Abstract
Microbialites, which are organosedimentary structures formed by microbial communities through binding and trapping and/or in situ precipitation, have a wide array of distinctive morphologies and long geologic record. The origin of morphological variability is hotly debated; elucidating the cause or causes of microfabric differences could provide insights into ecosystem functioning and biogeochemistry during much of Earth’s history. Although rare today, morphologically distinct, co-occurring extant microbialites provide the opportunity to examine and compare microbial communities that may be responsible for establishing and modifying microbialite microfabrics. Highborne Cay, Bahamas, has extant laminated (i.e., stromatolites) and clotted (i.e., thrombolites) marine microbialites in close proximity, allowing focused questions about how community composition relates to physical attributes. Considerable knowledge exists about prokaryotic composition of microbialite mats (i.e., stromatolitic and thrombolitic mats), but little is known about their eukaryotic communities, especially regarding heterotrophic taxa. Thus, the heterotrophic eukaryotic communities of Highborne stromatolites and thrombolites were studied. Here, we show that diverse foraminiferal communities inhabit microbialite mat surfaces and subsurfaces; thecate foraminifera are relatively abundant in all microbialite types, especially thrombolitic mats; foraminifera stabilize grains in mats; and thecate reticulopod activities can impact stromatolitic mat lamination. Accordingly, and in light of foraminiferal impacts on modern microbialites, our results indicate that the microbialite fossil record may reflect the impact of the radiation of these protists.
Keywords: Neoproterozoic, geobiology, sedimentology, ooid
Considerable disagreement clouds the definitions of stromatolites and thrombolites (1, 2). Both are microbialites under guidelines proposed by Burne and Moore (3), who favor, like most, the original definition of stromatolites as laminated structures of probable microbial origin. The redefinition of stromatolites as “organosedimentary structures formed by trapping, binding, and/or precipitation as a result of the growth and metabolic activity of microorganisms, principally cyanophytes” (4) is now more widely accepted despite no reference to lamination. Thrombolites are considered to have similar origins but lack lamination, having, instead, a clotted microstructure (5). Some have proposed that the lack of lamination in thrombolites to result from disruption by grazers, principally metazoans (6). Alternatively, others (7) consider thrombolite mesoclots to be a calcification response by certain cyanobacteria. Under that definition, thrombolites are considered confined to lower Paleozoic sediments. In contrast, many authors consider structures with clotted microfabrics that abound in some modern sedimentary environments (e.g., coastal Western Australian lakes; shallow Bahamian marine habitats) to be thrombolites (3, 8, 9). Also, clotted microfabrics may result from “remodeling of a precursor fabric” by a combination of processes, including physical and metazoan disruption (9).
Foraminiferal eukaryotes are a major component of most present-day marine benthic habitats. Benthic foraminifera can be important primary carbonate producers (10), hosts of photoautotrophic endobionts (11), and significant players in food-web dynamics (12). Although the most familiar foraminifera today have calcareous tests (shells), agglutinated and organic (thecate or allogromid) foraminifera also exist (13). Agglutinated foraminiferal species create tests by assembling inorganic grains using a bioadhesive, and thecate foraminifera have an organic test. Ancient oceans also supported foraminifera, evidenced by fossil occurrences from the Cambrian (14) and, most likely, earlier (15). Thecate forms, rather than agglutinated or carbonate-secreting forms, likely were the earliest evolving foraminiferal group (16). Thecate foraminifera, which are quite common in the deep sea and shallow Antarctic waters (17–19), are capable of bioturbating sediments (20).
Foraminifera extend reticulopods, which are anastomosing pseudopods, to perform a wide variety of cellular functions (21), including lateral and vertical migration in sediments and on hard substrates, as well as food capture. Foraminiferal reticulopods could significantly impact microbialite fabric. However, other than fossil and relict occurrences, little is known regarding microbialite foraminifera and their reticulopodial impact on microbialites to our knowledge. The purpose of this study was to compare and contrast microbialite foraminiferal communities and assess their possible impact on microbialite microfabric; such insights will provide context for future studies of fossil microbialites and future in-depth ecological and physiological investigations.
Results
Morphological analyses indicate that foraminifera occur in all examined microbialite types (Table 1). Although calcareous foraminifera were most abundant in each sample, thecate and agglutinated forms also occur, with both groups being relatively more abundant in thrombolites compared with most stromatolite samples (Table 1 and Table S1). Agglutinated foraminifera were relatively uncommon compared with their abundances in other marine habitats, although certain agglutinated taxa were moderately abundant in thrombolites (Table S1). The foraminiferal abundance (total number of stained foraminifera per cubic centimeter) did not differ significantly between thrombolites and all types of stromatolites (P > 0.05; pooled variance; df = 5). The percentage of thecate foraminifera was significantly higher in the thrombolites than in all types of stromatolites (P = 0.038; df = 5).
Table 1.
