Obesity is a pressing health problem affecting more than one-third of adults in the United States and Europe. Besides their increased risk to develop cardiovascular disease and type 2 diabetes, metabolic disturbances in overweight individuals affect sleeping behavior, promoting arousal and feeding (1). Task-dependent recruitment of diverse neuropeptidergic neurons in hypothalamic nuclei orchestrates distinct endocrine functions particularly relevant to maintain energy homeostasis (2). The interplay of neuropeptide Y (NPY)/agouti-related peptide and α-melanocyte–stimulating hormone (αMSH) neurons of the arcuate nucleus with orexin (hypocretin)-containing neurons in the lateral hypothalamus is central to the regulation of food intake and arousal. A key attribute of these hypothalamic circuits is their remarkable ability to undergo “synaptic rewiring” to maintain the body’s energy homeostasis (3, 4). In PNAS, Cristino et al. (5) provide fresh understanding of the molecular regulation of synaptic plasticity by showing how endocannabinoids control inhibitory synapses newly recruited to orexinergic neurons in obesity.
Orexin-containing neurons (up to ∼70,000 in human) (6–8) are mostly located in the lateral hypothalamic nucleus, and are thought to contribute to regulating food intake, wakefulness, and arousal (3, 9). Orexin-containing cells are innervated by NPY and αMSH-containing arcuate neurons (Fig. 1A). Neuropeptides typically coexist with fast neurotransmitters (2): NPY neurons contain the inhibitory neurotransmitter GABA, and αMSH can colocalize with excitatory glutamate (10). NPY itself is an orexinergic neuropeptide robustly inducing food intake (11, 12). In contrast, αMSH is anorexigenic, decreasing feeding (13). GABAergic and glutamatergic synaptic inputs to these arcuate neurons are prone to remodeling upon genetic or dietary manipulation of leptin (4), the adipocyte-derived satiety hormone, which critically impacts energy homeostasis and body weight (14). Nevertheless, a lack of consensus exists as to the neurophysiological requirements of neuropeptide and primary fast neurotransmitter corelease from hypothalamic neurons, and their endocrine action.
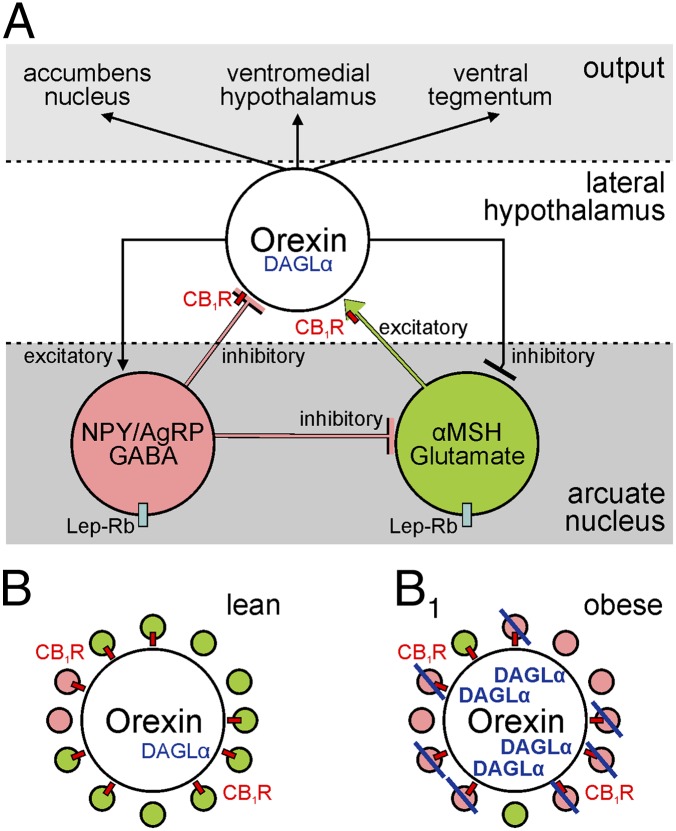
Fig. 1.
(A) Hierarchical depiction of synaptic connections allowing information flow among neuropeptidergic neurons in the arcuate mucleus and lateral hypothalamus. Lep-Rb denotes leptin receptor expression by neurons in the arcuate nucleus. (B) Orexin-containing neurons receive predominantly excitatory synaptic inputs in lean mice. (pink: inhibitory/GABA synapse; green: excitatory/glutamate synapse; red rectangles pinpoint CB1 cannabinoid receptors). (B1) In obesity, orexin-containing neurons up-regulate DAGLα to block surplus inhibition by retrograde endocannabinoid signals.
