Abstract
AIM: To retrospectively assess the effect of comprehensive cryosurgery (ablation of intra- and extra-hepatic tumors) plus dendritic cell-cytokine-induced killer cell immunotherapy in metastatic hepatocellular cancer.
METHODS: We divided 45 patients into cryo-immunotherapy (21 patients), cryotherapy (n = 12), immunotherapy (n = 5) and untreated (n = 7) groups. Overall survival (OS) after diagnosis of metastatic hepatocellular cancer was assessed after an 8-year follow-up.
RESULTS: Median OS was higher following cryo-immunotherapy (32 mo) or cryotherapy (17.5 mo; P < 0.05) than in the untreated group (3 mo) and was higher in the cryo-immunotherapy group than in the cryotherapy group (P < 0.05). In the cryo-immunotherapy group, median OS was higher after multiple treatments (36.5 mo) than after a single treatment (21 mo; P < 0.05).
CONCLUSION: Cryotherapy and, especially, cryo-immunotherapy significantly increased OS in metastatic hepatocellular cancer patients. Multiple cryo-immunotherapy was associated with a better prognosis than single cryo-immunotherapy.
Keywords: Cryoablation, Dendritic cell-cytokine-induced killer cell, Immunotherapy, Metastatic hepatocellular cancer, Survival time
Core tip: Fourty-five patients of metastatic hepatocellular cancer were divided into cryo-immunotherapy, cryotherapy, immunotherapy and untreated groups. Median overall survival (OS) was higher following cryo-immunotherapy (32 mo) or cryotherapy (17.5 mo) than in the untreated group (3 mo); In the cryo-immunotherapy group, median OS was higher after multiple treatments (36.5 mo) than after a single treatment (21 mo). In a word, cryotherapy and, especially, cryo-immunotherapy significantly increased OS in metastatic hepatocellular cancer patients. Multiple cryo-immunotherapy was associated with a better prognosis than single cryo-immunotherapy.
INTRODUCTION
Hepatocellular carcinoma (HCC), which is the fifth most common cancer worldwide, is usually discovered late and has a poor prognosis[1]. In about 80% of patients, HCC is associated with chronic liver disease (i.e., hepatitis and cirrhosis), with major implications for the prognosis and therapeutic options[2]. Many patients are unsuitable for tumor resection because of factors such as poor hepatic reserve (cirrhosis), multicentric tumors or extrahepatic disease[3,4]. Until recently, no systemic chemotherapy has significantly increased survival in patients with advanced HCC[5,6]. External beam radiation has had a limited role in the treatment of HCC because of radiation toxicity to the adjacent normal liver[7,8]. Percutaneous ablation is currently considered the best therapeutic modality for patients with early stage HCC who are not candidates for surgery; it principally involves percutaneous ethanol injection, radiofrequency ablation, microwave ablation, laser ablation or cryoablation[9].
Because cryoablation forms an ice ball that can be visualized by many imaging methods, it has been an attractive option for reasons of safety. Technically, cryoablation of tumors in multiple organs (i.e., liver, lung, kidney, breast, pancreas and prostate) has been proved to be safe and effective[10]. A long term study of medium to large tumors (more than 5 cm in diameter) treated with cryoablation and/or transarterial chemoembolization (TACE) showed a 5 year survival rate of 23% and local progression rate of 24%[11,12]. To our knowledge, there are currently no reports on the long term effects of simultaneous cryoablation of intra- and extra-hepatic tumors in metastatic HCC patients.
Another potential advantage of the in situ freezing of malignant disease is the cryo-immunologic response[13], which is an antitumor immune response triggered by the natural absorption of malignant tissue[14]. Immunotherapy mediated by autologous dendritic cells (DCs) is a promising treatment option for long lasting control of unresectable HCC[15-17]. Increased knowledge regarding vaccination with DCs co-cultured with cytokine-induced killer (CIK) cells has led to improved clinical treatment strategies[18]. Whether slow release of tumor antigen after cryoablation can improve the effect of immunotherapy remains unknown.
Here, we retrospectively compared the effects of comprehensive cryosurgery (simultaneous cryoablation of intra- and extrahepatic tumors and of liver tumors of diameter greater than 5 cm, with TACE performed once or twice before cryoablation to reduce the tumor to 5 cm) and/or DC-CIK immunotherapy in patients with untreated metastatic HCC. To measure the survival time of patients with metastatic cancer, overall survival (OS) after diagnosis of metastatic disease was the main evaluation index.