Foraminiferal abundances per microbialite type
| Microbialite type | Foraminifera | |||
| Calcareous | Agglutinated | Thecate | Total | |
| Type 1 stromatolitic mat | 40.3 ± 2.1 | 0 | 0.4 ± 0.5 | 40.7 ± 2.7 |
| Incipient type 2 stromatolitic mat | 17.5 ± 17.3 | 0.2 ± 0.3 | 1.0 ± 1.2 | 18.8 ± 18.7 |
| Type 2 stromatolitic mat | 9.9 | 0.1 | 0.1 | 10.1 |
| Thrombolitic mat | 24.4 ± 1.0 | 4.0 ± 1.2 | 3.4 ± 2.4 | 31.9 ± 4.7 |
Microbialite types investigated listed with mean abundance (per cm3) ± SD of rose Bengal-stained calcareous, agglutinated, and thecate foraminifera from Highborne Cay microbialite duplicates. Note there is no replicate for type 2 mat.
Similarly, 18S rDNA analyses show signatures of foraminifera in every sampled microbialite type (Fig. S1). These analyses show that thrombolitic mats had 1.5 to 2 times more diversity at the family level, and more thecate (“Allogromida”) forms compared with stromatolitic mats [20.7% of operational taxonomic units (OTUs) after sequences were clustered at 97% similarity vs. 0–5.6%]. Foraminiferal small subunit (SSU) rRNA gene sequences have been deposited in GenBank (accession nos. JX873274– JX873955).
Microelectrode profile results indicate that the oxygen concentrations in the four microbialite types studied varied greatly between night and day (Fig. S2), with the anoxic–oxic interface considerably deeper during the day than at night. During the day, oxygen becomes undetectable within the top 10 mm for all microbialite types.
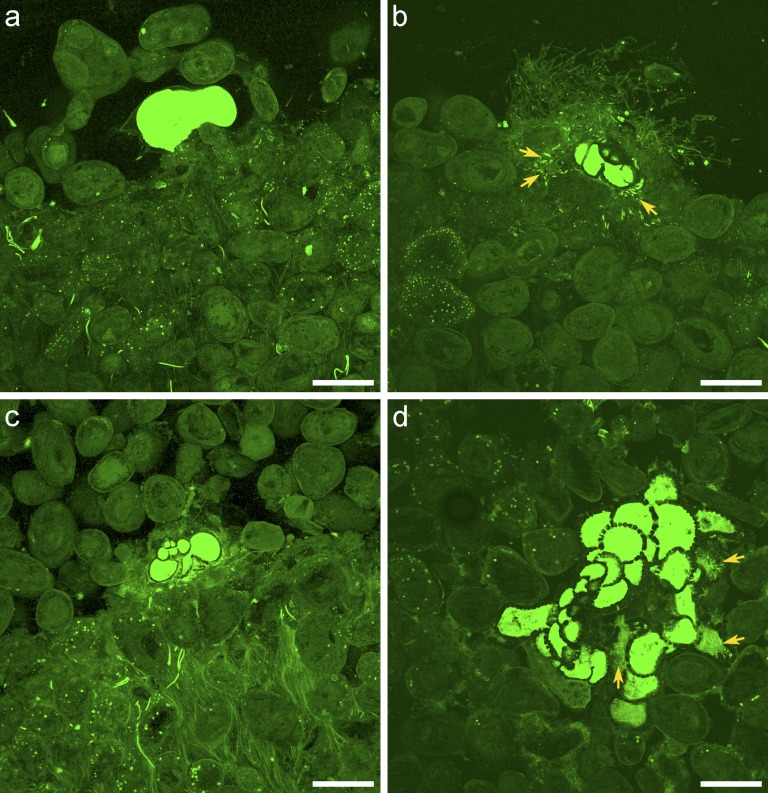
Fluorescently labeled embedded core (FLEC) analysis shows that foraminifera inhabited the sediment–water interface of all microbialites (Fig. 1 A and B), as well as within microbialites (Fig. 1 C and D), often >1 cm. Sometimes foraminifera affected microfabric by binding ooids, which are the predominant grains at Highborne; particularly striking examples are illustrated here. The ooids covering a monothalamous foraminifer (Fig. 1A) likely were bound by the foraminifer’s bioadhesive, as other monothalamids are known to bind detrital grains (19). A calcareous foraminifer was closely associated with tufted material extending into bottom waters and with diatoms among ooids below its test (Fig. 1B). Another calcareous specimen occurred along a seam between consolidated ooids and loosely consolidated overlying ooids (Fig. 1C); material adjacent to the foraminifer was tightly packed. Chambers of a large calcareous foraminifer appeared to displace ooids or grow between them; copious diatoms occurred inside some test chambers, suggesting active reticulopodial activities before and during sampling (Fig. 1D).
Fig. 1.
