Abstract
Inherited retinal degenerations, caused by mutations in over 100 individual genes, affect approximately 2 million people worldwide. Many of the underlying mutations cause protein misfolding or mistargeting in affected photoreceptors. This places an increased burden on the protein folding and degradation machinery, which may trigger cell death. We analyzed how these cellular functions are affected in degenerating rods of the transducin γ-subunit (Gγ1) knockout mouse. These rods produce large amounts of transducin β-subunit (Gβ1), which cannot fold without Gγ1 and undergoes intracellular proteolysis instead of forming a transducin βγ-subunit complex. Our data revealed that the most critical pathobiological factor leading to photoreceptor cell death in these animals is insufficient capacity of proteasomes to process abnormally large amounts of misfolded protein. A decrease in the Gβ1 production in Gγ1 knockout rods resulted in a significant reduction in proteasomal overload and caused a striking reversal of photoreceptor degeneration. We further demonstrated that a similar proteasomal overload takes place in photoreceptors of other mutant mice where retinal degeneration has been ascribed to protein mistargeting or misfolding, but not in mice whose photoreceptor degenerate as a result of abnormal phototransduction. These results establish the prominence of proteasomal insufficiency across multiple degenerative diseases of the retina, thereby positioning proteasomes as a promising therapeutic target for treating these debilitating conditions.
Keywords: neurodegenerative diseases, protein degradation
Inherited retinal degenerations are blinding diseases associated with the death of rod and/or cone photoreceptors. They are caused by mutations in over 100 individual genes (www.sph.uth.tmc.edu/RetNet/). At present, therapeutic options for patients suffering from retinal degenerations are extremely limited. The mechanisms by which this diverse set of mutations causes a similar endpoint pathology are poorly understood, but identification of these mechanisms could open the way to developing common treatment strategies for all of these related diseases. In many cases, the disease-causing mutations lead to misfolding or mistrafficking of photoreceptor-specific proteins (1), but the molecular pathways activated by these protein turnover abnormalities remain mostly obscure. Here we tested the idea that death of affected photoreceptors may originate from the stress associated with insufficient capacity of their protein folding and/or degradation machinery to process large amounts of abnormal proteins.
Previous studies in this direction were primarily focused on diseases associated with abnormal processing of photoreceptor-specific transmembrane proteins. For example, the thoroughly studied P23H mutation in rhodopsin (a single amino acid substitution of proline at position 23 for histidine), frequently encountered in retinitis pigmentosa patients (2, 3), was shown to cause rhodopsin misfolding and endoplasmic reticulum (ER) accumulation (4, 5), ultimately resulting in an unfolded protein response and cell death (6, 7). Protein misfolding is considered a therapeutic target in the ongoing clinical trial in which patients with retinitis pigmentosa are treated with valproic acid (http://clinicaltrials.gov/ct2/show/study/NCT01233609), the substance suggested to act as a pharmacological chaperone for unfolded proteins (8, 9).
In this study, we analyzed the pathobiological mechanisms of photoreceptor cell death associated with the production of a cytosolic protein, the β-subunit of transducin (Gβ1), in the absence of its constitutive partner, transducin γ-subunit (Gγ1). Previous work by us and others (10, 11) showed that Gγ1 knockout results in progressive photoreceptor loss. Even before degeneration begins, affected rods sustain a >90% loss of other transducin subunits: Gαt and Gβ1. This led us to hypothesize that Gγ1−/− rods degenerate as a result of ongoing cellular stress caused by their inability to form normal transducin heterotrimer and/or the requirement to proteolyze Gβ1 and Gαt instead of transporting them to the outer segment (10). Previously, we attempted to ameliorate this stress by knocking out Gαt in addition to Gγ1; however, rods of these double knockout mice degenerated at the same rate as in Gγ1−/− mice (10). We now report that a completely different effect is achieved by reducing the expression of Gβ1, a subunit distinguished from Gαt by its inability to fold without Gγ1: the photoreceptor loss in Gγ1−/− mice was nearly completely reversed in the animals expressing a single copy of the Gβ1 gene.
We explored whether Gβ1 unpaired by Gγ1 may act as a dominant negative pathobiological factor overwhelming the capacity of protein folding and/or degradation machinery in affected rods. Whereas evidence for both phenomena were revealed in these experiments, only the latter one manifesting itself as an overwhelmed capacity of proteasomes to degrade intracellular proteins correlated with the degree of Gβ1 expression and photoreceptor cell survival. Furthermore, a similar pattern of proteasomal insufficiency was ubiquitously observed in multiple mouse models of photoreceptor degeneration associated with protein misfolding or mistargeting, but not in the mouse whose photoreceptors degenerate due to aberrant phototransduction. Therefore, proteasomal insufficiency is a common, previously underappreciated stress factor in degenerative diseases of the retina, which may serve as a potential therapeutic target for treating these debilitating conditions.
