Abstract
A DNA macroarray was developed and evaluated for its potential to distinguish variants of the dinitrogenase reductase (nifH) gene. Diverse nifH gene fragments amplified from a clone library were spotted onto nylon membranes. Amplified, biotinylated nifH fragments from individual clones or a natural picoplankton community were hybridized to the array and detected by chemiluminescence. A hybridization test with six individual targets mixed in equal proportions resulted in comparable relative signal intensities for the corresponding probes (standard deviation, 14%). When the targets were mixed in unequal concentrations, there was a predictable, but nonlinear, relationship between target concentration and relative signal intensity. Results implied a detection limit of roughly 13 pg of target ml−1, a half-saturation of signal at 0.26 ng ml−1, and a dynamic range of about 2 orders of magnitude. The threshold for cross-hybridization varied between 78 and 88% sequence identity. Hybridization patterns were reproducible with significant correlations between signal intensities of duplicate probes (r = 0.98, P < 0.0001, n = 88). A mixed nifH target amplified from a natural Chesapeake Bay water sample hybridized strongly to 6 of 88 total probes and weakly to 17 additional probes. The natural community results were well simulated (r = 0.941, P < 0.0001, n = 88) by hybridizing a defined mixture of six individual targets corresponding to the strongly hybridizing probes. Our results indicate that macroarray hybridization can be a highly reproducible, semiquantitative method for assessing the diversity of functional genes represented in mixed pools of PCR products amplified from the environment.
The biochemical transformations of nitrogen have a major influence over biological productivity on Earth. The many pathways of the nitrogen cycle (nitrification, denitrification, ammonification, and nitrogen fixation) form a web of redox reactions catalyzed by specific groups of microorganisms. Biological nitrogen fixation, the enzyme-catalyzed reduction of dinitrogen (N2) to ammonium, is essential for maintaining fertility in many ecosystems (28) by making nitrogen available from the large gaseous atmospheric reservoir of N2, which is not directly accessible by eukaryotes or by many prokaryotes. Nitrogenase, the enzyme responsible for catalysis, is found in phylogenetically diverse groups of prokaryotes. In recent years, the molecular sequences of nitrogenase genes have been used to investigate the diversity of diazotrophs in marine (36), freshwater (13), estuarine (1), salt marsh (3, 11), hypersaline (24), and terrestrial (29) environments, including specialized habitats such as termite guts (9, 16). These studies have shown that there are diverse nitrogenase genes in natural environments and suggest that communities of N2-fixing microorganisms differ markedly among habitats (33).
Investigations of nitrogenase genes in the environment initially used PCR amplification with subsequent cloning and sequencing to catalog sequence diversity (17, 27, 34, 35). Analysis of clone libraries by exhaustive sequencing can be time-consuming and expensive, which severely restricts the number of samples that can be analyzed. Given the extensive nitrogenase sequence diversity being discovered in each new environment investigated (33), it is clear that quantitative comparisons among large numbers of samples will be necessary to understand the spatial and temporal variability of complex communities of diazotrophs and how this variability relates to ecosystem function.
To improve analytical throughput, a number of fingerprinting methods, such as denaturing gradient gel electrophoresis (DGGE) (14) and terminal restriction fragment length polymorphism (TRFLP) (10), have been adapted to the study of nitrogenase gene diversity (15, 18). These methods have shown promise, but the fingerprints provide either no (DGGE) or sometimes ambiguous (TRFLP) information about the phylogenetic composition of a community. In DGGE, different fragments are discriminated only by relative duplex stability, and in TRFLP, discrimination relies on a small subset of the information content of a sequence, namely, the location of specific restriction sites. An appealing feature of hybridization-based methods, such as DNA arrays, is that discrimination among sequences is primarily a function of their overall similarity to probes of known sequence. Initial applications of microarrays to the investigation of microbial diversity targeted 16S rRNA (7, 12, 23, 30), but microarrays are also being developed for assessing functional gene diversity (26, 31) and expression (6).
The goal of this study was to design simple, inexpensive DNA macroarrays that would facilitate the examination of nitrogenase gene diversity in the environment, in particular (nifH) genes in aquatic water samples. The arrays were designed to reflect the diversity of the nifH gene sequences we obtained from clone libraries generated by PCR amplification of genomic DNA samples obtained from the Choptank River and the Chesapeake Bay. We also included nifH genes from diverse reference microorganisms of known phylogenetic affiliation. In this report, we present an evaluation of the performance of the array with targets derived from individual sequences, defined mixtures of sequences, and a natural community. Our results indicate that the macroarray procedure is a highly reproducible and at least semiquantitative method for assessing the genetic diversity within pools of mixed PCR products.
MATERIALS AND METHODS
DNA extraction. (i) Culture collection isolates.
Cultured diazotrophs were obtained from the Pasteur Culture Collection of Cyanobacteria (PCC, Paris, France), the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ, Braunschweig, Germany), the Agriculture Research Services culture collection at the National Center for Agricultural Utilization Research (NRRL, Peoria, Ill.), and the Culture Collection of Algae at the University of Texas at Austin. Whenever feasible, microorganisms were grown in the appropriate medium prior to extraction of DNA. For microorganisms that we were not equipped to cultivate, the entire inoculum received from the supplier was extracted. For liquid cultures, an aliquot of 3 to 8 ml was centrifuged, and the resulting cell pellets were washed once with STE buffer (20% sucrose, 50 mM Tris-HCl, 50 mM EDTA), resuspended in 250 μl of STE buffer containing 5 mg of lysozyme ml−1, and incubated at 25°C for 30 min. Proteinase K (final concentration, 2 mg ml−1) and sodium dodecyl sulfate (SDS) (final concentration, 1%) were added to the lysate, and the resulting solutions were incubated for an additional 30 min at 65°C. Acid-washed, 0.1-mm-diameter beads were added (0.25 g per sample) with 250 μl of PCI (phenol, chloroform, isoamyl alcohol [25:24:1]). Tubes were shaken in a FastPrep bead-beating instrument (Q-Biogene, Carlsbad, Calif.) two times for 10 s each time at 6 m s−1 and then centrifuged to separate the phases. The aqueous phase was transferred to a new tube, and the organic phase was back extracted with 100 μl of TE (10 mM Tris-HCl [pH 8.0], 1 mM EDTA), and the second aqueous phase was pooled with the first aqueous phase. Samples were extracted once more with PCI and then extracted with chloroform. DNA was precipitated in 0.2 M NaCl and 2 volumes of ethanol overnight at −20°C (22). Samples were centrifuged for 30 min at 15,000 × g at 4°C. Pellets were washed with 70% ethanol and resuspended in 50 μl of TE.
(ii) Chesapeake Bay environmental samples.
