Abstract
The microbiota is pivotal in the pathogenesis of inflammatory bowel disease (IBD)-associated inflammation-induced colorectal cancer (CRC), yet mechanisms for these effects remain poorly characterized. Here, we demonstrate that aberrant inflammasome-induced microbiota plays a critical role in CRC development, where mice deficient in the NOD-like receptor family pyrin domain containing 6 (NLRP6) inflammasome feature enhanced inflammation-induced CRC formation. Intriguingly, WT mice cohoused either with inflammasome-deficient mice or with mice lacking IL-18 feature exacerbated inflammation-induced CRC compared with singly housed WT mice. Enhanced tumorigenesis is dependent on microbiota-induced chemokine (C-C motif) ligand 5 (CCL5)-driven inflammation, which in turn promotes epithelial cell proliferation through local activation of the IL-6 pathway, leading to cancer formation. Altogether, our results mechanistically link the altered microbiota with the pathogenesis of inflammation-induced CRC and suggest that in some conditions, microbiota components may transfer CRC susceptibility between individuals.
Keywords: colon cancer, microflora, ASC
Colorectal cancer (CRC) is one of the most common forms of cancer, yet much of the underlying molecular pathogenesis for CRC development remains unknown (1). A major risk factor for the development of CRC is prolonged IBD, with prevalence ranging from 2% of patients after 10 y of IBD to up to 18% after 30 y of disease (2, 3). The pathogenesis of inflammation-induced CRC was suggested to involve chronic exposure of epithelial cells to inflammatory stimuli (4–8). It was further demonstrated that activation of NF-κB by the classical inhibitor of NF-κB kinase-dependent pathway is crucial for tumor growth and progression, highlighting the importance of inflammation during the pathogenesis of CRC (9, 10).
The intestinal microflora make up a complex ecosystem that has been recently shown to greatly influence health and disease in both animal models and humans (11–14). The intestinal microbiota is believed to influence the pathogenesis of IBD, as antibiotic (Abx) treatment and probiotic therapy can ameliorate the disease in at least some subsets of patients with IBD (15). In addition, patients with IBD were recently demonstrated to have a reduced abundance of some members of the gut microbiota, including Firmicutes and Bacteroidetes (16, 17). Streptoccus bovis/gallolyticus, enterotoxigenic Bacterodies fragillis, and Escherichia coli NC101 have been implicated as risk factors for CRC (18–20). Furthermore, spontaneous intestinal auto-inflammation in several murine models fails to develop in the germ-free setting (21).
Alterations in the composition of the microflora have been also linked to a propensity for intestinal tumorigenesis. Human studies have revealed that high consumption of dietary fat and red meat are associated with high risk for colon cancer, which could potentially stem from the dietary-induced difference in composition and metabolic activity of microbiota (17, 22, 23). Bacteroides vulgatus and Bacteroides stercoris have been suggested to be linked to an increased risk for CRC, whereas low risk is associated with Lactobacillus acidophilus, Lactobacillus S06, and Eubacterium aerofaciens species in humans (24). Moreover, germ-free rats rarely develop colonic tumors in a chemically induced colon cancer model (17, 25). Likewise, T cell receptor beta chain (TCRβ)−/−tumor protein 53 (p53)−/− and transforming growth factor beta (TGFβ)−/−recombination activating gene 2 (Rag2)−/− mice rarely develop spontaneous colorectal adenocarcinoma under germ-free conditions (17, 26). Despite this emerging collateral evidence suggesting possible links between alterations in the composition of the microflora and risk for CRC, direct evidence for such a link remains sparse.
Inflammasomes are cytoplasmic multiprotein complexes that are composed of Nod-like receptors (NLRs), pro-caspase-1, and in some cases, the adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC). Known inflammasomes include the NOD-like receptor family pyrin domain containing 1 (NLRP1), NOD-like receptor family pyrin domain containing 3 (NLRP3), NOD-like receptor family CARD domain containing 4 (NLRC4), NLRP6, and absent in melanoma 2 (AIM2) inflammasomes (27, 28). Inflammasomes could be activated by a diverse range of microbial, stress, and damage signals, which leads to the activation of caspase-1 and the subsequent maturation and secretion of proinflammatory cytokines IL-1β and IL-18 (27-32). In addition to orchestrating multiple innate and adaptive immune responses, inflammasomes were demonstrated to mediate gut homeostasis and tumorigenesis (27, 33–41). Conflicting results show that deficiency in caspase-1, ASC, and NLRP3 in mice was associated with an altered severity of chemically induced colitis. Enhanced colitis in caspase-1−/− mice has been proposed to result from defects in epithelial cell regeneration and tissue repair, as well as enhanced NF-κB activation in the colon tissues (33). In other reports, by Allen et al (35) and Zaki et al (36), enhanced colitis in NLRP3−/− mice was suggested to be mediated by loss of epithelial barrier integrity or by reduction in local IL-1β, IL-18, or IFN-γ production. In contrast, Bauer et al reported an ameliorated severity of colitis in NLRP3−/− mice in the same dextran sodium sulfate (DSS)-induced colitis model, which was suggested to be mediated by the local reduction of proinflammatory cytokines IL-1β, TNF-α, and IFNγ (39).