LSCM images of foraminifera in FLEC sections. (A) Cross-section through sediment–water interface of type 1 stromatolitic mat showing cross-section through monothalamous foraminifer with a test composed of ooids. (B) Cross section through the sediment–water interface of a type 2 stromatolitic mat showing a rotalid calcareous foraminifera in cross-section. Note the abundant pennate diatoms (arrows) in the vicinity of the foraminifer as well as the tufted material extending above the substrate. (C) Calcareous foraminifer in cross-section along a bedding plane of type 1 stromatolitic mat 1.5 mm below the sediment–water interface. Note that the material adjacent to foraminifer is densely packed and bound, as are ooids below bedding plane. (D) Calcareous foraminifer (probably Planorbulina sp.) in tangential cross section in a thrombolitic mat. Note that foraminiferan had ingested pennate diatoms in multiple chambers (arrows). Specimen was 5 mm below the sediment–water interface. Number of images compiled (each with spacing of 1.38 µm) are as follows: a, n = 56; b, n = 44; c, n = 64; and d, n = 32. (Scale bars: 200 µm.)
With proper fixation, reticulopods of yet another foraminifer ramified (branched) among ooids at least 0.5 mm distant, contacting all visible adjacent grains, further implying that foraminifera can affect microbialite microfabric (Fig. 2). For example, 20 ooids were infiltrated by anastomosing reticulopods in a ∼43-µm stacked optical series (Fig. 2). On a different scale, results from our ∼6-mo experiment to assess the possible impact of thecate foraminifera on stromatolitic mat laminae show that laminations were not preserved, especially compared with those incubated with motility-inhibited foraminifera (Fig. 3). For comparison, Highborne Cay thrombolitic mat samples did not have laminae and showed only faint clotting (Fig. 4).
Fig. 2.
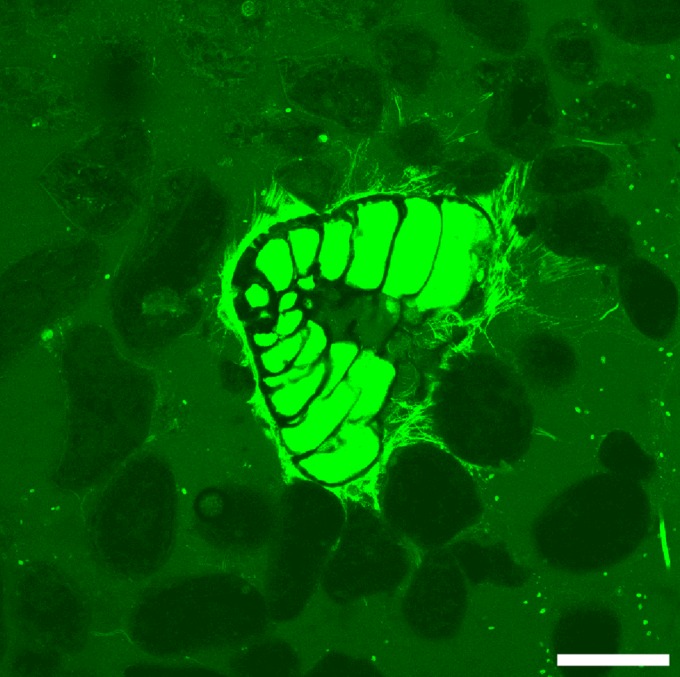
LSCM image of a calcareous foraminifer (possibly Cymbaloporetta sp.) with reticulopods infiltrating ooids of a type 2 stromatolitic mat, as part of the microfabric experiment. Specimen was 6 mm below the sediment–water interface; 32 images were compiled, each with 1.38-µm spacing. (Scale bar: 200 µm.)