MATERIALS AND METHODS
Patient selection
Between January, 2004 and October, 2011, 45 HCC patients with metastatic HCC met our inclusion criteria and were enrolled in this study. Surgery and chemotherapy were deemed unsuitable in any of the following situations: multifocal disease, unresectable HCC, patient refused to undergo surgery and chemotherapy or was seeking further treatment after failure of chemotherapy, severe complications (i.e., hypertension and ascites) and advanced age. Ideal patients for comprehensive cryoablation are those with: Karnofsky performance status (KPS) score ≥ 70; platelet count ≥ 80 × 109/L; white blood cell count ≥ 3 × 109/L; neutrophil count ≥ 2 × 109/L; hemoglobin ≥ 90 g/L; prothrombin time international normalized ratio ≥ 1.5; hepatic tumor not obviously invading the gallbladder, diaphragm or large vessels; absence of level 3 hypertension, severe coronary disease, myelosuppression, respiratory disease and acute or chronic infection; and adequate hepatic function (bilirubin < 30 μmol/L, aminotransferase < 60 U/L and Child-Pugh score A or B) and renal function (serum creatinine < 130 μmol/L, serum urea < 10 mmol/L).
The diagnosis of HCC was confirmed by liver pathology in 41 patients; in the remaining cases, HCC was diagnosed by classical imaging methods, including computed tomography (CT) or magnetic resonance imaging, or by biochemical markers such as increased alpha-fetoprotein. Twenty-four patients had only one mass in the liver, of 3.8-15 cm in diameter with an average of 6.5 cm. Twenty-one patients had two to four masses of 4.5-13 cm in diameter. There were a total of 71 masses in 45 patients. All except two cases had cirrhosis. Using the Child-Pugh score to assess the severity of cirrhosis, 25 patients were class A and 18 were class B. All patients received their final treatment in our hospital within an 8 year follow-up period.
TACE
The preferred treatment for 25 patients with a hepatic tumor of long diameter ≥ 5 cm was TACE[19,20], which was performed after cross-sectional imaging as previously described[21]. A French vascular sheath was placed into the femoral artery and a 0.035 inch diameter Mickaelson catheter was advanced into the celiac and superior mesenteric arteries. Contrast was injected into the arteries during rapid-sequence radiographic imaging. Arterial branches supplying the tumor were then located and the venous phase was examined carefully for patency of the portal veins. A 0.018 inch diameter Tracker catheter was advanced through the Mickaelson catheter to the arterial branches supplying the tumor. A mixture of doxorubicin (50 mg), mitomycin (10 mg) and lipiodol (4-15 mL) was injected into the arterial branches until hemostasis was achieved. If the tumor showed no shrinkage 2 wk after the procedure, a second TACE was performed.
Cryoablation procedure
Comprehensive cryosurgery was performed on 33 patients, with complete cryoablation of obvious intra- and extrahepatic masses. Each procedure comprised two freeze/thaw cycles accomplished using an argon gas-based cryosurgical unit (Endocare, Irvine, CA, United States). Cryoprobes (3, 5 or 8 mm) were inserted into the center of the tumor mass under ultrasonographic guidance and two freeze/thaw cycles were performed, each reaching a temperature of -180 °C at the tip of the probe. The duration of freezing was dependent on the achievement of an ice ball, visible as a hypoechoic region on ultrasonography. Generally, the maximal freezing time was 15 min, followed by thawing for 5 min; this cycle was then repeated. A margin of at least 1 cm of normal hepatic tissue was frozen circumferentially around the tumor. For masses larger than 5 cm in diameter, two or three cryoprobes were placed within the center and periphery of the tumor, to ensure freezing of the entire mass. The tracts formed were sealed with fibrin glue immediately after removal of the cryoprobes to ensure hemostasis.
Immunotherapy
Twenty-six patients opted for immunotherapy (adoptive transfer of DC-CIK cells performed four times). DC-CIK cells were generated according to previously published protocols[22,23]; 70 mL peripheral blood was drawn before cryosurgery and the treatment was given 3-5 d after cryosurgery. Using Ficoll-Hypaque density centrifugation, we harvested peripheral blood mononuclear cells (PBMCs) from peripheral blood samples (80 mL) collected from the 48 patients 2 d before cryosurgery.
For DC culture, PBMCs were resuspended in DC medium [X-VIVO 15 (Lonza, Basel, Switzerland), 25 ng/mL interleukin (IL)-4 (Peprotech, Rocky Hill, NJ, United States) and 30 ng/mL granulocyte macrophage colony stimulating factor (GM-CSF; Peprotech)], at a concentration of 1 × 106 to 2 × 106/mL. The cells were then allowed to adhere in two plastic flasks (T75; Corning Costa, Cambridge, MA, United States), each containing 50 mL DC medium and approximately 108 cells. After overnight culture at 37 °C with 5% CO2, the suspended cells were transferred to two fresh flasks. The cells sticking to the initial two flasks were continuously cultured in DC medium and a small amount of fresh medium was added daily to the cultures.
