Abstract
Clinical data showing correlations between low thyroid-stimulating hormone (TSH) levels and high bone turnover markers, low bone mineral density, and an increased risk of osteoporosis-related fractures are buttressed by mouse genetic and pharmacological studies identifying a direct action of TSH on the skeleton. Here we show that the skeletal actions of TSH deficiency are mediated, in part, through TNFα. Compound mouse mutants generated by genetically deleting the Tnfα gene on a Tshr−/− (homozygote) or Tshr+/− (heterozygote) background resulted in full rescue of the osteoporosis, low bone formation, and hyperresorption that accompany TSH deficiency. Studies using ex vivo bone marrow cell cultures showed that TSH inhibits and stimulates TNFα production from macrophages and osteoblasts, respectively. TNFα, in turn, stimulates osteoclastogenesis but also enhances the production in bone marrow of a variant TSHβ. This locally produced TSH suppresses osteoclast formation in a negative feedback loop. We speculate that TNFα elevations due to low TSH signaling in human hyperthyroidism contribute to the bone loss that has traditionally been attributed solely to high thyroid hormone levels.
Keywords: bone metabolism, thyroid disease, bone density
Clinical and biological observations underscore the importance of the direct effects of thyroid-stimulating hormone (TSH) on the skeleton as they relate to the pathogenesis of osteoporosis seen in human hyperthyroidism (1–7). First, we showed that mice lacking the TSH receptor (Tshr−/−) or expressing a signaling-deficient receptor (Tshrhyt/hyt) suffer from profound bone loss despite thyroid hormone supplementation (1, 2). Second, when rendered hyperthyroid, Tshr−/− mice lose more bone than wild-type hyperthyroid mice with a normal TSH axis (3); this suggests that TSH signaling deficiency contributes to the osteoporosis that has hitherto been attributed solely to thyroid hormone excess. Third, the greater bone loss in hyperthyroid Tshr−/− mice compared with wild-type hyperthyroid mice with undetectable serum Tsh levels is likely due to the osteoprotection exerted by a Tshβ variant (Tsh-βv) identified in bone marrow macrophages (4).
Consistent with the effect of Tshr deficiency, the administration of recombinant TSH to rodents or people prevents bone loss not only by inhibiting bone resorption, but also by stimulating bone formation (2, 8, 9). The antiresorptive effects are exerted through a low-abundance G protein-coupled Tshr on the osteoclast precursor, the activation of which suppresses c-Jun N-terminal kinase (Jnk) to directly inhibit osteoclastogenesis, as well as reduces the release of the osteoclastogenic cytokine Tnfα (1). TSH also promotes bone formation by stimulating osteoblast differentiation primarily through the activation of protein kinase Cδ and the up-regulation of the noncanonical Wingless/Integrase-1 (Wnt) components frizzled and Wnt5a (10). A Tsh-induced, fast-forward short loop in bone marrow stimulates the production of Wnt5a to enhance osteoblastogenesis, and of osteoprotegerin, to further inhibit bone resorption as a third mechanism for TSH-mediated osteoclast control (10).
Here, we focus on the central role of Tnfα in mediating the action of Tsh on the skeleton for several reasons. First, high circulating TNFα levels in patients with thyrotoxicosis with suppressed TSH levels have been implicated in the bone loss that accompanies the disease (11). Second, the TNFα elevation in human hyperthyroidism is recapitulated in Tshr−/− mice, in which CD11b+ macrophages seem to be the predominant source (12). Third, in preliminary studies, the deletion of Tnfα reversed the increased osteoclastogenesis in Tshr−/− and Tshr+/− mice in ex vivo cultures (12). Finally, Tsh inhibits cytokine-induced Tnfα expression by bone marrow macrophages, as does the overexpression of a constitutively active Tshr in transgenic mice (12). Taken together, the data strongly suggest a role for Tnfα, as well as other related downstream cytokines, such as IL-1 and IL-6, in Tsh action on bone.
In this study, we provide definitive evidence that the osteoporosis due to Tshr deficiency, at least in part, arises from elevated Tnfα expression. Notably, the genetic deletion of Tnfα fully rescues the reduced bone formation, increased bone resorption, and osteoporosis observed in Tshr−/− and Tshr+/− mice. We also explore in vitro whether bone-marrow-derived Tnfα and Tsh-βv interact in the local control of bone remodeling.
Results
To explore a pathophysiologic role for TNFα in causing the osteoporosis of TSHR deficiency, we crossed Tshr+/− and Tnfα+/− mice to yield compound mutants, the genotype of which was confirmed by PCR (Fig. 1A). The focus was to examine for reversal of the bone phenotype of Tshr deficiency in the absence of Tnfα. Mutants on the Tshr−/− background were fed on thyroid chow to ensure that they would remain euthyroid and grow and reproduce normally. All mice were on a B6/129 background. At 12 wk of age, the mice were injected with calcein (15 mg/kg) at days −7 and −2 before they were killed to label bone-forming surfaces. Structural and dynamic bone remodeling parameters were measured on trabecular bone by microtomography (L5) and histomorphometry (L1–L3), respectively (13).
