Abstract
Sepsis is a common life-threatening clinical syndrome involving complications as a result of severe infection. A cardinal feature of sepsis is inflammation that results in oxidative stress. Sepsis in wild-type mice induced oxidative activation of cGMP-dependent protein kinase 1 alpha (PKG Iα), which increased blood vessel dilation and permeability, and also lowered cardiac output. These responses are typical features of sepsis and their combined effect is a lowering of blood pressure. This hypotension, a hallmark of sepsis, resulted in underperfusion of end organs, resulting in their damage. A central role for PKG Iα oxidative activation in injury is supported by oxidation-resistant Cys42Ser PKG Iα knock-in mice being markedly protected from these clinical indices of injury during sepsis. We conclude that oxidative activation of PKG Iα is a key mediator of hypotension and consequential organ injury during sepsis.
Keywords: redox, cardiovascular function, endotoxin
Sepsis, a prevalent medical condition caused by severe infection with systemic inflammation, causes substantial morbidity and mortality (1). Prognosis is poor with 85% survival in uncomplicated sepsis, falling to 20% in those with multiorgan failure (2). The cost of acute care is enormous (3), but survivors often suffer long-term cognitive impairment generating a chronic health care burden (4). Sepsis is characterized by systemic inflammation (5), decreased peripheral vascular resistance (1), microvascular leak (6), and decreased cardiac output (1). The combined effect of these alterations is low blood pressure (hypotension), a major clinical feature of sepsis (1). This hypotension results in underperfusion of end organs that leads to their functional failure and too often patient death (1).
Oxidative stress is a hallmark of sepsis, consistent with the inflammatory respiratory burst by neutrophils generating high levels of oxidants (5). However, multiple oxidant–generating systems, including nicotinamide adenine dinucleotide phosphate oxidase, uncoupled nitric oxide synthase (NOS) (7), lysozyme-c (8), and mitochondria (9) are activated during