Water samples collected from the Choptank River in August 2000 and from the Chesapeake Bay and Choptank River in April 2001 (Table 1) were filtered through 2-μm Sterivex capsules (Millipore, Billerica, Mass.) using a peristaltic pump. The volume filtered per capsule ranged from 280 to 2,000 ml of water. Residual water was evacuated from the capsule by applying air pressure with a hand-operated syringe. Filter capsules were immediately placed in liquid nitrogen, then shipped on dry ice to the laboratory, and stored at −80°C prior to extraction. STE buffer (1.8 ml) containing 5 mg of lysozyme ml−1 was added to the filter. Samples were incubated at 25°C for 1 h, proteinase K (final concentration, 2 mg ml−1) and SDS (final concentration, 1%) were added to the samples, and the samples were incubated at 60°C for 1 h. Lysate was aspirated from the filter capsule with a syringe and transferred to a Corex tube (Corning, Acton, Mass.). Filter capsules were rinsed once with 1 ml of TE, and the rinse was pooled with the corresponding sample in the Corex tube. RNase cocktail (Ambion, Austin, Texas) (final concentration, 2.5 U ml−1) was added, and samples were incubated for 10 min at room temperature. Ammonium acetate was added to a final concentration of 2 M, and samples were centrifuged at 14,800 × g for 6 min to precipitate proteins. Supernatant was transferred to a new tube, and ethanol (2 volumes) was added. Nucleic acids were precipitated at −20°C for 30 min and pelleted by centrifugation at 12,000 × g for 35 min at 4°C. Pellets were washed with 70% ethanol and resuspended in 500 μl of TE. Samples were extracted first with an equal volume of PCI and then with an equal volume of chloroform and precipitated in ethanol and 0.3 M sodium acetate. Samples were resuspended in 10 μl of TE, 180 μl of Qiagen (Valencia, Calif.) ATL buffer, and 200 μl of Qiagen AL buffer and further purified using the DNeasy minikit (Qiagen) following the manufacturer's instructions.
TABLE 1.
Station names, locations, and sampling depths for water collected in the Choptank River and Chesapeake Bay for DNA extraction
| Stationa (date [yr/mo/day]) | Location (latitude, longitude) | Sampling depth (m) |
|---|---|---|
| CT100 (2000/7/11) | 38° 48′ N, 75° 55′ W | 1 |
| CT200 (2000/7/11) | 38° 37′ N, 76° 08′ W | 1 |
| 7.9 | ||
| CT100 (2001/4/3) | 38° 48′ N, 75° 55′ W | 1 |
| CB100 (2001/4/4) | 39° 21′N, 76° 11′ W | 1.8 |
| 7.7 | ||
| 9.7 | ||
| CB200 (2001/4/5) | 38° 34′ N, 76° 27′ W | 1.8 |
| 11.2 | ||
| 17.6 | ||
| CB300 (2001/4/6) | 37° 18′ N, 76° 09′ W | 1.9 |
| 8.3 | ||
| 11.3 |
Choptank River and Chesapeake Bay stations are indicated by CT and CB prefixes, respectively.
PCR amplification of nifH genes from genomic DNA extracts.
A fragment of the nifH gene (318 to 333 bp) was amplified by nested PCR with degenerate primers as described previously (32, 37), but with a new set of outer primers (24). Each 50-μl first-round PCR mixture contained 5 μl of 10× ExTaq buffer (Takara, Shuzo, Japan), 4 μl of 2.5 mM deoxynucleoside triphosphates, 37.5 μl of water, 0.5 μl of 100 μM CDHPnif53F (5′-TGAGACAGATAGCTATYTAYGGHAA-3′), 0.5 μl of 100 μM CDHPnif723R (5′-GATGTTCGCGCGGCACGAADTRNATSA-3′), 0.5 μl of ExTaq DNA polymerase (5 U μl−1), and 2 μl of template DNA. Thermal cycling consisted of the following steps: (i) an initial denaturation at 94°C for 5 min; (ii) 30 cycles, with 1 cycle consisting of denaturation at 94°C for 1 min, annealing at 50°C for 1 min, and extension at 72°C for 1 min; and (iii) a final 7-min extension at 72°C. For the second round of the nested amplification, one microliter of this reaction mixture was used as the template in a 50-μl reaction mixture containing the same reagent mixture described above, but with 38.5 μl of water and the inner primers nifH2 (5′-TGYGAYCCNAARGCNGA-3′) and nifH1 (5′-ADNGCCATCATYTCNCC-3′) (34). The thermal cycling protocol for the nested reactions was the same as above except that the annealing temperature was raised to 57°C. PCR products from the nested reaction were gel purified. A single dominant band of the expected size was observed in most cases using these conditions. Exceptions were amplifications for two isolates (Enterobacter aerogenes and Methanothermus fervidus) that yielded many nonspecific products of various sizes. Gel-purified fragments were quantified by fluorometry using the PicoGreen double-stranded DNA quantitation kit (Molecular Probes, Eugene, Oreg.) and a TD700 filter-based fluorometer (Turner Designs, Sunnyvale, Calif.) or a Cary spectrofluorometer (Varian, Palo Alto, Calif.).
Cloning and sequencing of amplified nifH gene fragments.
PCR products were cloned into a pGEM T-system II vector (Promega, Madison, Wis.) following the manufacturer's instructions. Plasmids were purified using a mini-spin plasmid purification kit (Qiagen), sequenced using BigDye version 3 cycle sequencing chemistry (Applied Biosystems, Foster City, Calif.) with T7 and Sp6 primers, and analyzed with ABI PRISM 310 or 3100 genetic analyzer.
Selection of clones for array construction.
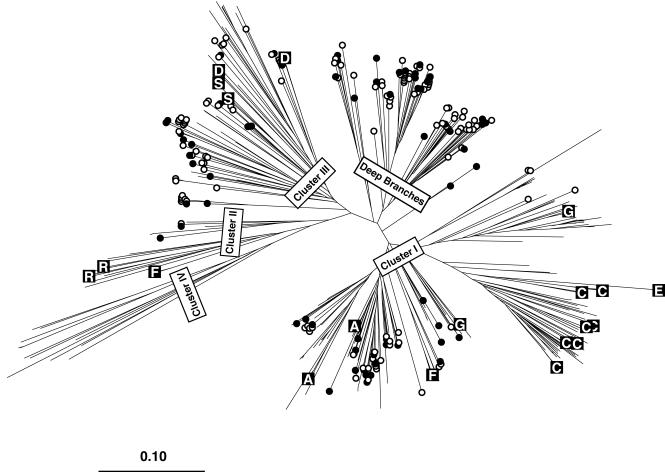
All of the sequences obtained in this study were combined into a database with other nitrogenase sequences retrieved from GenBank and used to build a distance tree using the neighbor-joining algorithm in order to identify representative clones for macroarray construction (Fig. 1). Sixty-five clones from the library of Chesapeake Bay and Choptank River clones were identified that represented the range of diversity found in those environments. Clones were chosen such that, on average, each shared 87% sequence identity with the next nearest selected clone (range, 73 to 96%). An additional three clones from a previous investigation in the Pacific Ocean (38) were also selected. A subset of the selected clones, primarily clones from cultivated isolates, was used to prepare an initial array (hereafter referred to as the test array) with 21 probes and 6 control DNA spots. A second larger array called the Chesapeake Bay version 1 (CBv1) array, was prepared with the 65 selected Chesapeake environmental clones, 2 Pacific Ocean environmental clones, clones from 21 cultivated microorganisms, and 8 control DNA spots (Table 2).
FIG. 1.
Neighbor-joining tree constructed from an uncorrected distance matrix among 447 nifH sequence fragments generated as part of this study (228 fragments) or retrieved from GenBank (219 fragments). For GenBank sequences, only those sequences from cultivated bacteria that spanned at least the region corresponding to our fragment were included. Sequences belonging to commonly recognized major clusters (clusters I to IV [5]) and some additional deep branches are labeled. Branches with open or closed circles contain sequences derived from Chesapeake Bay samples. Closed circles indicate those clones represented as probes on the array. Branches marked with a letter in a black box are sequences obtained from cultivated bacteria as part of this study that are also represented as probes on the array. The boxed letters indicate the phylogenetic designation of the bacterium from which the sequence was derived (A, D, E, and G indicate members of the alpha, delta, epsilon, and gamma subdivisions of the Proteobacteria, respectively; C, cyanobacteria; F, Firmicutes; S, green sulfur bacteria, R, Archaea).
