Abstract
Across the Canadian Arctic Archipelago, widespread ice retreat during the 20th century has sharply accelerated since 2004. In Sverdrup Pass, central Ellesmere Island, rapid glacier retreat is exposing intact plant communities whose radiocarbon dates demonstrate entombment during the Little Ice Age (1550–1850 AD). The exhumed bryophyte assemblages have exceptional structural integrity (i.e., setae, stem structures, leaf hair points) and have remarkable species richness (60 of 144 extant taxa in Sverdrup Pass). Although the populations are often discolored (blackened), some have developed green stem apices or lateral branches suggesting in vivo regrowth. To test their biological viability, Little Ice Age populations emerging from the ice margin were collected for in vitro growth experiments. Our results include a unique successful regeneration of subglacial bryophytes following 400 y of ice entombment. This finding demonstrates the totipotent capacity of bryophytes, the ability of a cell to dedifferentiate into a meristematic state (analogous to stem cells) and develop a new plant. In polar ecosystems, regrowth of bryophyte tissue buried by ice for 400 y significantly expands our understanding of their role in recolonization of polar landscapes (past or present). Regeneration of subglacial bryophytes broadens the concept of Ice Age refugia, traditionally confined to survival of land plants to sites above and beyond glacier margins. Our results emphasize the unrecognized resilience of bryophytes, which are commonly overlooked vis-a-vis their contribution to the establishment, colonization, and maintenance of polar terrestrial ecosystems.
Keywords: cryopreservation, subglacial ecosystems
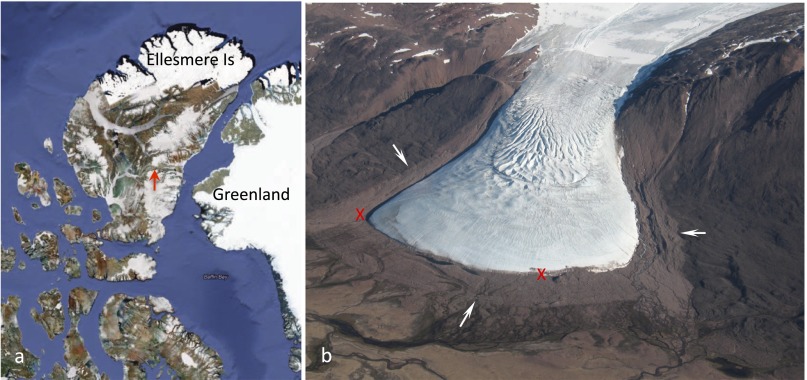
Throughout the Canadian Arctic Archipelago glaciers have undergone widespread retreat during the 20th century, sharply accelerating since 2004 (1, 2). In Sverdrup Pass, central Ellesmere Island, Nunavut, the Teardrop Glacier has retreated ∼200 m from its Little Ice Age limit (LIA, 1550–1850 AD) (Fig. 1). On the glacier foreland, this former margin is marked by a prominent trimline (3): the sharp outer edge of lighter-toned terrain containing sparse vegetation and scattered glacial erratics (Fig. 1B). Within the trimline, the vegetation includes both a chronosequence of recolonizing plants and exhumed, intact assemblages of vascular plants and bryophytes (4–6). The subfossil bryophyte assemblages are in pristine condition, resulting from prior entombment by a nonerosive, cold-based glacier margin, common at high latitudes (7).
Fig. 1.
Location of study site. (A) Map of the Canadian High Arctic and northwest Greenland with ice cover in white. Teardrop Glacier, Sverdrup Pass, Ellesmere Island, Nunavut, is indicated by a red arrow. (B) Oblique aerial view (from the north) of Teardrop Glacier, July 2009. Light-toned perimeter (white arrows) marks the trimline at the limit of the LIA advance. Subglacial samples were collected between the Xs (red) within 10 m of glacial margin.
Glaciers are known to contain diverse, cryophilic microbial communities in supra-, en-, and subglacial environments (8–10). Consequently, ongoing ice retreat in polar environments provides source populations for recolonization of raw terrain, including subglacial microbes (cyanobacteria, eukaryotic microalgae, and bacteria) (11). Viable subglacial ecosystems at the sediment/ice interface have focused on such microbial communities and their DNA signatures (9). In a novel approach to subglacial ecosystems, Willerslev et al. (12) described a diverse flora based on ancient biomolecules (aDNA) extracted from sediments at the base of the Greenland Ice Sheet. In contrast, there has been a limited amount of data that uses subglacial macrofossils. In terms of land plants (bryophytes and vascular plants), most studies of glacial ecosystems have focused on viable populations restricted to communities on supraglacial debris (e.g., refs. 13–15). Since the 1960s, numerous field studies have also reported emergent subglacial vegetation; however, all have concluded that the exhumed material was dead (4, 5, 16–18). Here, we present unique field and experimental evidence for the regeneration of subglacial populations of LIA bryophytes collected from the margin of the retreating Teardrop Glacier, and address the important biological implications for polar environments.