For culture of CIK cells, PBMCs were suspended in CIK medium [X-VIVO 15 (Lonza), 1000 U/mL IL-2 (Peprotech), 2.5 μg/mL monoclonal antibody to CD3 (OKT-3; Jansen-Kyowa, Tokyo, Japan), 25 μg/mL phytohemagglutinin (Peprotech) and 1000 U/mL interferon gamma (Peprotech)]. The CIK cells were allowed to grow and then continuously passaged. At approximately 7 d of culture, the CIK cells were passaged to fourteen T225 flasks. Cells adhering to the flasks were removed with a cell spatula, centrifuged and resuspended in DC-CIK medium [X-VIVO 15 (Lonza), 400 U/mL IL-2 and 0.5 μg/mL monoclonal antibody to CD3]. All DCs were distributed evenly in the 14 T225 flasks containing CIK cells (approximately 108 DCs per flask). After co-culture for 24-48 h, almost 1 wk after cryosurgery, the DC-CIKs were harvested and suspended in 100 mL saline for intravenous injection (cells were collected on four consecutive days; 6 × 109 to 10 × 109 cells were collected on each day). The final cell products were assessed for viability by the dye-exclusion test and checked twice for possible contamination by bacteria, fungi and endotoxins. All cell preparation processes were performed by the same technician and assessed by another technician.
Seven patients refused to undergo cryo- or immunotherapy owing to its cost or their health or age. These patients received no treatment and left the hospital.
Ethics
The study protocol received ethical approval from the Regional Ethics Committee of Guangzhou Fuda Cancer Hospital and conformed to the provisions of the World Medical Association’s Declaration of Helsinki in 1995 (as revised in Tokyo in 2004). Written informed consent was obtained from each participant.
Statistical analysis
Complications were recorded and classified in accordance with the Common Terminology Criteria of Adverse Events v4.0. Local tumor control and OS were also evaluated. Radiographic local tumor control was assessed using image-guided tumor ablation criteria[24]. Thoracic and/or abdominal ultrasonography was performed both 1 d and 1 wk after the minimally invasive treatment of primary and metastatic tumors. Follow-up dynamic CT was performed at 1 mo and then at 3-4 mo intervals. The revised Response Evaluation Criteria in Solid Tumors v1.1 were used to assess the response of the thoracic and abdominal tumors[25]. Three diagnostic radiologists reviewed CT scans for every case to determine whether progression or recurrence had occurred. Diagnoses were made independently, although the radiologists discussed cases over which they disagreed. Using the Dunnett test, we compared the OS of patients who had received cryo- and/or immunotherapy with that of untreated patients. The Kaplan-Meier test with log-rank analysis was used for comparison of OS between two groups. Significant differences were indicated by P < 0.05 or P < 0.01. All analyses were conducted using GraphPad software (San Diego, CA, United States).
RESULTS
Clinical data
Twenty-eight men and five women underwent comprehensive cryoablation and/or TACE. Their ages ranged from 29 to 79 years, with a mean age of 53 years. Twenty-eight patients had histories of hepatitis B infection and two had hepatitis C infection. Fifteen patients were from China and 18 were from Southeast Asia. Of these patients, 18 had initially been treated with surgery and 13 with systemic chemotherapy in other centers; a total of 22 patients came to our hospital for further treatment 1-7 mo after metastases were found and 11 patients came to our hospital for first treatment. Bone metastases (17 lesions) were found in 11 patients, lung metastases (21 lesions) in 15 and multiple organ metastases (18 lesions) in seven. Moderate/severe abdominal pain, evaluated as 5-10 on a visual analog scale (VAS) (17 patients), and mild/moderate ascites (15 patients) were common complaints. For metastasis or recurrence of HCC after treatment, 16 patients received multiple treatments (10 in the cryo-immunotherapy group and 6 in the cryotherapy group); 17 patients refused to continue treatment (11 in the cryo-immunotherapy group and 6 in the cryotherapy group).
The untreated group (those who refused cryoablation, TACE and immunotherapy for reasons of treatment concept, age or economic ability) comprised 12 patients (47-77 years of age, median age 63 years; 8 male, 4 female). All of these patients had histories of hepatitis B or C infection. Five patients were from China and seven were from Southeast Asia. Of these patients, eight had initially been treated with surgery or systemic chemotherapy in other centers; a total of seven patients came to our hospital for further treatment 1-6 mo after metastases were found and five patients came to our hospital for first treatment. Bone metastases (5 lesions) were found in three patients, lung metastases (12 lesions) in seven and multiple organ metastases (6 lesions) in two. These patients had complaints similar to those of the comprehensive treatment group.
Perioperative outcomes
Percutaneous cryoablation of primary and metastatic HCC was successful in every case. No severe complications, such as liver cracking and failure or acute renal failure with myoglobinuria, were discovered post-cryoablation. After the first comprehensive cryosurgery in 33 patients, many slight side effects of cryoablation were observed but recovered with or without symptomatic treatment. Slightly hepatorrhagia was found in six patients (18%) but all healed within 5 d, after injection of a hemostatic agent. Liver capsular cracking was found in one patient (3%) who recovered after blood transfusion. Transient thrombocytopenia occurred in seven patients (21%) within 1 wk after cryoablation; two received platelet transfusions. Two patients (6%) had tumor in the right lobe and developed asymptomatic right-sided pleural effusions close to the dome of the diaphragm; these disappeared spontaneously within 2-3 wk. Two patients (6%) developed liver abscess at the previous cryoablation site 2 and 4 d respectively following cryoablation, but recovered after antibiotic and drainage treatment. Four patients were found to have slight fever (body temperature less than 39 °C). No obvious side effects associated with TACE were found during the perioperative stage. In the first 2 wk after comprehensive cryosurgery, the VAS pain score decreased to 0-3 in 13 patients (76%) who had suffered pretreatment abdominal pain, with consumption of analgesics decreased by 50% and KPS score increased by ≥ 20.