TABLE 2.
Sources of the probes printed on the CBv1 nifH macroarraya
| Cluster or branch | Array positionb | Isolate or clonec | GenBank accession no. | Phylogenetic affiliation |
|---|---|---|---|---|
| Cluster I | A2 | Arcobacter nitrofigilis (DSM 7299) | AY221825 | ɛ Proteobacteriad |
| B2 | Synechocystis sp. (WH001) | AY221818 | Cyanobacteria | |
| C2 | Synechocystis sp. (WH8501) | AY221821 | Cyanobacteria | |
| D2 | Station ALOHA clone HT1903 | AF299422 | ||
| E2 | Station ALOHA clone HT1902 | AF299420 | ||
| F2 | Anabaena cylindrica (UTEX 629) | AY221813 | Cyanobacteria | |
| G2 | Tolypothrix sp. (PCC 7101) | AY221817 | Cyanobacteria | |
| H2 | Nostoc muscorum (UTEX 486) | AY221814 | Cyanobacteria | |
| A3 | Oscillatoria sancta (PCC 7515) | AY221815 | Cyanobacteria | |
| B3 | Symploca sp. (PCC 8002) | AY221816 | Cyanobacteria | |
| C3 | Vibrio diazotrophicus (DSM 2604) | AY221828 | γ Proteobacteria | |
| D3 | Paenibacillus azotofixans (DSM 5976) | AY221826 | Firmicutes | |
| E3 | CB894H2 | AY223945 | ||
| F3 | CB909H9 | AY224005 | ||
| G3 | Klebsiella oxytoca (NRRL B-199) | AY221827 | γ Proteobacteria | |
| H3 | CB909H5 | AY224002 | ||
| A4 | Azotobacter chroococcum (NRRL B-14637) | AY351672 | γ Proteobacteria | |
| B4 | CB894H8 | AY223950 | ||
| C4 | Frankia sp. (DSM 43829) | AY351671 | Actinobacteria | |
| D4 | CB911H3 | AY224015 | ||
| E4 | CB891H1 | AY223907 | ||
| F4 | CB891H4 | AY223912 | ||
| G4 | CB894H10 | AY223944 | ||
| H4 | CB914H1 | AY224026 | ||
| A5 | CB916H1 | AY224034 | ||
| B5 | CB895H8 | AY223957 | ||
| C5 | CB891H8 | AY223911 | ||
| D5 | CB907H2 | AY223968 | ||
| E5 | CB907H9 | AY223997 | ||
| F5 | Xanthobacter flavus (NRRL B-14838) | AY221812 | α Proteobacteria | |
| G5 | CB891H3 | AY223908 | ||
| H5 | CB921H3 | AY224040 | ||
| A6 | CB921H7 | AY224042 | ||
| B6 | CB909H7 | AY224003 | ||
| C6 | CB895H7 | AY223956 | ||
| D6 | CB914H5 | AY224029 | ||
| E6 | Sinorhizobium meliloti (NRRL L-45) | AY221811 | α Proteobacteria | |
| F6 | CB908H5 | AY223998 | ||
| Deep branches | ||||
| G6 | CB916H3 | AY224036 | ||
| H6 | CB916H7 | AY224037 | ||
| A7 | CB910H3 | AY224008 | ||
| B7 | CB910H8 | AY224012 | ||
| C7 | CB910H4 | AY224009 | ||
| D7 | CB910H6 | AY224011 | ||
| E7 | CB907H5 | AY223979 | ||
| F7 | CB912H4 | AY224020 | ||
| G7 | CB910H10 | AY224006 | ||
| H7 | CB911H10 | AY224013 | ||
| A8 | CB909H8 | AY224004 | ||
| B8 | CB911H1 | AY224014 | ||
| C8 | CB914H6 | AY224030 | ||
| D8 | CB907H10 | AY223960 | ||
| E8 | CB907H4 | AY223976 | ||
| F8 | CB894H4 | AY223946 | ||
| G8 | CB895H1 | AY223952 | ||
| H8 | CB921H1 | AY224039 | ||
| A9 | CB891H5 | AY223910 | ||
| B9 | CB894H6 | AY223948 | ||
| C9 | CB895H2 | AY223953 | ||
| D9 | CB895H4 | AY223955 | ||
| E9 | CB914H7 | AY224031 | ||
| F9 | CB895H9 | AY223958 | ||
| G9 | CB895H10 | AY223951 | ||
| H9 | CB894H5 | AY223947 | ||
| A10 | CB895H3 | AY223954 | ||
| Cluster II | B10 | Methanothermobacter thermoautotrophicus (DSM 1850) | AY221829 | Archaea |
| C10 | Methanococcus vannielli (DSM 1224) | AY221830 | Archaea | |
| D10 | CB921H10 | AY224038 | ||
| E10 | CB921H8 | AY224043 | /PICK> | |
| Cluster III | F10 | CB911H9 | AY224019 | |
| G10 | CB912H2 | AY224021 | ||
| H10 | CB911H7 | AY224018 | ||
| A11 | CB910H5 | AY224010 | ||
| B11 | CB914H8 | AY224032 | ||
| C11 | CB909H1 | AY224000 | ||
| D11 | CB914H3 | AY224028 | ||
| E11 | Desulfobacter latus (DSM 3381) | AY221822 | δ Proteobacteria | |
| F11 | Desulfotomaculum nigrificans (DSM 574) | AY221823 | δ Proteobacteria | |
| G11 | CB912H9 | AY224025 | ||
| H11 | CB921H4 | AY224041 | ||
| A12 | CB916H2 | AY224035 | ||
| B12 | CB907H6 | AY223986 | ||
| C12 | CB912H5 | AY224023 | ||
| D12 | Chlorobium limicola (DSM 245) | AY221831 | Green sulfur bacteria | |
| E12 | CB909H2 | AY224001 | ||
| F12 | Pelodictyon luteolum (DSM 273) | AY221832 | Green sulfur bacteria | |
| G12 | CB910H2 | AY224007 | ||
| H12 | CB912H1 | AY224020 |
Sources of the probes printed on the CBv1 nifH macroarray listed in order of array position along with GenBank sequence accession numbers and the phylogenetic affiliation of cultivated representatives.
Array positions as shown in Fig. 3.
Isolates are listed by their binomial designation followed by the specific culture collection designation in parentheses. All environmental clones from this study are shown with a CB prefix.
ɛ Proteobacteria, epsilon subdivision of the Proteobacteria.
PCR amplification of cloned nifH gene fragments for array probes.
For the test array, cloned nitrogenase gene fragments were amplified by nested PCR from plasmid minipreps for spotting as array elements (i.e., probes). First-round PCR mixtures contained the same constituents as described above for nifH amplification but with Sp6 and T7 (0.5 μM each) as the primers and 1 to 2 μl of miniprep plasmid DNA (ca. 9 to ≤200 ng) as the template. Thermal cycling conditions consisted of an initial denaturation at 96°C for 2 min, followed by 30 cycles, with 1 cycle consisting of denaturation at 96°C for 30 s, annealing at 50°C for 30 s, and extension at 72°C for 1 min. To increase the amount of product, a second-round, nested amplification was performed using nifH1 and nifH2 primers as described above. For the CBv1 array, a modified cycling program provided sufficient product with only a single round of amplification using the Sp6 and T7 primers. The modified program consisted of the following: (i) an initial denaturation at 94°C for 5 min; (ii) 30 cycles, with 1 cycle consisting of denaturation at 94°C, annealing at 42°C, and extension at 72°C (30 s at each temperature); and (iii) a final extension at 72°C for 3 min. Amplified DNA was gel purified using a QIAquick gel extraction kit (Qiagen). Concentration of the gel-purified DNA was determined using the PicoGreen assay as described above.