sepsis. Consistent with this, the levels of superoxide and hydroxyl radicals, hydrogen peroxide, peroxynitrite, nitrogen dioxide (7), nitroxyl (10), and nitrosothiols (11) can increase during sepsis. Because oxidants can activate cGMP-dependent protein kinase 1 alpha (PKG Iα) to lower blood pressure (12–14), we hypothesized this process underlies sepsis-induced hypotension and consequential organ injury. Because PKG couples to enhance endothelial permeability (6, 15), oxidative activation would also account for the enhanced microvascular leak that would further exacerbate the hypotension. PKG is also negatively inotropic (13, 16); therefore, oxidative activation might also explain the attenuated cardiac output characteristic of sepsis, further exacerbating the hypotension.
Results and Discussion
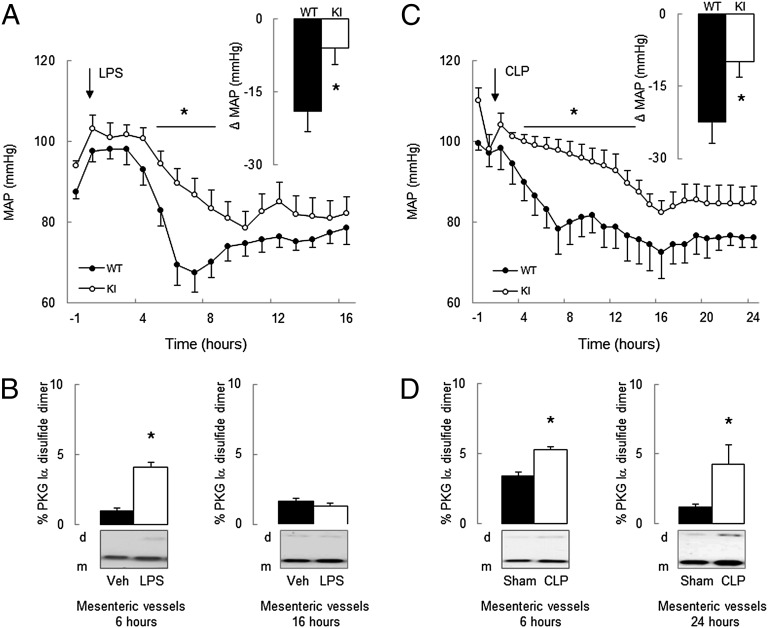
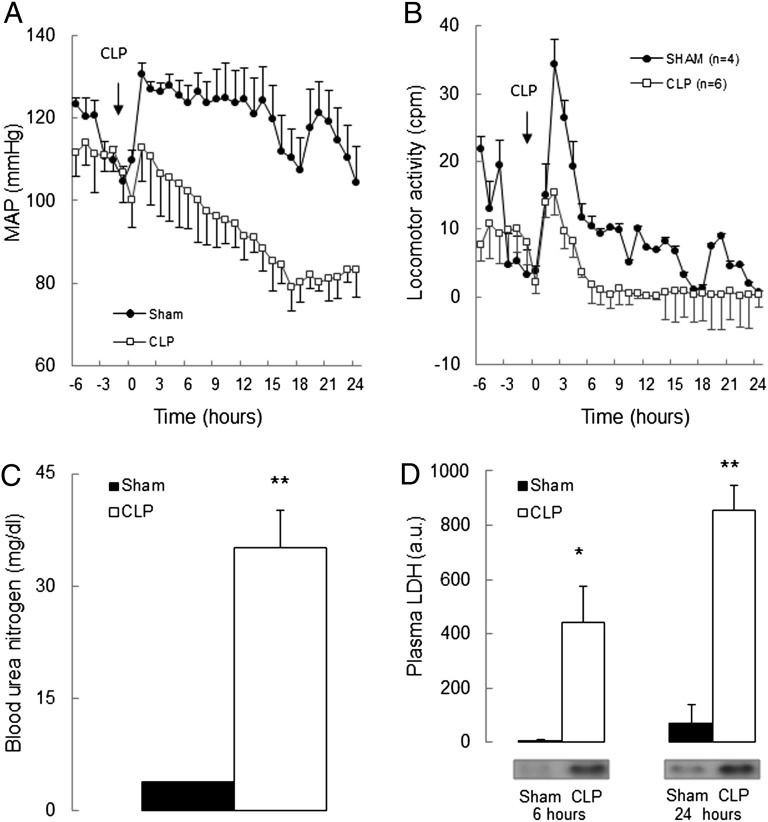
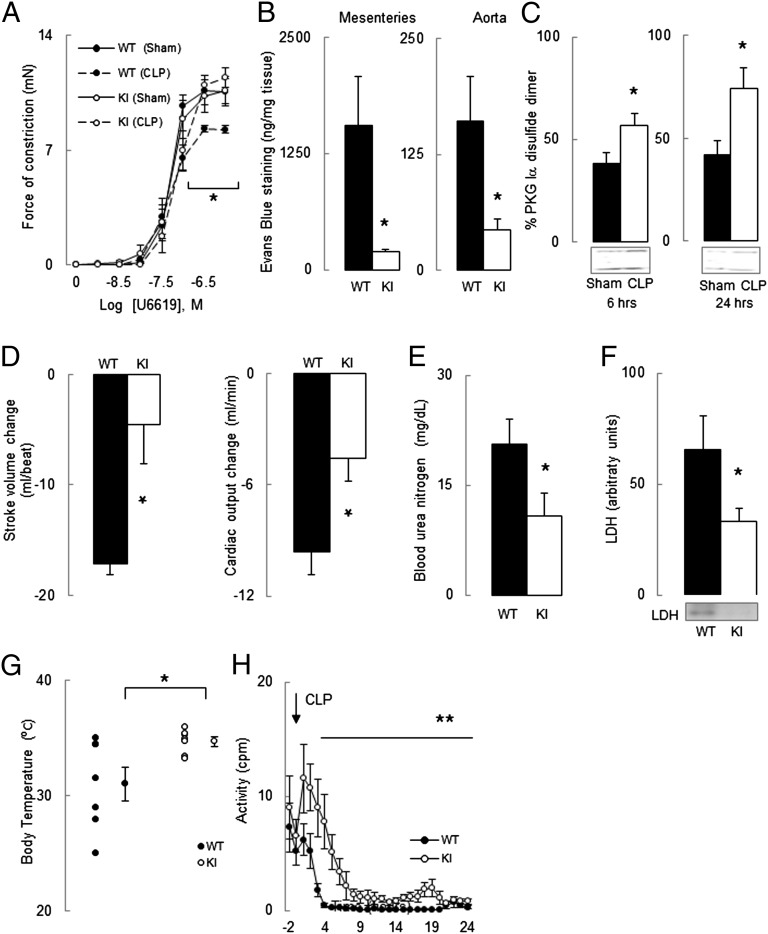
To investigate the hypothesis that PKG Iα oxidation mediates septic injury, we used a “redox-dead” Cys42Ser PKG Iα knock-in (KI) mouse that cannot be oxidant-activated (17). Two experimental models were used, comparing the responses of KI mice with wild-type (WT) littermate controls to endotoxemia or sepsis. Intraperitoneal injection of the bacterial endotoxin LPS caused a peak hypotension at ∼6–7 h that was threefold greater in WT than in KI (Fig. 1A). Thus, the peak mean arterial pressure (MAP) drop was 19 ± 4 mmHg in WT, whereas KI was substantively resistant and declined only 6 ± 3 mmHg Fig. 1A, Inset). Lipopolysaccharide (LPS) increased PKG Iα disulfide dimerization in WT mesenteric vessels at the time of maximal hypotension (∼6 h after LPS injection) compared with vehicle-treated controls (Fig. 1B). However, by 16 h, the MAP in WT had recovered toward basal, as had the redox state of PKG Iα in mesenteric vessels. Cecal ligation and puncture (CLP), a widely used model exhibiting many clinical features of sepsis, partially reflecting ruptured appendicitis or perforated diverticulitis in patients, were next studied. First, we validated the CLP model in WT mice in our laboratory, observing a peak