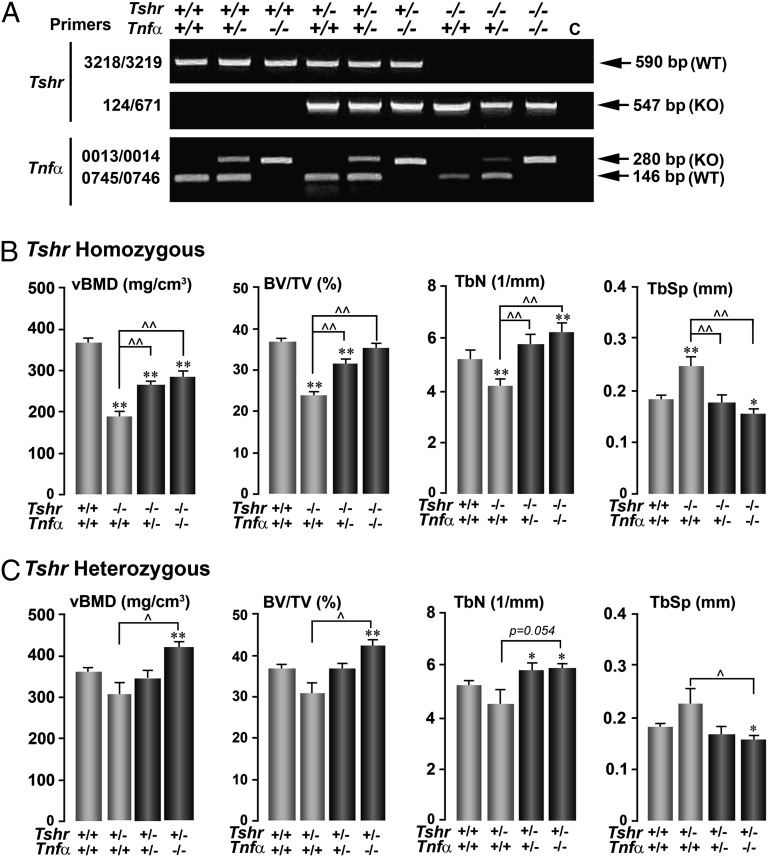
Fig. 1.
Deletion of Tnfα rescues the osteoporosis owing to Tshr deficiency. Agarose gels showing genotype of representative compound mutants, using specific primer sets to detect Tshr and Tnfα gene expression. (A) The effect of deleting the Tnfα gene on a homozygote (Tshr−/−) (B) or heterozygote (Tshr+/−) (C) background on microtomography-based parameters of trabecular architecture, namely vBMD, BV/TV, TbN, and TbSp. Statistics: Student t test with Bonferroni’s correction; comparisons as shown, *P ≤ 0.05; **P ≤ 0.01, compared with Tshr+/+/Tnfα+/+ mice; ^P ≤ 0.05; ^^P ≤ 0.01, comparisons with Tshr−/−/Tnfα+/+ (B) or Tshr+/−/Tnfα+/+ (C) mice; mean ± SEM is shown, up to eight mice per group.
We found that Tshr−/−/Tnfα+/+ mice showed a significantly lower volumetric bone mineral density (vBMD), bone volume/trabecular volume (BV/TV), and trabecular number (TbN) and a correspondingly higher trabecular spacing (TbSp) compared with wild-type controls (Fig. 1B). However, either a ∼50% reduction of Tnfα in Tshr−/−/Tnfα+/− mice or a complete loss of Tnfα in double-homozygote Tshr−/−/Tnfα−/− mice rescued this lower vBMD, BV/TV, and TbN and higher TbSp (Fig. 1B). The effect of deleting the Tshr on one allele was less marked than that noted in homozygous mice, confirming previous results (1). However, the osteopenic phenotype in the compound heterozygote Tshr+/−/Tnfα+/− was rescued fully, with an overshoot in Tshr+/−/Tnfα−/− mice (Fig. 1C).
Low bone mass occurs when the process of bone resorption exceeds bone formation (14). In the case of Tshr deficiency, there was an uncoupling between bone formation and resorption: Whereas bone formation was lower than in controls, resorption was higher, leading to lower BV/TV (Figs. 2 and 3).
