Abstract
Tinnitus, the perception of phantom sound, is often a debilitating condition that affects many millions of people. Little is known, however, about the molecules that participate in the induction of tinnitus. In brain slices containing the dorsal cochlear nucleus, we reveal a tinnitus-specific increase in the spontaneous firing rate of principal neurons (hyperactivity). This hyperactivity is observed only in noise-exposed mice that develop tinnitus and only in the dorsal cochlear nucleus regions that are sensitive to high frequency sounds. We show that a reduction in Kv7.2/3 channel activity is essential for tinnitus induction and for the tinnitus-specific hyperactivity. This reduction is due to a shift in the voltage dependence of Kv7 channel activation to more positive voltages. Our in vivo studies demonstrate that a pharmacological manipulation that shifts the voltage dependence of Kv7 to more negative voltages prevents the development of tinnitus. Together, our studies provide an important link between the biophysical properties of the Kv7 channel and the generation of tinnitus. Moreover, our findings point to previously unknown biological targets for designing therapeutic drugs that may prevent the development of tinnitus in humans.
Keywords: potassium channels, excitability, auditory brainstem, phantom perception
Tinnitus is a common auditory disorder that is often the result of extreme sound exposure. An estimated 5–15% of the population experiences chronic tinnitus, with many millions of those sufferers disabled by this condition (1–3). With an even higher prevalence of chronic tinnitus in recent war veterans (4), the personal and financial costs of tinnitus have expanded dramatically. Despite the high prevalence of tinnitus, the neuronal mechanisms that mediate the initiation (induction) and the maintenance (expression) of the disorder remain poorly understood. As a result there is no generally accepted treatment, cure, or preventive method for tinnitus.
Tinnitus is usually initiated by noise-induced cochlear damage that causes hair cell loss, ganglion cell degeneration, and reduced auditory nerve input to the central auditory system (5). Decreased peripheral input leads to pathogenic neuronal plasticity that results in subcortical hyperexcitability, increased neural synchrony, cortical reorganization, and ultimately stimulus-independent perception of sound (5–13). However, little is known about the plasticity mechanisms that initiate tinnitus. Elucidation of these mechanisms will lead to the development of drugs and therapies that can be applied soon after the acoustic trauma, thus preventing tinnitus from becoming permanent and irreversible.
The dorsal cochlear nucleus (DCN) is an auditory brainstem nucleus that is indispensable to the induction of tinnitus: ablation of the DCN before noise exposure prevents the induction of tinnitus (14). Consistent with its key role in tinnitus generation, the DCN is a site where robust tinnitus-related neuronal plasticity has been identified (15). Studies in animal models of noise-induced tinnitus have revealed that DCN principal neurons, fusiform cells, exhibit elevated spontaneous firing frequency (hyperactivity) that is correlated with the behavioral evidence of tinnitus (10, 12, 16). Although previous studies have suggested that a shift in the excitatory/inhibitory synaptic balance contributes to the DCN hyperactivity (12, 17, 18), direct evidence is lacking that these synaptic changes are crucial for the induction of tinnitus. Here we reveal that pathogenic plasticity of Kv7 (KCNQ) potassium channel establishes DCN hyperactivity and triggers the development of tinnitus. This significant role of KCNQ channels makes them promising targets for the development of therapeutic approaches for preventing the induction of tinnitus.
Results
Changes in the Intrinsic Properties of Fusiform Cells Mediate Tinnitus-Specific, DCN Hyperactivity.