Array construction.
Concentrations of the PCR-amplified, gel-purified nifH fragments from selected individual clones were adjusted to equal concentrations for spotting as probes on macroarrays. The concentration used for the test array was 5 ng μl−1. Subsequent tests showed no difference in signal with lower concentrations of probe (data not shown), so probes for the CBv1 array were normalized to 2.5 ng μl−1. Biotinylated phage lambda DNA was directly spotted as a dilution series onto arrays to serve as a positive control for signal detection. The control DNA was prepared by serial dilution of biotinylated lambda DNA (see biotinylation procedure below) in a diluent of 1× TE containing unlabeled lambda DNA such that the total DNA in each standard was always 5 ng (test array) or 2.5 ng (CBv1 array). DNA probes and controls were transferred to the wells of a 96-well, flat-bottom plate and replica spotted onto SuPerCharge nylon membranes (Schleicher & Schuell, Keene, N.H.) using a hand-operated, 96-pin tool with slot pins designed to deliver 1 μl per pin (V&P Scientific, San Diego, Calif.). The pin tool was pretreated with surfactant per the manufacturer's instructions to improve the reliability of spot delivery. The spotted DNA was denatured by incubating the membrane face up for 10 min on sheets of Whatman paper soaked with denaturation solution (3 M NaCl and 0.4 N NaOH). Membranes were neutralized by dipping them in two baths of 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and a final bath of 1× SSC. Membranes were exposed face down on a UV transilluminator for 2 min (irradiation time determined empirically; data not shown). Air-dried membranes were stored at room temperature between sheets of filter paper.
Biotinylation of target and control DNA.
Target DNA for hybridization to arrays was prepared by amplifying, gel purifying, and quantifying the nifH gene fragment from isolates or environmental genomic DNA as described above. Target DNA and phage lambda control DNA were biotinylated using the BrightStar psoralen-biotin kit (Ambion) according to the manufacturer's instructions. Briefly, psoralen-biotin reagent was added to heat-denatured DNA (20 to 50 ng μl−1 in 1× TE), and the mixture was irradiated with a long-wavelength (365-nm-wavelength) UV lamp for 45 min on ice while shielded from room light. The mixture was diluted to 100 μl with 1× TE, extracted twice with water-saturated butanol, and stored at −20°C.
Array hybridization and signal detection.
Membranes were incubated in hybridization buffer (1 mM EDTA, 6% SDS, 0.25 M sodium phosphate [pH 7.2], 0 to 40% formamide) at 65°C (test array) or 60°C (CBv1 array) for 1 to 2 h. The buffer was drained and replaced with 2 to 5 ml of hybridization buffer containing labeled, heat-denatured (99°C, 10 min) target at a final concentration of 25 ng ml−1 (test array) or 10 ng ml−1 (CBv1 array) (ca. 0.1 nM). Hybridization was performed in heat-sealed plastic pouches overnight (8 to 12 h) at 65°C (test array) or 60°C (CBv1 array). Hybridization buffer was drained, and the blots were transferred to trays and washed. The blots were washed twice for 5 min each time in 2× SSC plus 1% SDS at 65°C, twice for 15 min each time in 0.1× SSC plus 1% SDS at 65°C, and twice for 5 min each time in 1× SSC at room temperature. Bound biotinylated targets and biotinylated control DNA were detected using the Southern-Star chemiluminescence detection system (Applied Biosystems). Blots were incubated twice for 5 min each time in blocking buffer (1× phosphate-buffered saline, 0.2% I-Block reagent, 0.5% SDS) and then incubated for 20 to 30 min with a streptavidin-alkaline phosphatase conjugate (AvidX-AP; Applied Biosystems) diluted 1:5,000 (test array) or 1:2,000 (CBv1 array) in blocking buffer. Blots were washed four times in washing buffer (Applied Biosystems) and twice in assay buffer (Applied Biosystems), drained, and placed face up on a plastic sheet. Membranes were covered with a thin layer of CDP-Star chemiluminescence reagent (Applied Biosystems) and incubated for 5 to 30 min. Excess reagent was drained, and membranes were sandwiched between plastic sheets. Signal was recorded by exposure of the membranes to X-ray film (Biomax Light; Kodak, Rochester, N.Y.). Film images were scanned, and signal intensities for spots were extracted from the scanned images using ImageQuant software package (Amersham Biosciences, Sunnyvale, Calif.).
Nucleotide sequence accession numbers.
Sequences of nifH fragments from cultivated isolates were submitted to GenBank and assigned accession numbers AY351671, AY351672, and AY221811 to AY221832. Sequences from the Chesapeake Bay and Choptank River nifH clone libraries were submitted to GenBank and assigned accession numbers AY223907 to AY224045.
RESULTS
Cross-hybridization experiments with the test array.
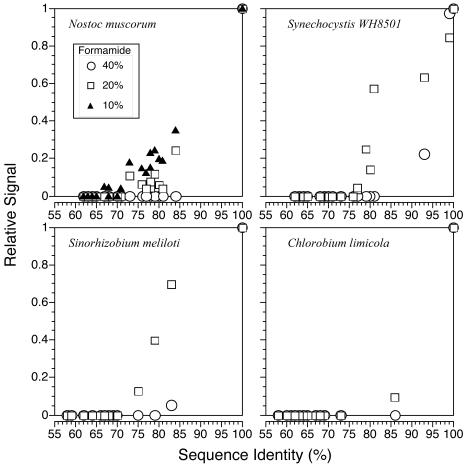
Single-target hybridization to the test array with the nifH fragment from the cyanobacterium Nostoc muscorum showed that target cross-hybridization varied as a function of percent similarity to the probe and formamide concentration (Fig. 2). At 10% formamide, the target cross-hybridized with probes with >67% sequence identity. Cross-hybridization occurred primarily with other cyanobacterial probes, but some cross-hybridization was detected with a probe from a member of the gamma subdivision of Proteobacteria (gamma proteobacterium) (Vibrio diazotrophicus). At 20% formamide, the similarity required for cross-hybridization increased to ≥71%. At 40% formamide, self-hybridization was still strong, but hybridization to the next most similar probe (Anabaena cylindrica, 84% identity) was not detected even with prolonged exposures of up to 2 min (data not shown). Three additional hybridizations were performed with single targets prepared from the alpha proteobacterium Sinorhizobium meliloti, the cyanobacterium Synechocystis (Crocosphaera) sp. strain WH8501 (38), and the green sulfur bacterium Chlorobium limicola. For each experiment, the relative signal intensity for each probe was quantified and plotted against percent sequence identity shared with the target (Fig. 2). Considering the results for all four targets at the highest stringency (40% formamide) together, no cross-hybridization was detected for any probe sharing ≤79% identity with the target. Conversely, there were no cases where a probe sharing ≥86% identity with the target failed to cross-hybridize.
FIG. 2.