We recently offered a mechanistic explanation to the above conflicting and at times opposing results by showing that components of the NLRP6 inflammasome are regulators of the gut microflora and/or the host response toward different components of the microbiota. We demonstrated that NLRP6−/− and ASC−/− mice harbor a colitogenic gut microflora that is transmissible to cohoused WT mice, resulting in an exacerbated colitis phenotype (34). Here, using the azoxymethane (AOM)-DSS induced colitis-associated CRC model (CAC), we demonstrate that WT mice cohoused with ASC−/− and NLRP6−/− mice develop a dramatically enhanced tendency for inflammation-induced CRC formation, which is mediated through IL-18-induced alterations in the microflora and resultant induction of CCL5-dependent colonic inflammation and activation of the IL-6 pathway.
Results and Discussion
To study the role of inflammasome-deficient microflora in inflammation-induced tumorigenesis, we induced colorectal tumorigenesis using the AOM-DSS model, with the former initiating mutation by alkylating DNA and the later causing disruption of the colonic mucosa (42). Colitis in this model, similar to in its human IBD counterpart, is believed to be induced by the approximation of members of the intestinal microflora, through the permeabilized mucosa, with the colonic mucosal innate immune arm, inducing a vicious cycle of exaggerated immune response resulting in enhanced tissue damage and repair.
Inflammasomes were recently shown to play pivotal roles in both colitis and inflammation-induced cancer. We recently showed that NLRC4 inflammasome deficiency contributes to enhanced inflammation-induced tumorigenesis through altered epithelial cell proliferation and death (37). This phenotype was independent of the effects of the NLRP3 or NLRP6 inflammasome-associated microflora, as caspase-1−/− or NLRC4−/− mice, but not NLRP3−/−, ASC−/−, or NLRP6−/− mice, featured enhanced tumorigenesis compared with cohoused WT mice, even after fully acquiring their microflora by prolonged cohousing. Indeed, NLRC4 contains a Caspase recruitment domain (CARD) that enables direct binding to caspase-1 through CARD–CARD interactions, so assembly of the NLRC4 inflammasome in mice may be achieved independent of the adaptor protein ASC (43, 44). Similar to other described inflammasomes, the NLRC4 inflammasome formation results in secretion of IL-1β and IL-18, but it can also induce caspase-1-mediated cell death (43, 45).
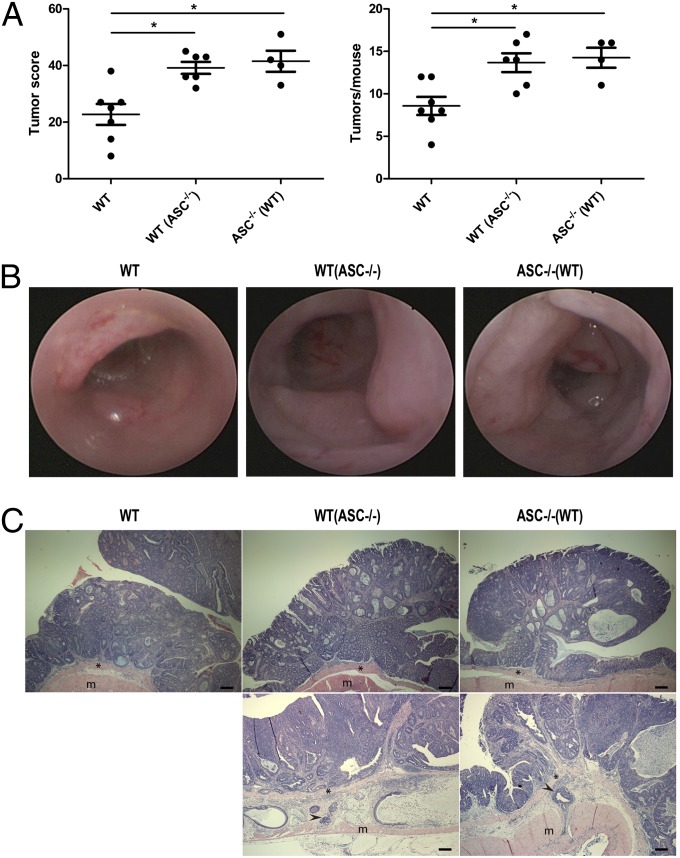
To study the role of the inflammasome-deficient colitogenic microflora on CRC formation, we applied the AOM-DSS induced CAC model to mice deficient in ASC, a common adaptor for most other inflammasomes. We found that ASC−/− mice exhibited significantly more tumorigenesis than singly housed WT mice (Fig. 1A). This led us to hypothesize that the aberrant composition of the colonic microflora in ASC−/− mice may represent a major inducer of colorectal tumorigenesis in these mice. Indeed, WT mice cohoused for 3 mo (1 mo cohousing followed by 2 mo more cohousing during AOM-DSS treatment and follow-up) with ASC−/− mice [WT(ASC−/−)] developed significantly enhanced tumorigenesis in the AOM-DSS model compared with singly housed WT mice, which was characterized by increased tumor numbers and tumor scores that even reached levels similar to those of ASC−/− mice (Fig. 1 A and B). Histopathologically, tumors from ASC−/− mice were indistinguishable from those of singly housed or cohoused WT mice, with all tumors composed of crypt and glandular structures consistent with a well-differentiated adenocarcinoma (Fig. 1C). Together, these results suggest that the enhanced tendency for inflammation induced tumorigenesis in ASC−/− mice is driven by their altered microflora.
Fig. 1.