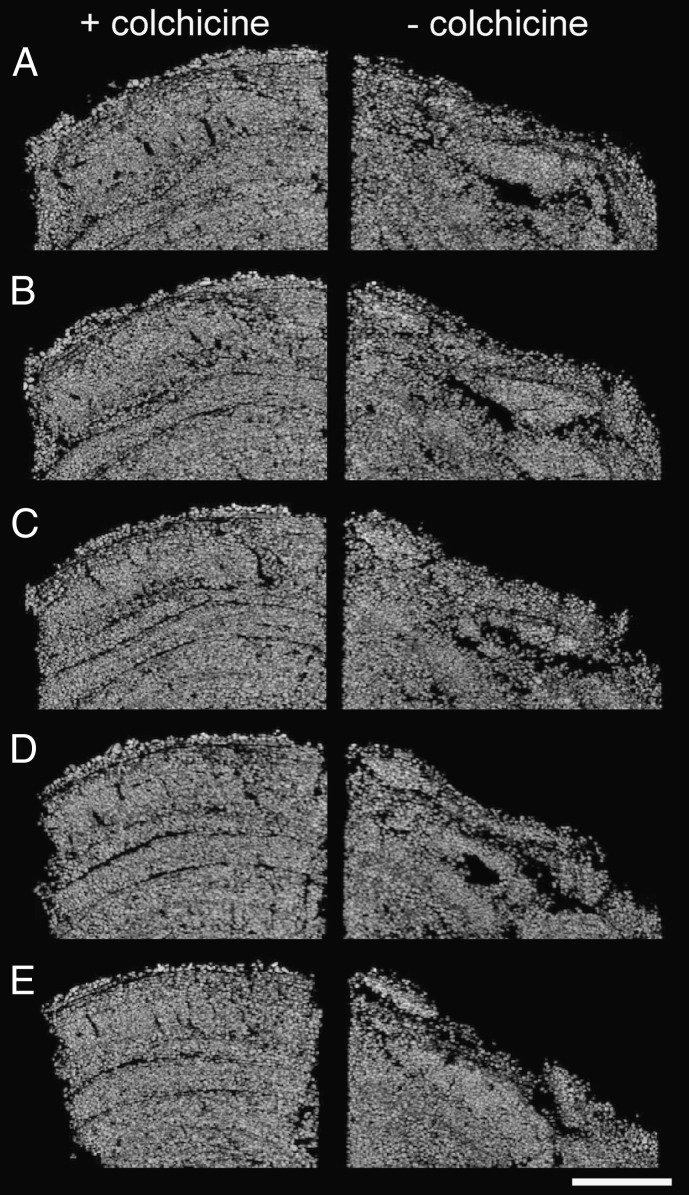
Fig. 3.
Series of micro-CT scans of two FLEC cores taken from the same type 2 stromatolitic mat surface after 6-mo incubation with A. laticollaris. (Left) Colchicine-treated core (reticulopod activity inhibited). (Right) Core lacking colchicine treatment (reticulopod activity uninhibited). Cores were oriented to show cross-sections bisecting the sediment–water interface. Each of the images (A–E) were sequentially separated by 50 frames (1 mm), beginning ∼4 mm (A) into the core. (Scale bar: 5 mm.)
Fig. 4.
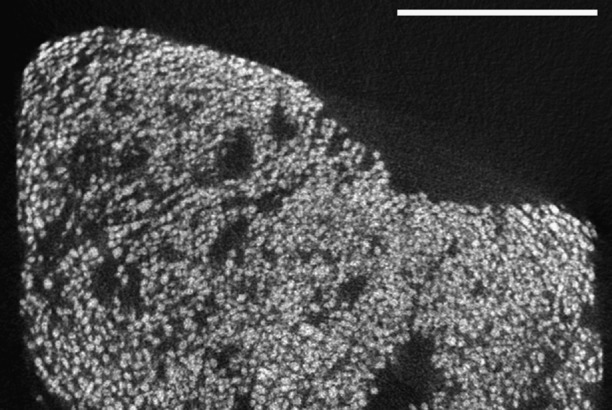
Micro-CT scan of FLEC core taken from a Highborne Cay thrombolitic mat. Note that the microfabric of this thrombolitic mat is similar to that shown in Fig. 3 (see especially Fig. 3D, Right), which was a strombolitic mat exposed to foraminiferal activity. Core was oriented to show cross section bisecting the sediment–water interface. (Scale bar: 5 mm.)
Discussion
Our documentation of foraminifera living in stromatolitic and thrombolitic mats indicates that these protists live on and within both types of microbialites. Our experimental approach showed that foraminifera can impact stromatolitic mat microfabric (Fig. 3), causing microfabric in the stromatolitic mats to have fewer laminae and incipient clotting akin to that observed in Highborne Cay thrombolitic mats (Fig. 4), where some lamination has been documented (22). Given that foraminifera ingest diatoms (Fig. 1D) and bacteria (23) as well as rend biofilms (24), stromatolite and/or thrombolite biomass is likely ingested by foraminifera; future dedicated studies will resolve foraminiferal trophic dynamics in microbialites.
Foraminifera inhabiting the microbialite subsurface are exposed to strong daily oxygen- and sulfide-concentration gradients (Fig. S2) in response to the balance of cyanobacterial photosynthesis and heterotrophic microbial respiration (1). In our samples, the oxic–anoxic interface existed within the surface 1 cm. If the foraminifera migrate daily to remain in oxygenated pore waters, they could disturb microbialite fabrics with their copious reticulopods (Figs. 2 and 3). If deeply infaunal foraminifera do not migrate, they likely rely on anaerobic metabolism, such as denitrification, which is documented in a considerable variety of foraminifera and/or their symbionts (25, 26). It is not known at this time if any of the Highborne Cay microbialite foraminifera perform complete denitrification.