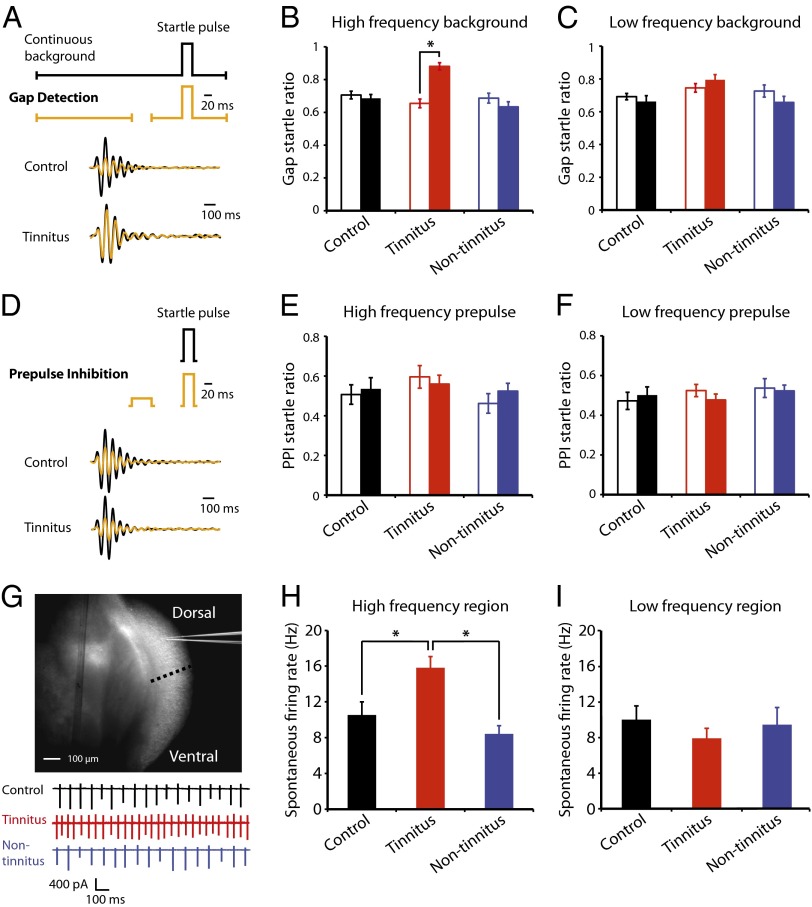
To determine the cellular mechanisms underlying the induction of tinnitus, we used an animal model that allows us to assess whether a mouse experiences tinnitus 1 wk after being exposed to a loud sound (noise exposure; SI Materials and Methods and Fig. S1). This animal model is based on the inhibition of an acoustic startle response by a silent gap that is embedded in a constant background sound (19); the silent gap is placed 130 ms before the startle stimulus (gap detection; Fig. 1A). Control mice and mice that do not experience tinnitus after noise exposure (nontinnitus mice) detect the gap and show inhibition of the startle response; mice with behavioral evidence of tinnitus after noise exposure (tinnitus mice) show reduced inhibition of the startle response, because their tinnitus fills the gap. One week after noise exposure, 51.4% of noise-exposed mice (18 of 35) showed behavioral evidence of tinnitus, with significantly increased gap startle ratio (startle response to gap and startle stimulus/response to startle stimulus alone) revealed only by high- (≥20 kHz) but not low-frequency background sounds (Fig. 1B, sham-exposed mice (control): n = 16, P = 0.55; tinnitus: n = 18, P < 0.001; nontinnitus: n = 17, P = 0.20; Fig. 1C, control: n = 16, P = 0.47; tinnitus: n = 18, P = 0.17; nontinnitus: n = 17, P = 0.14; Fig. S2). The development of the high-frequency tinnitus is consistent with previous studies that have used similar noise exposure (12, 17). Importantly, gap detection deficits of tinnitus mice are not due to temporal processing impairment or inability to hear the background sounds, because prepulse inhibition (PPI, inhibition of startle response by a preceding nonstartling sound; Fig. 1D) was identical among control, tinnitus, and nontinnitus mice (Fig. 1E, control: n = 18, P = 0.72; tinnitus: n = 16, P = 0.61; nontinnitus: n = 17, P = 0.32; Fig. 1F, control: n = 18, P = 0.62; tinnitus: n = 18, P = 0.26; nontinnitus: n = 17, P = 0.83; Figs. S3–S5). The behavioral distinction of tinnitus from nontinnitus mice enables us to identify the induction mechanisms that are tinnitus-specific and that are not general markers of noise exposure or hearing loss.
Fig. 1.