The increased tumorigenesis in ASC−/− mice is transmissible to cohoused WT mice. (A) Tumor score and tumor numbers/mouse in singly housed WT mice, WT mice cohoused with ASC−/− mice [designated WT(ASC−/−)], and ASC−/− mice cohoused with WT mice [designated ASC−/−(WT)]. Data are mean ± SEM; n ≥ 4; *P < 0.05 by one-way ANOVA. The experiments were repeated three times. (B) Representative colonoscopic appearance of WT, WT(ASC−/−), and ASC−/−(WT) mice colon in AOM-DSS-induced CRC. (C) Representative histopathologic sections of colon adenocarcinomas from WT, WT(ASC−/−), and ASC−/−(WT) mice. Invasive tumor foci (arrowhead) were surrounded by abundant amounts of pale blue mucin. *, muscularis mucosae; m, mascularis externa. (Scale bars, 200 µm.)
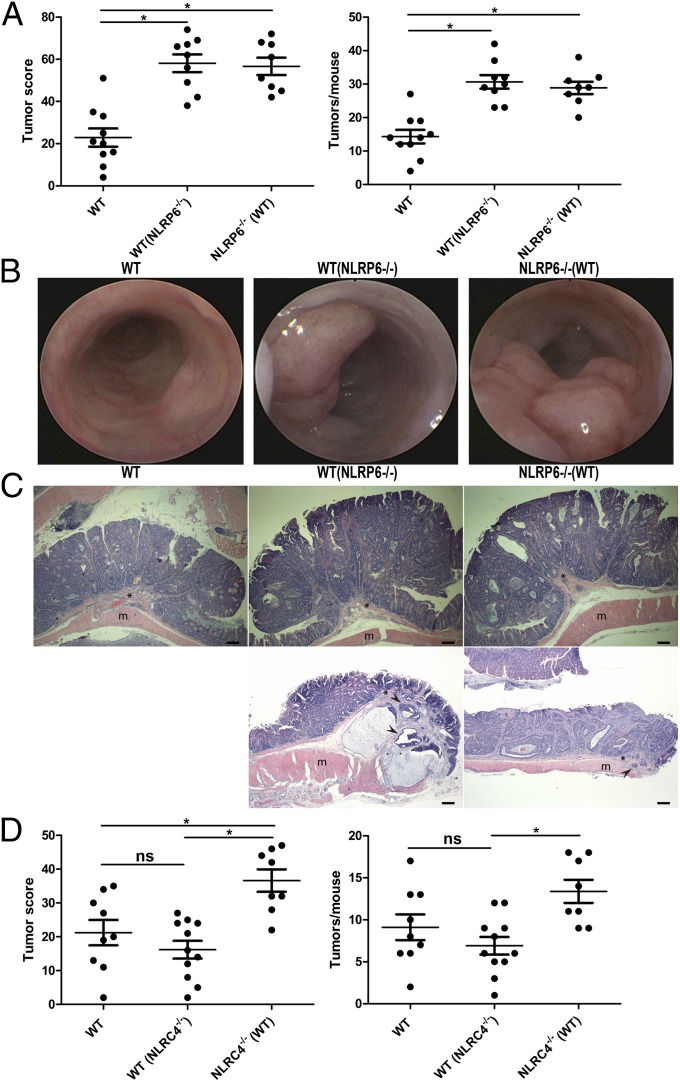
To study the roles of upstream NLRs in this microflora-induced tumorigenesis, we repeated the cohousing experiments using the NLRP6−/− mice as donors of the aberrant microflora. As seen in Fig. 2 A and B, NLRP6−/− mice, and their cohoused WT partners [WT(NLRP6−/−)], featured significantly greater tumorigenesis than singly housed WT mice, with tumors from both NLRP6−/−, singly housed, and cohoused WT mice again featuring features of well-differentiated adenocarcinoma (Fig. 2C). As controls, we performed similar experiments in NLRC4−/− mice that do not feature enhanced DSS-induced colitis or transferability of exacerbated colitis on cohousing with WT mice (34). As is seen in Fig. 2D, prolonged cohousing of WT mice with NLRC4−/− mice [WT(NLRC4−/−)] was not associated with enhanced transferrable colitis-induced tumorigenesis in these mice, as featured in similar tumor score and numbers compared with singly housed WT mice, supporting our hypothesis that microflora transferred from the NLRP6−/− mice plays a critical role in the enhanced susceptibility to inflammation-induced tumorigenesis.
Fig. 2.
The increased tumorigenesis in NLRP6−/− mice is transmissible to cohoused WT mice. (A) Tumor score and tumor numbers/mouse in singly housed WT mice, WT mice cohoused with NLRP6−/− mice [designated WT(NLRP6−/−)], and NLRP6−/− mice cohoused with WT mice [designated NLRP6−/−(WT)]. Data are mean ± SEM; n ≥ 8; *P < 0.05 by one-way ANOVA. The experiments were repeated three times. (B) Representative colonoscopic appearance of WT, WT(NLRP6−/−), and NLRP6−/−(WT) mice colon in AOM-DSS-induced CRC. (C) Representative histopathologic sections of colon adenocarcinomas from WT mice, WT(NLRP6−/−) mice, and NLRP6−/−(WT) mice. Invasive tumor foci (arrowhead) were surrounded by abundant amounts of pale blue mucin. *, muscularis mucosae; m, mascularis externa. (Scale bars, 200 µm.) (D) Tumor score and tumor numbers/mouse in singly housed WT mice, WT mice cohoused with NLRC4−/− mice [designated WT(NLRC4−/−)], and NLRC4−/− mice cohoused with WT mice [designated NLRC4−/−(WT)]. Data are mean ± SEM; n ≥ 8; ns, not significant; *P < 0.05 by one-way ANOVA.