Influence of treatment method and frequency on OS
In our therapeutic protocol, large hepatic tumors (long diameter ≥ 5 cm) were treated by TACE first and considerably reduced in size before cryoablation. Whether patient life span is significantly affected by liver tumor size and additional TACE treatment remains to be determined. Of the 33 patients who received comprehensive cryosurgery, the median OS of those who underwent TACE first was 29 mo; those who received cryoablation directly had a median OS of 26 mo. There was no difference in the OS of these two groups according to the log-rank test (P = 0.798, Kaplan-Meier test with log-rank analysis; Figure 1A). Thus, a large hepatic tumor successfully shrunk by TACE can be treated as a small tumor, with no difference in the results of cryoablation.
Figure 1.
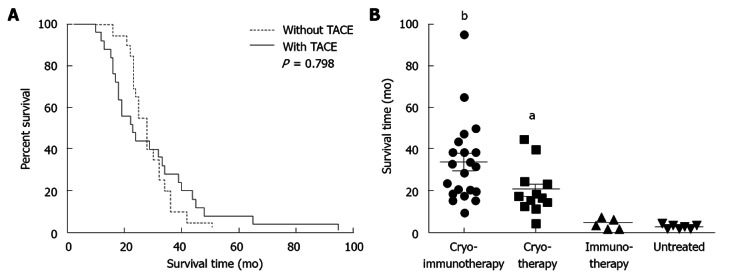
Correlation of overall survival with type of treatment. All 45 patients with metastatic hepatocellular carcinoma died before June, 2012. There were 21 patients in the cryo-immunotherapy group, 12 in the cryotherapy group, five in the immunotherapy group and seven in the untreated group. The overall follow-up period was 8 years. A: Overall survival (OS) of patients who underwent comprehensive cryosurgery with or without transarterial chemoembolization (TACE). Thirty-three patients were enrolled; based on the long diameter of their hepatic tumors (≥ 5 cm), 25 underwent TACE before hepatic cryoablation. Kaplan-Meier test with long-rank analysis; B: OS in the cryo-immunotherapy, cryotherapy and/or immunotherapy groups vs that in the untreated group using the Dunnett test. Horizontal lines represent the average and standard deviation. aP < 0.05, bP < 0.01 vs untreated group.
To the date of the last follow-up, the median OS of all patients was 18 mo (25% percentile, 6 mo; 75% percentile, 33.5 mo). Median OS in the cryo-immunotherapy, cryotherapy, immunotherapy and untreated groups was 32, 17.5, 4 and 3 mo, respectively. OS was significantly higher in the cryo-immunotherapy (P < 0.01) and cryotherapy (P < 0.05) groups than in the untreated group (by the Dunnett test, with the untreated group as the control group; Figure 1B).
Comparing the two groups in which there were obvious therapeutic effects, OS was higher in the cryo-immunotherapy group than in the cryotherapy group (P = 0.024, Kaplan-Meier test with log-rank analysis; Figure 2).
Figure 2.
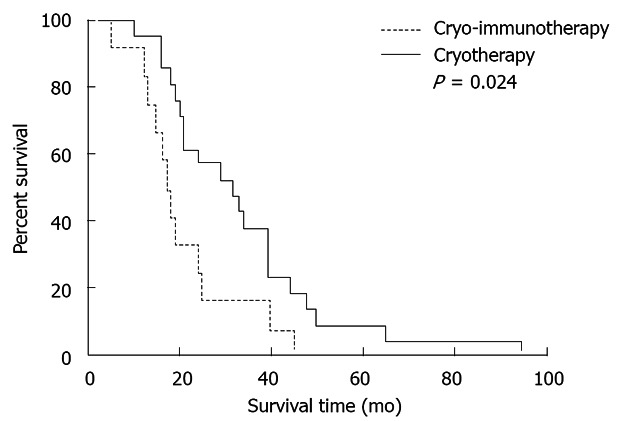
Overall survival of patients who underwent comprehensive cryosurgery with or without immunotherapy. The Kaplan-Meier test with log-rank analysis was used to compare the overall survival of 21 patients in the cryo-immunotherapy group with that of 12 patients in the cryotherapy group.