Fusiform cells recorded from DCN areas representing high-frequency sounds display increased spontaneous firing frequency in mice with tinnitus. (A) (Upper) Diagram illustrating the gap detection protocol (black trace: a startle sound stimulus preceded by a constant background sound; yellow trace: a startle sound stimulus preceded by a constant background sound with a brief gap). (Lower) Startle responses elicited by gap detection protocol were recorded as a downward pressing force on a mechanical platform. (B and C) Summary graphs of gap startle ratio (response to gap and startle stimulus/response to startle stimulus alone) for high- and low-frequency background sounds (high-frequency background, 20–32 kHz, control: n = 18, tinnitus: n = 18, nontinnitus: n = 17; low frequency background, 10–16 kHz, control: n = 17, tinnitus: n = 17, nontinnitus: n = 15). Open bars represent gap startle ratio before sham- or noise exposure; filled bars represent gap startle ratio 1 wk later. (D) (Upper) Diagram illustrating the PPI protocol; (Lower) startle responses elicited by a loud sound (black trace) or by a loud sound preceded by a brief nonstartling sound (yellow trace). (E and F) Summary graphs of prepulse startle ratio (response to prepulse and startle stimulus/response to startle stimulus alone) for high- and low-frequency prepulse (high-frequency prepulse, control: n = 18, tinnitus: n = 16, nontinnitus: n = 17; low-frequency prepulse, control: n = 17, tinnitus: n = 17, nontinnitus: n = 15). (G) (Upper) Light microscopic image of a coronal section of DCN from a P25 ICR mouse. The dotted line indicates the boundary that was used for dividing DCN areas that respond to high- (∼ ≥20 kHz, dorsal) or low-frequency sounds (ventral). (Lower) Representative cell-attached recordings from fusiform cells in the high-frequency region of the DCN from control (black), tinnitus (red), and nontinnitus mice (blue). (H and I) Summary graphs of spontaneous firing rate of fusiform cells from control, tinnitus, and nontinnitus mice in the presence of excitatory and inhibitory receptor antagonists [10 μM 2,3-dihydroxy-6-nitro- 7-sulfamoylbenzo[f]quinoxaline (NBQX), 20 μM SR95531, and 0.5 μM strychnine] (high-frequency region, control: n = 14, tinnitus: n = 16, nontinnitus: n = 12; low-frequency region, control: n = 17, tinnitus: n = 7, nontinnitus: n = 8). *P < 0.05. Error bars indicate SEM. Detailed values in SI Materials and Methods (Values for main figures).
Previous studies have shown that DCN fusiform cells exhibit elevated spontaneous firing frequency (hyperactivity) that could underlie the triggering of tinnitus (10, 16). Given that spontaneous firing of fusiform cells is dependent on their intrinsic ionic conductances (20), we blocked excitatory and inhibitory synaptic transmission to study the role of intrinsic conductances on the observed tinnitus-related DCN hyperactivity. Using whole-cell and cell-attached recordings in DCN slices, we recorded from fusiform cells in control, tinnitus, and nontinnitus mice 1 wk after noise exposure. We revealed that when synaptic transmission is blocked, only fusiform cells from tinnitus mice showed increased spontaneous activity (Fig. 1H, control: 9.7 ± 1.8 Hz, n = 14, tinnitus: 15.9 ± 1.0 Hz, n = 16, nontinnitus: 8.4 ± 0.9 Hz, n = 12, P = 0.0004). Moreover, this hyperactivity is observed only in DCN areas that represent high (∼ ≥20 kHz, dorsal part) but not low sound frequencies (21) (<20 kHz, ventral part) (Fig. 1 G and I, control: 10.0 ± 1.5 Hz, n = 17, tinnitus: 7.9 ± 1.1 Hz, n = 7, nontinnitus: 9.5 ± 1.9 Hz, n = 8, P = 0.71). Thus, our results suggest that noise-induced DCN hyperactivity is tinnitus-specific and is mediated by changes in the intrinsic ionic conductances of fusiform cells.
Decreased KCNQ Channel Activity Causes Tinnitus-Specific, DCN Hyperactivity.