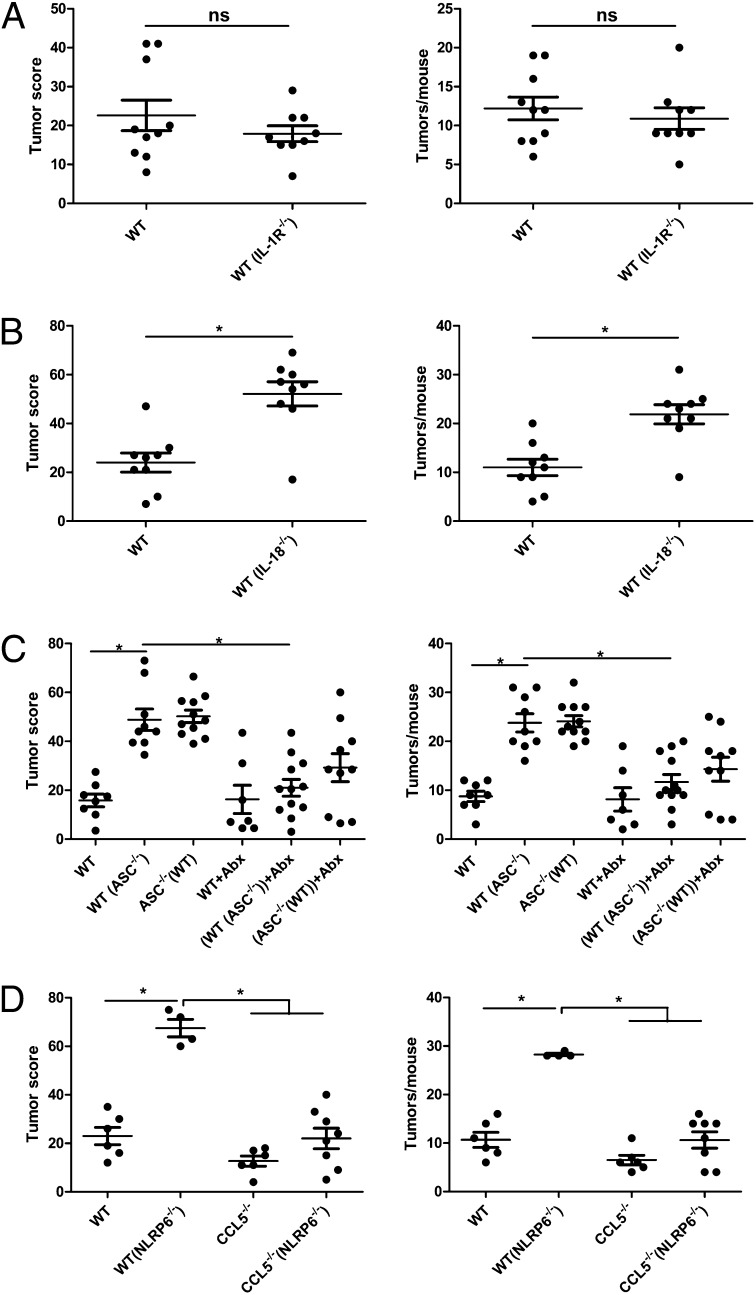
The well-characterized effector functions of inflammasomes includes activation, by cleavage, of the cytokines IL1-β and IL-18, as well as promotion of an inflammation-driven form of cell death termed pyroptosis (27). To test the involvement of the IL-1β and IL-18 axis in the enhanced inflammation-induced tumorigenesis in inflammasome-deficient mice, we performed cohousing experiments using either IL-1R−/− or IL-18−/− mice. As seen in Fig. 3A, WT cohoused with IL-1R−/− mice [WT(IL-1R−/−)] featured a comparable tumor score and tumor number compared with singly housed WT mice. In contrast, WT cohoused with IL-18−/− mice [WT(IL-18−/−)] developed an increase in tumor score and number compared with singly housed WT mice (Fig. 3B), indicating that IL-18, but not IL-1β, is responsible for the aberrant microflora that is associated with the enhanced tendency for inflammation-induced tumorigenesis.
Fig. 3.
Enhanced tumorigenesis in cohoused WT mice is driven by microbiota and mediated by IL-18 and CCL5. (A) Tumor score and tumor numbers/mouse in singly housed WT mice and WT mice cohoused with IL-1R−/− mice [designated WT(IL-1R−/−)]. Data are mean ± SEM; n ≥ 9; ns, not significant. The experiments were repeated two times. (B) Tumor score and tumor numbers/mouse in singly housed WT mice and WT mice cohoused with IL-18−/− mice [designated WT(IL-18−/−)]. Data are mean ± SEM; n = 9; *P < 0.05 by one-way ANOVA. The experiments were repeated two times. (C) Singly housed WT mice, WT mice cohoused with ASC−/− mice [designated WT(ASC−/−)], and ASC−/− mice were treated with 4 wk of metronidazole and ciprofloxacin. In parallel, non-Abx-treated singly housed WT mice, WT mice cohoused with ASC−/− mice, and ASC−/− mice were set up. Subsequently, AOM-DSS treatment was induced, and tumor score and tumor numbers/mouse were recorded. Data are mean ± SEM; n ≥ 7; *P < 0.05 by one-way ANOVA. The experiments were repeated two times. (D) Tumor score and tumor numbers/mouse in separately housed WT mice, WT mice cohoused with NLRP6−/− mice [designated WT(NLRP6−/−)], CCL5−/− mice cohoused with NLRP6−/− mice [designated CCL5−/−(NLRP6−/−)], and separately housed CCL5−/− mice. Data are mean ± SEM; n ≥ 4; *P < 0.05 by one-way ANOVA. The experiments were repeated two times.