Results and Discussion
Reduction in the Expression Level of Gβ1 Slows Retinal Degeneration in Gγ1−/− Mice.
Pathological changes in the retina of the Gγ1−/− mouse begin at ∼1 mo with a subtle rod outer segment shortening, followed by a progressive photoreceptor cell loss initiated at ∼2 mo (10) (Fig. 1A) (a phenotypically similar but somewhat slower pathology was reported for an alternatively produced Gγ1−/− strain) (11). We first established that photoreceptor loss in these mice is indeed originating from the production of Gβ1 unpaired with Gγ1 by reducing the expression level of Gβ1. Whereas homozygous Gβ1−/− mice are not viable, Gβ1+/− mice are healthy (12) and display no photoreceptor abnormality (Fig. 1A). Their retinas contain ∼50% of WT Gβ1 mRNA (Fig. S1) and roughly half-normal contents of each transducin subunit (Fig. 1B). Remarkably, the photoreceptor loss in Gγ1−/− mice was nearly reversed in Gγ1−/−Gβ1+/− animals (Fig. 1A). The earliest morphological sign of degeneration in Gγ1−/−Gβ1+/− mice—a loss of one row of nuclei from the outer nuclear layer—was detected at 6 mo (Fig. 1C). In comparison, Gγ1−/− mice have lost five rows of nuclei at this age. Further progression of degeneration in the Gγ1−/−Gβ1+/− mice was slow, such that only three rows of nuclei were gone at 12 mo. This is in striking contrast to the complete devastation of the photoreceptor layer that occurs in 1-y-old Gγ1−/− mice. As might be expected, we also rescued degeneration in Gγ1−/− mice by transgenic expression of Gγ1 (trGγ1/Gγ1−/− mice; Fig. 1 A–C), which restored the levels of all three transducin subunits to roughly half-normal. A minor nuclear loss in these animals became evident only at 12 mo.
Fig. 1.
Photoreceptor degeneration in Gγ1−/− mice is rescued by deleting one copy of the Gβ1 gene or by expressing the Gγ1 transgene. (A) Comparative analysis of retinal morphology in mice of indicated genotypes. (B) Expression levels of transducin Gαt, Gβ1, and Gγ1 subunits in the retinas of 1-mo-old mice. The data were obtained by quantifying and averaging the corresponding Western blot bands after normalizing them to the values obtained for WT retinas. The number of experiments varied between four and eight for different mouse lines. (C) Average number of nuclei in the outer nuclear layer columns of the central retina at 6 and 12 mo of age. Color coding of individual mouse lines for B and C is indicated (C, Right); error bars represent SEM. See also Fig. S1 for mRNA levels of transducin subunit in the retinas of Gβ1+/− mice. (D) The mechanism of transducin βγ-subunit assembly in WT rods and its alterations by the Gγ1 knockout (see text for details). The synthesis of Gβ1 in the absence of Gγ1 may cause cellular stress by either occupying a significant portion of the chaperonin CCT or by overloading the capacity of proteasomes to conduct proteolytic digestion of misfolded proteins.
Importantly, both rescue strategies reduced the amount of Gβ1 that is synthetized but unable to form a dimer with Gγ1. Therefore, survival of rods lacking Gγ1 is defined by a fine balance between acceptable and excessive cellular stress, ultimately determined by the amount of unpaired Gβ1 produced in these cells. Mechanistically, this stress may originate from Gβ1 acting as a dominant negative factor overwhelming the cellular capacity to conduct protein folding and/or protein degradation.
Before describing the experiments addressing each of these possibilities, we briefly summarize the normal mechanism of transducin βγ-subunit folding and its putative aberrations in Gγ1−/− rods (Fig. 1D). Newly synthetized G-protein β-subunit polypeptides assume their final shape inside the cytosolic chaperonin containing T-complex protein 1 (CCT), whereas Gγ subunits (aided by the phosducin-like protein, PhLP) (13) play a critical role in stabilizing and releasing Gβ from CCT to yield mature Gβγ complexes (14–17). Therefore, the lack of Gγ1 in rods may result in Gβ1 retention inside CCT, ultimately reducing the CCT availability to process the dozens of other cytosolic proteins normally folded inside this chaperonin (18). This possibility is consistent with our observation that normal photoreceptors contain a large, ∼100-fold excess of Gβ1 over CCT (Fig. S2). Such a CCT “jamming” may eventually cause cell death, similarly to that in rods expressing the dominant negative PhLP mutant (19). Given that the ubiquitin–proteasome system plays a critical role in intracellular degradation of both G-protein βγ-complexes and unfolded Gβ subunits (20, 21), it is likely that the bulk of Gβ1 synthetized in Gγ1−/− rods is eventually targeted to proteasomes, either directly or following a stage of CCT association (Fig. 1D, Lower). This increased load on proteasomes may also be stressful for the cells because impaired proteasomal function is a well-recognized proapoptotic factor (22).