Prior botanical studies in Sverdrup Pass include biological soil crusts, ecological succession, and floristic inventories (extant and subglacial). However, the bryophyte record has not been the focus of a systematic study (4–6, 19–22). In high arctic environments the bryophyte flora is comprised of mosses and hepatics, lacking hornworts. Given that bryophytes form a major component of arctic plant diversity, a thorough investigation is warranted to enhance our understanding of their role in polar terrestrial ecosystems. To complement our research on bryophyte systematics and floristics in Sverdrup Pass, the exhumed assemblages from the Teardrop Glacier foreland were carefully sampled. Their structural preservation is exceptional and indicates ice-entombment under ideal conditions (Fig. 2). For example, gametophytic tissues have stems with intact leaves (often one cell layer thick), specialized stem organelles (e.g., paraphyllia, tomentum, rhizoids), and more rarely, sporophytic tissues consisting of setae or capsules. Furthermore, their occurrence is widespread along ∼1 km of the glacier margin (Fig. 1B). Initial examination in 2007 of exhumed specimens revealed samples with green lateral branches or stem apices that suggested regrowth (or in vivo regeneration). These field observations stimulated three research objectives: (i) to conduct a systematic inventory of the exhumed bryophyte assemblages; (ii) to determine their age; and (iii) to culture (in vitro) subglacial bryophytes to test their potential for regrowth after ice-entombment. These objectives address long-standing hypotheses concerning recolonization of deglaciated landscapes (23, 24) and provide fresh insights on totipotent bryophytes in extreme environments.
Fig. 2.
Subglacial LIA bryophyte populations emerging from Teardrop Glacier margin. (A) Intact population of P. alpinum at glacier margin. (Scale bar, 10 cm.) (B) Corresponding detail of same P. alpinum population (red arrow). (C) Populations of A. turgidum < 1 m from glacier margin. (Scale bar, 20 cm). (D) Corresponding detail of same of A. turgidum (red arrow) showing intact stems and leaves.
Results
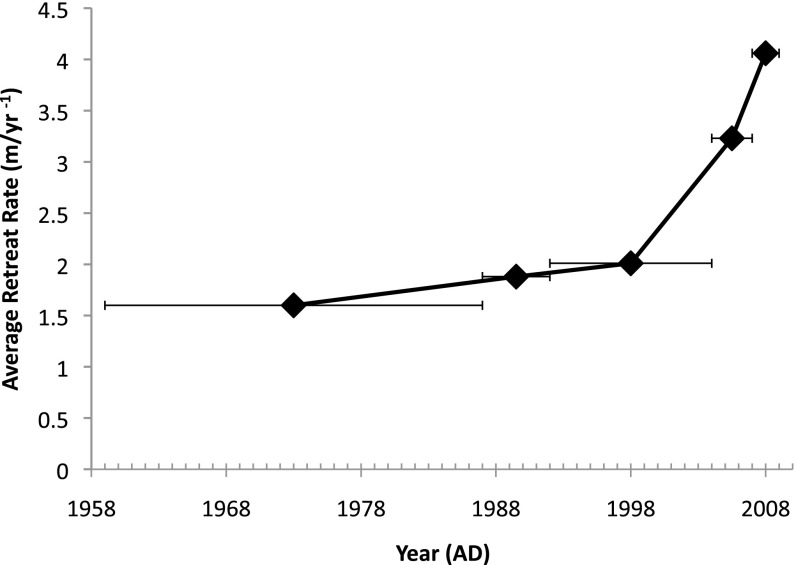
Median radiocarbon dates (2σ) on three subglacial samples from the Teardrop Glacier foreland ranged from 404.5 to 614.5 calibrated years Before Present (cal y BP), documenting LIA assemblages inside the trim-line (Fig.1B and Table S1). Aulacomnium turgidum provides the youngest date, which is the best maximum age of the subsequent entombment by ice. Recent retreat rates of the Teardrop Glacier averaged 3.2 m/y (n = 1) between 2004 and 2007 and 4.1 m/y (n = 1) between 2007 and 2009, documenting rapid exposure of subglacial bryophyte assemblages along the margin (Figs. 2 and 3). These rates double earlier calculations (1.6 ± 0.3 SE m/y, n = 9) made by comparing the 1986 glacier margin to that recorded in the 1959 aerial photograph (19). As well, retreat rates for the 1987–1992 interval averaged 1.88 m/y (n = 1) that increased to 2.01 ± 0.13 SE m/y (n = 7) from 1992–2004 (5). Despite multiple measurements for two of the intervals (n = 7 or 9), ice retreat rates along the entire margin were similar (SE ≤ 0.3 m/y). Since 2004, the accelerated exposure of the foreland coincides with the sharp increase in negative mass balance of glaciers and ice caps across the Canadian Arctic (2).