To further validate the causative role of the aberrant microflora on tumor development in inflammasome-deficient mice, we treated ASC−/− mice and their WT cohabitants with 1 g/L metronidazole and 200 mg/L ciprofloxacin in the drinking water for a period of 4 wk during cohousing. This Abx protocol was recently demonstrated to inhibit the microflora elements responsible for enhanced colonic inflammation (34). As shown in Fig. 3C, Abx-treated ASC−/− mice and their cohoused WT cohabitants featured a reduced level of tumor formation compared with control ASC−/− mice and their cohoused WT mice. Tumor score and tumor numbers in WT mice cohoused with Abx-treated ASC−/− mice were indistinguishable from those of singly housed WT mice, supporting the role of the ASC-deficient microflora in the pathogenesis of enhanced tumorigenesis.
The aberrant microflora in the NLRP6 inflammasome-deficient mice was recently shown to induce spontaneous or chemically induced colonic inflammation through induction of the chemokine CCL5 [also known as regulated on activation, normal T cell expressed and secreted (RANTES)]. In the absence of CCL5, mice failed to develop microflora-dependent exacerbation in colitis despite full acquisition of the aberrant microflora from the NLRP6−/− mice (34). We thus sought to examine whether CCL5 was also necessary for inflammation-induced tumor formation. Indeed, as is shown in Fig. 3D, CCL5−/− mice cohoused with NLRP6−/− mice [CCL5(NLRP6−/−)] developed significantly fewer tumors than WT mice cohoused with NLRP6−/− mice [WT(NLRP6−/−)], indicating that in the absence of CCL5, reduced colitis leads to reduced tumor formation in mice exposed to the aberrant inflammasome-deficient microflora.
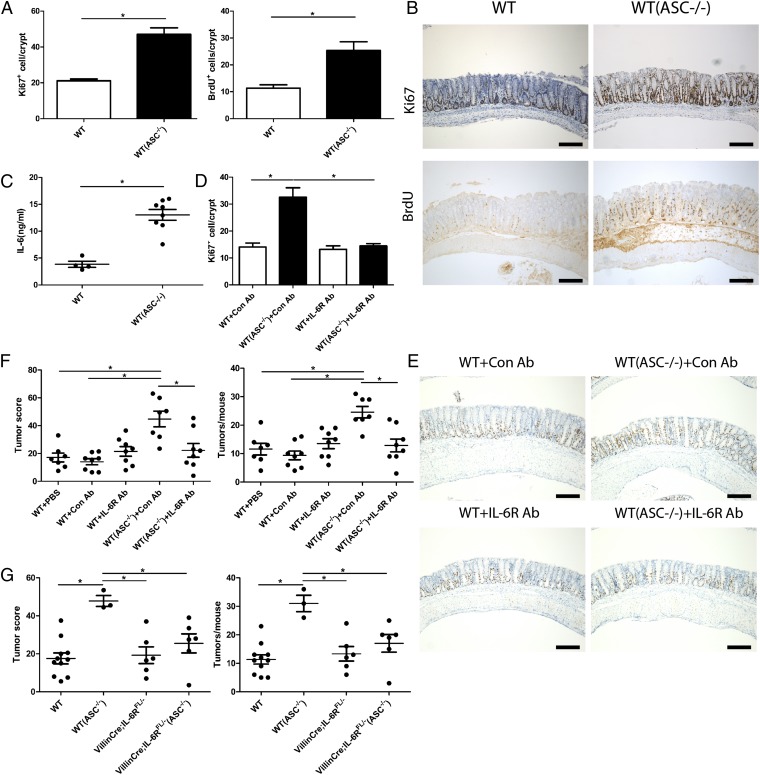
Intestinal inflammation and associated recurrent mucosal damage have been suggested to enhance colonic epithelial proliferation, ultimately resulting in epithelial dysplasia (6, 7, 9). Thus, we determined the level of epithelial cell proliferation in WT cohoused with ASC−/− mice [WT(ASC−/−)] compared with singly housed WT mice (Fig. 4 A and B) at day 15 after administration of AOM, when no tumors were yet detectable by murine colonoscopy. Intriguingly, epithelial cell proliferation, determined by both antigen KI-67 (Ki67) and BrdU staining, was significantly higher in WT mice cohoused with ASC−/− mice [WT(ASC−/−)] compared with singly housed WT mice, suggesting that alterations in the microflora drive excessive epithelial cell proliferation, possibly leading to enhanced tumorigenesis.
Fig. 4.