Characterization of the Gβ1–CCT Complex in Gγ1−/− Mice.
To seek evidence for the CCT jamming by Gβ1, we obtained soluble protein extracts from WT and Gγ1−/− retinas at an early stage of pathology (1 mo old) and analyzed the degree of Gβ1–CCT association in each case using size-exclusion chromatography. In a typical chromatogram of WT retina, CCT was eluted in a single sharp peak in the middle of the run (Fig. 2A; elution volume ∼11–12 mL), whereas the bulk of Gβ1 was eluted toward the end of the run, likely representing G-protein trimers and/or Gβ1 dimers with various Gγ subunits. A small amount of Gβ1 was also detected in the void volume (∼7 mL; likely as aggregates). In contrast, the chromatogram from a Gγ1−/− retina contained an additional distinct peak of Gβ1 coeluting with CCT. Despite an ∼10-fold higher total amount of Gβ1 in the WT extract, the amount of Gβ1 found in the CCT-containing fractions was smaller than in the Gγ1−/− extract and did not appear to exceed light background smear persisting in adjacent fractions. These data suggest that Gγ1 knockout causes a distinct increase in the CCT occupancy by Gβ1, consistent with our hypothesis.
Fig. 2.
Coelution of CCT and Gβ1 in retinal extracts from Gγ1−/− and Gγ1−/−Gβ1+/− mice. (A) Retinal extracts from 1-mo-old WT and Gγ1−/− mice (240 μg total protein) were fractionated by size-exclusion chromatography on a Superose-6 column. Proteins in 1-mL fractions were probed by Western blotting using antibodies against Gβ1 and the α- and β-subunits of CCT. Positions of elution peaks for three molecular weight standards (ferritin, BSA, and ribonuclease) are indicated by arrows. (B) Percentile fractions of CCT occupied by Gβ1 in extracts from Gγ1−/− and Gγ1−/−Gβ1+/− retinas were calculated after measuring absolute amounts of each protein in the CCT-containing chromatography peaks by quantitative mass spectrometry (mean ± SEM). (C) Relative amounts of CCTα, CCTβ, and PhLP in the retinas of 1-mo-old Gγ1−/−, Gγ1−/−Gβ1+/−, and wild-type retinas determined by Western blotting (5 μg protein/lane). (D) Retinal lysates from Gγ1−/− and Gγ1−/−Gβ1+/− littermates (275 μg protein) were processed as in A and CCTα, CCTβ, and Gβ1 in 0.5-mL fractions around the CCT elution peak were detected on the same Western blot. (E) Intensities of Gβ1 bands in chromatography fractions in D quantified using a Li-Cor instrument. Data in A represent one of four replicates; C, D, and E represent one of three replicates. See also Fig. S2 for details on quantification of Gβ1:CCT ratios in whole retinas.
Using quantitative mass spectrometry with isotope-labeled peptide standards, we determined that approximately one-third of CCT is occupied by Gβ1 in Gγ1−/− extracts (Fig. 2B). Considering that the photoreceptor layer contains ∼65% of total retinal CCT (Fig. S2) and the contents of CCT in 1-mo-old Gγ1−/− retinas are normal (Fig. 2C), and assuming that CCT jamming occurs mostly in rods, the upper limit of the CCT occupation by Gβ1 in Gγ1−/− rods is ∼50%. To assess the pathobiological significance of this phenomenon, we repeated gel-filtration experiments using age-matched Gγ1−/−Gβ1+/− littermates (Fig. 2D) and found that the levels of CCT occupation by Gβ1 in Gγ1−/−Gβ1+/− and Gγ1−/− mice were essentially identical (38.9 ± 2.7% vs. 34.4 ± 6.8%; Fig. 2 B and E). Such a surprising lack of correlation between the degree of CCT jamming and the status of photoreceptor health suggests that partial CCT blockade by Gβ1 has little if any pathological consequence. This result redirected our efforts to elucidating the effect of Gγ1 knockout on the status of photoreceptor protein degradation.
Gγ1−/− Rods Suffer from Proteasomal Overload.