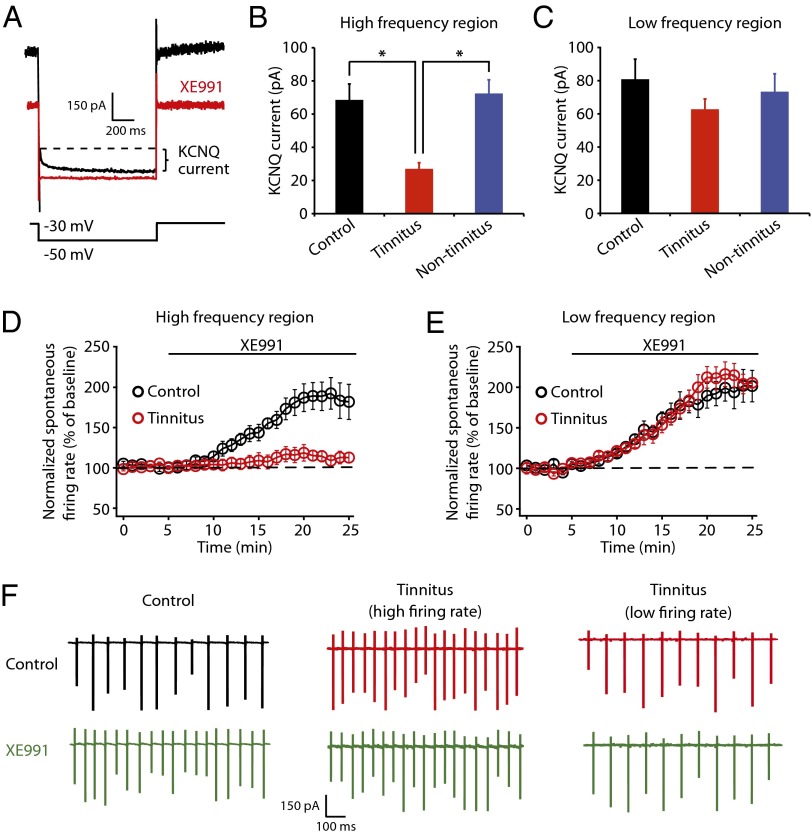
To determine the ionic conductances that are associated with the tinnitus-specific hyperactivity, we examined the intrinsic properties of fusiform cells. Our studies revealed that reduction in KCNQ (M) current—a subthreshold, noninactivating K+ current (22, 23)—is responsible for the detected DCN hyperactivity. To quantify KCNQ channel activity we held fusiform cells at −30 mV for 5 s and then stepped the voltage to −50 mV for 1 s to unmask their slow deactivation (Fig. 2A). In agreement with previous studies, this protocol revealed a slowly deactivating current that is blocked by XE991 (10 μΜ), a specific KCNQ channel blocker (Fig. 2A). By recording from control, tinnitus, and nontinnitus mice, we found that XE991-sensitive KCNQ currents are specifically reduced in tinnitus mice (Fig. 2B, control: 68.6 ± 9.6 pA, n = 7, tinnitus: 27.0 ± 3.7 pA, n = 8, nontinnitus: 72.4 ± 8.2 pA, n = 7, P = 0.004). Importantly, the reduction of KCNQ currents in tinnitus mice is observed only in fusiform cells that represent high but not low sound frequencies (Fig. 2C, control: 80.9 ± 12.0 pA, n = 6; tinnitus: 62.8 ± 6.2 pA, n = 6, nontinnitus: 73.4 ± 10.7 pA, n = 6, P = 0.45). Thus, the frequency-dependent reduction of KNCQ currents corresponds to the frequency dependence of tinnitus-specific hyperactivity (Fig. 1 H and I), as well as to the frequency dependence of tinnitus behavior (Fig. 1 B and C). Together, these results suggest that the reduction of KCNQ channel activity is associated with the tinnitus behavior and the tinnitus-specific DCN hyperactivity.
Fig. 2.
Decreased KCNQ channel activity is responsible for tinnitus-specific DCN hyperactivity. (A) Current elicited by 1 s hyperpolarization to −50 mV from a holding potential of −30 mV before (black) and after XE991 (10 μM) (red). Curly bracket indicates the XE991-sensitive, slowly deactivating, KCNQ current. (B and C) Summary graphs showing KCNQ currents as measured by protocol in A (high-frequency region, control: n = 7, tinnitus: n = 8, nontinnitus: n = 7; low-frequency region, control: n = 6, tinnitus: n = 6, nontinnitus, n = 6). (D and E) Summary graphs showing the time course of normalized spontaneous firing frequency of fusiform cells before and after XE991 in high- (control: n = 6, tinnitus: n = 7) and low-frequency DCN region (control: n = 6, tinnitus: n = 6). (F) Representative cell-attached recordings showing spontaneous firing of fusiform cell in high-frequency region of the DCN before (control: black; tinnitus: red) and after XE991 (green). All experiments were performed in the presence of excitatory and inhibitory receptor antagonists as in Fig. 1 H and I. *P < 0.05. Error bars indicate SEM.
