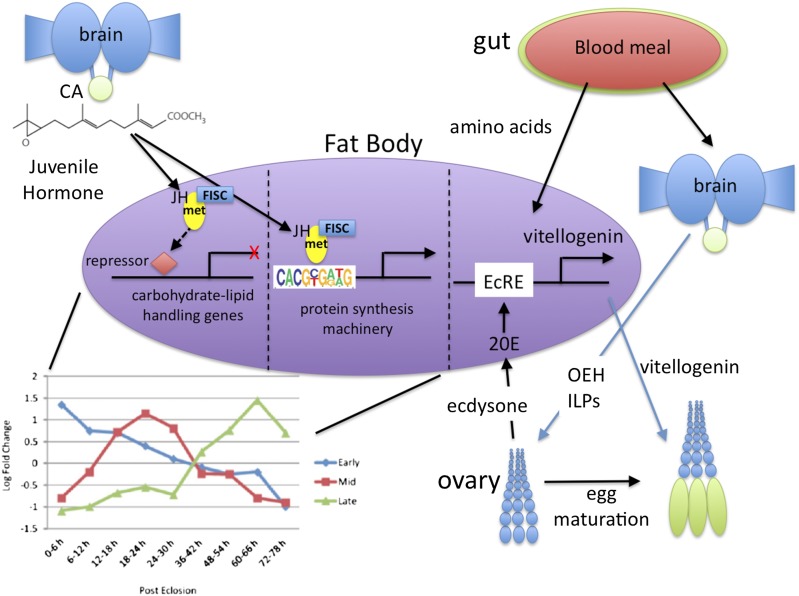
Mosquitoes are vectors of some of the world’s most devastating human diseases: malaria, dengue fever, and yellow fever in the tropics, and encephalitis and West Nile virus in the United States. The female mosquito usually requires a blood meal before she makes a batch of eggs, and one for each subsequent batch thereafter. This repeated blood feeding makes her an exceptional disease vector, picking up a pathogen with one blood meal and then transmitting it on successive meals. After emergence (eclosion) as an adult, the female must mature before she can produce eggs in response to the blood meal. This maturation is initiated by an insect-specific juvenile hormone (JH), a sesquiterpenoid that acts on the fat body (the liver and the adipose tissue of the insect) to increase both its metabolic machinery and its protein-synthesizing capacity so that it can synthesize the yolk protein vitellogenin in response to the blood meal (Fig. 1) (1). JH also directs previtellogenic development of the ovary. In PNAS, Zou et al. (2) show, using microarray analysis, that JH controls the maturation of the fat body of the yellow fever, dengue mosquito Aedes aegypti in a time-dependent manner by regulating three major gene clusters. Early-posteclosion (EPE) gene expression peaks at 6 h, mid-posteclosion (MPE) genes at 24 h, and late-posteclosion (LPE) genes at 66 h after eclosion (Fig. 1). These times correspond to low, intermediate, and high JH titers because JH secretion begins at eclosion and peaks at 36 h (3). The EPE and MPE genes are primarily involved in carbohydrate and lipid metabolism, whereas the late genes are primarily for the buildup of the protein-synthesizing machinery based on gene ontology, as discussed below.
Fig. 1.
Diagram of the hormonal control of reproductive maturation in the female mosquito. The corpora allata (CA) secrete JH III that causes posteclosion development of the fat body by regulating the expression of three gene clusters, as portrayed in figure 1C from Zou et al. (2). The early and middle gene clusters regulate carbohydrate and lipid metabolism and are suppressed by JH, whereas the late genes are activated by JH and the JH receptor Met, binding directly to the genes via the consensus sequence shown (from figure 4A of ref. 2). These genes make the protein synthesizing machinery so that after the blood meal the fat body responds to the ecdysteroid 20-hydroxyecdysone (20E) binding with Ultraspiracle (USP) to the ecdysone response element (EcRE) and activating vitellogenin production for yolk in the eggs. The blood meal provides amino acids for this production and signals the brains to secrete the neuropeptides ovarian ecdysteroidogenic hormone (OEH) and several insulin-like peptides (ILPs), which cause the ovarian follicle cells to secrete ecdysone that is converted to the active 20E in the fat body and other peripheral tissues. [Figure prepared by James W. Truman (Howard Hughes Medical Institute, Ashburn, VA).]
Insects have an open circulatory system and breathe through a series of tracheal tubes that bring oxygen to every cell and get rid of waste carbon dioxide, so they can live perfectly well when headless or even as an isolated abdomen. Zou et al. (2) isolated abdomens just after eclosion to deprive the mosquitoes of JH, which is secreted by a gland at the base of the head (Fig. 1). In these abdomens 24 h later, representative EPE gene transcripts were increased, whereas those of the LPE genes were suppressed. When these abdomens were treated with JH III, the natural JH of mosquitoes (3), the EPE genes were suppressed and the LPE genes activated. Thus, JH suppressed at least a subset of the early genes and activated a subset of the late genes.