hypotension at ∼7–8 h with reduced locomotor activity compared with sham-operated controls (Fig. 2 A and B). As anticipated, CLP decreased kidney function, evidenced by an eightfold increase in blood urea nitrogen (BUN, Fig. 2C). A global marker of tissue injury, plasma lactate dehydrogenase (LDH), was also markedly increased 6 and 24 h after CLP, but not in sham-operated controls (Fig. 2D). Having validated the CLP model, we compared the responses of KI and WT littermates in this model. There was an initial 22 ± 5 mmHg peak decline in MAP in WT after CLP, whereas the KI was protected from this hypotension and only declined by 10 ± 3 mmHg (Fig. 1C, Inset). CLP-induced a sustained hypotension over the entire 24-h observation period, not just the initial phase as occurred in the endotoxemia model. The KI mice were resistant to the hypotension induced by CLP compared with WT. CLP, as in LPS-treated mice, also increased PKG Iα disulfide dimerization in WT mesenteries compared with controls by 6 and 24 h (Fig. 1D). There is a temporal correlation between PKG Iα disulfide levels and sepsis-induced hypotension, which is consistent with kinase oxidation significantly mediating the blood pressure lowering. This helps substantiate the cause-and-effect relationship between kinase oxidation and hypotension and is consistent with previous studies demonstrating that PKG Iα oxidation lowers blood pressure (17, 18). We compared the dose–response of mesenteric vessels from septic or nonseptic mice to the vasoconstrictor drug U46619 using myography. The maximal U46619-induced vasoconstriction in WT nonseptic vessels was 10.0 ± 0.9 mN, whereas sepsis reduced this to 8.3 ± 0.2 mN (Fig. 3A). This is consistent with the widely acknowledged fact that sepsis generates a potent “vasodilatory signal” to cause hypotension, which our observations suggest are mediated by PKG Iα disulfide activation. Indeed, sepsis did not lower the constriction induced by U46619 in KI, which is consistent with their protection from hypotension in vivo. This supports the premise that PKG Iα oxidation underlies the injurious hypotension during sepsis.
Fig. 1.