Given that the ubiquitin–proteasome system plays a critical role in intracellular degradation of unfolded Gβ subunits (20, 21), we assessed the functional status of this system in Gγ1−/− and Gγ1−/−Gβ1+/− mice by crossing them with transgenic mice expressing an in vivo reporter of proteasome activity, UbG76V–GFP (a fusion of ubiquitin with GFP containing a G76V mutation in the linker) (23). The lifetime of this reporter in healthy cells is short, whereas its intracellular accumulation is indicative of proteasomal insufficiency (24).
Retinal cross-sections from 1-mo-old Gγ1−/− mice displayed intense reporter fluorescence in rods (Fig. 3A). Fluorescence persisted as photoreceptor loss progressed with age, whereas no detectable fluorescence was observed in rods of age-matched WT controls. Remarkably, the UbG76V–GFP fluorescence in Gγ1−/−Gβ1+/− rods barely exceeded the background level in 1- to 3-mo-old animals. However, it was clearly detectable at 6 mo, when morphological features of photoreceptor degeneration in these mice become evident. A more sensitive and linear UbG76V–GFP detection by Western blotting revealed that 1- to 3-mo-old Gγ1−/−Gβ1+/− mice actually accumulated small amounts of nonproteolyzed reporter above WT levels, which increased at 6 mo, yet not reaching the prominence observed in Gγ1−/− mice (Fig. 3B). These experiments established a strong correlation between proteasomal insufficiency and progression of photoreceptor degeneration in affected animals and further stress that the photoreceptor health in Gγ1−/− mice is defined by a subtle difference between tolerable and nontolerable stress imposed by the level of Gβ1 expression.
Fig. 3.
Gγ1 knockout rods suffer from proteasomal overload, which can be alleviated by reduced Gβ1 expression. (A) UbG76V–GFP reporter fluorescence (green) in retinal cross-sections from Gγ1−/−, Gγ1−/−Gβ1+/−, and WT mice. Rod outer segments are stained with wheat germ agglutinin (WGA) conjugated to Alexa 594 (red). (B) Western blot detection of the UbG76V–GFP reporter using an anti-GFP antibody in retinal lysates from mice of indicated genotypes (25 μg protein/lane); β-actin was used as a loading control. (C) Three enzymatic activities of the 26S proteasome—chymotrypsin-like (CT-L), trypsin-like (T-L), and caspase-like (C-L)—were measured in retinal extracts from 1-mo-old Gγ1−/− and WT mice (mean ± SEM; n = 3). (D) Western blot detection of proteasome subunits β1, proteasome activator PA28 alpha (PA28α), and 26S proteasome non-ATPase regulatory subunit 11 (PSMD11) representing the 20S core and the 11S and 19S caps in retinal extracts from 1-mo-old Gγ1−/−, Gγ1−/−Gβ1+/−, and WT mice. A total of 30 μg of protein was loaded on each lane. Data represent one of three similar experiments.
We next addressed whether proteasomal insufficiency caused by the Gγ1 knockout reflects direct proteasomal impairment (i.e., a reduction in their maximal activity and/or amount) or whether it represents proteasomal overload (i.e., proteasome saturation by an unusually large amount of unfolded Gβ1 substrate). All three proteolytic activities of 26S proteasomes were measured with fluorescent peptide substrates (25) in retinal extracts of 1-mo-old Gγ1−/− mice, the age at which photoreceptor loss is minimal, although proteasomal insufficiency is prominent. We found no reduction of any proteolytic activity in Gγ1−/− mice compared with WT controls (Fig. 3C). The contents of proteasomal subunits, representing the 20S core and the 19S and 11S caps, were also identical in Gγ1−/− and WT retinas (Fig. 3D). Together, these measurements indicate that both the amount and the functionality of proteasomes in young Gγ1−/− rods remain normal despite the ongoing degeneration. Therefore, the accumulation of UbG76V–GFP in these cells is indicative of proteasomal overload, i.e., their insufficient capacity to process an increased amount of unfolded proteins. A complimentary possibility is that proteasomal insufficiency in affected rods is caused by misbalance in the protein ubiquitination/deubiquitination system. These data further indicate that photoreceptors experiencing proteasomal insufficiency in the course of their progressive degeneration are essentially defenseless against this condition, due to a lack of compensatory mechanisms that allow proteasomal activity enhancement, such as those used in response to acute oxidative stress (26).
Proteasomal Insufficiency Is Encountered in Multiple Mouse Models of Photoreceptor Degeneration.
