1007/s10620-008-0651-x. [DOI] [PubMed] [Google Scholar]
- 41.Sahai AV, Lemelin V, Lam E, Paquin SC. Central vs. bilateral endoscopic ultrasound-guided celiac plexus block or neurolysis: a comparative study of short-term effectiveness. Am J Gastroenterol. 2009;104:326–329. doi: 10.1038/ajg.2008.64. [DOI] [PubMed] [Google Scholar]
- 42.Günter E, Lingenfelser T, Eitelbach F, Müller H, Ell C. EUS-guided ethanol injection for treatment of a GI stromal tumor. Gastrointest Endosc. 2003;57:113–115. doi: 10.1067/mge.2003.39. [DOI] [PubMed] [Google Scholar]
- 43.Barclay RL, Perez-Miranda M, Giovannini M. EUS-guided treatment of a solid hepatic metastasis. Gastrointest Endosc. 2002;55:266–270. doi: 10.1067/mge.2002.120784. [DOI] [PubMed] [Google Scholar]
- 44.Hu YH, Tuo XP, Jin ZD, Liu Y, Guo Y, Luo L. Endoscopic ultrasound (EUS)-guided ethanol injection in hepatic metastatic carcinoma: a case report. Endoscopy. 2010;42 Suppl 2:E256–E257. doi: 10.1055/s-0030-1255653. [DOI] [PubMed] [Google Scholar]
- 45.Artifon EL, Lucon AM, Sakai P, Gerhardt R, Srougi M, Takagaki T, Ishioka S, Bhutani MS. EUS-guided alcohol ablation of left adrenal metastasis from non-small-cell lung carcinoma. Gastrointest Endosc. 2007;66:1201–1205. doi: 10.1016/j.gie.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 46.DeWitt J, Mohamadnejad M. EUS-guided alcohol ablation of metastatic pelvic lymph nodes after endoscopic resection of polypoid rectal cancer: the need for long-term surveillance. Gastrointest Endosc. 2011;74:446–447. doi: 10.1016/j.gie.2011.01.064. [DOI] [PubMed] [Google Scholar]