Differential responses of WT and KI mice to sepsis-induced hypotension. (A) In vivo telemetric blood pressure monitoring comparing KI mice with WT controls before and during LPS-induced sepsis. (Inset) The difference between baseline MAP and the initial hypotensive response to LPS. (B) Comparison of PKG Iα disulfide formation in mesenteric vessels at 6 and 16 h time points from control vehicle– or LPS-treated mice. (C) Telemetric blood pressure monitoring comparing KI mice with WT controls before and during CLP-induced sepsis. (Inset) The difference between baseline MAP and initial hypotension in response to CLP. (D) Comparison of PKG Iα disulfide formation in mesenteric vessels at 6 and 24 h time points from sham-operated or CLP-treated mice. Δ MAP, change in MAP. *P < 0.05, comparing WT to KI by repeated measures analysis of variance (RM ANOVA) or t test. Data are mean ± SEM.
Fig. 2.
Validation of sepsis-induced hypotension and organ damage in WT mice after CLP. (A and B) Comparison of in vivo telemetric blood pressure and locomotor activity monitoring in WT mice before and during 24 h of CLP-induced sepsis versus sham-operated WT mice. (C) Plasma level of LDH as an indirect measure of multiple organ injury in WT mice in 6 and 24 h after CLP-induced sepsis or sham operation. (D) Comparison of BUN level in WT mice after 24 h CLP-induced sepsis or sham-operated WT mice. *P < 0.05, **P < 0.01 comparing WT CLP with WT sham-operated mice by t test. Data are mean ± SEM.
Fig. 3.
Comparison of vascular function and permeability, cardiac function and well being of WT and KI mice after CLP-induced sepsis. (A) Dose-dependent constriction of mesenteric vessels from sham-operated or CLP-operated WT and KI mice in response to U-46619. (B) Evans blue dye staining (an index vascular permeability) of mesenteric vessels and aorta of WT and KI mice after CLP-induced sepsis. (C) Comparison of PKG Iα disulfide formation in cardiac tissue at 6 and 24 h time points from sham-operated or CLP-treated mice. (D) Comparison of stroke volume and cardiac output changes after CLP-induced sepsis in WT and KI mice. (E and F) Comparison of BUN as a marker of acute kidney injury and plasma LDH level as a measure of multiple organ injury in WT and KI mice after CLP-induced sepsis. (G) Comparison of rectal body temperature in anesthetized WT and KI mice after CLP-induced sepsis. (H) Comparison of locomotor activity assessed by telemetry before and after CLP-induced sepsis in WT and KI mice. *P < 0.05, comparing WT with KI by t test, **P < 0.01 comparing WT with KI by RM ANOVA. Data are mean ± SEM.
Evans blue staining of blood vessels was used to index vascular permeability. CLP prominently increased vascular leak eight- and fourfold in WT mesenteries and aorta respectively compared with vessels from identically treated KI littermates (Fig. 3B). This concurs with recent work showing PKG Iα disulfide formation increased kidney podocyte permeability (15), further supporting the idea that oxidative activation is a crucial, causative mediator of this classical feature of sepsis. CLP resulted in PKG Iα disulfide dimerization in cardiac tissue at 6 and 24 h (Fig. 3C). Cardiac output was also compared between genotypes before and after CLP using echocardiography. Again the KI was protected, with a better preserved stroke volume and cardiac output relative to WT (Fig. 3D). This is consistent with our previous finding that PKG Iα oxidation in isolated heart preparations is negatively inotropic (13). Mice without the nitrosothiol-reducing enzyme S-nitrosoglutathione reductase have increased systemic S-nitrosylation, tissue damage, and mortality following septic shock (11). This is notable in the context of this study because nitrosothiols induce PKG Iα disulfide formation (13).
It is evident that PKG Iα oxidation during sepsis reduces blood pressure. This hypotension during sepsis ensues because oxidant-activated PKG increases the dilation and permeability of blood vessels as well as attenuating cardiac output. All three of these effected processes are not only prominent clinical features of sepsis, but each of them is also known to be regulated by PKG, further supporting the cause-and-effect nature of our observations evident from the “protected phenotype” of the KI. The consequent hypotension and resulting organ underperfusion is anticipated to be systemically injurious. Indeed, KI mice subjected to CLP have less renal damage (indexed by plasma BUN levels, Fig. 3E) as well as less global tissue damage (plasma LDH levels, Fig. 3F) compared with WT. Both BUN and LDH negatively correlate with animal survival after sepsis (19), consistent with our synopsis that KI mice are protected compared with WT. Indeed, KI mice subjected to CLP have attenuated hypothermia (Fig. 3G) and preserved locomotor activity (Fig. 3H), indicating better systemic well being compared with WT. The KI locomotor activity compared with WT is markedly preserved during the initial 8 h after CLP. However, between 9 and 24 h, the protection in the KI is relatively small. These data are significantly complicated by the diurnal variation, but at the later phase perhaps the protection is lost because it is mediated by different mechanisms, such as cognitive impairment, which is a major consequence of sepsis in human (4), and mice (20).