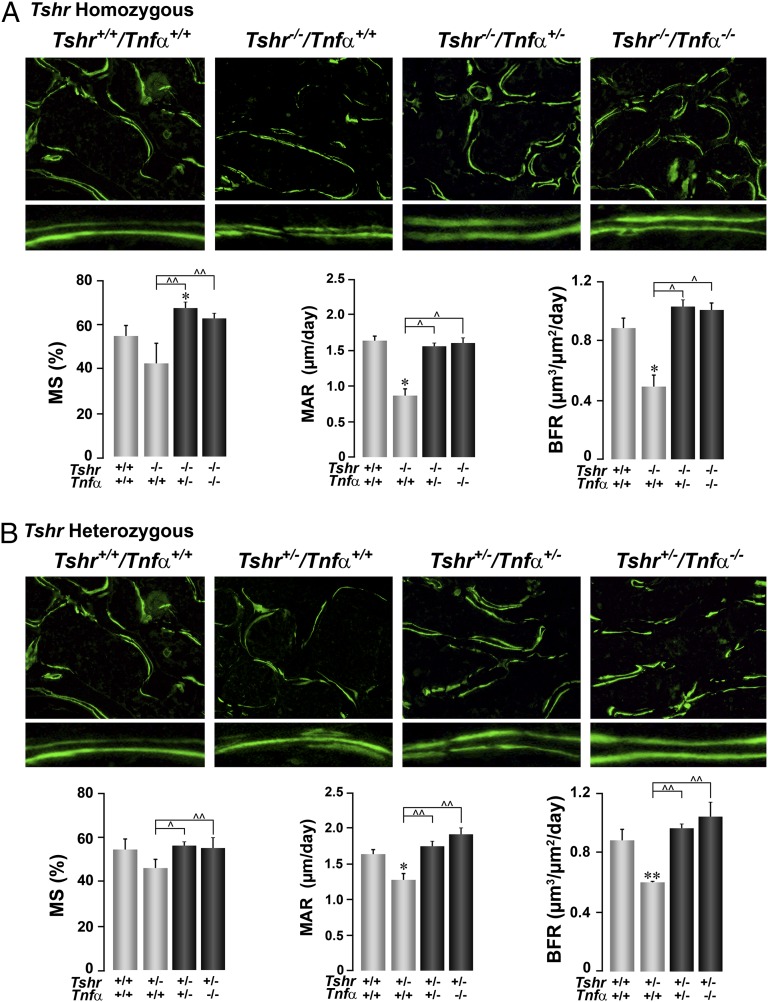
Fig. 2.
Deletion of Tnfα rescues the low bone formation owing to Tshr deficiency. The effect of deleting the Tnfα gene on a homozygote (Tshr−/−) (A) or heterozygote (Tshr+/−) (B) background on bone formation, shown either as double-labeled surfaces (10×, Lower: magnified digitally to show differences in interlabel distances) or as quantitative estimates of MS, MAR, and BFR (units as shown). Statistics by Student t test with Bonferroni’s correction; comparisons as shown, *P ≤ 0.05; **P ≤ 0.01, compared with Tshr+/+/Tnfα+/+ mice; ^P ≤ 0.05; ^^P ≤ 0.01, comparisons with Tshr−/−/Tnfα+/+ (A) or Tshr+/−/Tnfα+/+ (B) mice; mean ± SEM is shown, 9–57 sections from up to six mice per group.
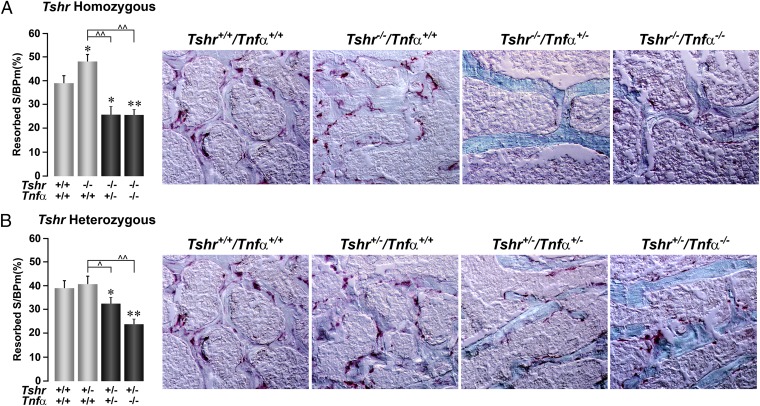
Fig. 3.
Deletion of Tnfα suppresses resorption. The effect of deleting the Tnfα gene on a homozygote (Tshr−/−) (A) or heterozygote (Tshr+/−) (B) background on bone resorption, shown either as TRAP-positive surfaces (10×; dark pink) or as quantitative estimates of resorption surfaces (resorbed S/BPm). Statistics by Student t test with Bonferroni’s correction; comparisons as shown, *P ≤ 0.05; **P ≤ 0.01, compared with Tshr+/+/Tnfα+/+ mice; ^P ≤ 0.05; ^^P ≤ 0.01, compared with Tshr−/−/Tnfα+/+ (A) or Tshr+/−/Tnfα+/+ (B) mice; mean ± SEM is shown, 10–134 sections from up to 12 mice per group.
Parameters of bone formation, notably bone formation rate (BFR) and mineral apposition rate (MAR), were significantly lower in both heterozygote (Tshr+/−/Tnfα+/+) and homozygote (Tshr−/−/Tnfα+/+) mice, compared with wild-type littermates (Fig. 2). Mineralizing surfaces (MSs) showed only a trend (Fig. 2). However, even a 50% deletion of Tnfα in either genotype reversed the decrements in MSs, MAR, and BFR to levels that were not significantly different from those of control littermates, with the exception of an overshoot in MSs in Tshr−/−/Tnfα+/− mice and MAR and BFR in Tshr+/−/Tnfα−/− mice. That the deletion of Tnfα rescued the Tshr−/− phenotype in a graded, dose-related manner suggested that the low bone formation in Tshr deficiency was caused by elevated Tnfα levels. This is consistent with the fact that Tnfα is known to cause osteoblast apoptosis (15).