Some foraminiferal reticulopods have high tensile strength that facilitates rending of prey (27); such strength would anchor individuals during extreme hydrodynamic conditions that are typical for this site (28, 29), perhaps minimizing microbialite scour (Fig. 1C). The degree of scour is likely impacted by foraminiferal density and extent and consistency of reticulopodial deployment; differences in foraminiferal abundances and reticulopod extent could affect microbialite fabric, as our experimental results indicate. The observation that foraminifera stabilize sediment grains (Fig. 1 A–C) does not necessarily exclude that they alter microfabric. Stabilization of grains near the foraminifer’s body or test (shell) and disruption of the microfabric (e.g., during migration) are two different processes.
Our microscopy and molecular observations (SSU rRNA gene diversity; Fig. S1) that thecate foraminifera are perhaps more abundant and more diverse in thrombolites compared with stromatolites led us to consider the microbialite fossil record. The oldest fossil stromatolites are >3.4 billion years old and are the most visible manifestations of pervasive microbial life on early Earth (30). Changes in stromatolite abundance and morphology over time document complex interplays between biological, geological, and chemical processes (1). Details of fossil stromatolite formation and preservation are unclear; a decline in stromatolite occurrence and diversity in the late Precambrian has long been a conundrum (2, 31). A popular hypothesis to explain stromatolite decline is the radiation of eukaryotic predators (6). The most commonly proposed predatory culprits are unspecified metazoa, although fossil evidence is nonexistent. Many protists are not expected to leave obvious fossils, but are also possible stromatolite predators, although largely ignored in this context. Possibly, foraminifera were an elusive taxon responsible for Neoproterozoic stromatolite decline because of their impressive reticulopodial reach, which is commonly many times an individual’s cell body diameter (24), and the estimated foraminiferal origin based on molecular clock methods is 650 to 920 Mya (32), whereas the earliest suspected foraminiferal fossils comprise agglutinated tubes dated at ∼635 to 716 Mya (15). Thus, independent assessments indicate that the origin of basal foraminifera roughly coincides with the described changes in Proterozoic stromatolitic sediment fabric and their decline. As noted, the very earliest foraminifers, like modern thecate forms, probably lacked a mineralized shell (16), and thus are not expected to have produced obvious or easily identifiable microfossils. Importantly, it is certainly feasible that other heterotrophic protists including flagellates and ciliates and even metazoans were also associated with changes in Neoproterozoic stromatolite occurrences. The concentrations of the microtubule inhibitor colchicine that was used in our ∼6-mo-long microfabric experiment, however, is not thought to prevent ciliate and metazoan activities (33).
It may ultimately prove to be impossible to definitively determine the exact organisms that may have been responsible for observed changes to microbialite microfabric in the late Precambrian. However, our laboratory experiments and microscopy, coupled with molecular data on eukaryotic diversity in extant microbialites, have at least demonstrated that foraminifera (and possibly other taxa) could have been responsible. Our observations show that foraminifera inhabit modern microbialites and may impact their microfabric. These observations cause the following testable hypotheses to be posed: (i) foraminiferal reticulopods are capable of producing grain movements during vertical migration (diel or otherwise); and (ii) such disturbances directly disrupt laminated fabrics and produce clotted fabrics. Alternative hypotheses are that (i) microbialite foraminifera do not vertically migrate or (ii) foraminifera vertically migrating through microbialites can maneuver although grains without causing disturbance. Ultimately, future comparative studies between modern and fossil microbialites, especially in light of our findings, may provide insights on this longstanding conundrum.
Methods
Sampling and Environmental Measurements.
Suites of samples were collected from the intertidal zone of Exuma Sound, along the southern part of the beach at Highborne Cay (24°43.5′N, 76°49′W) in March 2010 (sites 2, 4, and 8 in ref. 34). Site 10, which is furthest north, lies ∼215 m north of site 8, which is ∼290 m north of site 2. Highborne stromatolitic mat types were designated according to Reid et al. (28), and thrombolitic mats were button types, classified according to Myshrall et al. (8). In brief, stromatolitic mats have a type 1 mat (binding and trapping by Schizothrix filaments, aligned vertically), a type 2 mat (dense biofilm community of cyanobacteria and heterotrophic bacteria, with horizontally oriented filaments that forms a thin micritic crust through microbial calcium carbonate precipitation), or a type 3 mat (in which ooids are cemented together as a result of microboring activity of the endolithic cyanobacterium Solentia). These three surface mat types cycle and form the stromatolite microfabric (28). One type of mat we sampled had a noncontinuous crust, being between a type 1 and type 2 stromatolitic mat (i.e., a microcrystalline CaCO3 crust was starting to form). We refer to this as an incipient type 2 stromatolitic mat. The thrombolitic button mats used in this study contained clusters of calcified and noncalcified filaments of Dichothrix spp.; button mats were found on the exposed side of thrombolitic platforms.
The salinity at the time of collection was 33 to 35.5 practical salinity units. Profiles of dissolved oxygen were measured using polarographic needle microelectrodes (0.1-mm outer diameter) (34). Daytime readings were made during peak photosynthesis, and nighttime observations were made at the end of the dark period.
In Situ Abundances Based on Morphology.