Repeated cryo- and immunotherapy for tumor progression and/or recurrence was performed in 10 patients in the cryo-immunotherapy group (twice in five patients, thrice in four patients and four times in one patient) and 6 patients in the cryotherapy group (twice in four patients and thrice in two patients); the remaining patients refused repeat treatments. Due to the shorter survival time, all patients in the immunotherapy group received one treatment. In the cryo-immunotherapy group, the median OS of the patients who underwent repeated treatment (36.5 mo) was higher than that of those who underwent a single treatment (21 mo; P = 0.039, Kaplan-Meier test with log-rank analysis; Figure 3A). In the cryotherapy group, the median OS after repeated treatment was 21.5 mo, whereas that after a single treatment was 14 mo (P = 0.035, Kaplan-Meier test with log-rank analysis; Figure 3B).
Figure 3.
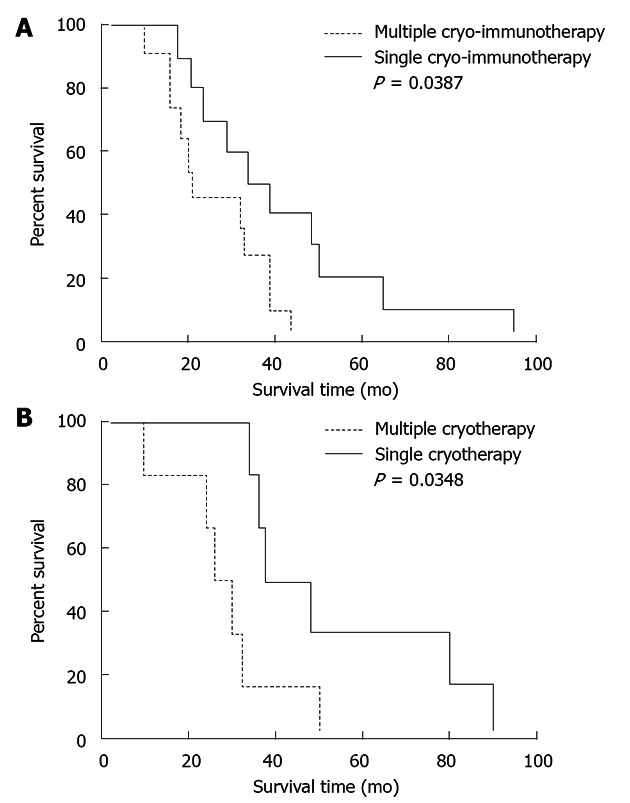
Correlation of overall survival with number of cryo- and/or immunotherapy procedures, using the Kaplan-Meier test with long-rank analysis. A: Comparison of overall survival (OS) between 10 patients who underwent repeated treatments and 11 patients who underwent a single treatment in the cryo-immunotherapy group; B: Comparison of OS between six patients who underwent repeated cryoablation and six who underwent a single cryoablation in the cryotherapy group.
DISCUSSION
In this study, we retrospectively reviewed our hospital’s database to evaluate the survival time of patients with metastatic HCC. These patients had received various therapies in different medical centers before the metastases were found, and our treatment program directly determined their survival time in the metastatic stage. Increasing numbers of patients are undergoing cryoablation of their primary tumor and metastases, termed comprehensive cryoablation. With skilled operators and strict patient selection, this combined technology can be effective in preventing the occurrence of severe complications (i.e., liver cracking and failure, acute renal failure with myoglobinuria), reducing the probability of side effects (i.e., hepatorrhagia, liver capsular cracking, thrombocytopenia and liver abscess) and provide guarantee for the success of cryotherapy. Theoretically, most of the side effects can be further reduced, with the exception of thrombocytopenia. Development of systemic thrombocytopenia after cryosurgery is associated with excessive platelet trapping and destruction within the cryolesion[26]. This symptom is difficult to avoid simply through improved care, but can heal spontaneously or with platelet supplements.
It is increasingly clear that immunotherapy can be useful in cancer therapy, but there are also obstacles that need to be overcome. Due to their organ-like structural environment, these tumors are able to escape immune surveillance[27], and immunotherapy for HCC must therefore be combined with additional therapy to disrupt this structure. Adoptive transfer of CIK cells along with DCs has been shown to be efficacious when the tumor burden is relatively low or when used as an adjuvant therapy rather than as a treatment for bulky tumors[18], indicating the importance of cytoreductive cryoablation before immunotherapy. DCs have been the subject of much research in the last decade and are widely used in immunotherapy protocols. These bone marrow-derived cells have been identified as the most potent immune-stimulatory cells known and are specialized for the initiation and shaping of immune responses. Activated DCs after cryoablation are potent stimulators of both CD4+ and CD8+ T cells, as supported by evidence from experimental[28] and human[29,30] studies. DCs are often pulsed with synthetic peptides derived from known tumor antigens[31], tumor cell lysates[32], apoptotic tumor cells[33] or RNA derived from tumor antigens[34] and transfected with whole tumor cell DNA[35] or RNA[36]. Moreover, DCs have been fused with tumor cells to induce antigen-specific, polyclonal cytotoxic T lymphocyte responses[37].