In contrast to its proapoptotic action on osteoblasts (15), Tnfα increases osteoclast formation (16, 17). We have previously shown that increases in osteoclastogenesis ex vivo in bone marrow cultures derived from Tshr-deficient mice is reversed in the absence of Tnfα (12). Here, we show that whereas tartrate-resistant acid phosphatase (TRAP)-positive resorbed surfaces in vivo are significantly elevated in Tshr−/− mice (P = 0.019), there is strong inhibition of resorption in Tshr−/−/Tnfα+/− and Tshr−/−/Tnfα−/− mice (Fig. 3A). Notable is that the deletion of Tnfα not only rescued the hyperresorption phenotype of Tshr−/− mice, but also suppressed resorption to levels lower than control littermates in both Tshr homozygote and heterozygote mice (Fig. 3). Overall, therefore, elevated Tnfα levels in Tshr deficiency (1) not only seem to inhibit bone formation, but also to elevate resorption, thus causing osteopenia. Deletion of Tnfα on a Tshr-deficient background reverses the phenotype. These genetic rescue studies implicate Tnfα in the pathophysiology of bone loss arising from low Tsh signaling.
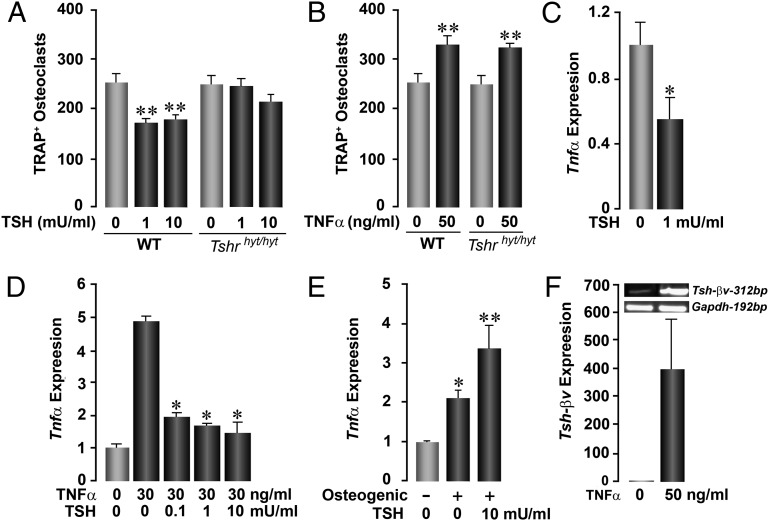
We carried out in vitro assays to examine individual interactions between Tsh and Tnfα. We found that recombinant TSH (1 and 10 mU/mL) inhibited osteoclastogenesis in bone marrow cell cultures derived from wild-type mice, but the effect was expectedly abolished in cultures from Tshrhyt/hyt mice that express a signaling-deficient Tshr (Fig. 4A). We next examined whether the action of Tnfα on osteoclastogenesis was exerted independently of the Tshr. Fig. 4B shows that Tnfα (50 ng/mL) stimulated osteoclast formation both in wild-type and Tshrhyt/hyt mice.
Fig. 4.
Interactions between Tsh and Tnfα. Effect of Tsh (A) and Tnfα (B) on TRAP-positive osteoclast formation in ex vivo bone marrow cell cultures derived from WT mice or Tshrhyt/hyt mice, which harbor a mutated, signaling-deficient Tshr. Whereas TSH inhibits osteoclast formation in WT cultures, the effect is lost in cultures from Tshrhyt/hyt mice (A). In contrast, Tnfα stimulates osteoclastogenesis in both WT and Tshrhyt/hyt mice (B). TSH inhibits both basal (C) and Tnfα-induced (D) Tnfα expression in osteoclasts, whereas it stimulates Tnfα expression by osteoblasts differentiating in osteogenic media (10) (E) (by quantitative PCR). Tnfα stimulates the expression of Tsh-βv in RAW264.7 macrophages, shown as 312-bp product on agarose gel and quantitated by quantitative PCR (F). Statistics by Student t test with Bonferroni’s correction; all comparisons with zero dose, *P ≤ 0.05; **P ≤ 0.01.
We showed that TSH (0.1–10 mU/mL) inhibited both basal and Tnfα-induced Tnfα expression (Fig. 4 C and D). However, TSH stimulated, rather than inhibited, Tnfα expression from embryonic stem (ES) cell-derived osteoblasts (10) (Fig. 4E). These data argue for a counterregulatory mechanism in which Tsh will paradoxically inhibit and stimulate Tnfα expression from osteoclasts and osteoblasts, respectively. Finally, Tnfα markedly stimulated the production of the alternatively spliced product of the Tsh gene, Tsh-βv, from macrophages (Fig. 4F). We thus surmise that Tnfα produced by the osteoblast in response to Tsh could potentially stimulate the production of Tsh-βv as a feedback mechanism for osteoprotection.