One sample set was collected for a microscopic survey of eukaryotic communities, concentrating on foraminifera. These samples consisted of the surface of 2.6-cm (inner diameter) syringe cores preserved in 3% (vol/vol) formalin. The depth interval of these samples varied because some cores were inclined and others were too indurated to core >0.5 cm; core lengths (depths) were recorded. Rose Bengal was introduced into samples for at least 24 h before sieving and picking to enable easier identification of biological material. Specifically, thecate foraminifera, which are often whitish spherical to oblong objects similar in appearance to ooids, are more easily distinguished in these carbonate samples after staining with rose Bengal. Foraminiferal populations were isolated and/or studied from quantitative aliquots of the >63-µm fraction; in some cases, an entire sample was picked. Two replicates were analyzed for three of the four microbialite mat habitats; the fourth habitat (type 2 stromatolite) lacked replication. By using MyStat (systat.com), two-sample t tests were performed to determine whether the foraminiferal abundance (total number of stained foraminifera per cubic centimeter) and the percentage of thecate foraminifera differed significantly between thrombolites and all types of stromatolites combined.
SSU rRNA Gene Sequencing and Analysis.
A parallel suite of samples was collected and preserved in RNAlater (Ambion) for 18S SSU rRNA gene sequencing and analyses. Samples stored in RNAlater (three pooled replicates of 0.5 g of preserved material) were rinsed three times with 1× PBS solution before RNA extraction. RNA was extracted using the FastRNA Pro Soil-Direct Kit (MP Biomedical). The manufacturer’s extraction protocol was modified to include the addition of 2 M sodium acetate immediately following cell lysis and a Turbo DNase (Ambion) treatment before the RNA Matrix cleanup included in the extraction kit. DNA removal was confirmed by using prepared RNA as template and by using primers described later in a 45-cycle PCR. RNA was transcribed by using the SuperScript One-Step RT-PCR kit (Invitrogen) and general primers for foraminifera S14F1/S17. PCR products were purified from 1% agarose gels by using the Zymoclean Gel DNA Recovery Kit (Zymo Research) and cloned into the pCR4-TOPO TA cloning vector using the TOPO TA Cloning Kit (Invitrogen) for Sanger sequencing (one 96-well plate per sample). Sequences were clustered into OTUs at 97% similarity in QIIME (35), and taxonomy of OTU representatives was assigned using JAguc (36). We present unweighted data because these clone libraries may under-sample diversity. Sizes of individual colored blocks in the stacked bar graph therefore reflect the proportion of unique OTUs recovered within each taxonomic group (Fig. S1).
In Situ Life Positions.
Habitats were also sampled for analysis with the FLEC method (37), which fluorescently labels organisms with active esterase activity in their life position within sediments. Syringe cores (1.5-cm inner diameter) were incubated in CellTracker Green 5-chloromethylfluorescein diacetate (CMFDA) (Invitrogen) and preserved by using our standard protocols (37). Cores were embedded with Spurr resin. After polymerization, cores were sectioned perpendicular to the sediment–water interface by using a low-speed Isomet rock saw (0.4-mm-thick blade), resulting in ∼18 sections of ∼0.5-mm thickness per core. Both sides of each section were scanned with a Leica FLIII stereomicroscope equipped with epifluorescence capabilities to identify fluorescent objects suggestive of foraminifera and other eukaryotes (size, shape). Promising targets were imaged with an Olympus Fluoview 300 laser scanning confocal microscope (LSCM). Some thrombolitic samples were analyzed by using a high-resolution desktop micro-CT system (as detailed later).
Experimental Design.
An experiment was performed to assess the microfabric response of subtidal stromatolites to the presence of thecate foraminifera. Syringe cores (1.5-cm inner diameter) were collected from a type 2 stromatolitic mat on March 21, 2010. The cores were exposed to a ∼12-h light/dark cycle during transport from Highborne Cay to Woods Hole. At the experiment start, cultured Allogromia laticollaris were seeded onto the core surfaces while maintaining the light/dark cycle. The use of this species is justified because a thrombolite sample from Highborne Cay produced partial SSU rRNA gene sequences (e.g., GenBank accession no. JX873904) that were 97% to 99% identical to the SSU rRNA gene sequence from the A. laticollaris culture used in our experiment (GenBank accession no. KC790540), and 96% to 100% identical to the four additional A. laticollaris partial SSU rRNA sequences available in GenBank. Based on these results, we conclude that at least some of the Highborne Cay and cultured A. laticollaris sequences likely represent the same species. Half of the cores, each housed individually, were bathed in normal seawater, whereas the remainder, also housed individually, were bathed in seawater containing 1 mM colchicine to prevent reticulopod extension (38). After ∼6 mo, colchicine-free cores were bathed in calcium-free artificial seawater, which allows preservation of extended reticulopods, for 3 d (38), and then processed for FLEC (as described earlier). A subset of polymerized cores was analyzed by using a high-resolution desktop micro-CT system (μCT40; Scanco Medical). Scans were acquired by using an isotropic voxel size of 20 μm3, with the following settings: X-ray energy of 70 kVp, intensity of 114 μA, 200-ms integration time, and a beam hardening correction of 1,200 mgHA/cm3. Because all core surfaces were not flat (i.e., some cores were inclined), the scanning region for each core included at least 1.5 cm at the core’s shortest point. In addition, the native foraminiferal community of selected colchicine-free cores was screened with LSCM to document extent of reticulopodial deployment.