Fig. 3.
Average retreat rates of Teardrop Glacier, Sverdrup Pass, central Ellesmere Island. Initial rates were determined for intervals of 1959–1986, 1986–1992, 1992–2004, and extended in our study for 2004–2007 and 2007–2009 (see text). Each, averaged interval is represented by a horizontal line with a diamond representing the midpoint. The line of connected diamonds shows the increasing retreat rate. Note the abrupt acceleration in retreat rates post-2004, which coincides with increased rates of net mass loss reported for ice caps from Arctic Canada.
In addition to the radiocarbon dates from the LIA populations, the distinction between extant, colonizing bryophytes and the exhumed subglacial assemblages is conspicuous on recently exposed terrain (Fig. 4). Nonetheless, examination of subglacial samples indicated lateral branch development or apical stem regeneration from LIA populations, suggesting unprecedented regrowth (Fig. 5). In vitro culture assays of the subglacial bryophytes were analyzed and a conservative interpretation of the results has been made. Only cultures that contained the same species present in the original subglacial sample are considered unequivocal examples of regeneration. Furthermore, we excluded all weedy, prolific taxa that occur in the extant flora of the foreland, regardless of whether they are also represented in the LIA sample (Materials and Methods). Based on these criteria, our results indicate regrowth from 11 cultures derived from seven subglacial specimens, representing four distinct taxa (Table S2). Our most consistent regenerate was A. turgidum, which produced seven in vitro cultures from three subglacial specimens (SBG2: D4, D5, D20, J9; SBG27: D7; SBG44: D10, D12) (Fig. 6 B–D). Three additional species, Distichium capillaceum (SBG8: D30), Encalypta procera (SBG–CLF13145: D69) (Fig. 6A), and Syntrichia ruralis (SBG37: D42, D43) were also cultured from parental subglacial samples.
Fig. 4.
Examples of extant, pioneer species growing on exhumed LIA plant material. (A) Extensive populations of LIA A. turgidum used as a colonizing substrate for P. cavifolium (a common weedy species) ∼6 m from glacier margin. (B) P. cavifolium growing on blackened mats of LIA populations ∼10 m from glacier margin.
Fig. 5.
Examples of LIA field specimens showing apparent regrowth (in vivo) of exhumed bryophytes, Ellesmere Island, Nunavut. (A) B. ithyphylla (SBG 43, Sverdrup Pass). (Scale bar, 1.5 cm.) (B) A. turgidum (CLF 13131, Sverdrup Pass). (LIA growth = Scale bar, 4 mm.) (C) R. lanuginosum (CLF 12575, Alexandra Fiord). (Scale bar, 5 mm.) (D) Hygrohypnum polare (CLF 12574, Alexandra Fiord). (Scale bar, 4 mm.)
Fig. 6.
Cultures of LIA bryophytes from the Tear Drop glacial foreland demonstrating regrowth (in vitro). (A) Prolific protonemal gemmae growth and gametophore development from exhumed E. procera material (CLF-13145). (Scale bar, 5 mm.) (B) Dense, pure development of A. turgidum (SBG44–D12). (Scale bar, 2.4 mm.) (C) A. turgidum with D. capillaceum and Ptychostomum sp. (SBG27–D7) growing intermixed. (Scale bar, 15 mm.) (D) Development of gametophore (Inset) of A. turgidum on ground leaf tissue of parent LIA specimen (SBG44–D12). (Scale bar, 1.5 mm.)
Of the regenerated taxa, the hydric species A. turgidum produced the most luxuriant growth in vitro. We emphasize that D. capillaceum, a mesic widespread species, was grown from LIA material chipped from the ice margin and selectively sown as a single species. S. ruralis represents the most xeric member of the regenerated taxa. In the high arctic, it is a widespread and abundant species that is not known to produce sporophytes, and therefore its regrowth is dependent on clonal reproduction. The culture of E. procera is particularly noteworthy, given that specialized asexual propagules (axillary gemmae) were selectively sown from a single stem (Fig. 6A), collected 1.5 m from the ice margin, indicating <1 y of exposure. This LIA specimen contained axillary gemmae that are specifically developed for rapid establishment of new populations. The culture produced new stems with the typical axillary gemmae, and protonema covering the culture substrate with prolific amounts of desiccation resistant, protonemal gemmae. This type of gemmae has not been reported previously for Encalypta and is identified by specialized abscission (tmema) cells. A recent systematic study of Ptychostomum (commonly referred to as Bryum) has shown that the protonemal gemmae are in fact the most ubiquitous type of propagule (vs. rhizoidal tubers, axillary gemmae or bulbils, foliar gemmae) for the genus, but are often overlooked in the field (25). This finding is based on the fact that they occur on the soil, which often is not examined when determining a taxon. The capacity to retain E. procera’s specialized clonal strategy on a temporal scale (i.e., 400 y) demonstrates a remarkable biological advantage within land plants.