Discussion
We have shown that Tshr deficiency up-regulates and Tsh down-regulates Tnfα expression by modulating activator protein 1- and NF-κB-mediated transcriptional activation of the Tnfα gene (1, 12). This action is mediated through a receptor activator of NF-κB ligand (RANKL)-responsive cis-acting regulatory element (CCG AGA CAG AGG TGT AGG GCC) spanning from −157 to −137 bp of the 5′-flanking region of the Tnfα gene (18). The latter sequence binds two high-mobility group box proteins, Hmgb1 and Hmgb2, that are down-regulated by Tsh (18). Here, we show that the genetic ablation of Tnfα in Tshr-deficient mice rescues, in a graded, dose-dependent manner, the osteoporosis arising from increased bone resorption and decreased bone formation. Together, the data establish a fundamental role for Tnfα in the bone loss that accompanies Tshr deficiency. In contrast, we have shown previously that the related glycoprotein Fsh directly stimulates Tnfα production, and that mice lacking Fshβ have low circulating Tnfα levels (17). This would implicate TNFα as being central to the opposing actions of the two glycoprotein hormones Tsh and Fsh.
There is no doubt that Tnfα increases osteoclastic bone resorption: Most Tnfα-driven diseases, such as rheumatoid arthritis and Crohn disease, are characterized by profound local osteolysis, as well as systemic osteoporosis (19). One mechanism is the direct stimulatory action of Tnfα on osteoclast precursor differentiation, so that more mature, bone-resorbing cells are produced, as is noted in the Tnfα transgenic mouse (16, 20). In addition, we find that Tnfα increases the size of the osteoclast precursor pool—otherwise, the pool will ultimately deplete as a function of enhanced differentiation, a premise that is not supported either by mathematical modeling or by examining experimental data in Tnfα transgenic mice (17).
High T-cell-derived Tnfα levels have also been implicated in the pathogenesis of ovariectomy-induced bone loss in mice and in postmenopausal osteoporosis. Notably, Tnfα null mice are resistant to ovariectomy-induced bone loss (21), and elevated TNFα production from T cells has been documented in postmenopausal women (22). Furthermore, FSH, which is elevated postovariectomy and in postmenopausal women, stimulates Tnfα production (17), suggesting that Tnfα may be a common signal that drives, at least part, the bone loss seen postmenopause and in hyperthyroid states. This may be of therapeutic relevance because more than 10% of postmenopausal women receive thyroid hormone replacement therapy, which often causes subclinical hyperthyroidism, essentially characterized by low TSH levels (23). Furthermore, hyperthyroidism is also 10-fold more common in women (23). The question thus arises whether the two diseases together could produce more profound bone loss, and whether, by implication, anti-TNF therapies may have a role. Alternatively, in view of our observation that Tsh is both antiresorptive and anabolic (2), although admittedly ambitious, it is possible that novel thyroid-sparing TSH analogs may become therapeutic options for human osteoporosis.
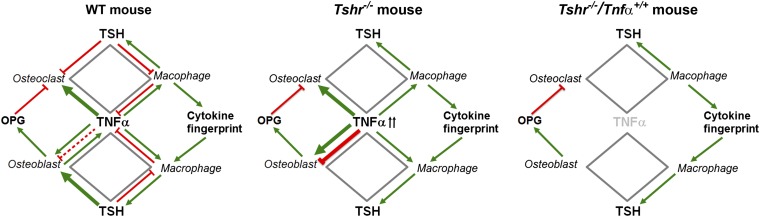
Mechanistically, we surmise that the elevated Tnfα in Tshr−/− mice will increase osteoclast formation and contribute to bone loss via several interacting mechanisms (Fig. 5). First, it will act directly on precursors to stimulate osteoclastogenesis (16, 17). Second, it will create a footprint for the release of other pro-osteoclastogenic cytokines, such as IL-1 and IL-6, toward a feed-forward loop to enable further Tnfα production and osteoclast formation (24). Third, we show that although excessive Tnfα can stimulate Tsh-βv production from macrophages (Fig. 4F); because of the absence of the Tshr, the released molecule will fail to stimulate the production of osteoprotegerin, a Rank-l inhibitor, from osteoblasts, an action that we have previously documented (10). Finally, released Tsh-βv will itself not be able to inhibit osteoclastic bone resorption in Tshr−/− mice. Hence, profound hyperresorption and bone loss should, and does, follow. When Tnfα is removed from the Tshr−/− background, its effect on osteoclastogenesis is removed, resulting in a greater than full recovery, as noted, of the hyperresorption.
Fig. 5.
A central role for Tnfα in hyperthyroid bone loss. Elevated Tnfα in Tshr−/− mice increases osteoclastogenesis by acting directly on the osteoclast precursor and by creating a footprint for the release of other pro-osteoclastogenic cytokines, such as IL-1 and IL-6. Excessive Tnfα also stimulates Tsh-βv production from macrophages, but in Tshr−/− mice the released molecule fails to inhibit osteoclastic bone resorption or to stimulate the production of osteoprotegerin, a Rank-l inhibitor. The Tsh-βv also fails to stimulate osteoblastic bone formation in Tshr−/− mice. Hence, profound hyperresorption, reduced bone formation, and bone loss ensue in Tsh, signaling deficiency. When Tnfα is removed from the Tshr−/− background, full recovery occurs.