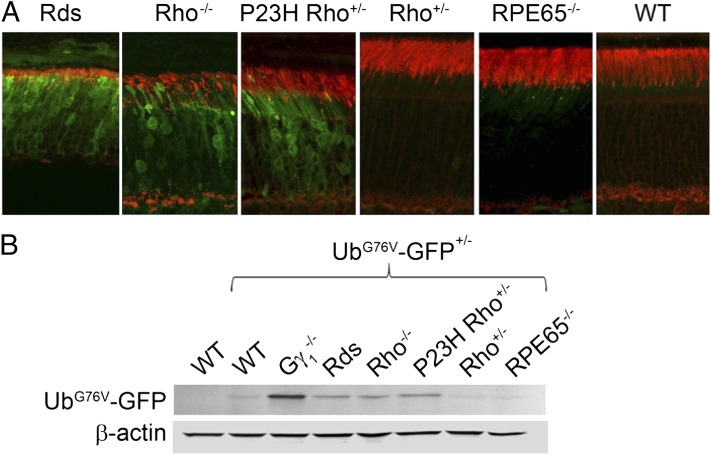
In the final set of experiments, we expanded our findings with Gγ1−/− mice to explore whether proteasomal insufficiency is encountered in other mouse models of retinal degeneration. The UbG76V–GFP transgene was introduced into four mouse lines and reporter fluorescence was analyzed at 1 mo of age. Prominent fluorescence was observed in three models, retinal degeneration slow (Rds), rhodopsin knockout (Rho−/−), and P23H, each facing a requirement to degrade abnormal amounts of misfolded or mistargeted proteins (Fig. 4A).
Fig. 4.
Proteasomal insufficiency is encountered in multiple mouse models of photoreceptor degeneration. (A) UbG76V–GFP reporter fluorescence (green) in retinal cross-sections from 1-mo-old mice of indicated genotypes. Rod outer segments are stained with WGA (red). (B) Western blot detection of the UbG76V–GFP reporter in retinal lysates from 1-mo-old mice of indicated genotypes using an anti-GFP antibody (30 μg protein/lane); β-actin was used as a loading control. Note that following the original report (2), we maintained the P23H transgenic mice as heterozygotes on the Rho+/− background and therefore used Rho+/− mice as the corresponding control.
Relatively slowly degenerating photoreceptors of Rds mice do not form outer segments due to the lack of structural protein, peripherin-2 (27, 28); this requires continuous degradation of multiple proteins otherwise residing in this cellular compartment. Similarly, more rapidly degenerating rods of Rho−/− mice form only rudimentary outer segments, also necessitating the degradation of outer segment-specific proteins (29, 30). P23H mice, already mentioned in the Introduction, contain a point mutation in rhodopsin causing its misfolding and ER accumulation (4, 6). Large quantities of misfolded P23H rhodopsin are thought to be extruded via the ER-associated degradation pathway and ultimately targeted to proteasomes (4, 31) similarly to other cells undergoing ER stress (32); this likely explains proteasomal insufficiency observed in rods of P23H mice. Western blot analysis (Fig. 4B) revealed comparable levels of nonproteolyzed UbG76V–GFP reporter accumulation in all three mouse lines. Interestingly, it was below the level observed in the age-matched Gγ1−/− mice, suggesting the latter being a particularly attractive and sensitive tool for further exploration of this type of pathology.
In contrast, no significant increase in UbG76V–GFP fluorescence was detected in rods of young, age-matched knockout mice lacking the retinal pigment epithelium 65 (RPE65) protein (Fig. 4 A and B), a model of type 2 Leber’s congenital amaurosis (33, 34). Unlike the other mice analyzed in this study, the primary cause of their photoreceptor degeneration is not thought to relate to abnormal protein synthesis, folding, or trafficking. Rather, RPE65−/− rods die, due to the lack of rhodopsin regeneration by 11-cis-retinal, which leads to persistent phototransduction activation (35), producing a functional equivalent of constant light exposure—an alternative stressor causing photoreceptor degeneration, particularly in rodents (36).
Concluding Remarks.
Our data provide unique in vivo evidence that proteasomal insufficiency is ubiquitously encountered in various forms of inherited retinal degeneration associated with misfolding or mistargeting of both cytosolic and membrane proteins. Whereas the studies of other neurodegenerative diseases revealed a strong association between the underlying pathology and impaired proteasomal function (37–40), this important stress factor largely escaped the attention of researchers studying retinitis pigmentosa and allied diseases. Given that inherited retinal disorders are typically diagnosed at their early stages, the emerging therapeutic strategies targeting proteasomes in neurodegeneration (41) could be particularly efficacious in treating these blinding conditions.
Materials and Methods
Mouse care and experiments were performed in accordance with procedures approved by the Institutional Animal Care and Use Committee of Duke University. The Gγ1 knockout and Gβ1 heterozygous mice were licensed from Deltagen and the former was characterized previously (10). Details on other animal strains, antibodies, immunoblotting, histological techniques, and immunohistochemical procedures are available in SI Materials and Methods.
Size-Exclusion Chromatography.