Enhanced tumorigenesis in cohoused WT mice is mediated by IL-6. (A) Ki67 (Left) and BrdU (Right) proliferation index in colonic epithelial cells of separately housed WT mice and WT mice cohoused with ASC−/− mice [designated WT(ASC−/−)] at day 15 of the CAC regimen. Data are mean ± SEM; n ≥ 6; *P < 0.05 by t test. The experiments were repeated two times. (B) Representative Ki67 (Upper) and BrdU (Lower) immunohistochemistry of singly housed WT mice and WT mice cohoused with ASC−/− mice [designated WT(ASC−/−)] at day 15 of the CAC regimen. (Scale bars, 200 µm.) (C) IL-6 concentration in colonic tissue explant of separately housed WT mice and WT mice cohoused with ASC−/− mice [designated WT(ASC−/−)] harvested at day 15 of the CAC regimen. Data are mean ± SEM; n ≥ 5; *P < 0.05 by one-way ANOVA. The experiments were repeated three times. (D) Singly housed WT mice and WT mice cohoused with ASC−/− mice [designated WT(ASC−/−)] were treated with IL-6R Ab or isotype control Ab at day 7 of the CAC regimen. Ki67 proliferation index in colonic epithelial cells of each group of mice was quantified at day 15 of the CAC regimen. Data are mean ± SEM; n ≥ 5; *P < 0.05 by one-way ANOVA. The experiments were repeated two times. (E) Representative Ki67 immunohistochemistry of singly housed WT mice and WT mice cohoused with ASC−/− mice [designated WT(ASC−/−)] with IL-6R Ab (Lower) or isotype control Ab (Upper) treatment at day 15 of the CAC regimen. (Scale bars, 200 µm.) (F) Singly housed WT mice and WT mice cohoused with ASC−/− mice [designated WT(ASC−/−)] were treated with IL-6R Ab, isotype control Ab, or PBS weekly after the last cycle of DSS. Tumors were analyzed after 4 wk Ab or PBS treatment. Data are mean ± SEM; n ≥ 7; *P < 0.05 by one-way ANOVA. The experiments were repeated two times. (G) Tumor score and tumor numbers/mouse in separately housed WT mice, WT mice cohoused with ASC−/− mice [designated WT(ASC−/−)], VillinCre;IL-6Rf/f mice cohoused with ASC−/− mice [designated VillinCre;IL-6RF/-−ASC−/−)], and singly housed VillinCre;IL-6RF/− mice. Data are mean ± SEM; n ≥ 3; *P < 0.05 by one-way ANOVA. The experiments were repeated two times.
Finally, we sought to determine the mechanisms by which microflora-induced inflammation promotes excessive epithelial cell proliferation. The IL-6 axis has been shown to be central in the pathogenesis of CRC through enhancing proliferation of tumor progenitor cells (6, 7, 9). In addition, IL-6 produced by lamina propria myeloid cells has been suggested to protect normal and premalignant intestinal epithelial cells from apoptosis through signal transducer and activator of transcription 3 (Stat3) signaling (6, 7, 9). Indeed, colonic IL-6 levels were markedly increased in WT mice cohoused with ASC−/− mice [WT(ASC−/−)] and NLRP6−/− mice [WT(NLRP6−/−)] compared with singly housed WT mice, but not in CCL5−/− mice cohoused with NLRP6−/− mice [CCL5−/−(NLRP6−/−)], suggesting that transfer of the aberrant microflora and the associated CCL5-induced enhanced inflammation induced increased local IL-6 secretion in these mice (Fig. 4C and Fig. S1A). In addition, during the late CAC regimen, IL-6 mRNA expression levels in both the normal colonic tissue and tumor tissue of WT mice cohoused with NLRP6−/− mice [WT(NLRP6−/−)] and NLRP6−/− mice were up-regulated compared with those of singly housed WT mice, which could potentially stem from the enhanced infiltration of IL-6 secreting inflammatory cells (Fig. S1 B and C). Furthermore, systemic administration of neutralizing anti-IL-6 receptor Ab (46) completely abolished both the cohousing-associated epithelial cell proliferation, as evident by Ki67 labeling (Fig. 4 D and E), and cohousing-associated tumor promotion with continuous IL-6R Ab treatment (Fig. 4F), establishing the IL-6 axis as a major mechanism for microflora-enhanced inflammation-induced CRC. Moreover, the effect of IL-6 on the colonic epithelium is direct, as cohousing of mice with conditional deletion of IL-6R in the intestinal epithelial compartment (VillinCre;IL-6RFL/-) with ASC−/− mice resulted in abrogation of the microbiota-mediated transferrable cancer phenotype. In fact, tumorigenesis in VillinCre;IL-6RFL/−(ASC−/−) mice was comparable to that of singly housed VillinCre;IL-6RFL/− and WT mice (Fig. 4G). This near-total abolishment of the microbiota-mediated tumor phenotype on deletion of IL-6R from intestinal epithelial cells makes unlikely the possibility of a contribution of IL-6 transsignaling through soluble IL-6 receptors. Furthermore, we suggest that IL-6-related mucosal phenotypes in our work, as well as in previous other studies, may be dependent on the presence of an underlying dysbiotic microbiota configuration, as we observed no effects of IL-6 blockade when applied to singly housed WT mice. Thus, dysbiosis in IL-6-dependent models should be taken into consideration and further characterized in future studies. Altogether, microbiota-induced activation of IL-6 signaling in intestinal epithelial cells is a critical step in the induction of inflammation-induced CRC.