Cross-hybridization experiments using four targets hybridized individually at different stringencies to test arrays composed of 21 diverse nifH probes. Relative hybridization signal is plotted against percent sequence identity between probe and target. The source organisms for the targets are shown in each graph. Symbols indicating formamide concentrations are the same for all panels. The 10% formamide treatment was performed only with the Nostoc muscorum target.
Experiments with the CBv1 array.
A cross-hybridization test was performed with a single target (S. meliloti) on a copy of the Chesapeake Bay macroarray printed with duplicate spots (data not shown). Self-hybridization was detected to the duplicated S. meliloti probe spots, but no cross-hybridization to the next closest probe represented on the array (Xanthobacter flavus, 83% identity) was detected.
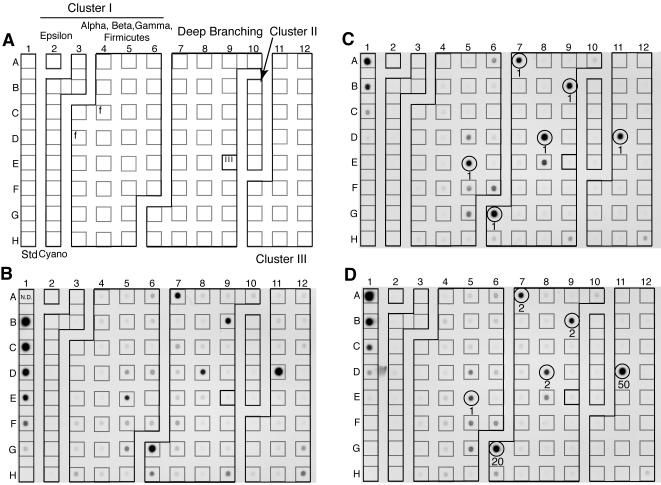
A mixed nifH amplification product from a DNA sample collected from Chesapeake Bay Station CB200 (38° 34′ N, 76° 27′ W) at a depth of 11.2 m was hybridized to the CBv1 array (Fig. 3A and B). Probe CB914H3 (position D11) in cluster III yielded the highest signal, and five additional probes (four deep branching and one in cluster I) had signals >25% of that maximum. The most similar sequences to these six probes (excluding other clones generated in this study) were determined by BLAST analysis (2) using the GenBank database (Table 3). The probe with the highest signal showed 91% sequence identity with a clone derived from the Neuse River estuary. Two of the four positive, deeply branching probes (CB916H3 and CB910H3) were most similar (89 and 88%) to a clone derived from bacteria associated with decaying sea grass, and a third probe was most similar (89%) to a clone derived from soil. The fourth probe was most similar (96%) to an alpha proteobacterium (Bradyrhizobium sp.), but the match extended over less than two-thirds of the fragment (194 of 324 bp). The sixth positive probe from cluster I was most similar (94%) to an uncultivated bacterium associated with a sweet potato plant but was also similarly related (94%) to nifH genes from an alpha proteobacterium (Bradyrhizobium sp.).
FIG. 3.
Map illustrating the arrangement of probe types on the CBv1 nifH array (A) and autoradiographs showing chemiluminescence signal resulting from hybridizations with natural (B) or simulated (C and D) community targets. Cluster I includes members of the epsilon, alpha, beta, and gamma subdivisions of the Proteobacteria and cyanobacteria (Cyano). In panel A, the two sequences from Firmicutes (f) and a misplaced cluster III probe (III) are indicated. The template grid is laid over each autoradiograph to aid in identification of corresponding probes among arrays. The natural community target was prepared by direct PCR amplification from a community genomic DNA sample from central Chesapeake Bay. The simulated community targets were prepared by mixing amplification products from six individual nifH clones. Clones were chosen to correspond with the probes yielding the highest signal in the natural community hybridization. Probes that are perfect matches to the six targets are circled, and the relative concentrations of the targets within each experiment are indicated under the circled probe. Column 1 in each case contains a dilution series of biotinylated lambda DNA in a background of unlabeled lambda DNA as a positive control. The amounts of biotinylated DNA in the standards (Std) are (from top to bottom) no data (spot misprint), 0.83, 0.28, 0.093, 0.031, 0.010, 0.0034, and 0 ng (panel B) or 2.5, 0.62, 0.16, 0.039, 0.0098, 0.0024, 0.00061, and 0 ng (panels C and D).
TABLE 3.
Summary information for the six probes yielding the highest signals when the CBv1 array was hybridized with a target derived from a natural Chesapeake Bay picoplankton community
| Clone (probe locationa) | Signal (% of peak) | Closest match (GenBank accession no.)b | % Similarityc |
|---|---|---|---|
| CB914H3 (D11) | 100 | Uncultivated; clone NRS1C607 from Neuse River Estuary (AF518561) | 91 (323/327) |
| CB916H3 (G6) | 74 | Uncultivated; clone SIS2-7 from decaying sea grass (AF389736) | 89 (321/327) |
| CB894H6 (B9) | 50 | Uncultivated; clone g1-HW4 from soil (AY196408) | 89 (327/327) |
| CB910H3 (A7) | 48 | Uncultivated; clone SIS2-7 from decaying sea grass (AF389736) | 88 (320/327) |
| CB907H10 (D8) | 30 | Bradyrhizobium sp. strain IRBG 230 (AB079617) | 96 (194/324) |
| CB907H9 (E5) | 26 | Uncultivated; clone nifH51 from sweet potato (AY159593) | 94 (324/324) |
Array location (as in Fig. 3).
The most similar sequence in GenBank as determined by the BLAST algorithm (2).
Similarities between the probe and the top-ranked BLAST match are presented as a percentage followed, in parentheses, by the ratio of the length of the matching region to the total length of the submitted fragment.
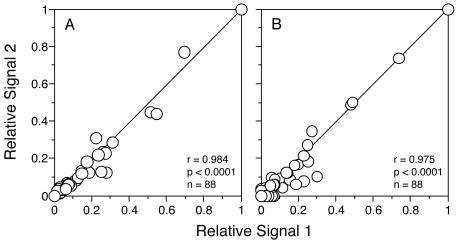
An independent hybridization of the nifH amplified target from the sample collected from CB200 at a depth of 11.6 m was performed using a second array that was printed with duplicate, slightly offset spots (autoradiograph not shown). Relative signal intensities from corresponding probes displayed significant (P < 0.0001, n = 88) positive correlations whether considering duplicate spots on one array (Fig. 4A, r = 0.984) or corresponding probes on duplicate arrays hybridized independently (Fig. 4B, r = 0.975).
FIG. 4.
Correlation of relative signal intensities between duplicate probes spotted on a single array (A) and between corresponding probes on duplicate arrays hybridized independently. The same natural community target was used in each hybridization. The plotted lines represent a 1:1 relationship.
Two defined target mixtures (10 ng ml−1) were prepared by mixing amplification products from six clones that yielded the highest hybridization signals in the natural community hybridizations (above). For target mixture A (ratio of 1:1:1:1:1:1 [Fig. 3C]), the signal from each perfect-match probe was normalized to the mean value. The observed relative signal ratios ranged from 0.8 to 1.54, with a mean and standard deviation of 1 ± 0.18 or an error of 14%, and the results were similar for duplicate experiments (Fig. 5). Signal intensities for all probes with a signal detected in at least one of the experiments were significantly correlated (r = 0.96, P < 0.0001, n = 15). These reproducible differences in relative signal intensity among the six probes were not attributable to differences in G+C content (data not shown). A single experiment with target mixture B (ratio of 50:20:2:2:2:1 [Fig. 3D]) indicated that the relationship between relative signal intensity for the perfect-match probes and the relative target concentration was nonlinear, with signal saturating at higher target concentrations (Fig. 6). Cross-hybridization to non-perfect-match probes in these two experiments varied as a function of percent identity to the next most similar target (Fig. 7). No signal was detected for any probe sharing <78% identity with one of the targets (n = 64). Conversely, a signal was detected for all probes with >88% identity to at least one of the six targets (n = 15).