Collectively, the paleoecological reconstruction of these subglacial taxa represents a broad range of habitats (hydric to xeric) based on the extant assemblages of Sverdrup Pass, as well as a quantitative study of High Arctic bryophytes (26). These LIA assemblages form either large populations comprised of few species or diverse, mixed populations comparable to the well-established assemblages of Sverdrup Pass. The four regenerated species (above) do not represent weedy, pioneer colonizers on the foreland that are typified by prolific spore production (e.g., Funaria arctica, Psilopilum cavifolium, Leptobryum pyriforme, Ptychostomum spp., and Pohlia spp.) (Fig. 4). The remaining cultures of these subglacial specimens (22 of 32) (Table S2) lacked growth or produced fungal hyphae, green algae, weedy taxa (see above), or local taxa not recorded in the original sample (Materials and Methods). L. pyriforme was recorded as the only contaminant in the control dishes; it grows in Sverdrup Pass, but is also a common greenhouse invader.
The diversity of bryophytes exhumed from beneath the Teardrop Glacier provides a window into the communities present during the LIA. This subglacial flora (60 spp.: 52 mosses, 8 hepatics) (Table S3) is exceptional, representing 42% of the extant flora (144 spp., 133 mosses and 11 hepatics) of Sverdrup Pass based on our current surveys (2007 and 2009). The extant bryophyte flora also exceeds the reported lichen (115 spp.), algal (84 spp.), and cyanobacteria (52 spp.) floras of Sverdrup Pass (21, 22), emphasizing the prominence of bryophytes within the modern Canadian arctic floras. Furthermore, the subfossil species richness approaches that of the extant vascular plants (75 spp.) within Sverdrup Pass (20).
Discussion
The uniqueness of this study is the regeneration of exhumed LIA bryophytes after 400 y of ice entombment, demonstrating their remarkable biological resilience in the terrestrial ecology of polar environments. Often ignored in ecosystem studies, this ancient (470 Ma) group of land plants has developed unique evolutionary solutions (27, 28). Bryophytes are predominantly clonal organisms with cells that are: (i) totipotent: the capacity of a living cell to dedifferentiate into a meristematic state that can then reprogram the cell for development of the organism (29, 30); and (ii) poikilohydric: lacking the ability to control water content. Hence, bryophyte cells can shut down physiologically during desiccation and revive when conditions are favorable (31). Together, these attributes enable fragmentary gametophytic (i.e., leaf, rhizoid, stem) or more rarely sporophytic (i.e., seta, capsule) tissue to regenerate new plants initiated from dedifferentiated cells. Recent genomic research on Physcomitrella patens suggests a genetic basis for totipotent cells, which have a similar function to stem cells in faunal organisms (32). The ability of exhumed bryophyte tissue (gametophytic) to regenerate emphasizes their successful adaptation to extreme polar environments.
Bryophytes, constrained by their structural and reproductive simplicity, are not just reduced vascular plants but have evolved a unique course in evolution. For example, Pleistocene (31,800 y BP) seed (sporophytic) tissue of Silene stenophylla from Siberian permafrost (20–40 m in depth) was recently cultured (33). However, this process required that placental tissue from the seeds was first extracted, carefully cloned, and then grown on specialized nutrient media (in vitro) to stimulate development of adventitious shoots. In contrast, regeneration of subglacial bryophytes from Teardrop Glacier was accomplished by simply grinding gametophytic tissue (primarily stems and leaves) and sowing it on potting soil or growth media, followed by frequent watering (Materials and Methods). Our culture experiments are reinforced by field observations of in situ samples. For example, Bartramia ithyphylla—collected within 0.59 m of the ice margin that represents <1 y of exposure—showed spontaneous regeneration (Fig. 5A). This result further supports the biological capacity of bryophyte tissue for cellular dedifferentiation and clonal reproduction after ice entombment. In contrast, regeneration of vegetative (sporophytic) tissue of vascular plants occurring with the bryophytes at Teardrop Glacier has not been reported or suggested.
An early, salient study at Tiger Ice Cap, Baffin Island, Nunavut, documented LIA populations of the moss Polytrichum juniperinum emerging from the ice margin (16). Falconer (16) further stated that: “Outside the estimated position of the 1949 margin vigorous new moss shoots appear in places to be growing directly out of the underlying dead moss.” He concluded that the new growth was a result of germination of either dormant or migrant spores on the “dead moss mats.” Although Falconer cites earlier observations of “apparently living” exhumed vegetation, his interpretation that the LIA populations were dead has been the consensus of subsequent studies (4–6, 17, 18). For example, Anderson et al. (18) also investigated ice caps on northern Baffin Island, reporting that “new growth was observed to occur on previously dead and buried vegetation only meters beyond the ice margin.” A more specific biological investigation of vegetation radiocarbon dated to the LIA tested chlorophyll activity of emergent, green plants from Twin Glacier, Alexandra Fiord, 90 km east of Sverdrup Pass (17). The moss Racomitrium lanuginosum retained the highest chlorophyll content (55%) relative to extant populations; however, their observations indicated that rapid degradation of the chlorophyll content occurred after a few days. What is clear from previous studies on emergent vegetation is that the paleomaterial is considered subfossil (or dead); hence, it could only serve as an organic substrate for subsequent colonization.