Our results demonstrate that altered elements in the microbiota of inflammasome-deficient mice drive inflammation-induced CRC. This microbiota-mediated effect is dependent on the induction of local colonic inflammation through epithelial reprogramming and induction of CCL5 transcription. This, in turn, results in local induction of IL-6 secretion, resulting in enhanced epithelial cell proliferation culminating in tumor formation. We suggest that alterations in the microbiota ecosystem be added to factors regulating tumorigenesis and, in particular, in the inflammatory setting. Further studies are needed to characterize components in the altered microbiota in inflammasome-deficient animals that are responsible for transferable inflammation-induced tumorigenesis and the possible implications for human IBD-associated CRC. Nonetheless, recognition that dysbiosis arising from inflammasome dysregulation may be a central component in carcinogenesis, as well as the remarkable transmissibility of this tendency from one mouse to another, are intriguing and merit further investigation in mice and humans.
Materials and Methods
Mice.
ASC−/−, NLRP6−/−, NLRC4−/−, IL-18−/−, IL-1R−/−, CCL5−/−, and VillinCre;IL-6RF/− mice on C57BL/6 background were bred and maintained under specific pathogen-free conditions at the animal facility of Yale University School of Medicine.
For cohousing experiments, age- and sex-matched WT and knockout mice were cohoused in new cages at 1:1 ratios for 4 wk.
For Abx treatment, mice were given a combination of ciprofloxacin (0.2 g/L) and metronidazole (1 g/L) for 4 wk in their drinking water. All Abx were obtained from Sigma Aldrich.
For IL-6R Ab and isotype control Ab (Bioxcell, BE0047 and BE0090) treatment, mice were injected intraperitoneally with Ab at a dose of 5 mg/kg body weight at day 7 of the CAC regimen or weekly 4 d after the last cycle of DSS for 4 wk.
All experimental procedures were approved by Yale University's Institutional Animal Care and Use Committee.
Tumor Induction and Analysis.
Age- and sex-matched cohoused 6–8-wk-old mice were injected with AOM (Sigma) intraperitoneally at a dose of 12.5 mg/kg body weight. After 5 d, mice were treated with 2% (wt/vol) DSS (MP Biomedicals; molecular weight 36,000–50,000 Da) in the drinking water for 5 d, followed by 16 d of regular water. This cycle was repeated twice (9).
Endoscopic Procedures.
Colonoscopy was performed at indicated points to monitor for severity of colitis and tumorigenesis. According to the murine endoscopic index of colitis severity system (47), tumor size was graded from 1 to 5 as follows: grade 1 (very small but detectable tumor), grade 2 (tumor covering up to one-eighth of colonic circumference), grade 3 (tumor covering up to one-fourth of the colonic circumference), grade 4 (tumor covering up to half of the colonic circumference), and grade 5 (tumor covering more than half of the colonic circumference) (47). Tumors observed during endoscopy were counted to obtain the total number of tumors per animal. The size of each tumor in every mouse was recorded, and the sum of all tumor sizes per mouse was calculated to be the tumor score.
Histopathology.
Organs were removed and fixed in 10% neutral formalin solution overnight at 4°C, then embedded in paraffin, sectioned, and stained with H&E. Slides were prepared at the Yale University Department of Pathology.
Immunohistochemistry.
Paraffin-embedded sections were rehydrated, subjected to heat-induced epitope retrieval, and incubated with the following antibodies: anti-BrdU (1:4,000; Sigma), anti-Ki67 (1:100; Lab Vision). The DAKO EnVision System was used for detection. All sections were counterstained with hematoxylin. For the BrdU proliferation assay, mice were injected intraperitoneally with 1 mL BrdU (Sigma) solution (1 mg/mL) and killed 24 h after injection. Ki67+ and BrdU+ cells were quantitated from ≥30 crypts of each mouse at day 15 of the CAC regimen at the magnification of 10 × 40.
Colon Culture and ELISA.
Sections of 1 cm of the proximal colon were cut, removed of feces, washed with PBS, and then cut in half longitudinally. The colon sections were placed into culture in Roswell Park Memorial Institute (RPMI)-1640 media (Sigma) with penicillin, streptomycin, and gentamicin and cultured at 37°C with 5% CO2. Supernatants were harvested after 24 h, and the concentration of cytokine was determined by ELISA. IL-6 antibody pair was purchased from BD Pharmingen, and ELISA was performed according to the manufacturer's protocol.
Statistical Analysis.
Data are expressed as mean ± SEM. Differences were analyzed by Student t test and one-way ANOVA and post hoc analysis for multiple group comparison. P values < 0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Lauren Zenewicz, Jon Alderman, Elizabeth Eynon, Adam Williams, William O'Connor, Yasushi Kobayashi, Jorge Henao Mejia, and all members of the R.A.F. laboratory for technical help and scientific discussion; Fran Manzo and Caroline Lieber for technical assistance; Ruslan Medzhitov, David Schatz, and Patrick Sung for scientific discussions and critique of the work; and Dr. Jens C. Brüning, Dr. F. Thomas Wunderlich, Dr. Ursula Lichtenberg, and Dr. Claudia Wunderlich (University of Cologne) for the VillinCre;IL-6RF/− mice. E.E. is supported by the Cancer Research Institute (2010–2012) and by a supplementary grant from the Israel–US Educational Foundation (2009) and is also a recipient of the Claire and Emmanuel G. Rosenblatt Award from the American Physicians for Medicine in Israel Foundation (2010–2011). S.H. was supported by the Deutsche Forschungsgemeinschaft (SFB841) and by a Crohn’s and Colitis Foundation of America fellowship. S.C.E. is supported by National Institutes of Health Grants K08AI085038 and UL1 RR024139. This work was supported, in part, by the Howard Hughes Medical Institute (R.A.F.) and a United States–Israel Binational Foundation grant (to E.E. and R.A.F.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1307575110/-/DCSupplemental.