On account of continued antigen release after cryoablation[14], in vitro activation of DCs was omitted and the DCs were stimulated in vivo in the present study. We found that combined cryo- and immunotherapy extended the median OS of metastatic HCC patients from 3 to 32 mo (Figure 1B). Desirable results were achieved, and OS was longer in the cryo-immunotherapy group than in the cryotherapy group, demonstrating the synergistic effect of these two therapies (Figure 2). Owing to procedural costs, age or health, some of our patients underwent cryo-immunotherapy only once. We found that, compared with a single treatment, multiple cytoreductive cryoablation combined with immunotherapy was therapeutically valuable (Figure 3A) and prolonged survival time. Continued cryotherapy delayed disease progression, maintained function of multiple organs and improved quality of life and KPS scores, thereby achieving a better effect than single cryotherapy (Figure 3B).
In studies of the sequential use of TACE and percutaneous cryosurgery for unresectable HCC, pre-cryosurgical TACE was shown to increase the efficacy of cryoablation and decrease its adverse effects in patients with large HCCs (> 5 cm in diameter)[19]. It is well known that the presence of large HCCs often predicts rapid loss of liver function and a poor prognosis, and reducing their size before treatment is more effective than direct treatment of a large tumor. Data are available from two studies on the possible effect of TACE on immune stimulation[38,39], which may further increase the therapeutic effect of combination therapy. Depending on whether single or multiple TACE is performed, a large HCC can first be reduced to 5 cm in diameter and then completely ablated by the combined application of multiple cryoprobes[19]. Consistent with the 2009 report of Shibata et al[40], treatment of larger tumors with sequential TACE and cryoablation can achieve significantly better effects than TACE or cryoablation alone. The findings of these authors and our own results indicate that not the frequency of TACE but the shrinkage of large HCCs contributed to the increase in median OS of about 30 mo, and differences due to HCC diameter can be eliminated by additional TACE procedures (median OS was 29 and 26 mo, respectively; P = 0.798; Figure 1A).
In conclusion, we combined a minimally invasive procedure (percutaneous cryoablation of primary and metastatic tumors) with a common immunotherapy method (DC-CIK) to treat metastatic HCC. This new strategy extended the median OS from 3 to 32 mo. Better outcomes are expected as more patients undergo cryo-immunotherapy.
COMMENTS
Background
Hepatocellular carcinoma (HCC), which is the fifth most common cancer worldwide, is usually discovered late and has a poor prognosis. In about 80% of patients, HCC is associated with chronic liver disease (i.e., hepatitis and cirrhosis), with major implications for the prognosis and therapeutic options. Many patients are unsuitable for tumor resection because of factors such as poor hepatic reserve (cirrhosis), multicentric tumors or extrahepatic disease. Until recently, no systemic chemotherapy has significantly increased survival in patients with advanced HCC. External beam radiation has had a limited role in the treatment of HCC because of radiation toxicity to the adjacent normal liver
Research frontiers
The effects of comprehensive cryosurgery (simultaneous cryoablation of intra- and extrahepatic tumors and of liver tumors of diameter greater than 5 cm, with transarterial chemoembolization (TACE) performed once or twice before cryoablation to reduce the tumor to 5 cm) and/or dendritic cells - cytokine-induced killer (DC-CIK) immunotherapy in patients with untreated metastatic HCC.
Innovations and breakthroughs
Cryotherapy and, especially, cryo-immunotherapy significantly increased overall survival in metastatic hepatocellular cancer patients. Multiple cryo-immunotherapy was associated with a better prognosis than single cryo-immunotherapy.
Applications
For metastatic HCC, comprehensive cryotherapy and cryo-immunotherapy can help patients improve symptoms, reduce pain and prolong life.
Terminology
Comprehensive cryotherapy: simultaneous cryoablation of intra- and extrahepatic tumors and of liver tumors of diameter greater than 5 cm, with TACE performed once or twice before cryoablation to reduce the tumor to 5 cm; cryo-immunotherapy: Immunotherapy is performed shortly after comprehensive cryosurgery, breakong products of tumor may continually stimulate immune cells to clean up the systemic metastases lesions.
Peer review
In this study, authors combined a minimally invasive procedure (percutaneous cryoablation of primary and metastatic tumors) with a common immunotherapy method (DC-CIK) to treat metastatic HCC. This new strategy extended the median overall surviva from 3 mo to 32 mo. Better outcomes are expected as more patients undergo cryo-immunotherapy.