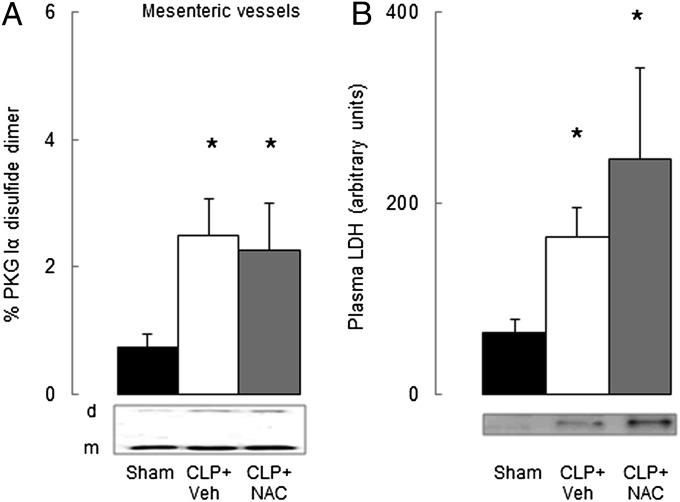
We examined the ability of N-acetylcysteine (NAC) to prevent sepsis-induced PKG Iα oxidation. The antioxidant capacity of NAC is due to it containing a thiol, which can react with (scavenge) oxidants such as hydrogen peroxide and nitrosothiols. Because PKG Iα oxidation is also thiol-dependent, NAC is considered a rationale choice of antioxidant because it will potentially scavenge the same molecular species that oxidize Cys42 in the kinase. However, we found NAC did not prevent CLP-induced disulfide formation (Fig. 4A) and also failed to reduce organ damage as indexed by plasma LDH levels (Fig. 4B).
Fig. 4.
Effect of N-acetylcysteine (NAC) treatment on PKG Iα oxidation or tissue LDH release. (A) Comparison of PKG Iα disulfide formation in WT mesenteric vessels 6 h after a sham operation, or CLP in the presence of NAC or a vehicle control. (B) Comparison of plasma LDH levels in WT mice 6 h after a sham operation, or CLP in the presence of NAC or a vehicle control. *P < 0.05, comparing WT CLP with WT sham operated by t test. Data are mean ± SEM.
Although antioxidants historically have been considered a panacea for disease, there are several factors that may limit their effectiveness. Although compounds such as NAC can scavenge thiol-oxidizing species such as hydrogen peroxide or nitrosothiols to prevent PKG Iα oxidation, one limitation is that thiol oxidant sensors may be significantly more reactive. So a very high concentration of an antioxidant may not efficiently compete with a low pKa protein thiol to prevent its oxidation. Even if the antioxidant did compete, at some point it will become exhausted (i.e., fully oxidized). Unlike endogenous antioxidant thiols (e.g., glutathione, thioredoxin), reduced NAC may not be regenerated by cellular reducing enzymes. Even if NAC can be recycled to the antioxidant reduced form by the cell, during sepsis the cellular reducing capacity of the cell is compromised—hence the oxidative stress. A further consideration is that antioxidants may also prevent oxidation of sensor proteins that couple to protective responses. In this scenario, antioxidants could be harmful by preventing appropriate adaptive cellular responses. Indeed, it is now understood that antioxidants are injurious in many disease scenarios (21, 22). Furthermore, because antioxidants are reducing molecules, they could directly or indirectly serve as electron donors for cellular oxidases. Because these enzymes generate oxidant species, antioxidants can therefore counterintuitively have pro-oxidant effects and exacerbate target oxidation and worsen disease progression. Indeed, a meta-analysis of N-acetylcysteine during sepsis concluded that it certainly did not reduce mortality and cautioned that it could worsen the outcome (23).