References
- 1.Weir HK, et al. Annual report to the nation on the status of cancer, 1975-2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst. 2003;95(17):1276–1299. doi: 10.1093/jnci/djg040. [DOI] [PubMed] [Google Scholar]
- 2.Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: A meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10(6):639–645. doi: 10.1016/j.cgh.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: A meta-analysis. Gut. 2001;48(4):526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Popivanova BK, et al. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118(2):560–570. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker C, et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21(4):491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 6.Bollrath J, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15(2):91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Grivennikov S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huber S, et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature. 2012;491(7423):259–263. doi: 10.1038/nature11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greten FR, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118(3):285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henao-Mejia J, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482(7384):179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361(9356):512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 13.Wen L, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455(7216):1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gionchetti P, et al. Prophylaxis of pouchitis onset with probiotic therapy: A double-blind, placebo-controlled trial. Gastroenterology. 2003;124(5):1202–1209. doi: 10.1016/s0016-5085(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 16.Frank DN, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104(34):13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis CD, Milner JA. Gastrointestinal microflora, food components and colon cancer prevention. J Nutr Biochem. 2009;20(10):743–752. doi: 10.1016/j.jnutbio.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodwin AC, et al. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc Natl Acad Sci USA. 2011;108(37):15354–15359. doi: 10.1073/pnas.1010203108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdulamir AS, Hafidh RR, Abu Bakar F. The association of Streptococcus bovis/gallolyticus with colorectal tumors: The nature and the underlying mechanisms of its etiological role. J Exp Clin Cancer Res. 2011;30:11. doi: 10.1186/1756-9966-30-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arthur JC, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338(6103):120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elson CO, et al. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 22.Hambly RJ, Rumney CJ, Fletcher JM, Rijken PJ, Rowland IR. Effects of high- and low-risk diets on gut microflora-associated biomarkers of colon cancer in human flora-associated rats. Nutr Cancer. 1997;27(3):250–255. doi: 10.1080/01635589709514534. [DOI] [PubMed] [Google Scholar]
- 23.Muegge BD, et al. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science. 2011;332(6032):970–974. doi: 10.1126/science.1198719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy BS, el-Bayoumy K, Louis YM. Germfree animal as a tool to study role of gut microflora and nutrition in cancer. Prog Clin Biol Res. 1985;181:293–296. [PubMed] [Google Scholar]
- 25.Reddy BS, Weisburger JH, Narisawa T, Wynder EL. Colon carcinogenesis in germ-free rats with 1,2-dimethylhydrazine and N-methyl-n’-nitro-N-nitrosoguanidine. Cancer Res. 1974;34(9):2368–2372. [PubMed] [Google Scholar]
- 26.Fukata M, Abreu MT. Microflora in colorectal cancer: A friend to fear. Nat Med. 2010;16(6):639–641. doi: 10.1038/nm0610-639. [DOI] [PubMed] [Google Scholar]
- 27.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 28.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481(7381):278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 29.Martinon F, Burns K, Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 30.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453(7198):1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440(7081):228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 32.Jin C, et al. NLRP3 inflammasome plays a critical role in the pathogenesis of hydroxyapatite-associated arthropathy. Proc Natl Acad Sci USA. 2011;108(36):14867–14872. doi: 10.1073/pnas.1111101108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dupaul-Chicoine J, et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32(3):367–378. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Elinav E, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145(5):745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen IC, et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207(5):1045–1056. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaki MH, et al. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32(3):379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hu B, et al. (2010) Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci USA 107(50):21635–21640. [DOI] [PMC free article] [PubMed]
- 38.Chen GY, Liu M, Wang F, Bertin J, Núñez G. A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. J Immunol. 2011;186(12):7187–7194. doi: 10.4049/jimmunol.1100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bauer C, et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59(9):1192–1199. doi: 10.1136/gut.2009.197822. [DOI] [PubMed] [Google Scholar]
- 40. Allen IC, et al. (2012) NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-κB signaling. Immunity 36(5):742–754. [DOI] [PMC free article] [PubMed]
- 41.Zaki MH, et al. The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell. 2011;20(5):649–660. doi: 10.1016/j.ccr.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papanikolaou A, et al. Initial levels of azoxymethane-induced DNA methyl adducts are not predictive of tumor susceptibility in inbred mice. Toxicol Appl Pharmacol. 1998;150(1):196–203. doi: 10.1006/taap.1998.8393. [DOI] [PubMed] [Google Scholar]
- 43.Mariathasan S, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430(6996):213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 44.Miao EA, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7(6):569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 45.Sutterwala FS, Flavell RA. NLRC4/IPAF: A CARD carrying member of the NLR family. Clin Immunol. 2009;130(1):2–6. doi: 10.1016/j.clim.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mihara M, et al. Anti-interleukin 6 receptor antibody inhibits murine AA-amyloidosis. J Rheumatol. 2004;31(6):1132–1138. [PubMed] [Google Scholar]
- 47.Becker C, Fantini MC, Neurath MF. High resolution colonoscopy in live mice. Nat Protoc. 2006;1(6):2900–2904. doi: 10.1038/nprot.2006.446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.