In contrast to its action on the osteoclast, the effect of Tnfα on the osteoblast is conflicting. Tnfα has been shown to inhibit osteoblast differentiation to a mineralizing phenotype and to stimulate apoptosis in vitro (15, 25). Specifically, Tnfα inhibits osteoblastic function by activating NF-κB and repressing transcription of the osteocalcin gene (26, 27). However, these effects cannot explain the high bone formation noted in Tnfα transgenic mice (20). In these mice, osteoblast numbers are doubled, suggesting that excessive Tnfα can be pro-osteoblastic in vivo. Unexpectedly, we note a reduction, rather than a stimulation, of bone formation in Tshr−/− mice that overexpress Tnfα. This may result from the absence of the direct osteoblastogenic action of Tsh, or be due to predominance of potential antiosteoblastic effects of Tnfα (15, 25) in the face of Tshr deficiency. Although unlikely, the latter mechanism could explain full rescue of the bone formation phenotype in the Tshr−/−/Tnfα−/− double-mutant mice.
Another important consideration relates to the critical role of Tsh-βv produced in bone marrow in skeletal regulation. Our observation that hyperthyroid Tshr−/− mice lost more bone than hyperthyroid mice with suppressed circulating Tsh levels argues strongly for osteoprotection by a Tsh-like molecule locally within bone marrow. This makes biological sense because, unlike pituitary Tsh, Tsh-βv in macrophages is regulated positively, rather than negatively, by thyroid hormones. Thus, we find that excess thyroid hormone stimulates Tsh-βv expression, which then protects the skeleton by acting on bone-cell Tshrs (Fig. 5). The effects of this osteoprotection are lost in Tshr−/− mice, culminating in significant bone loss.
Finally, the expression of so-called pituitary hormones and their receptors in bone, and at other hitherto unrecognized sites, has only been recognized recently. Osteoblast-like cells were shown to possess Tshrs, as were T cells and enterocytes, mainly through early radiolabeling studies (28, 29). However, the significance of Tshrs in nonthyroid tissues remained unclear until 2003, when we showed that functional Tshrs were indeed expressed on bone cells (1). Thereafter, we and others showed that functionally active Fshrs (30), melanocortin 2 receptors (31, 32), and oxytocin (33, 34) receptors are expressed on bone cells. Notably, the bone cell Fshr is a shorter isoform missing exon 9 (35). Likewise, and importantly, we also found that the ligands of certain of these receptors, such as oxytocin and Tsh-βv, are expressed in bone cells (34, 36). We believe, therefore, that it is critical to begin to study local regulatory control mechanisms through which “pituitary” glycoprotein hormones interact with cytokines, such as Tnfα and others, in the local, as opposed to the central, control of bone remodeling and bone mass. Important therapeutic implications may eventually follow.
Materials and Methods
All experimental protocols were approved by the Institutional Animal Care and Use Committee of Mount Sinai School of Medicine. We genotyped groups of compound mutant mice on a B6/129 background in which the Tshr and/or Tnfα genes were deleted (37) (Jackson Laboratory) (Fig. S1). For microtomography measurements, the L5 vertebra was scanned nondestructively, as before (13), by using a Scanco microtomography scanner (µCT-40; Scanco Medical) at 12-µm isotropic voxel size, with an X-ray source power of 55 kV and 145 µA, and integration time of 300 ms. The trabecular microstructure of the entire secondary spongiosa of L5 between the cranial and the caudal area was evaluated. The scanned grayscale images were processed by using a low-pass Gaussian filter to remove noise, and a fixed bone mineral density (BMD) threshold of 220 mg/cm3 was used to extract the mineralized bone from soft tissue and the marrow phase. The same settings for scan and analysis were used for all samples. Trabecular bone parameters included volumetric BMD, BV/TV, TbN, and TbSp (units in Fig. 1).
Bone formation and resorption rates were quantified by dynamic histomorphometry following two sequential injections of calcein (15 mg/kg) 5 d apart before animals were killed (at days −7 and −2). Measured parameters included MS, MAR, BFR, and TRAP surfaces (resorbed S/BPm) (as in ref. 31). Histological sections were photographed on a fluorescent microscope (Observer Z1; Zeiss) (34).
For the ex vivo studies, bone marrow was isolated and stromal cell cultures performed in the presence of ascorbate-2-phosphate (1 mM) for 10 d (30). Additionally, osteoclast formation assays were performed following Ficoll purification of bone marrow and incubation with macrophage colony-stimulating factor (M-CSF) (30 ng/mL) and RANK-L (50 ng/mL) for 5 d (30). For RAW264.7 macrophages, we excluded M-CSF from the 5-d cultures. Quantitative PCR was performed on extracted RNA using appropriate primer sets, as described previously (34). Mouse ES cell cultures were performed as described previously (10, 38).