Given their structural simplicity and adaptation to diverse microhabitats, bryophytes are ideal candidates for biological experiments, especially in extreme environments. Our contribution demonstrates that bryophytes buried by ice 400 y ago can remain dormant and provide an unrecognized pathway for recolonization of deglaciated terrains (recent and ancient). Of the cultures containing parental material, 30% were successful in producing regenerated species. Based on these results, landscapes exposed by ice retreat should no longer be assumed to be barren (tabula rasa) of land plants (Embryophyta: bryophytes and vascular plants). Biotic sources for recolonization must include contributions from subglacial environments, as well as traditionally recognized refugia above and beyond the glacial margins. Furthermore, the viability of cryopreserved vegetation should not be temporally limited to LIA material, amplifying the implications of our experiments. Regrowth of bryophytes after ice entombment is relevant to understanding basic life systems, including biological development in extraterrestrial environments (34) and evolution of plant terrestrialization (35). In a world of diminishing biological diversity, our study shows that preservation of subglacial bryophytes serves as an unrecognized genetic reservoir that manifests the resilience of land plants and the emerging richness of glacial ecosystems as polar glaciers retreat.
Materials and Methods
Ice Retreat Rate.
Glacial retreat rates for the Teardrop Glacier have been reported for the past several decades. Our study used the 12-m stake established by Breen and Lèvesque in 2004 as the benchmark to calculate more recent retreat rates (5). Furthermore, two new stakes were placed 5 m from the ice margin, one in 2007 and the other in 2009, each with GPS reference. These surveys indicate a clear increase in the retreat rates, reaching 3.2 m/y (n = 1) from 2004 to 2007 and 4.1 m/y (n = 1) from 2007 to 2009 (Fig. 3). On July 1, 2009, 23 GPS way-points were established to document the Teardrop Glacier margin (1.2-km-wide) for future reference.
Subglacial Sample Collections and Preparation.
During the 2007 and 2009 field seasons, 140 LIA samples were collected within 10 m of the glacial margin where the distinction between colonizing species and subglacial assemblages was unequivocal (Figs. 2 and 4). This distance from the glacier margin is equivalent to 2.6-y exposure time, based on our retreat rates. Exhumed LIA subglacial material is comprised of large discolored or blackened populations that are sometimes diverse, containing up to 15 species. In contrast, pioneer or colonizing species observed within 10 m of the glacier margin are either distinct from the LIA substrate species, or form sparse, homogeneous populations on raw, deglaciated substrates that lack assemblage diversity, given the short time for establishment. The growing season is restricted to 12–16 wk/y (May through August) depending on microhabitat, limiting the development rate (size) of any colonizing population. A subset of 24 selected samples for the culture experiments were collected within 5.7 m from the glacier margin, which represents 1.4-y exposure time (Fig. 3). Some samples were excavated directly from the ice. If wet, the subglacial samples were collected and placed in polyethylene Nasco Whirl-Pak* bags (Fisher Scientific), or if dry, placed in 2-lb Standard Kraft paper bags, then stored in a cooler until frozen in Resolute Bay (Cornwallis Island, Nunavut) for transport south. The specimens were kept frozen for subsampling.
In the laboratory, field specimens were identified and examined for evidence of in vivo regrowth with compound and dissecting microscopes (Fig. 5). To further test the potential regeneration of subglacial bryophytes, we conducted multiple in vitro cultures. Growth-chamber experiments were assayed on 24 subglacial specimens (Fig. 6). Subsamples consisted of either individual stems or stems with some of the original substrate. These subsamples were ground and sown onto solid nutrient medium (below) in sterilized jars or onto potting soil/soil-sand in Petri dishes (36). Duplicate subsamples were sown to test the consistency of results. Vouchers of each subglacial species were deposited in the Cryptogamic Herbarium (ALTA), Department of Biological Sciences, University of Alberta, Edmonton, AB, Canada.
The majority of subglacial specimens consisted of large populations of taxa, not representing the early pioneer (colonizing) species in the foreland today (see species in text). The life strategies of these pioneer taxa are conspicuously shortened and typically prolific. In contrast, the LIA samples represent later, successional species on the foreland >20 m from the glacier margin that have had >5 y of establishment and growth (e.g., Niphotrichum panschii, A. turgidum, Hylocomium splendens, Sanionia uncinata, Polytrichastrum alpinum). Two species, Pohlia cruda and P. cavifolium, were regenerated from subglacial samples containing parental material, but these data are considered equivocal, given that they are weedy species present in the extant bryoflora of the foreland.