We conclude that sepsis, long associated with oxidative stress, induces disulfide-activated PKG Iα to lower blood pressure. This hypotension results in underperfusion of organs and systemic dysfunction. These findings provide insight for rational therapy design, for example drugs that limit PKG Iα disulfide formation may be protective. Although antioxidant therapies can be envisaged as a potential therapeutic strategy to attenuate sepsis-induced injury by limiting PKG Iα oxidation, as discussed previously, this approach may not be viable.
Materials and Methods
Animal Study.
All procedures were performed in accordance with the Home Office Guidance on the Operation of the Animals (Scientific Procedures) Act 1986 in the United Kingdom and were approved by the King's College Animal Welfare and Ethical Review Body. Mice constitutively expressing PKG Iα Cys42Ser were generated on a pure C57BL/6 background by TaconicArtemis as described elsewhere (17). Only male mice were used in this study. C57BL/6 mice were used for sepsis validation studies. Age- and body weight–matched WT and PKG Iα Cys42Ser KI mice were used in all other studies. All studies were performed at the same time of day to obviate circadian influences in pair-matched littermates (i.e., WT and KIs). All animals had ad libitum access to standard chow and water. Mice were kept under specific pathogen-free conditions and under a 12-h day/night cycle.
Systemic Blood Pressure Measurements.
Mean arterial pressure and the locomotor activity were assessed by telemetry in conscious, freely moving mice as described elsewhere (14, 24). Briefly, mice were anesthetized with 2% (vol/vol) isoflurane (Centaur Services) in 1 L of oxygen per minute with pre- and postoperative analgesia (buprenorphine, 0.1 mg per kilogram of body weight, Abbot Laboratories), and a TA11PA-C10 probe catheter (Data Science International) was implanted into the aortic arch via the left carotid artery. Following up to 10 d recovery, mice were placed above the telemetric receivers and the blood pressure was recorded by scheduled sampling.
Induction of Endotoxemia or Sepsis.
Endotoxemia was induced in mice by i.p. injection of Escherichia coli bacterial LPS (9 mg/kg) as previously described (24). This dose of LPS is not typically associated with mortality before 24 h, but at longer time points, survival is low. Sepsis was induced in mice by CLP (25, 26). Briefly, mice were anesthetized with 2% (vol/vol) isoflurane in 1 L of oxygen per minute, the cecum was then subjected to a single “through and through” perforation with a 19-gauge needle and gently squeezed to exteriorize 1–2 mm of cecum content. Sham-operated mice underwent the same procedure except for ligation and perforation of the cecum. Analgesia (buprenorphine, 0.1 mg/kg) was applied immediately after the induction of sepsis and volume resuscitation [NaCl 0.9% (wt/vol), 0.03 mL/g body weight] was administered into four body quadrants through s.c. injection (n = 10–11 per group). After recovery, mice were placed back over the telemetry receivers to enable recording of blood pressure during sepsis development. The room temperature was maintained at 25 °C before and after induction of sepsis; all mice had unlimited access to chow and water. Based on the studies of others (27), the CLP model we used has significant mortality after 24 h. Under the terms and conditions of the United Kingdom Home Office license that authorizes these studies, we cannot extend LPS or CLP studies beyond 24 h and are obliged for welfare reasons to euthanize mice whose clinical signs indicate they are moribund. In a separate cohort, septic mice were treated with NAC (150 mg/kg in saline, pH 7.4) immediately after CLP procedure by IP injection, followed by the second injection 3 h after CLP.
Small Vessels Myography.
Third-order mesenteric vessels were mounted for isometric tension recordings in a tension myograph [Danish Myo Technology (DMT)], stretched to the optimal pretension condition with DMT normalization module and bathed in Krebs solution at 37 °C with a 95% (vol/vol) O2:5% (vol/vol) CO2 environment. Constricting dose–responses to thromboxane mimetic compound U46619 were assessed with endothelium-intact isolated mouse vessels.
Microvascular Leak Assessment.