To determine how JH was regulating these genes, the authors took advantage of the recent identification of the JH receptor as the basic helix–loop–helix (bHLH) protein, Methoprene-tolerant (Met) (4, 5), a member of the Period (Per)-Aryl hydrocarbon nuclear translocator (Arnt)-Singleminded (Sim) (PAS) domain family of transcription factors. JH binds to a Met homo- or heterodimer, which then binds with another bHLH-PAS domain protein such as the steroid receptor coactivator FISC (βFTZ-F1 interacting steroid receptor coactivator) (6) or Cycle (7) in mosquitoes and Taiman in Drosophila. When Met RNAi was injected shortly after eclosion, Met was suppressed and no ovarian development was seen 72 h later at the end of the posteclosion period, similarly to mosquitoes deprived of JH (8). By exposing the fat body from these Met RNAi-treated mosquitoes to JH in culture, Zou et al. (2) show that the subsets of early and late genes described above were unchanged, indicating that JH was acting through Met. In contrast, the fat body exposed to the control Luc RNAi responded normally to JH, such that the EPE genes were suppressed and the LPE genes were activated. When the transcriptome of the Met RNAi-treated fat bodies was analyzed, 1,385 genes were up-regulated (including 27% of the EPE genes and 40% of the MPE genes) and 1,169 were down-regulated (including 36% of the LPE genes), with fewer than 5% of the subsets being suppressed or activated, respectively. The ontology of these genes placed them into 13 functional groups, with the EPE and MPE genes primarily involved in carbohydrate and lipid metabolism and xenobiotic degradation, whereas the majority of the LPE genes were involved in translation, transcription, DNA replication and repair, and folding and sorting. Genes involved in amino acid metabolism were found in both sets.
Previous studies in the mosquito (6) and the commercial silkworm Bombyx mori (9) had identified similar Met DNA binding motifs in the promoters of the early trypsin and Krüppel-homolog 1 genes, respectively, which were necessary for direct up-regulation of the respective genes by the JH-Met-coactivator complex. Zou et al. (2) identified a consensus sequence for the JH-Met–regulated LPE genes (Fig. 1) and validated it by gel-shift assays with fat body nuclear extracts. This consensus sequence is similar to the Met DNA binding motifs seen in the other directly JH-regulated genes above, and more importantly to the E box motif bound by other bHLH transcription factors. Only the
Zou et al. identified a consensus sequence for the JH-Met–regulated LPE genes and validated it by gel-shift assays with fat body nuclear extracts.
fifth position in the nine-base motif shows degeneracy. It will be interesting to know how this motif agrees with those in promoters of genes in other insects that are directly regulated by JH.
These studies clearly show that JH is acting via Met to regulate posteclosion development of the fat body and has dual roles. (i) The EPE and MPE gene clusters are active when the JH titer is low and then are suppressed by the rising JH. This suppression appears to be indirect, likely requiring synthesis of repressive transcription factors that then act on these genes. One candidate is the known repressive bHLH transcription factor Hairy, which is known to be activated by the JH-Met-Cycle complex in the mosquitoes (7). How Hairy might suppress these genes is not known. (ii) The LPE genes appear when the JH titer is high and are activated directly by the JH-Met-FISC complex. The genes are then involved in producing the protein synthesis machinery necessary for vitellogenesis after the blood meal brings in the amino acids necessary for yolk protein production. This blood meal also triggers the release of the ovary ecdysteroidogenic hormone and several insulin-like peptides from the brain to stimulate ecdysone synthesis by the ovaries (1, 10) (Fig. 1). Ecdysone then is converted by the fat body to the active hormone 20-hydroxyecdysone to activate vitellogenin synthesis in the fat body. More studies are necessary to work out the details of this fascinating dual coordinating role of JH in the fat body development of the mosquito. The complex regulation of reproductive maturation in blood-feeding female mosquitoes offers new avenues that might be exploited in the control of this important disease vector.
Footnotes
The author declares no conflict of interest.
See companion article on page E2173.
References
- 1.Raikhel AS. Vitellogenesis of disease vectors, from cell biology to genes. In: Beaty BJ, Marquardt W, editors. Biology of Vectors. New York: Elsevier, Academic; 2004. pp. 329–346. [Google Scholar]
- 2.Zou Z, et al. Juvenile hormone and its receptor, methoprene-tolerant, control the dynamics of mosquito gene expression. Proc Natl Acad Sci USA. 2013;110:E2173–E2181. doi: 10.1073/pnas.1305293110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro AB, et al. Juvenile hormone and juvenile esterase in adult females in Aedes aegypti. J Insect Physiol. 1986;32(10):867–877. [Google Scholar]
- 4.Charles JP, et al. Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc Natl Acad Sci USA. 2011;108(52):21128–21133. doi: 10.1073/pnas.1116123109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jindra M, Palli SR, Riddiford LM. The juvenile hormone signaling pathway in insect development. Annu Rev Entomol. 2013;58:181–204. doi: 10.1146/annurev-ento-120811-153700. [DOI] [PubMed] [Google Scholar]
- 6.Li M, Mead EA, Zhu J. Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proc Natl Acad Sci USA. 2011;108(2):638–643. doi: 10.1073/pnas.1013914108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin SW, Zou Z, Saha TT, Raikhel AS. bHLH-PAS heterodimer of methoprene-tolerant and Cycle mediates circadian expression of juvenile hormone-induced mosquito genes. Proc Natl Acad Sci USA. 2012;109(41):16576–16581. doi: 10.1073/pnas.1214209109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raikhel AS, Lea AO. Hormone-mediated formation of the endocytic complex in mosquito oocytes. Gen Comp Endocrinol. 1985;57(3):422–433. doi: 10.1016/0016-6480(85)90224-2. [DOI] [PubMed] [Google Scholar]
- 9.Kayukawa T, et al. Transcriptional regulation of juvenile hormone-mediated induction of Krüppel homolog 1, a repressor of insect metamorphosis. Proc Natl Acad Sci USA. 2012;109(29):11729–11734. doi: 10.1073/pnas.1204951109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen Z, et al. Two insulin-like peptide family members from the mosquito Aedes aegypti exhibit differential biological and receptor binding activities. Mol Cell Endocrinol. 2010;328(1–2):47–55. doi: 10.1016/j.mce.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]