Footnotes
P- Reviewers Gokhan K, Mario K S- Editor Zhai HH L- Editor A E- Editor Xiong L
References
- 1.Said A, Wells J. Management of hepatocellular carcinoma. Minerva Med. 2009;100:51–68. [PubMed] [Google Scholar]
- 2.Turner PM, Turner TJ. Validation of the crisis triage rating scale for psychiatric emergencies. Can J Psychiatry. 1991;36:651–654. doi: 10.1177/070674379103600905. [DOI] [PubMed] [Google Scholar]
- 3.Hodgson HJ. Primary hepatocellular carcinoma. Br J Hosp Med. 1983;29:240, 246, 250 passim. [PubMed] [Google Scholar]
- 4.Dusheiko GM, Hobbs KE, Dick R, Burroughs AK. Treatment of small hepatocellular carcinomas. Lancet. 1992;340:285–288. doi: 10.1016/0140-6736(92)92367-o. [DOI] [PubMed] [Google Scholar]
- 5.Burroughs A, Hochhauser D, Meyer T. Systemic treatment and liver transplantation for hepatocellular carcinoma: two ends of the therapeutic spectrum. Lancet Oncol. 2004;5:409–418. doi: 10.1016/S1470-2045(04)01508-6. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 7.Davis CR. Interventional radiological treatment of hepatocellular carcinoma. Cancer Control. 2010;17:87–99. doi: 10.1177/107327481001700204. [DOI] [PubMed] [Google Scholar]
- 8.Fuss M, Salter BJ, Herman TS, Thomas CR. External beam radiation therapy for hepatocellular carcinoma: potential of intensity-modulated and image-guided radiation therapy. Gastroenterology. 2004;127:S206–S217. doi: 10.1053/j.gastro.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 9.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 10.Chen JB, Li JL, He LH, Liu WQ, Yao F, Zeng JY, Zhang Y, Xu KQ, Niu LZ, Zuo JS, et al. Radical treatment of stage IV pancreatic cancer by the combination of cryosurgery and iodine-125 seed implantation. World J Gastroenterol. 2012;18:7056–7062. doi: 10.3748/wjg.v18.i47.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orlacchio A, Bazzocchi G, Pastorelli D, Bolacchi F, Angelico M, Almerighi C, Masala S, Simonetti G. Percutaneous cryoablation of small hepatocellular carcinoma with US guidance and CT monitoring: initial experience. Cardiovasc Intervent Radiol. 2008;31:587–594. doi: 10.1007/s00270-008-9293-9. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu T, Sakuhara Y, Abo D, Hasegawa Y, Kodama Y, Endo H, Shirato H, Miyasaka K. Outcome of MR-guided percutaneous cryoablation for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2009;16:816–823. doi: 10.1007/s00534-009-0124-4. [DOI] [PubMed] [Google Scholar]
- 13.Sabel MS. Cryo-immunology: a review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology. 2009;58:1–11. doi: 10.1016/j.cryobiol.2008.10.126. [DOI] [PubMed] [Google Scholar]
- 14.Rovere-Querini P, Manfredi AA. Tumor destruction and in situ delivery of antigen presenting cells promote anti-neoplastic immune responses: implications for the immunotherapy of pancreatic cancer. JOP. 2004;5:308–314. [PubMed] [Google Scholar]
- 15.Palmer DH, Midgley RS, Mirza N, Torr EE, Ahmed F, Steele JC, Steven NM, Kerr DJ, Young LS, Adams DH. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology. 2009;49:124–132. doi: 10.1002/hep.22626. [DOI] [PubMed] [Google Scholar]
- 16.Lee WC, Wang HC, Hung CF, Huang PF, Lia CR, Chen MF. Vaccination of advanced hepatocellular carcinoma patients with tumor lysate-pulsed dendritic cells: a clinical trial. J Immunother. 2005;28:496–504. doi: 10.1097/01.cji.0000171291.72039.e2. [DOI] [PubMed] [Google Scholar]
- 17.Iwashita Y, Tahara K, Goto S, Sasaki A, Kai S, Seike M, Chen CL, Kawano K, Kitano S. A phase I study of autologous dendritic cell-based immunotherapy for patients with unresectable primary liver cancer. Cancer Immunol Immunother. 2003;52:155–161. doi: 10.1007/s00262-002-0360-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thanendrarajan S, Nowak M, Abken H, Schmidt-Wolf IG. Combining cytokine-induced killer cells with vaccination in cancer immunotherapy: more than one plus one? Leuk Res. 2011;35:1136–1142. doi: 10.1016/j.leukres.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Fazio MJ, Olsen DR, Uitto JJ. Skin aging: lessons from cutis laxa and elastoderma. Cutis. 1989;43:437–444. [PubMed] [Google Scholar]
- 20.Azizi A, Naguib NN, Mbalisike E, Farshid P, Emami AH, Vogl TJ. Liver metastases of pancreatic cancer: role of repetitive transarterial chemoembolization (TACE) on tumor response and survival. Pancreas. 2011;40:1271–1275. doi: 10.1097/MPA.0b013e318220e5b9. [DOI] [PubMed] [Google Scholar]
- 21.Liaw YF, Lin DY. Transcatheter hepatic arterial embolization in the treatment of hepatocellular carcinoma. Hepatogastroenterology. 1990;37:484–488. [PubMed] [Google Scholar]