Vascular permeability was assessed by visualizing and quantifying the leakage of Evans Blue dye (EBD) into the vascular wall as described (28). Briefly EBD [0.1 mL of 1% (wt/vol) dye in PBS] was injected into the inferior vena cava. After 15min, mice were euthanized with anesthetic overdose and perfused through the left ventricle with 4% (wt/vol) formaldehyde in PBS; the main mesenteric vessel with small branches and a segment containing the aortic arch, the brachiocephalic and carotid arteries were isolated, dried, and weighed. EBD was extracted by incubation in formamide for 24 h at 60 °C, and the absorbance was measured at 620 nm. Standard curves for pure EBD were used to calculate the total amount of dye in the tissue (n = 7 per group).
Echocardiography.
Twenty-four hours after sepsis induction by CLP, mice were anesthetized and examined by echocardiography at the body temperature 36 °C using a high resolution Vevo 770 echocardiography system (VisualSonics) with a RMV-707B transducer running at 30 MHz. High-resolution, 2D B-mode and M-mode images at the level of the papillary muscles were obtained for offline stroke volume and cardiac output measurements with Vevo Software (VisualSonics) (n = 10 per group).
Plasma Analysis and Immunoblotting.
After completion of echocardiography imaging, abdominal dissection was performed; blood was sampled from inferior vena cava. Fresh blood was used for rapid biochemical analysis of BUN by using an iSTAT blood biochemistry analyzer (courtesy of Manasi Nandi, King’s College London) (n = 10 per group). The rest of the blood was centrifuged and plasma was frozen for measurement of LDH level. Immunoblotting for PKG Iα disulfide dimer was performed as described previously (14), with maleimide (100 mmol/L) used in preparation buffers to alkylate thiols and prevent thiol disulfide exchange. Antibodies used in these studies included cGKIα (ADI-KAP-PK005; Enzo Life Science) and LDH (ab7638, Abcam). HRP-linked secondary antibody (Cell Signaling) and ECL reagent (GE Healthcare) were used. Digitized immunoblots were analyzed quantitatively with Gel-Pro Analyzer 3.1 software.
Acknowledgments
We thank Dr. Manasi Nandi and Dr. Anna Starr for training in the CLP model and expert advice and Dr. James Clark for helpful assistance with echocardiography measurements. This work was supported by Medical Research Council UK, the British Heart Foundation, the Fondation Leducq, and the UK Department of Health through the National Institute for Health Research comprehensive Biomedical Research Centre award to Guy's and St Thomas' National Health Service Foundation Trust.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. T.M. is a guest editor invited by the Editorial Board.
References
- 1.Remick DG. Pathophysiology of sepsis. Am J Pathol. 2007;170(5):1435–1444. doi: 10.2353/ajpath.2007.060872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wheeler AP, Bernard GR. Treating patients with severe sepsis. N Engl J Med. 1999;340(3):207–214. doi: 10.1056/NEJM199901213400307. [DOI] [PubMed] [Google Scholar]
- 3.Talmor D, et al. The costs and cost-effectiveness of an integrated sepsis treatment protocol. Crit Care Med. 2008;36(4):1168–1174. doi: 10.1097/CCM.0b013e318168f649. [DOI] [PubMed] [Google Scholar]
- 4.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nat Med. 2011;17(11):1381–1390. doi: 10.1038/nm.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durán WN, Breslin JW, Sánchez FA. The NO cascade, eNOS location, and microvascular permeability. Cardiovasc Res. 2010;87(2):254–261. doi: 10.1093/cvr/cvq139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berg RM, Møller K, Bailey DM. Neuro-oxidative-nitrosative stress in sepsis. J Cereb Blood Flow Metab. 2011;31(7):1532–1544. doi: 10.1038/jcbfm.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gotes J, Kasian K, Jacobs H, Cheng ZQ, Mink SN. Mechanisms of systemic vasodilation by lysozyme-c in septic shock. J Appl Physiol. 2012;112(4):638–650. doi: 10.1152/japplphysiol.00707.2011. [DOI] [PubMed] [Google Scholar]
- 9.Galley HF. Oxidative stress and mitochondrial dysfunction in sepsis. Br J Anaesth. 2011;107(1):57–64. doi: 10.1093/bja/aer093. [DOI] [PubMed] [Google Scholar]