Bryophyte diaspore banks play a critical role in ecosystem maintenance and warrant consideration in the interpretation of our results. In situ diaspores include bryophyte propagules produced by former generations. As an assemblage develops or changes, different diaspores accumulate in the substrate and can remain dormant until environmental conditions stimulate germination. These biological stores are well-known components of floristic diversity (e.g., refs. 37–39). For example, taxa from earlier successional stages can be stored and germinate when conditions are favorable, despite their absence in the extant communities (40). In our culture assays we not only exclude weedy species but also species not identified in the original LIA sample, which could be because of diaspores of unknown age (extant or LIA).
Subglacial Sample Age.
To constrain the age of the emergent subglacial vegetation, three samples were selected for radiocarbon (14C) dating on individual fragments using Accelerator Mass Spectrometry (AMS). The three AMS 14C dates were determined on two subglacial moss samples (A. turgidum and B. ithyphylla) collected within 40 cm from glacier margin, and a woody stem (Salix arctica) collected within 180 cm of the ice margin. Subsamples from these original specimens were identified using dissecting and compound microscopes, rinsed in doubled-distilled H2O, then wrapped in tinfoil. Care was taken that the selected material showed no indication of regrowth. The submitted moss samples included the stem apices of each taxon to ensure that the youngest material was dated. The youngest date from the three samples provides the best, maximum limiting age on subsequent ice entombment. Samples were submitted to W. M. Keck Carbon Cycle Accelerator Mass Spectrometry Laboratory, University of California at Irvine. All dates were calibrated using CALIB 6.0 (41). The ages presented in the text use a 2σ range, from which a median value was calculated. The median calendar age, expressed as both Cal BP and Cal AD is summarized in Table S1.
Laboratory Experiments in Growth Chamber.
In vitro cultures were grown under controlled conditions with a mean irradiance of 74 ± 9.3 SD μmol−2⋅s−1, simulating 16 h of daylight and 8 h of darkness with 215 W cool-white fluorescent bulbs, with temperature maintained at 15 °C. All samples were placed in shallow trays and repositioned every 30 d to reduce effects of any variations in light intensity within the chamber. Double-distilled milliQ water, used for hydrating the specimens, was autoclaved for 20 min at 121 °C. Sample hydration was maintained by misting each specimen with a hand-held spray bottle every 2–3 d throughout the 12 mo in the growth chamber. Regeneration of subglacial samples on soil with misting was more successful than in sealed jars on nutrient medium, which was susceptible to desiccation during long-term growth.
A total of 32 sterilized Petri dishes (60 or 100 mm in diameter, 15-mm deep) contained soil substrates for the cultures (37). The dishes were filled 7-mm deep with premium commercial potting soil, consisting of peat moss, humus, compost, and perlite (sterile CIL-Gro, weed-free). A different substrate was assayed for taxa with mesic to xeric habitat preferences, consisting of 50% potting soil and 50% sterilized sand. The ground bryophyte material was seeded on substrates with different start dates between July 6, 2011 and March 9, 2012. The growth chamber assays were ended July 26, 2012 (Table S2).
An additional assay used six glass jars filled with sterilized nutrient medium. The cultures consisted of White’s Basal Salt nutrient medium (0.45 g) solidified with Phytagel (7.5g) (Sigma) dissolved in 500 mL of double-distilled milliQ water. The nutrient medium was autoclaved for 20 min at 121 °C, then 40 mL was poured into 100-mL glass tissue-culture jars with vented Magenta B-cap closures (Sigma), then sealed with Parafilm “M” (Bemis) until the bryophyte samples were sown and the jars resealed. These samples were not misted during the trials given the closed, but breathable systems.
Throughout the experiment, all substrate types (nutrient medium, potting soil and potting soil/sand) were tested for contaminants. A total of 17 controls (six dishes and one jar) were placed in the growth chamber to test for contaminants during the growth experiment. Initially, three unautoclaved dishes with potting soil and one jar with growth medium were placed in the growth chamber (July 2011). Three dishes with potting soil and six with a mixture of half potting soil and half sand were autoclaved and placed in the growth chamber as additional control samples (January 2012). An additional four, unautoclaved control dishes were added in January (2012) for the mixed substrate dishes.