Supplementary Material
Acknowledgments
We thank Dr. Jay Cao for help with microtomography measurements. This work was supported by National Institutes of Health Grants DK80459 (to L.S., T.F.D., and M.Z.), AG23176 (to M.Z.), and AG40132 (to M.Z.). T.F.D. acknowledges support from the Department of Veterans Affairs (Veterans Affairs Merit Award). M.I.N. is supported by the Maria I. New Children’s Hormone Research Foundation and the Chinese University of Hong Kong.
Footnotes
Conflict of interest statement: M.Z. is a named inventor of a pending patent application related to osteoclastic bone resorption filed by the Mount Sinai School of Medicine.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1308336110/-/DCSupplemental.
References
- 1.Abe E, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115(2):151–162. doi: 10.1016/s0092-8674(03)00771-2. [DOI] [PubMed] [Google Scholar]
- 2.Sun L, et al. Intermittent recombinant TSH injections prevent ovariectomy-induced bone loss. Proc Natl Acad Sci USA. 2008;105(11):4289–4294. doi: 10.1073/pnas.0712395105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baliram R, et al. Hyperthyroid-associated osteoporosis is exacerbated by the loss of TSH signaling. J Clin Invest. 2012;122(10):3737–3741. doi: 10.1172/JCI63948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vincent BH, et al. Bone marrow cells produce a novel TSHbeta splice variant that is upregulated in the thyroid following systemic virus infection. Genes Immun. 2009;10(1):18–26. doi: 10.1038/gene.2008.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grimnes G, Emaus N, Joakimsen RM, Figenschau Y, Jorde R. The relationship between serum TSH and bone mineral density in men and postmenopausal women: The Tromsø study. Thyroid. 2008;18(11):1147–1155. doi: 10.1089/thy.2008.0158. [DOI] [PubMed] [Google Scholar]
- 6.Bauer DC, Ettinger B, Nevitt MC, Stone KL. Study of Osteoporotic Fractures Research Group Risk for fracture in women with low serum levels of thyroid-stimulating hormone. Ann Intern Med. 2001;134(7):561–568. doi: 10.7326/0003-4819-134-7-200104030-00009. [DOI] [PubMed] [Google Scholar]
- 7.Zofkova I, Hill M. Biochemical markers of bone remodeling correlate negatively with circulating TSH in postmenopausal women. Endocr Regul. 2008;42(4):121–127. [PubMed] [Google Scholar]
- 8.Sampath TK, et al. Thyroid-stimulating hormone restores bone volume, microarchitecture, and strength in aged ovariectomized rats. J Bone Miner Res. 2007;22(6):849–859. doi: 10.1359/jbmr.070302. [DOI] [PubMed] [Google Scholar]
- 9.Mazziotti G, et al. Recombinant human TSH modulates in vivo C-telopeptides of type-1 collagen and bone alkaline phosphatase, but not osteoprotegerin production in postmenopausal women monitored for differentiated thyroid carcinoma. J Bone Miner Res. 2005;20(3):480–486. doi: 10.1359/JBMR.041126. [DOI] [PubMed] [Google Scholar]
- 10.Baliram R, et al. TSH induces a Wnt-dependent, feed-forward loop for osteoblastogenesis in ES cell cultures. Proc Natl Acad Sci USA. 2011;108:16277–16282. doi: 10.1073/pnas.1110286108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Senturk T, Kozaci LD, Kok F, Kadikoylu G, Bolaman Z. Proinflammatory cytokine levels in hyperthyroidism. Clin Invest Med. 2003;26(2):58–63. [PubMed] [Google Scholar]
- 12.Hase H, et al. TNFalpha mediates the skeletal effects of thyroid-stimulating hormone. Proc Natl Acad Sci USA. 2006;103(34):12849–12854. doi: 10.1073/pnas.0600427103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu LL, et al. Vitamin C prevents hypogonadal bone loss. PLoS ONE. 2012;7(10):e47058. doi: 10.1371/journal.pone.0047058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13(7):791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 15.Byun CH, et al. Alpha-lipoic acid inhibits TNF-alpha-induced apoptosis in human bone marrow stromal cells. J Bone Miner Res. 2005;20(7):1125–1135. doi: 10.1359/JBMR.050302. [DOI] [PubMed] [Google Scholar]
- 16.Kitaura H, et al. Marrow stromal cells and osteoclast precursors differentially contribute to TNF-alpha-induced osteoclastogenesis in vivo. J Immunol. 2004;173(8):4838–4846. doi: 10.4049/jimmunol.173.8.4838. [DOI] [PubMed] [Google Scholar]
- 17.Iqbal J, Sun L, Kumar TR, Blair HC, Zaidi M. Follicle-stimulating hormone stimulates TNF production from immune cells to enhance osteoblast and osteoclast formation. Proc Natl Acad Sci USA. 2006;103(40):14925–14930. doi: 10.1073/pnas.0606805103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamoah K, et al. High-mobility group box proteins modulate tumor necrosis factor-alpha expression in osteoclastogenesis via a novel deoxyribonucleic acid sequence. Mol Endocrinol. 2008;22(5):1141–1153. doi: 10.1210/me.2007-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldmann M, Maini RN. Anti-TNF therapy, from rationale to standard of care: what lessons has it taught us? J Immunol. 2010;185(2):791–794. doi: 10.4049/jimmunol.1090051. [DOI] [PubMed] [Google Scholar]