FIG. 5.
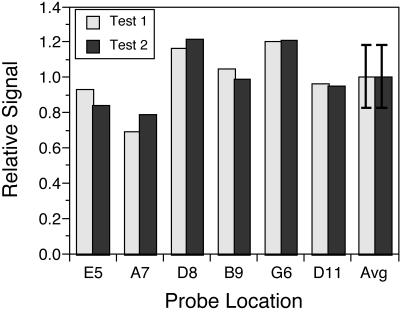
Relative signal intensity for the six perfect-match probes corresponding to six targets mixed in equal ratios and hybridized to the CBv1 array. Signal intensities were normalized to the average intensity. Error bars for the average (Avg) intensity are the standard deviation among the six probes.
FIG. 6.
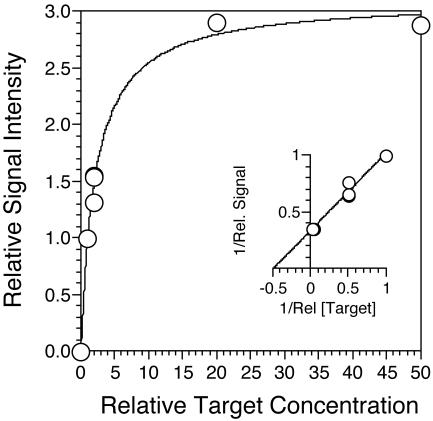
Relative signal intensity for perfect-match probes as a function of relative concentration of the corresponding six targets composing an artificial community. The insert shows data presented as a double-reciprocal plot with a line fit by linear regression (Rel, relative).
FIG. 7.
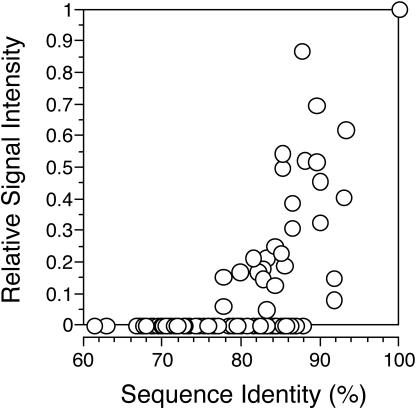
Cross-hybridization data for all probes in two artificial community hybridization experiments. Within each experiment, signal from each cross-hybridizing probe was normalized to the signal from the most similar probe having a perfect match to one of the targets. Relative signal is plotted as a function of the percent identity between probe and the most similar target.
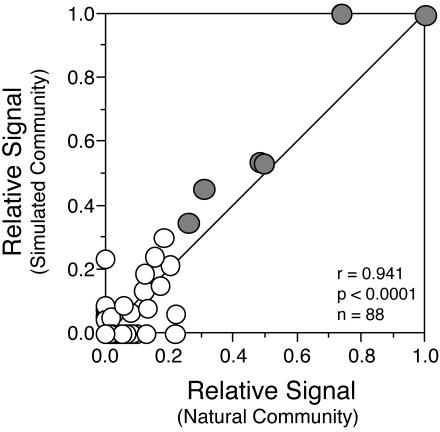
The target ratios in mixture B were chosen to roughly mimic the relative signal intensity ratios obtained with the natural community hybridization, at least in rank order. Quantitative comparison of relative probe intensities resulting from the hybridizations with natural (Fig. 3B) and artificial (Fig. 3D) communities revealed a significant (P < 0.0001, n = 88) positive correlation (Fig. 8, r = 0.941).
FIG. 8.
Correlation between relative signal intensities on arrays hybridized with a natural community target and a simulated community composed of six individual targets. Signals from the probes that are perfect matches to the six targets in the simulated community are indicated by the shaded circles. Within each experiment, signals were normalized to peak probe intensity. The plotted line represents a 1:1 relationship.
DISCUSSION
Performance of the array.
Our results suggest that the DNA macroarray procedure which we have developed for profiling mixtures of amplified nifH gene fragments is highly reproducible, and to some extent, quantitative. A high degree of reproducibility was illustrated by the significant correlations observed for intra- and interarray comparisons for simple mixtures of six targets and for a complex target mixture derived from a natural community. We note that these experiments demonstrate only the reproducibility of the macroarray procedure itself (array printing and hybridization and signal detection). As our procedure employs PCR-amplified targets, reproducibility for field applications will ultimately be influenced by all of the potential biases in the PCR (4, 8, 19, 21, 25). With some care taken in the amplification procedure, bias can be minimized (19). In practice, good reproducibility has been observed for arrays hybridized with targets amplified independently from the same DNA sample (8a).
Experiments with targets at known concentrations showed that the signal intensity of perfect-match probes was a roughly predictable function of target concentration. The relative signals from six targets that were mixed in equal concentrations were similar. The variability among probe signals was reproduced in two independent experiments, suggesting that there may be inherent differences in the hybridization kinetics of the six probes. The differences were not explained by variations in G+C content, however, and may simply reflect errors in the determination of the individual target concentrations in the stock solutions. Results obtained with unequal target ratios indicated that the relationship between target concentration and relative signal intensity is predictable, but nonlinear, with signal saturation occurring at the highest concentrations (ca. 2 ng/ml). Analysis of the double-reciprocal plot of the data in Fig. 6 and conversion into absolute concentrations indicates that the half-saturation of signal occurred at an absolute target concentration of approximately 0.26 ng ml−1. The lowest known concentration of target tested was 0.13 ng ml−1, which was readily detected. However, extrapolation from the relationship between concentration and signal in Fig. 6 to the lowest signals reliably detected suggests that the detection limit is an order of magnitude lower. The dynamic range for the assay thus appears to be roughly 2 orders of magnitude.
A dilution series of biotinylated lambda DNA was included to compensate for nonlinearity in the response of the X-ray film in recording the chemiluminescence signal. The standard curves showed that in some cases the response was linear over the entire standard range, but in some cases saturation was apparent at higher standards (data not shown). This standard curve controls only for detection system response (any nonlinearity of the chemiluminescence reaction and film response) and not for nonlinearity in hybridization kinetics. In practice, and perhaps for the aforementioned reason, normalization to the standard curve provided little or no improvement in interpreting the hybridization results, and probe signals were instead normalized within an experiment to the average or lowest perfect-match probe signal or, for natural samples, simply to peak probe signal. The standard curve did prove useful, however, as a positive control for the signal detection system and for gauging appropriate exposure times.