- 22.Li H, Wang C, Yu J, Cao S, Wei F, Zhang W, Han Y, Ren XB. Dendritic cell-activated cytokine-induced killer cells enhance the anti-tumor effect of chemotherapy on non-small cell lung cancer in patients after surgery. Cytotherapy. 2009;11:1076–1083. doi: 10.3109/14653240903121252. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura M, Wada J, Suzuki H, Tanaka M, Katano M, Morisaki T. Long-term outcome of immunotherapy for patients with refractory pancreatic cancer. Anticancer Res. 2009;29:831–836. [PubMed] [Google Scholar]
- 24.Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, Dupuy DE, Gervais D, Gillams AR, Kane RA, Lee FT, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2005;16:765–778. doi: 10.1097/01.RVI.0000170858.46668.65. [DOI] [PubMed] [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Pistorius GA, Alexander C, Krisch CM, Feifel G, Schilling MK, Menger MD. Local platelet trapping as the cause of thrombocytopenia after hepatic cryotherapy. World J Surg. 2005;29:657–660; discussion 661. doi: 10.1007/s00268-005-7543-4. [DOI] [PubMed] [Google Scholar]
- 27.Plate J. Clinical trials of vaccines for immunotherapy in pancreatic cancer. Expert Rev Vaccines. 2011;10:825–836. doi: 10.1586/erv.11.77. [DOI] [PubMed] [Google Scholar]
- 28.Walker AR, Walker BF, Vorster HH. Functional significance of mild-to-moderate malnutrition. Am J Clin Nutr. 1990;52:178–179. doi: 10.1093/ajcn/52.1.178. [DOI] [PubMed] [Google Scholar]
- 29.Schueller G, Stift A, Friedl J, Dubsky P, Bachleitner-Hofmann T, Benkoe T, Jakesz R, Gnant M. Hyperthermia improves cellular immune response to human hepatocellular carcinoma subsequent to co-culture with tumor lysate pulsed dendritic cells. Int J Oncol. 2003;22:1397–1402. [PubMed] [Google Scholar]
- 30.Ali MY, Grimm CF, Ritter M, Mohr L, Allgaier HP, Weth R, Bocher WO, Endrulat K, Blum HE, Geissler M. Activation of dendritic cells by local ablation of hepatocellular carcinoma. J Hepatol. 2005;43:817–822. doi: 10.1016/j.jhep.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 31.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 32.Mackensen A, Herbst B, Chen JL, Köhler G, Noppen C, Herr W, Spagnoli GC, Cerundolo V, Lindemann A. Phase I study in melanoma patients of a vaccine with peptide-pulsed dendritic cells generated in vitro from CD34(+) hematopoietic progenitor cells. Int J Cancer. 2000;86:385–392. doi: 10.1002/(sici)1097-0215(20000501)86:3<385::aid-ijc13>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 33.Palucka AK, Ueno H, Connolly J, Kerneis-Norvell F, Blanck JP, Johnston DA, Fay J, Banchereau J. Dendritic cells loaded with killed allogeneic melanoma cells can induce objective clinical responses and MART-1 specific CD8+ T-cell immunity. J Immunother. 2006;29:545–557. doi: 10.1097/01.cji.0000211309.90621.8b. [DOI] [PubMed] [Google Scholar]
- 34.Nair SK, Boczkowski D, Morse M, Cumming RI, Lyerly HK, Gilboa E. Induction of primary carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes in vitro using human dendritic cells transfected with RNA. Nat Biotechnol. 1998;16:364–369. doi: 10.1038/nbt0498-364. [DOI] [PubMed] [Google Scholar]
- 35.Zhu CZ, Norris JW. Seizures after stroke. Arch Neurol. 1991;48:18–19. doi: 10.1001/archneur.1991.00530130026009. [DOI] [PubMed] [Google Scholar]
- 36.Gilboa E, Vieweg J. Cancer immunotherapy with mRNA-transfected dendritic cells. Immunol Rev. 2004;199:251–263. doi: 10.1111/j.0105-2896.2004.00139.x. [DOI] [PubMed] [Google Scholar]
- 37.Gong J, Chen D, Kashiwaba M, Kufe D. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nat Med. 1997;3:558–561. doi: 10.1038/nm0597-558. [DOI] [PubMed] [Google Scholar]
- 38.Akizuki S, Magara T, Tanaka T. [Diminution of the number of gamma delta T lymphocytes in hepatocellular carcinoma patients treated with transcatheter arterial embolization] Nihon Rinsho Meneki Gakkai Kaishi. 1998;21:108–117. doi: 10.2177/jsci.21.108. [DOI] [PubMed] [Google Scholar]
- 39.Ayaru L, Pereira SP, Alisa A, Pathan AA, Williams R, Davidson B, Burroughs AK, Meyer T, Behboudi S. Unmasking of alpha-fetoprotein-specific CD4(+) T cell responses in hepatocellular carcinoma patients undergoing embolization. J Immunol. 2007;178:1914–1922. doi: 10.4049/jimmunol.178.3.1914. [DOI] [PubMed] [Google Scholar]
- 40.Shibata T, Isoda H, Hirokawa Y, Arizono S, Shimada K, Togashi K. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology. 2009;252:905–913. doi: 10.1148/radiol.2523081676. [DOI] [PubMed] [Google Scholar]