Retinal lysates were cleared of debris by centrifugation and subjected to gel filtration on a Superose-6 column (Amersham) attached to the FPLC system (Pharmacia) at a flow rate of 500 μL/min in PBS. Proteins in 0.5- or 1-mL fractions were precipitated with acetone and analyzed either by Western blotting or quantitative mass spectrometry. Additional details on processing of samples are provided in SI Materials and Methods.
mRNA Purification and Quantitation by Real-Time Reverse Transcription-PCR.
The levels of Gαt, Gβ1, and Gγ1 transcripts in Gβ1+/− and WT mice were measured using quantitative reverse transcription (qRT)-PCR as described earlier (10).
Proteasomal Activity Measurements.
Caspase-like, trypsin-like, and chymotrypsin-like proteolytic activities of proteasomes were measured with the fluorogenic 7-Amino-4-methylcoumarin (AMC)-conjugated peptide substrates following the protocols described in ref. 25. Details on retina sample processing, specific fluorogenic substrates, and data analysis are described in SI Materials and Methods.
Mass Spectrometry.
Absolute amounts of CCT and Gβ1 retinal lysates and chromatography fractions were determined by quantitative mass spectrometry (42) using isotope-labeled peptide standards. For CCT, the standards were: the EQLA[15N]IA[15N]EFA[15N]R peptide from CCTα and the GA[15N]TQQILDEA[15N]ER peptide from CCTβ (synthesized by GenScript). Two peptide standards for Gβ1 were LLVSASQDGR[13C6;15N4] and AGVLAGHDNK[13C6;15N2] (synthesized by ThermoFisher). Peptide dilutions were performed in a solution of leucine enkephalin in 3% (vol/vol) acetonitrile and 0.1% formic acid (500 fmol/μL; Sigma-Aldrich) to reduce their nonspecific binding to test tubes. Each peptide standard was chosen after LC/MS/MS identification of all CCT- and Gβ1-derived peptides in a whole retina digest, based on their good ionization and chemical stability. The consistency between absolute CCT quantifications using the CCTα and CCTβ peptides was 12% (n = 4); the consistency between Gβ1 quantifications with the corresponding peptides was 4% (n = 6). The data were averaged between measurements obtained with two peptides representing each protein. Proteins from retinal lysates or chromatography fractions were separated by SDS/PAGE and gel slices containing CCT subunits or Gβ1 were excised and subjected to in-gel tryptic digestion. Proteolytic peptides were extracted, solubilized in 10–20 μL of 3% acetonitrile/0.1% formic acid, mixed with various amounts of peptide standards, and subjected to LC/MS analysis. Peptide ion chromatograms for the first isotope in the cluster were integrated using MassLynx 4.1 software (Waters) and the peptide amounts were calculated from the ratios between the peak areas of endogenous and standard peptides (for CCT peptides, isotope correction was made to account for the overlap between the second isotope of the endogenous peptide and first isotope of the peptide standard).
Supplementary Material
Acknowledgments
We thank N. Katsanis, A. S. Lewin, T. M. Redmond, J. Lem, and R. K. Crouch for providing mouse lines; P. Ferry-Leeper for assistance with maintaining mouse colonies; Y. Hao for preparing histological specimens; and S. A. Baker, J. N. Pearring, J. N. Kay, and C. Bowes Rickman for critical reading of the manuscript. This work was supported by National Institutes of Health Grants EY10336 (to V.Y.A.) and EY05722 (to Duke University), and an unrestricted grant from Research to Prevent Blindness.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305521110/-/DCSupplemental.