Analyses of the cross-hybridization of targets to non-perfect-match probes indicate that, for the stringency conditions that we routinely employed, the threshold for hybridization of target to probe lies between 78 and 88% sequence identity. This is similar to that reported for oligonucleotide (26) and clone-based arrays (31). The cross-hybridization threshold was readily controlled by adjusting stringency with formamide concentration. Analysis of changes in hybridization pattern in response to deliberate manipulation of stringency may be useful for extracting more information from a sample, since perfect-match signal declines less than signals from cross-hybridization (Fig. 2) (26, 31). Comparison of hybridization signals at several stringencies could thus reveal the presence of sequences having different degrees of similarity to each probe. This phenomenon is currently being exploited with technology for monitoring real-time denaturation patterns on microarrays (7). Signal from cross-hybridizing probes above the threshold is positively correlated with percent identity to the target, but significant variability was evident both for single-target (Fig. 2) and mixed-target (Fig. 7) experiments. The scatter in the relationship between percent similarity and relative cross-hybridization intensity suggests considerable probe-to-probe variability. Possible contributing factors include variations in G+C content and variations in the distribution of mismatches, both of which will affect duplex stability. This was illustrated in a recent study using an oligonucleotide functional gene array where prediction of cross-hybridization was improved if the free energy of binding was considered in addition to overall sequence similarity (26).
The cross-hybridization threshold achievable with the DNA array has implications for array design and interpretation of hybridization patterns. Using higher levels of stringency improves resolution among closely related sequence types but simultaneously increases the number of probes required to detect the same range of genetic diversity. We had intended to design the CBv1 array to minimize the number of probes needed to assure that every sequence in the Chesapeake Bay nifH sequence library could hybridize (or cross-hybridize) to at least one probe. However, subsequent analysis of nifH diversity turned up new sequences, many with much less than 85% similarity to any probe on the array. This highlights one of the difficulties one may encounter in the development of clone-based arrays, particularly in environments with high diversity. Exhaustive assessment of the diversity must be made to ensure a truly representative array, but this can be difficult to achieve. Oligonucleotide-based arrays are not constrained by clone library diversity, since any desired probe sequence can be synthesized. High-density arrays covering all known (and even as yet unknown) variants of a particular gene region can be created to make potentially universally applicable arrays. Some attempts in this direction have been made for examining 16S rRNA gene diversity (30). The results have been promising, but the major drawback to oligonucleotide arrays at present is their expense. Like clone-based arrays, the results are still only semiquantitative (26, 31).
Potential for application of the array in the field.
Our results highlight the reproducibility and potential for quantification of the macroarray approach. Interpreting the quantitative information obtained from complex environmental samples can be confounded by a number of nonlinear factors that influence hybridization. Even with only a single, perfectly matched probe-target pair, the relationship between concentration of target in solution and amount of target hybridized to a membrane-bound probe is nonlinear due to the underlying kinetics (e.g., Fig. 6). Competition for binding sites among perfectly matched and slightly mismatched targets will contribute to nonlinear relationships between target concentration and probe signal, as will sequence-dependent variables such as G+C content and the distribution of mismatches.
Our experiments were not designed to explicitly investigate these finer scale issues, but systematic differences in melting temperature among the probes on the CBv1 array seems likely, since the G+C content of the probes varies from 37 to 68%. We did not observe a significant correlation (r = 0.32, P = 0.57, n = 6) between G+C content and the relative signal intensity for the six perfect match probes in mixture A, but the power of this test was limited because of the few probes analyzed and the limited range of G+C content represented (56 to 65%). Comparison of distance matrices using only the 5′ or 3′ halves of the nifH sequences generated for this study indicates that there is significant variability in the distribution of mismatches (data not shown). In the most extreme case, CB907H9 and CB907H10 share 73% identity over their 5′ halves compared to 97% for their 3′ halves. Such extreme disparity could be indicative of chimeras (20) but may also simply reflect differences in constraints on evolution over different regions of the molecule. A number of these complications can be circumvented. The influence of G+C content on binding kinetics, for example, might be minimized by the addition of tetramethylammonium chloride to the hybridization buffer (22). Uneven distribution of mismatches (and perhaps differences in G+C content) may be minimized by careful selection of the fragment location used for the probe. This is easier to achieve with oligonucleotide arrays, since clone-based arrays are constrained by the availability of conserved sequences flanking the selected region to allow PCR amplification.
As a semiquantitative tool, macroarray analyses may serve as an alternative or a supplement to other fingerprinting methods, such as DGGE or analysis of TRFLP. An advantage of the macroarray approach over these other methods is that hybridization to a particular probe provides a more direct, robust indication of phylogenetic affinity based on overall sequence similarity. A disadvantage is that a large amount of work can be required at the outset to establish a clone library, characterize the diversity, and design a useful array. Any diversity not represented on the array will go undetected. Once a comprehensive array is developed for a particular environment, however, analysis of samples appears to be relatively straightforward. Our ability to closely replicate the hybridization or cross-hybridization pattern of a natural sample suggests that the relative probe signals obtained from the natural sample provided at least a semiquantitative representation of the identity and relative abundance of targets in the PCR mixture. In this case, the natural target mixture was dominated by six clones representing three divergent clusters.
The reliable performance of the macroarray and its lower cost compared to microarrays suggest that this is a viable method for conducting spatial or temporal surveys of functional gene diversity in the environment. The usefulness of the macroarray in such applications has been demonstrated in a parallel study (8a). By substituting templates derived from RNA rather than DNA, the macroarray procedure we have developed here may also be used to study patterns of nitrogenase gene expression among complex communities in the environment.
Acknowledgments
M. A. Voytek and J. L. Collier are gratefully acknowledged for assistance with the collection of field samples. We thank G. A. Jackson for creating and maintaining the Biocomplexity project web site (http://snow.tamu.edu/).
This research was supported in part by NSF Biocomplexity grants to J.P.Z. (OCE 9981437) and B.B.W. (OCE 9981482).