Next we examined whether the decrease of KCNQ channel activity is causally linked to the tinnitus-specific hyperactivity. If hyperactivity of fusiform cell in tinnitus mice is caused by decreases in KCNQ channel activity, then the pharmacological blockade of KCNQ channel activity is expected to have a smaller effect on increasing the spontaneous firing activity in tinnitus mice compared with control mice. Moreover, we expect to observe this occluding effect in recordings from the high-frequency but not the low-frequency region of the DCN. Indeed, in cell-attached recordings from high-frequency DCN regions, XE991 did not affect spontaneous firing rates of fusiform cells in tinnitus mice, but it significantly increased spontaneous firing rates of fusiform cells in control mice (Fig. 2D, at 15–20 min after XE991 application, control: 184.9% ± 1.9% of baseline, n = 6, tinnitus: 111.3% ± 1.1% of baseline, n = 7, P < 0.05). Moreover, application of XE-991 equalizes the spontaneous firing rate of fusiform cells of control and tinnitus mice (Fig. S6), which further supports the hypothesis that a decrease in KCNQ channel activity is responsible for the tinnitus-specific hyperactivity. Consistent with our hypothesis, in recordings from low-frequency DCN regions, blockade of KCNQ channels with XE991 revealed a similar enhancing effect on the spontaneous firing frequency of fusiform cells in control and tinnitus mice (Fig. 2E, control: 199.8% ± 1.6% of baseline, n = 6, tinnitus: 208.8% ± 3.0% of baseline, n = 6, P > 0.05). The lack of effect of XE991 on fusiform cells in tinnitus mice is not due to a “ceiling” effect, because XE991 had a similar effect in fusiform cells with both low (<12 Hz, n = 2) and high (>12 Hz, n = 5) spontaneous firing frequency (Fig. 2F, center and right traces). Together, these results demonstrate that reduction of KCNQ channel activity is correlated and causally linked to the DCN, tinnitus-specific hyperactivity. This previously unknown plasticity of KCNQ channels reveals the importance of KCNQ channels in the generation of the neural correlates of tinnitus.
Reduced KCNQ Channel Activity Enhances Subthreshold Excitability in Tinnitus Mice.
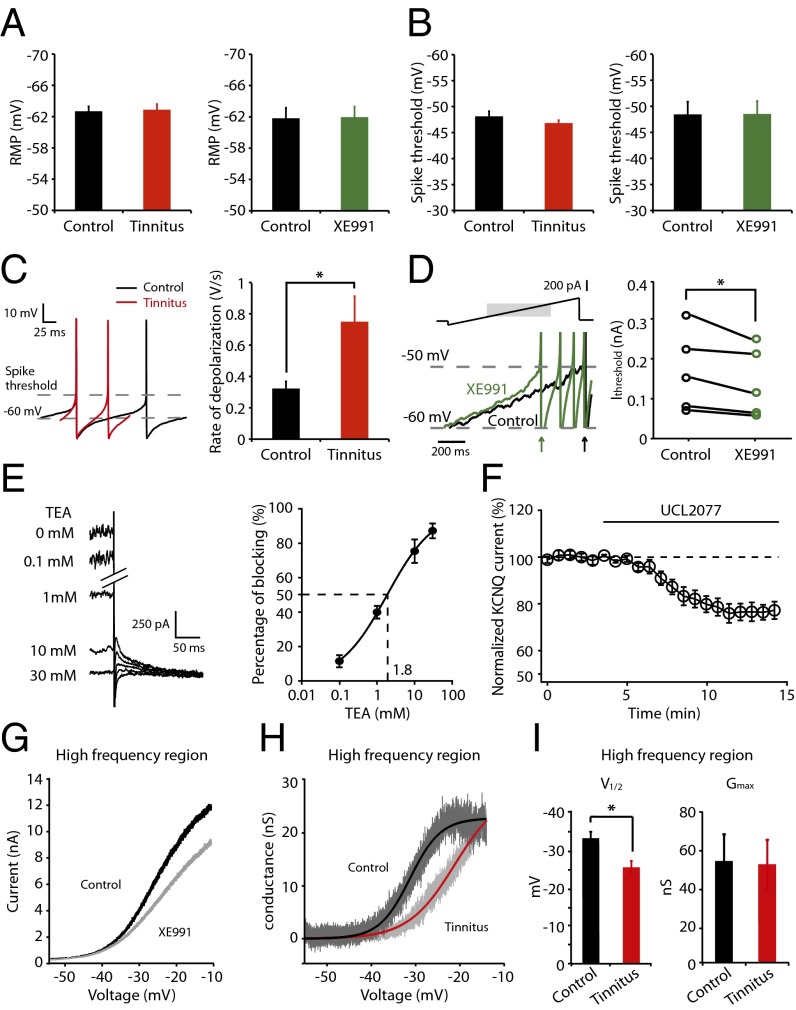
Previous studies in hippocampal neurons have shown that KCNQ channel activity reduces neuronal excitability by modulating resting membrane potential (RMP), action potential threshold, spike afterdepolarization, and subthreshold excitability (24–28). To investigate the mechanism through which KCNQ channels modulate fusiform cell excitability, we examined these parameters in control and tinnitus mice. Our results show that