Supplementary Material
Acknowledgments
We thank the captain, crew, and science party of research vessel Walton Smith; Sabine Mehay, Sara Dilegge, Phil Forte, Dave Beaudoin, Daniel Brooks for technical support; and Sam Bowser and Susan Goldstein for discussions. Collections for the present study were supported by National Science Foundation American Recovery and Reinvestment Act of 2009, Ocean Sciences Grants OCE-0926421 (to J.M.B. and V.P.E.) and OCE-0926372 (to R.E.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. JX873274–JX873955 and KC790540).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221721110/-/DCSupplemental.
References
- 1.Dupraz C, et al. Processes of carbonate precipitation in modern microbial mats. Earth Sci Rev. 2009;96(3):141–162. [Google Scholar]
- 2.Riding R. The nature of stromatolites: 3,500 million years of history and a century of research. In: Reitner J, et al., editors. Advances in Stromatolite Geobiology, Lecture Notes in Earth Sciences. Vol 131. Berlin: Springer-Verlag; 2011. [Google Scholar]
- 3.Burne RV, Moore LS. Microbialites: Organosedimentary deposits of benthic microbial communities. Palaios. 1987;2(3):241–254. [Google Scholar]
- 4.Walter MR. Introduction. In: Walter MR, editor. Stromatolites, Developments in Sedimentology. Vol 20. Amsterdam: Elsevier; 1976. pp. 1–3. [Google Scholar]
- 5.Aitken JD. Classification and environmental significance of cryptalgal limestones and dolomites, with illustrations from the Cambrian and Ordovician of southwestern Alberta. J Sediment Petrol. 1967;37:1163–1178. [Google Scholar]
- 6.Walter MR, Heys GR. Links between the rise of the metazoa and the decline of stromatolites. Precambrian Res. 1985;29:149–174. [Google Scholar]
- 7.Kennard JM, James NP. Thrombolites and stromatolites: Two distinct types of microbial structures. Palaios. 1986;1(5):492–503. [Google Scholar]
- 8.Myshrall KL, et al. Biogeochemical cycling and microbial diversity in the thrombolitic microbialites of Highborne Cay, Bahamas. Geobiology. 2010;8(4):337–354. doi: 10.1111/j.1472-4669.2010.00245.x. [DOI] [PubMed] [Google Scholar]
- 9.Planavsky N, Ginsburg RN. Taphonomy of modern marine Bahamian microbialites. Palaios. 2009;24(1-2):5–17. [Google Scholar]
- 10.Langer MR. Assessing the contribution of foraminiferan protists to global ocean carbonate production. J Eukaryot Microbiol. 2008;55(3):163–169. doi: 10.1111/j.1550-7408.2008.00321.x. [DOI] [PubMed] [Google Scholar]
- 11.Hallock P. Symbiont-bearing foraminifera. In: Gupta BKS, editor. Modern Foraminifera. Dordrecht, The Netherlands: Kluwer; 1999. pp. 123–139. [Google Scholar]
- 12.van Oevelen D, et al. Carbon flows through a benthic food web: Integrating biomass, isotope and tracer data. J Mar Res. 2006;64(3):453–482. [Google Scholar]
- 13.Goldstein ST. Foraminifera: A biological overview. In: Sen Gupta BK, editor. Modern Foraminifera. Dordrecht, The Netherlands: Kluwer Academic; 1999. pp. 37–55. [Google Scholar]
- 14.Culver SJ. Early Cambrian foraminifera from west Africa. Science. 1991;254(5032):689–691. doi: 10.1126/science.254.5032.689. [DOI] [PubMed] [Google Scholar]
- 15.Bosak T, et al. Possible early foraminiferans in post-Sturtian (716-635 Ma) cap carbonates. Geology. 2012;40(1):67–70. [Google Scholar]
- 16.Pawlowski J, et al. The evolution of early Foraminifera. Proc Natl Acad Sci USA. 2003;100(20):11494–11498. doi: 10.1073/pnas.2035132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gooday AJ, et al. Soft-walled, monothalamous benthic foraminiferans in the Pacific, Indian and Atlantic Oceans: Aspects of biodiversity and biogeography. Deep Sea Res Part I Oceanogr Res Pap. 2004;51(1):33–53. [Google Scholar]
- 18.Bowser SS, Bernhard JM, Habura A, Gooday AJ. Structure, taxonomy and ecology of Astrammina triangularis (Earland), an allogromiid-like agglutinated foraminifer from Explorers Cove, Antarctica. J Foraminiferal Res. 2002;32(4):364–374. [Google Scholar]