Excitatory synapses onto orexin-containing neurons vastly outnumber inhibitory terminals (3). The number of these excitatory inputs increases upon food deprivation (3). However, synapse remodeling in the lateral hypothalamus in obesity remains unknown. Using a multiparametric approach encompassing systems neuroanatomy, neurophysiology, molecular pharmacology, and mouse genetics, Cristino et al. (5) identify an excitatory-to-inhibitory switch of synapses impacting orexin-containing neurons in genetically leptin deficient ob/ob mice (11). Here, a substantial subset of new inhibitory terminals from NPY-containing neurons replaced excitatory inputs formed by αMSH cells (Fig. 1 B and B1). Significantly, high-fat diet, evoking leptin resistance in arcuate, but not lateral hypothalamic neurons, replicated circuit remodeling. This synaptology was unequivocally driven by the lack of leptin because leptin signaling through the mammalian target of rapamycin rapidly normalized synapse composition.
Orexin, like NPY, is up-regulated upon decreased leptin availability (15). The formation of new inhibitory synapses terminating on orexin-containing neurons in ob/ob mice, which lack leptin, would suggest increased synaptic inhibition, decreasing the excitability and perhaps even reducing neuropeptide release from orexin-containing neurons. However, this is clearly not the case because orexin expression, axonal transport, and accumulation in terminals increased in ob/ob mice.
Early work by Di Marzo et al. revealed increased concentrations of hypothalamic endocannabinoids in ob/ob mice (16). Endocannabinoids, particularly 2-arachidonoyl glycerol (2-AG), are produced upon neuronal activity and released from subsynaptic dendrites to presynaptically reduce neurotransmitter release (17). Thus, and by adopting a “retrograde” mode of action, endocannabinoid signaling is a form of feedback control of synaptic neurotransmission. 2-AG is thought to be primarily produced by sn-1-diacylglycerol lipase α (DAGLα) in the adult brain (18). Cristino et al. (5) exploit this knowledge to demonstrate that the synaptic rewiring of orexin neurons in ob/ob mice coincides with their increased DAGLα expression. Because 70% of all synaptic inputs to orexin neurons, including many NPY- and αMSH-containing afferents, contain presynaptic CB1 cannabinoid receptors (CB1Rs), Cristino et al. hypothesize that orexin neurons in obese mice can successfully eliminate surplus inhibition by using 2-AG as retrograde messenger. The authors address the functional significance of synapse reorganization by showing that somatic depolarization of orexin-containing neurons, a means to induce endocannabinoid release (17), in ob/ob mice suppressed presynaptic GABA release. The lack of retrograde signaling at inhibitory synapses converging onto orexin-containing neurons in lean mice highlights the network-level significance of the glutamate-to-GABA switch.
Mice lacking monoacylglycerol lipase (MAGL), a key 2-AG–degrading enzyme (19), are lean even though presenting many-fold increased brain 2-AG levels. Similarly, synaptic afferentation of orexin neurons is unchanged in the lateral hypothalamus of MAGL−/− mice. These data clearly establish a hierarchical relationship between leptin and endocannabinoids, with leptin signaling (or the lack thereof) driving synapse remodeling. However, orexin expression was dramatically reduced by a single dose of a CB1R antagonist in ob/ob mice. This result suggests that CB1Rs on orexin-containing neurons are poised to regulate orexin expression via a hitherto unexplained mechanism.
This research, like any other comprehensive report, inspires a number of radical hypotheses, and certainly calls for further analysis. Neuropeptides coexist with fast neurotransmitters in this arcuate nucleus-lateral hypothalamus circuit (10). A key take-home message of Cristino et al. (5) and earlier studies (3, 4) is that fast neurotransmission, once reorganized, can reset the excitability of neuropeptidergic neurons. Although the relationship of neuropeptide and fast neurotransmitter action remains elusive, an appealing hypothesis is that a floating circuit code driven by the extreme plasticity of GABA/glutamate synapses (3, 4) encodes a form of “metabolic memory” to set the threshold for neuropeptide release.
High-fat diet-induced leptin resistance was found restricted to arcuate neurons, even though the leptin receptor is expressed in many neurons of the lateral hypothalamus (20). This differential response may be a result of the molecular diversity of signal transduction cascades, differences in excitability in vivo, or feedback coupling between leptin and endocannabinoid signaling systems.
The balance between excitation and inhibition on orexin neurons hinges on enhanced DAGLα synthesis in ob/ob mice. The fundamental importance of DAGLα in this monosynaptic circuitry could be tested in DAGLα null mice, where synaptic reorganization but not muted inhibition onto orexin-containing neurons would be the anticipated phenotype. More importantly, mechanistic insights in
Cristino et al. identify an excitatory-to-inhibitory switch of synapses impacting orexin-containing neurons in genetically leptin deficient ob/ob mice.
this report suggest that DAGLα inhibitors, rather than CB1R antagonists, could be used to reinstate inhibition of orexin-containing neurons. If so, this process could facilitate a sea-change in existing “CB1R-centric” views of weight control, and identify DAGLα as an equally potent molecular target. The ultimate benefit, learning from the failure of rimonabant, is that depressive/anxiety side-effects might be reduced and drug dosing made safer.