References
- 1.Kosmaoglou M, Schwarz N, Bett JS, Cheetham ME. Molecular chaperones and photoreceptor function. Prog Retin Eye Res. 2008;27(4):434–449. doi: 10.1016/j.preteyeres.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao HY, et al. AAV delivery of wild-type rhodopsin preserves retinal function in a mouse model of autosomal dominant retinitis pigmentosa. Hum Gene Ther. 2011;22(5):567–575. doi: 10.1089/hum.2010.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dryja TP, et al. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990;343(6256):364–366. doi: 10.1038/343364a0. [DOI] [PubMed] [Google Scholar]
- 4.Illing ME, Rajan RS, Bence NF, Kopito RR. A rhodopsin mutant linked to autosomal dominant retinitis pigmentosa is prone to aggregate and interacts with the ubiquitin proteasome system. J Biol Chem. 2002;277(37):34150–34160. doi: 10.1074/jbc.M204955200. [DOI] [PubMed] [Google Scholar]
- 5.Saliba RS, Munro PMG, Luthert PJ, Cheetham ME. The cellular fate of mutant rhodopsin: Quality control, degradation and aggresome formation. J Cell Sci. 2002;115(Pt 14):2907–2918. doi: 10.1242/jcs.115.14.2907. [DOI] [PubMed] [Google Scholar]
- 6.Lin JH, et al. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318(5852):944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorbatyuk MS, et al. Restoration of visual function in P23H rhodopsin transgenic rats by gene delivery of BiP/Grp78. Proc Natl Acad Sci USA. 2010;107(13):5961–5966. doi: 10.1073/pnas.0911991107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mockel A, et al. Pharmacological modulation of the retinal unfolded protein response in Bardet-Biedl syndrome reduces apoptosis and preserves light detection ability. J Biol Chem. 2012;287(44):37483–37494. doi: 10.1074/jbc.M112.386821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemson CM, et al. Therapeutic potential of valproic acid for retinitis pigmentosa. Br J Ophthalmol. 2011;95(1):89–93. doi: 10.1136/bjo.2009.175356. [DOI] [PubMed] [Google Scholar]
- 10.Lobanova ES, et al. Transducin γ-subunit sets expression levels of α- and β-subunits and is crucial for rod viability. J Neurosci. 2008;28(13):3510–3520. doi: 10.1523/JNEUROSCI.0338-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolesnikov AV, et al. G-protein betagamma-complex is crucial for efficient signal amplification in vision. J Neurosci. 2011;31(22):8067–8077. doi: 10.1523/JNEUROSCI.0174-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okae H, Iwakura Y. Neural tube defects and impaired neural progenitor cell proliferation in Gbeta1-deficient mice. Dev Dyn. 2010;239(4):1089–1101. doi: 10.1002/dvdy.22256. [DOI] [PubMed] [Google Scholar]
- 13.Willardson BM, Howlett AC. Function of phosducin-like proteins in G protein signaling and chaperone-assisted protein folding. Cell Signal. 2007;19(12):2417–2427. doi: 10.1016/j.cellsig.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubota S, Kubota H, Nagata K. Cytosolic chaperonin protects folding intermediates of Gbeta from aggregation by recognizing hydrophobic beta-strands. Proc Natl Acad Sci USA. 2006;103(22):8360–8365. doi: 10.1073/pnas.0600195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells CA, Dingus J, Hildebrandt JD. Role of the chaperonin CCT/TRiC complex in G protein betagamma-dimer assembly. J Biol Chem. 2006;281(29):20221–20232. doi: 10.1074/jbc.M602409200. [DOI] [PubMed] [Google Scholar]
- 16.Marrari Y, Crouthamel M, Irannejad R, Wedegaertner PB. Assembly and trafficking of heterotrimeric G proteins. Biochemistry. 2007;46(26):7665–7677. doi: 10.1021/bi700338m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dingus J, Hildebrandt JD. Synthesis and assembly of G protein βγ dimers: Comparison of in vitro and in vivo studies. Subcell Biochem. 2012;63:155–180. doi: 10.1007/978-94-007-4765-4_9. [DOI] [PubMed] [Google Scholar]
- 18.Kabir MA, et al. Functional subunits of eukaryotic chaperonin CCT/TRiC in protein folding. J Amino Acids. 2011;2011:843206. doi: 10.4061/2011/843206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Posokhova E, et al. 2011. Disruption of the chaperonin containing TCP-1 function affects protein networks essential for rod outer segment morphogenesis and survival. Mol Cell Proteomics 10(1):M110.000570.