Supplementary Material
Acknowledgments
We thank D. Wilkie for assistance in the field; D. Haughey, L. M. Heaman, and R. S. Bradley for constructive comments on earlier versions of the manuscript; and two formal anonymous reviews for additional critiques. Logistical support for fieldwork was provided by the Polar Continental Shelf Program, Natural Resources Canada, Ottawa; funding was provided by Natural Sciences and Engineering Research Council Discovery Grant G121211007 (to C.L.F.) and Natural Sciences and an Engineering Research Council Northern Research Chair (J.H.E.); and additional support was provided by the Canadian Circumpolar Institute, University of Alberta, Edmonton.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.H.V. is a guest editor invited by the Editorial Board.
Data deposition: Voucher specimens are deposited in the Cryptogamic Herbarium (ALTA), Department of Biological Sciences, University of Alberta, Edmonton T6G 2E9.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1304199110/-/DCSupplemental.
References
- 1.Kaufman DS, et al. Arctic Lakes 2k Project Members Recent warming reverses long-term arctic cooling. Science. 2009;325(5945):1236–1239. doi: 10.1126/science.1173983. [DOI] [PubMed] [Google Scholar]
- 2.Gardner AS, et al. Sharply increased mass loss from glaciers and ice caps in the Canadian Arctic Archipelago. Nature. 2011;473(7347):357–360. doi: 10.1038/nature10089. [DOI] [PubMed] [Google Scholar]
- 3.Wolken GJ, England JH, Dyke AS. Re-evaluating the relevance of vegetation trimlines in the Canadian Arctic as an indicator of Little Ice Age paleoenvironments. Arctic. 2005;58(4):342–353. [Google Scholar]
- 4.Jones GA, Henry GHR. Primary plant succession on recently deglaciated terrain in the Canadian High Arctic. J Biogeogr. 2003;30(2):277–296. [Google Scholar]
- 5.Breen K, Lèvesque E. Proglacial succession of biological soil crusts and vascular plants: Biotic interactions in the High Arctic. Can J Bot. 2006;84(11):1714–1731. [Google Scholar]
- 6.Breen K, Lèvesque E. The influence of biological soil crusts on soil characteristics along a High Arctic glacier foreland, Nunavut, Canada. Arct Antarct Alp Res. 2008;40(2):287–297. [Google Scholar]
- 7.Dyke AS. Landscapes of cold-centred late Wisconsinan ice caps, Arctic Canada. Prog Phys Geogr. 1993;17(2):223–247. [Google Scholar]
- 8.Bhatia M, Sharp M, Foght JM. Distinct bacterial communities exist beneath a high Arctic polythermal glacier. Appl Environ Microbiol. 2006;72(9):5838–5845. doi: 10.1128/AEM.00595-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodson A, et al. Glacial ecosystems. Ecol Monogr. 2008;78(1):41–67. [Google Scholar]
- 10.Humlum O, et al. Late-Holocene glacier growth in Svalbard, documented by subglacial relict vegetation and living soil microbes. Holocene. 2005;15(3):396–407. [Google Scholar]
- 11.Kastovská K, Elster J, Stibal M, Santrůcková H. Microbial assemblages in soil microbial succession after glacial retreat in Svalbard (high arctic) Microb Ecol. 2005;50(3):396–407. doi: 10.1007/s00248-005-0246-4. [DOI] [PubMed] [Google Scholar]
- 12.Willerslev E, et al. Ancient biomolecules from deep ice cores reveal a forested southern Greenland. Science. 2007;317(5834):111–114. doi: 10.1126/science.1141758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fickert T, Friend D, Grüninger F, Molnia B, Richter M. Did debris-covered glaciers serve as Pleistocene refugia for plants? A new hypothesis derived from observations of recent plant growth on glacier surfaces. Arct Antarct Alp Res. 2007;39(2):245–257. [Google Scholar]
- 14.Porter PR, Evans AJ, Hodson AJ, Lowe AT, Crabtree MD. Sediment-moss interactions on a temperate glacier: Falljökull, Iceland. Ann Glaciol. 2008;48(1):25–32. [Google Scholar]
- 15.Pelfini M, et al. The influence of glacier surface processes on the short-term evolution of supraglacial tree vegetation: The case study of the Miage Glacier, Italian Alps. Holocene. 2012;22(8):847–856. [Google Scholar]
- 16.Falconer G. Preservation of vegetation and patterned ground under thin ice body in northern Baffin Island, N.W.T. Geogr Bull. 1966;8(2):194–200. [Google Scholar]
- 17.Bergsma BM, Svoboda J, Freedman B. Entombed plant communities by a retreating glacier at central Ellesmere Island, Canada. Arctic. 1984;37(1):49–52. [Google Scholar]