- 19.Bowser SS, Gooday AJ, Alexander SP, Bernhard JM. Larger agglutinated foraminifera of McMurdo Sound, Antarctica: Are Astrammina rara and Notodendrodes antarctikos allogromiids incognito? Mar Micropaleontol. 1995;26(1-4):75–88. [Google Scholar]
- 20.Gross O. Sediment interactions of foraminifera: Implications for food degradation and bioturbation processes. J Foraminiferal Res. 2002;32(4):414–424. [Google Scholar]
- 21.Travis JL, Bowser SS. The motility of foraminifera. In: Lee JJ, Anderson OR, editors. Biology of Foraminifera. London: Academic; 1991. pp. 91–155. [Google Scholar]
- 22.Mobberley JM, Ortega MC, Foster JS. Comparative microbial diversity analyses of modern marine thrombolitic mats by barcoded pyrosequencing. Environ Microbiol. 2012;14(1):82–100. doi: 10.1111/j.1462-2920.2011.02509.x. [DOI] [PubMed] [Google Scholar]
- 23.Muller WA, Lee JJ. Apparent indispensability of bacteria in foraminiferan nutrition. J Protozool. 1969;16(3):471. [Google Scholar]
- 24.Bernhard JM, Bowser SS. Bacterial biofilms as a trophic resource for certain benthic foraminifera. Mar Ecol Prog Ser. 1992;83(2-3):263–272. [Google Scholar]
- 25.Risgaard-Petersen N, et al. Evidence for complete denitrification in a benthic foraminifer. Nature. 2006;443(7107):93–96. doi: 10.1038/nature05070. [DOI] [PubMed] [Google Scholar]
- 26.Bernhard JM, Edgcomb VP, Casciotti KL, McIlvin MR, Beaudoin DJ. Denitrification likely catalyzed by endobionts in an allogromiid foraminifer. ISME J. 2012;6(5):951–960. doi: 10.1038/ismej.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowser SS, Alexander SP, Stockton WL, Delaca TE. Extracellular matrix augments mechanical properties of pseudopodia in the carnivorous foraminiferan Astrammina rara - Role in prey capture. J Protozool. 1992;39(6):724–732. [Google Scholar]
- 28.Reid RP, et al. The role of microbes in accretion, lamination and early lithification of modern marine stromatolites. Nature. 2000;406(6799):989–992. doi: 10.1038/35023158. [DOI] [PubMed] [Google Scholar]
- 29.Bowlin EM, et al. Environmental controls on microbial community cycling in modern marine stromatolites. Sediment Geol. 2012;263:45–55. [Google Scholar]
- 30.Allwood AC, Walter MR, Kamber BS, Marshall CP, Burch IW. Stromatolite reef from the Early Archaean era of Australia. Nature. 2006;441(7094):714–718. doi: 10.1038/nature04764. [DOI] [PubMed] [Google Scholar]
- 31.Grotzinger JP, Knoll AH. Stromatolites in Precambrian carbonates: Evolutionary mileposts or environmental dipsticks? Annu Rev Earth Planet Sci. 1999;27:313–358. doi: 10.1146/annurev.earth.27.1.313. [DOI] [PubMed] [Google Scholar]
- 32.Groussin M, Pawlowski J, Yang ZH. Bayesian relaxed clock estimation of divergence times in foraminifera. Mol Phylogenet Evol. 2011;61(1):157–166. doi: 10.1016/j.ympev.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Shimeta J, Cook PLM. Testing assumptions of the eukaryotic inhibitor method for investigating interactions between aquatic protozoa and bacteria, applied to marine sediment. Limnol Oceanogr Methods. 2011;9:288–295. [Google Scholar]
- 34.Stolz JF, et al. The microbial communities of the modern marine stromatolites at Highborne Cay, Bahamas. Journal of Atoll Research. 2009;567:1–29. [Google Scholar]
- 35.Caporaso JG, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nebel ME, et al. JAguc—a software package for environmental diversity analyses. J Bioinform Comput Biol. 2011;9(6):749–773. doi: 10.1142/s0219720011005781. [DOI] [PubMed] [Google Scholar]
- 37.Bernhard JM, Visscher PT, Bowser SS. Submillimeter life positions of bacteria, protists, and metazoans in laminated sediments of the Santa Barbara Basin. Limnol Oceanogr. 2003;48(2):813–828. [Google Scholar]
- 38.Travis JL, Bowser SS. A new model of reticulopodial motility and shape: Evidence for a microtubule-based motor and an actin skeleton. Cell Motil Cytoskeleton. 1986;6(1):2–14. doi: 10.1002/cm.970060103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.