- 20.Wan Y, et al. Misfolded Gβ is recruited to cytoplasmic dynein by Nudel for efficient clearance. Cell Res. 2012;22(7):1140–1154. doi: 10.1038/cr.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moulédous L, et al. Long-term morphine treatment enhances proteasome-dependent degradation of G beta in human neuroblastoma SH-SY5Y cells: Correlation with onset of adenylate cyclase sensitization. Mol Pharmacol. 2005;68(2):467–476. doi: 10.1124/mol.105.013391. [DOI] [PubMed] [Google Scholar]
- 22.Wójcik C. Role of ubiquitin- and proteasome system in neuronal apoptosis. In: Di Napoli M, Wójcik C, editors. The Ubiquitin Proteasome System in the Central Nervous System: From Physiology to Pathology. New York: Nova Biomedical Books; 2007. pp. 513–536. [Google Scholar]
- 23.Lindsten K, Menéndez-Benito V, Masucci MG, Dantuma NP. A transgenic mouse model of the ubiquitin/proteasome system. Nat Biotechnol. 2003;21(8):897–902. doi: 10.1038/nbt851. [DOI] [PubMed] [Google Scholar]
- 24.Dantuma NP, Lindsten K, Glas R, Jellne M, Masucci MG. Short-lived green fluorescent proteins for quantifying ubiquitin/proteasome-dependent proteolysis in living cells. Nat Biotechnol. 2000;18(5):538–543. doi: 10.1038/75406. [DOI] [PubMed] [Google Scholar]
- 25.Kisselev AF, Goldberg AL. Monitoring activity and inhibition of 26S proteasomes with fluorogenic peptide substrates. Methods Enzymol. 2005;398:364–378. doi: 10.1016/S0076-6879(05)98030-0. [DOI] [PubMed] [Google Scholar]
- 26.Pickering AM, Linder RA, Zhang H, Forman HJ, Davies KJ. Nrf2-dependent induction of proteasome and Pa28αβ regulator are required for adaptation to oxidative stress. J Biol Chem. 2012;287(13):10021–10031. doi: 10.1074/jbc.M111.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Travis GH, Brennan MB, Danielson PE, Kozak CA, Sutcliffe JG. Identification of a photoreceptor-specific mRNA encoded by the gene responsible for retinal degeneration slow (rds) Nature. 1989;338(6210):70–73. doi: 10.1038/338070a0. [DOI] [PubMed] [Google Scholar]
- 28.Sanyal S, Jansen HG. Absence of receptor outer segments in the retina of rds mutant mice. Neurosci Lett. 1981;21(1):23–26. doi: 10.1016/0304-3940(81)90051-3. [DOI] [PubMed] [Google Scholar]
- 29.Lem J, et al. Morphological, physiological, and biochemical changes in rhodopsin knockout mice. Proc Natl Acad Sci USA. 1999;96(2):736–741. doi: 10.1073/pnas.96.2.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee ES, Burnside B, Flannery JG. Characterization of peripherin/rds and rom-1 transport in rod photoreceptors of transgenic and knockout animals. Invest Ophthalmol Vis Sci. 2006;47(5):2150–2160. doi: 10.1167/iovs.05-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiang WC, Messah C, Lin JH. IRE1 directs proteasomal and lysosomal degradation of misfolded rhodopsin. Mol Biol Cell. 2012;23(5):758–770. doi: 10.1091/mbc.E11-08-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menéndez-Benito V, Verhoef LG, Masucci MG, Dantuma NP. Endoplasmic reticulum stress compromises the ubiquitin-proteasome system. Hum Mol Genet. 2005;14(19):2787–2799. doi: 10.1093/hmg/ddi312. [DOI] [PubMed] [Google Scholar]
- 33.Redmond TM, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20(4):344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 34.Cremers FP, van den Hurk JA, den Hollander AI. Molecular genetics of Leber congenital amaurosis. Hum Mol Genet. 2002;11(10):1169–1176. doi: 10.1093/hmg/11.10.1169. [DOI] [PubMed] [Google Scholar]
- 35.Woodruff ML, et al. Spontaneous activity of opsin apoprotein is a cause of Leber congenital amaurosis. Nat Genet. 2003;35(2):158–164. doi: 10.1038/ng1246. [DOI] [PubMed] [Google Scholar]
- 36.Fain GL. Why photoreceptors die (and why they don’t) Bioessays. 2006;28(4):344–354. doi: 10.1002/bies.20382. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz AL, Ciechanover A. Targeting proteins for destruction by the ubiquitin system: Implications for human pathobiology. Annu Rev Pharmacol Toxicol. 2009;49:73–96. doi: 10.1146/annurev.pharmtox.051208.165340. [DOI] [PubMed] [Google Scholar]
- 38.Dantuma NP, Lindsten K. Stressing the ubiquitin-proteasome system. Cardiovasc Res. 2010;85(2):263–271. doi: 10.1093/cvr/cvp255. [DOI] [PubMed] [Google Scholar]
- 39.Ortega Z, et al. Acute polyglutamine expression in inducible mouse model unravels ubiquitin/proteasome system impairment and permanent recovery attributable to aggregate formation. J Neurosci. 2010;30(10):3675–3688. doi: 10.1523/JNEUROSCI.5673-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheroni C, et al. Functional alterations of the ubiquitin-proteasome system in motor neurons of a mouse model of familial amyotrophic lateral sclerosis. Hum Mol Genet. 2009;18(1):82–96. doi: 10.1093/hmg/ddn319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang QA, Figueiredo-Pereira ME. Ubiquitin/proteasome pathway impairment in neurodegeneration: Therapeutic implications. Apoptosis. 2010;15(11):1292–1311. doi: 10.1007/s10495-010-0466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerber SA, Rush J, Stemman O, Kirschner MW, Gygi SP. Absolute quantification of proteins and phosphoproteins from cell lysates by tandem MS. Proc Natl Acad Sci USA. 2003;100(12):6940–6945. doi: 10.1073/pnas.0832254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.