A remarkable finding is that orexin expression in various target areas of the brain was vastly enhanced, fueling the hypothesis that increased orexin release from tegmental and hypothalamic projections will exacerbate obesity. Nevertheless, orexins are primarily implicated in the regulation of arousal and sleep (21), and perhaps in reward aspects via the mesolimbic system (22). Thus, obesity-driven synaptic reorganization in the hypothalamus could also influence narcolepsy (6) and cyclic or bipolar vegetative functions, providing stepping stones to understand the molecular pathology of obesity-linked psychiatric disorders.
Exquisitely designed and executed experiments in rodents, such as the study by Cristino et al. (1), are indispensable to unravel key pathomechanisms of human diseases. Nevertheless, their human relevance, ingrained in potential evolutionary differences in the complexity of underlying neuronal circuitries, must be addressed. Does synapse remodeling on orexin neurons occur in obese humans? The discovery of equivalent patterns and mechanisms will ultimately define the clinical relevance of this study. Alas, the proof of the pudding, or rather a good burger, will be eating it.
Acknowledgments
This work was supported by the Swedish Medical Research Council and the NovoNordisk Foundation.
Footnotes
The authors declare no conflict of interest.
See companion article on page E2229.
References
- 1.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1(3):e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hökfelt T, Johansson O, Ljungdahl A, Lundberg JM, Schultzberg M. Peptidergic neurones. Nature. 1980;284(5756):515–521. doi: 10.1038/284515a0. [DOI] [PubMed] [Google Scholar]
- 3.Horvath TL, Gao XB. Input organization and plasticity of hypocretin neurons: Possible clues to obesity’s association with insomnia. Cell Metab. 2005;1(4):279–286. doi: 10.1016/j.cmet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Pinto S, et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304(5667):110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 5.Cristino L, et al. Obesity-driven synaptic remodeling affects endocannabinoid control of orexinergic neurons. Proc Natl Acad Sci USA. 2013;110:E2229–E2238. doi: 10.1073/pnas.1219485110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thannickal TC, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27(3):469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thannickal TC, Nienhuis R, Siegel JM. Localized loss of hypocretin (orexin) cells in narcolepsy without cataplexy. Sleep. 2009;32(8):993–998. doi: 10.1093/sleep/32.8.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aziz A, et al. Hypocretin and melanin-concentrating hormone in patients with Huntington disease. Brain Pathol. 2008;18(4):474–483. doi: 10.1111/j.1750-3639.2008.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leinninger GM. Lateral thinking about leptin: A review of leptin action via the lateral hypothalamus. Physiol Behav. 2011;104(4):572–581. doi: 10.1016/j.physbeh.2011.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collin M, et al. Plasma membrane and vesicular glutamate transporter mRNAs/proteins in hypothalamic neurons that regulate body weight. Eur J Neurosci. 2003;18(5):1265–1278. doi: 10.1046/j.1460-9568.2003.02840.x. [DOI] [PubMed] [Google Scholar]
- 11.Erickson JC, Hollopeter G, Palmiter RD. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science. 1996;274(5293):1704–1707. doi: 10.1126/science.274.5293.1704. [DOI] [PubMed] [Google Scholar]
- 12.Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115(1):427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- 13.Huszar D, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88(1):131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 15.Diano S, Horvath B, Urbanski HF, Sotonyi P, Horvath TL. Fasting activates the nonhuman primate hypocretin (orexin) system and its postsynaptic targets. Endocrinology. 2003;144(9):3774–3778. doi: 10.1210/en.2003-0274. [DOI] [PubMed] [Google Scholar]
- 16.Di Marzo V, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410(6830):822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 17.Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296(5568):678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- 18.Harkany T, Mackie K, Doherty P. Wiring and firing neuronal networks: Endocannabinoids take center stage. Curr Opin Neurobiol. 2008;18(3):338–345. doi: 10.1016/j.conb.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dinh TP, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA. 2002;99(16):10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leinninger GM, et al. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 2011;14(3):313–323. doi: 10.1016/j.cmet.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anaclet C, et al. Orexin/hypocretin and histamine: Distinct roles in the control of wakefulness demonstrated using knock-out mouse models. J Neurosci. 2009;29(46):14423–14438. doi: 10.1523/JNEUROSCI.2604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baldo BA, Daniel RA, Berridge CW, Kelley AE. Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol. 2003;464(2):220–237. doi: 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]