- 20.Li P, et al. Systemic tumor necrosis factor alpha mediates an increase in peripheral CD11bhigh osteoclast precursors in tumor necrosis factor alpha-transgenic mice. Arthritis Rheum. 2004;50(1):265–276. doi: 10.1002/art.11419. [DOI] [PubMed] [Google Scholar]
- 21.Kimble RB, Bain S, Pacifici R. The functional block of TNF but not of IL-6 prevents bone loss in ovariectomized mice. J Bone Miner Res. 1997;12(6):935–941. doi: 10.1359/jbmr.1997.12.6.935. [DOI] [PubMed] [Google Scholar]
- 22.Cenci S, et al. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest. 2000;106(10):1229–1237. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawin CT, Geller A, Hershman JM, Castelli W, Bacharach P. The aging thyroid. The use of thyroid hormone in older persons. JAMA. 1989;261(18):2653–2655. doi: 10.1001/jama.261.18.2653. [DOI] [PubMed] [Google Scholar]
- 24.Sun L, Yang G, Zaidi M, Iqbal J. TNF-induced gene expression oscillates in time. Biochem Biophys Res Commun. 2008;371(4):900–905. doi: 10.1016/j.bbrc.2008.03.114. [DOI] [PubMed] [Google Scholar]
- 25.Schett G, et al. Osteoprotegerin protects against generalized bone loss in tumor necrosis factor-transgenic mice. Arthritis Rheum. 2003;48(7):2042–2051. doi: 10.1002/art.11150. [DOI] [PubMed] [Google Scholar]
- 26.Chang J, et al. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat Med. 2009;15(6):682–689. doi: 10.1038/nm.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li YP, Stashenko P. Characterization of a tumor necrosis factor-responsive element which down-regulates the human osteocalcin gene. Mol Cell Biol. 1993;13(6):3714–3721. doi: 10.1128/mcb.13.6.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar RS, et al. Cloning and functional expression of a thyrotropin receptor from the gonads of a vertebrate (bony fish): Potential thyroid-independent role for thyrotropin in reproduction. Mol Cell Endocrinol. 2000;167(1-2):1–9. doi: 10.1016/s0303-7207(00)00304-x. [DOI] [PubMed] [Google Scholar]
- 29.Ishikawa Y, Genge BR, Wuthier RE, Wu LN. Thyroid hormone inhibits growth and stimulates terminal differentiation of epiphyseal growth plate chondrocytes. J Bone Miner Res. 1998;13(9):1398–1411. doi: 10.1359/jbmr.1998.13.9.1398. [DOI] [PubMed] [Google Scholar]
- 30.Sun L, et al. FSH directly regulates bone mass. Cell. 2006;125(2):247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 31.Blair HC, et al. Skeletal receptors for steroid-family regulating glycoprotein hormones: A multilevel, integrated physiological control system. Ann N Y Acad Sci. 2011;1240:26–31. doi: 10.1111/j.1749-6632.2011.06287.x. [DOI] [PubMed] [Google Scholar]
- 32.Zaidi M, et al. ACTH protects against glucocorticoid-induced osteonecrosis of bone. Proc Natl Acad Sci USA. 2010;107(19):8782–8787. doi: 10.1073/pnas.0912176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamma R, et al. Oxytocin is an anabolic bone hormone. Proc Natl Acad Sci USA. 2009;106(17):7149–7154. doi: 10.1073/pnas.0901890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colaianni G, et al. Bone marrow oxytocin mediates the anabolic action of estrogen on the skeleton. J Biol Chem. 2012;287(34):29159–29167. doi: 10.1074/jbc.M112.365049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson LJ, et al. FSH-receptor isoforms and FSH-dependent gene transcription in human monocytes and osteoclasts. Biochem Biophys Res Commun. 2010;394(1):12–17. doi: 10.1016/j.bbrc.2010.02.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colaianni G, et al. Regulated production of the pituitary hormone oxytocin from murine and human osteoblasts. Biochem Biophys Res Commun. 2011;411(3):512–515. doi: 10.1016/j.bbrc.2011.06.158. [DOI] [PubMed] [Google Scholar]
- 37.Marians RC, et al. Defining thyrotropin-dependent and -independent steps of thyroid hormone synthesis by using thyrotropin receptor-null mice. Proc Natl Acad Sci USA. 2002;99(24):15776–15781. doi: 10.1073/pnas.242322099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma R, Morshed S, Latif R, Zaidi M, Davies TF. The influence of thyroid-stimulating hormone and thyroid-stimulating hormone receptor antibodies on osteoclastogenesis. Thyroid. 2011;21(8):897–906. doi: 10.1089/thy.2010.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.