- 18.Anderson RK, Miller GH, Briner JP, Lifton NA, DeVogel SB. A millennial perspective on Arctic warming from 14C in quartz and plants emerging from beneath ice caps. Geophys Res Lett. 2008;35:L01502. [Google Scholar]
- 19.Fahselt D, Maycock PF, Svoboda J. Initial establishment of saxicolous lichens following recent glacial recession in Sverdrup Pass, Ellesmere Island, Canada. Lichenologist. 1988;20(3):253–268. [Google Scholar]
- 20.Bergeron JF, Svoboda J. Plant communities of Sverdrup Pass, Ellesmere Island, N.W.T. Musk-Ox. 1989;37:76–85. [Google Scholar]
- 21.Maycock PF, Fahselt D. Vegetation of stressed calcareous screes and slopes in Sverdrup Pass, Ellesmere Island, Canada. Can J Bot. 1992;70(12):2359–2377. [Google Scholar]
- 22.Elster J, Lukesova A, Svoboda J, Kopecky J, Kanda H. Diversity and abundance of soil algae in the polar desert, Sverdrup Pass, central Ellesmere Island. Polar Rec (Gr Brit) 1999;35(194):231–254. [Google Scholar]
- 23.Fernald ML. Persistence of plants in unglaciated areas of boreal America. Mem Am Acad Arts Sci. 1925;15(3):239–342. [Google Scholar]
- 24.Brochmann C, Gabrielsen TM, Nordal I, Landvik JY, Elven R. Glacial survival or tabula rasa? The history of North Atlantic biota revisited. Taxon. 2003;52(3):417–450. [Google Scholar]
- 25.Pressel S, Matcham HW, Duckett JG. Studies of protonemal morphogenesis in mosses. XI. Bryum and allied genera: A plethora of propagules. J Bryol. 2007;29(4):241–258. [Google Scholar]
- 26.La Farge-England C. The contemporary moss assemblages of a high arctic upland, Northern Ellesmere Island, N.W.T. Can J Bot. 1989;67(2):491–504. [Google Scholar]
- 27.Kendrick P, Crane PR. The Origin and Early Diversification of Land Plants: A Cladistic Study. Washington, DC: Smithsonian Institution; 1997. [Google Scholar]
- 28.Shaw AJ, Szövényi P, Shaw B. Bryophyte diversity and evolution: Windows into the early evolution of land plants. Am J Bot. 2011;98(3):352–369. doi: 10.3732/ajb.1000316. [DOI] [PubMed] [Google Scholar]
- 29.Menon MKC, Lal M. Problems of development in mosses and moss allies. Proc Natl Acad Sci USA. 1981;B47(1):115–152. [Google Scholar]
- 30.Lal M. In: The Experimental Biology of Bryophytes. Dyer AF, Duckett JG, editors. London: Academic; 1984. pp. 97–115. [Google Scholar]
- 31.Proctor MC, Tuba Z. Poikilohydry and homoihydry: Antithesis or spectrum of possibilities? New Phytol. 2002;156(3):327–349. doi: 10.1046/j.1469-8137.2002.00526.x. [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa M, et al. Physcomitrella cyclin-dependent kinase A links cell cycle reactivation to other cellular changes during reprogramming of leaf cells. Plant Cell. 2011;23(8):2924–2938. doi: 10.1105/tpc.111.088005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yashina S, et al. Regeneration of whole fertile plants from 30,000-y-old fruit tissue buried in Siberian permafrost. Proc Natl Acad Sci USA. 2012;109(10):4008–4013. doi: 10.1073/pnas.1118386109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kern VD, et al. Gravitropic moss cells default to spiral growth on the clinostat and in microgravity during spaceflight. Planta. 2005;221(1):149–157. doi: 10.1007/s00425-004-1467-3. [DOI] [PubMed] [Google Scholar]
- 35.Kenrick P, Wellman CH, Schneider H, Edgecombe GD. A timeline for terrestrialization: Consequences for the carbon cycle in the Palaeozoic. Philos Trans R Soc Lond B Biol Sci. 2012;367(1588):519–536. doi: 10.1098/rstb.2011.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaw AJ. A new approach to the experimental propagation of bryophytes. Taxon. 1986;35(4):671–675. [Google Scholar]
- 37.During H. Bryophyte diaspore banks. Adv Bryol. 1997;6(6):103–134. [Google Scholar]
- 38.During H. Diaspore banks. Bryologist. 2001;104(1):92–97. [Google Scholar]
- 39.Hock Z, Szövényi P, Schneller JJ, Tóth Z, Urmi E. Bryophyte diaspore bank: A genetic memory? Genetic structure and genetic diversity of surface populations and diaspore bank in the liverwort Mannia fragrans (Aytoniaceae) Am J Bot. 2008;95(5):542–548. doi: 10.3732/ajb.2007283. [DOI] [PubMed] [Google Scholar]
- 40.Caners RT, Macdonald SE, Belland RJ. Recolonization potential of bryophyte diaspore banks in harvested boreal mixed-wood forest. Plant Ecol. 2009;204:55–68. [Google Scholar]
- 41.Stuiver M, Reimer PJ, Reimer RW. 2010. CALIB 6.0. Available at http://calib.qub.ac.uk/calib/. Accessed April 13, 2011.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.