REFERENCES
- 1.Affourtit, J., J. P. Zehr, and H. W. Paerl. 2001. Distribution of nitrogen-fixing microorganisms along the Neuse River Estuary, North Carolina. Microb. Ecol. 41:114-123. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Bagwell, C. E., and C. R. Lovell. 2000. Persistence of selected Spartina alterniflora rhizoplane diazotrophs exposed to natural and manipulated environmental variability. Appl. Environ. Microbiol. 66:4625-4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandler, D. P., J. K. Frederickson, and F. J. Brockman. 1997. Effect of PCR template concentration on the composition and distribution of total community 16S rDNA libraries. Mol. Ecol. 6:475-483. [DOI] [PubMed] [Google Scholar]
- 5.Chien, Y. T., and S. H. Zinder. 1996. Cloning, functional organization, transcript studies, and phylogenetic analysis of the complete nitrogenase structural genes (nifHDK2) and associated genes in the archaeon Methanosarcina barkeri 227. J. Bacteriol. 178:143-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dennis, P., E. A. Edwards, S. N. Liss, and R. Fulthorpe. 2003. Monitoring gene expression in mixed microbial communities by using DNA microarrays. Appl. Environ. Microbiol. 69:769-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Fantroussi, S., H. Urakawa, A. E. Bernhard, J. J. Kelly, P. A. Noble, H. Smidt, G. M. Yershov, and D. A. Stahl. 2003. Direct profiling of environmental microbial populations by thermal dissociation analysis of native rRNAs hybridized to oligonucleotide microarrays. Appl. Environ. Microbiol. 69:2377-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrelly, V., F. A. Rainey, and E. Stackebrandt. 1995. Effect of genome size and rrn gene copy number on PCR amplification of 16S rRNA genes from a mixture of bacterial species. Appl. Environ. Microbiol. 61:2798-2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Jenkins, B. D., G. F. Steward, S. M. Short, B. B. Ward, and J. P. Zehr. 2004. Fingerprinting diazotroph communities in the Chesapeake Bay by using a DNA macroarray. Appl. Environ. Microbiol. 70:1767-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lilburn, T. C., K. S. Kim, N. E. Ostrom, K. R. Byzek, J. R. Leadbetter, and J. A. Breznak. 2001. Nitrogen fixation by symbiotic and free-living spirochetes. Science 292:2495-2498. [DOI] [PubMed] [Google Scholar]
- 10.Liu, W.-T., T. L. Marsh, H. Cheng, and L. J. Forney. 1997. Characterization of microbial diversity by determining terminal restriction fragment length polymorphisms of genes encoding 16S rRNA. Appl. Environ. Microbiol. 63:4516-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovell, C. R., Y. M. Piceno, J. M. Quattro, and C. E. Bagwell. 2000. Molecular analysis of diazotroph diversity in the rhizosphere of the smooth cordgrass, Spartina alterniflora. Appl. Environ. Microbiol. 66:3814-3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loy, A., A. Lehner, N. Lee, J. Adamczyk, H. Meier, J. Ernst, K.-H. Schleifer, and M. Wagner. 2002. Oligonucleotide microarray for 16S rRNA gene-based detection of all lineages of sulfate-reducing prokaryotes in the environment. Appl. Environ. Microbiol. 68:5064-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacGregor, B. J., B. Van Mooy, B. J. Baker, M. Mellon, P. H. Moisander, H. W. Paerl, J. Zehr, D. Hollander, and D. A. Stahl. 2001. Microbiological, molecular biological and stable isotopic evidence for nitrogen fixation in the open waters of Lake Michigan. Environ. Microbiol. 3:205-219. [DOI] [PubMed] [Google Scholar]
- 14.Muyzer, G., E. C. De Wall, and A. G. Uitterlinden. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noda, S., M. Ohkuma, R. Usami, K. Horikoshi, and T. Kudo. 1999. Culture-independent characterization of a gene responsible for nitrogen fixation in the symbiotic microbial community in the gut of the termite Neotermes koshunensis. Appl. Environ. Microbiol. 65:4935-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohkuma, M., S. Noda, and T. Kudo. 1999. Phylogenetic diversity of nitrogen fixation genes in the symbiotic microbial community in the gut of diverse termites. Appl. Environ. Microbiol. 65:4926-4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohkuma, M., S. Noda, R. Usami, K. Horikoshi, and T. Kudo. 1996. Diversity of nitrogen fixation genes in the symbiotic intestinal microflora of the termite Reticulitermes speratus. Appl. Environ. Microbiol. 62:2747-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poly, F., L. J. Monrozier, and R. Bally. 2001. Improvement in the RFLP procedure for studying the diversity of nifH genes in communities of nitrogen fixers in soil. Res. Microbiol. 152:95-103. [DOI] [PubMed] [Google Scholar]
- 19.Polz, M. F., and C. M. Cavanaugh. 1998. Bias in template-to-product ratios in multitemplate PCR. Appl. Environ. Microbiol. 64:3724-3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu, X. Y., L. Y. Wu, H. S. Huang, P. E. McDonel, A. V. Palumbo, J. M. Tiedje, and J. Z. Zhou. 2001. Evaluation of PCR-generated chimeras, mutations, and heteroduplexes with 16S rRNA gene-based cloning. Appl. Environ. Microbiol. 67:880-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reysenbach, A.-L., L. J. Giver, G. S. Wickham, and N. Pace. 1992. Differential amplification of rRNA genes by polymerase chain reaction. Appl. Environ. Microbiol. 58:3417-3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 23.Small, J., D. R. Call, F. J. Brockman, T. M. Straub, and D. P. Chandler. 2001. Direct detection of 16S rRNA in soil extracts by using oligonucleotide microarrays. Appl. Environ. Microbiol. 67:4708-4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steward, G. F., J. P. Zehr, R. Jellison, J. P. Montoya, and J. T. Hollibaugh. Vertical distribution of nitrogen-fixing phylotypes in a meromictic, hypersaline lake. Microb. Ecol., in press. [DOI] [PubMed]
- 25.Suzuki, M. T., and S. J. Giovannoni. 1996. Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl. Environ. Microbiol. 62:625-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taroncher-Oldenburg, G., E. M. Griner, C. A. Francis, and B. B. Ward. 2003. Oligonucleotide microarray for the study of functional gene diversity in the nitrogen cycle in the environment. Appl. Environ. Microbiol. 69:1159-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ueda, T., Y. Suga, N. Yahiro, and T. Matsuguchi. 1995. Genetic diversity of N2-fixing bacteria associated with rice roots by molecular evolutionary analysis of a nifD library. Can. J. Microbiol. 41:235-240. [DOI] [PubMed] [Google Scholar]
- 28.Vitousek, P. M., and R. W. Howarth. 1991. Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13:87-115. [Google Scholar]
- 29.Widmer, F., B. T. Shaffer, L. A. Porteous, and R. J. Seidler. 1999. Analysis of nifH gene pool complexity in soil and litter at a Douglas fir forest site in the Oregon Cascade Mountain Range. Appl. Environ. Microbiol. 65:374-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson, K. H., W. J. Wilson, J. L. Radosevich, T. Z. DeSantis, V. S. Viwanathan, T. A. Kuczmarski, and G. L. Andersen. 2002. High-density microarray of small-subunit ribosomal DNA probes. Appl. Environ. Microbiol. 68:2535-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu, L. Y., D. K. Thompson, G. Li, R. A. Hurt, J. M. Tiedje, and J. Zhou. 2001. Development and evaluation of functional gene arrays for detection of selected genes in the environment. Appl. Environ. Microbiol. 67:5780-5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zani, S., M. T. Mellon, J. L. Collier, and J. P. Zehr. 2000. Expression of nifH genes in natural microbial assemblages in Lake George, New York, detected with reverse transcriptase PCR. Appl. Environ. Microbiol. 66:3119-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zehr, J. P., B. D. Jenkins, S. M. Short, and G. F. Steward. 2003. Nitrogenase gene diversity and microbial community structure: a cross-system comparison. Environ. Microbiol. 5:539-554. [DOI] [PubMed] [Google Scholar]
- 34.Zehr, J. P., and L. A. McReynolds. 1989. Use of degenerate oligonucleotides for amplification of the nifH gene from the marine cyanobacterium Trichodesmium thiebautii. Appl. Environ. Microbiol. 55:2522-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zehr, J. P., M. Mellon, S. Braun, W. Litaker, T. Steppe, and H. W. Paerl. 1995. Diversity of heterotrophic nitrogen fixation genes in a marine cyanobacterial mat. Appl. Environ. Microbiol. 61:2527-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zehr, J. P., M. T. Mellon, and S. Zani. 1998. New nitrogen-fixing microorganisms detected in oligotrophic oceans by amplification of nitrogenase (nifH) genes. Appl. Environ. Microbiol. 64:3444-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zehr, J. P., and P. J. Turner. 2001. Nitrogen fixation: nitrogenase genes and gene expression, p. 271-286. In J. H. Paul (ed.), Methods in marine microbiology. Academic Press, New York, N.Y.
- 38.Zehr, J. P., J. B. Waterbury, P. J. Turner, J. P. Montoya, E. Omoregie, G. F. Steward, A. Hansen, and D. M. Karl. 2001. Unicellular cyanobacteria fix N-2 in the subtropical North Pacific Ocean. Nature 412:635-638. [DOI] [PubMed] [Google Scholar]