- 10.Crawford JH, et al. Transduction of NO-bioactivity by the red blood cell in sepsis: Novel mechanisms of vasodilation during acute inflammatory disease. Blood. 2004;104(5):1375–1382. doi: 10.1182/blood-2004-03-0880. [DOI] [PubMed] [Google Scholar]
- 11.Liu L, et al. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116(4):617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 12.Burgoyne JR, et al. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science. 2007;317(5843):1393–1397. doi: 10.1126/science.1144318. [DOI] [PubMed] [Google Scholar]
- 13.Burgoyne JR, Eaton P. Transnitrosylating nitric oxide species directly activate type I protein kinase A, providing a novel adenylate cyclase-independent cross-talk to beta-adrenergic-like signaling. J Biol Chem. 2009;284(43):29260–29268. doi: 10.1074/jbc.M109.046722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudyk O, Prysyazhna O, Burgoyne JR, Eaton P. Nitroglycerin fails to lower blood pressure in redox-dead Cys42Ser PKG1α knock-in mouse. Circulation. 2012;126(3):287–295. doi: 10.1161/CIRCULATIONAHA.112.101287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piwkowska A, Rogacka D, Jankowski M, Kocbuch K, Angielski S. Hydrogen peroxide induces dimerization of protein kinase G type Iα subunits and increases albumin permeability in cultured rat podocytes. J Cell Physiol. 2012;227(3):1004–1016. doi: 10.1002/jcp.22810. [DOI] [PubMed] [Google Scholar]
- 16.Yang L, et al. Protein kinase G phosphorylates Cav1.2 alpha1c and beta2 subunits. Circ Res. 2007;101(5):465–474. doi: 10.1161/CIRCRESAHA.107.156976. [DOI] [PubMed] [Google Scholar]
- 17.Prysyazhna O, Rudyk O, Eaton P. Single atom substitution in mouse protein kinase G eliminates oxidant sensing to cause hypertension. Nat Med. 2012;18(2):286–290. doi: 10.1038/nm.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burgoyne JR, Prysyazhna O, Rudyk O, Eaton P. cGMP-dependent activation of protein kinase G precludes disulfide activation: Implications for blood pressure control. Hypertension. 2012;60(5):1301–1308. doi: 10.1161/HYPERTENSIONAHA.112.198754. [DOI] [PubMed] [Google Scholar]
- 19.Gao M, et al. Use of blood urea nitrogen, creatinine, interleukin-6, granulocyte-macrophage colony stimulating factor in combination to predict the severity and outcome of abdominal sepsis in rats. Inflamm Res. 2012;61(8):889–897. doi: 10.1007/s00011-012-0481-3. [DOI] [PubMed] [Google Scholar]
- 20.Chavan SS, et al. HMGB1 mediates cognitive impairment in sepsis survivors. Mol Med. 2012;18:930–937. doi: 10.2119/molmed.2012.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2008;(2):CD007176. doi: 10.1002/14651858.CD007176. [DOI] [PubMed] [Google Scholar]
- 22.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297(8):842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 23.Szakmany T, Hauser B, Radermacher P. N-acetylcysteine for sepsis and systemic inflammatory response in adults. Cochrane Database Syst Rev. 2012;9:CD006616. doi: 10.1002/14651858.CD006616.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgoyne JR, Rudyk O, Mayr M, Eaton P. Nitrosative protein oxidation is modulated during early endotoxemia. Nitric Oxide. 2010;25(2):118–124. doi: 10.1016/j.niox.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4(10):854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- 26.Merx MW, et al. Statin treatment after onset of sepsis in a murine model improves survival. Circulation. 2005;112(1):117–124. doi: 10.1161/CIRCULATIONAHA.104.502195. [DOI] [PubMed] [Google Scholar]
- 27.Ebong SJ, et al. Critical role of CD14 for production of proinflammatory cytokines and cytokine inhibitors during sepsis with failure to alter morbidity or mortality. Infect Immun. 2001;69(4):2099–2106. doi: 10.1128/IAI.69.4.2099-2106.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phinikaridou A, et al. Noninvasive magnetic resonance imaging evaluation of endothelial permeability in murine atherosclerosis using an albumin-binding contrast agent. Circulation. 2012;126(6):707–719. doi: 10.1161/CIRCULATIONAHA.112.092098. [DOI] [PubMed] [Google Scholar]