RMP, spike threshold, and other suprathreshold parameters are not different either between control and tinnitus mice or in control mice before and after XE991 application (Fig. 3A, RMP, control: n = 6, tinnitus: n = 11, P = 0.87; control: n = 6, XE991: n = 6, P = 0.74; Fig. 3B, spike threshold, control: n = 11, tinnitus: n = 11, P = 0.26; control: n = 6; XE991: n = 6, P = 0.85; Table S1). However, we observed a significant increase in the subthreshold excitability of tinnitus mice (Fig. 3C, depolarization rate from −60 mV to spike threshold, control: 0.32 ± 0.05 V/s, n = 11, tinnitus: 0.75 ± 0.16 V/s, n = 12, P = 0.02). Moreover, application of XE991 in control mice mimics the effect of reduced KCNQ channel activity in tinnitus mice (Fig. 3D, Left): XE991 speeds up the rate of subthreshold depolarization and reduces the threshold current (Ithreshold) needed to elicit a spike (Fig. 3D, Ithreshold, control: 0.13 ± 0.05 nA, n = 5, after XE991: 0.10 ± 0.04 nA, n = 5, P = 0.045). Together, our results suggest that the reduced KCNQ channel activity in tinnitus mice increases spontaneous firing rate of fusiform cells by increasing subthreshold excitability.
Fig. 3.
Reduced KCNQ2/3 channel activity leads to increased subthreshold excitability in tinnitus mice; this reduction is due to a depolarizing shift of V1/2. All recordings were performed in fusiform cells from the high-frequency region of the DCN. (A) (Left) RMP of fusiform cells from control and tinnitus mice (control: n = 11; tinnitus: n = 11). (Right) RMP of fusiform cells from control mice before and after the effect of XE991 (10 μΜ) (n = 6). (B) (Left) Spike threshold of fusiform cells from control and tinnitus mice (control: n = 11; tinnitus: n = 11). (Right) Spike threshold of fusiform cells from control mice before and after the effect of XE991 (n = 6). (C) Representative traces (Left) and summary graph (Right) of rate of subthreshold depolarization (from −60 mV to spike threshold) during spontaneous firing of fusiform cell in control (black: n = 11) and tinnitus mice (red: n = 12). (D) (Left) Representative traces of voltage response of fusiform cell (Lower) to injection of a current ramp (top, 0.2 nA/s) before (black) and after the effect of XE991 (green) (only response to shadowed region of stimulus is shown). Arrows indicate the times of the peak of the first spike response in each case. (Right) Currents needed to evoke the first spike during the current ramp (Ithreshold) before (black, n = 5) and after XE991 application (green, n = 5). (E) Representative traces (Left) and summary graph (Right) showing block of KCNQ currents by 0.1, 1, 10, and 30 mM TEA (0.1 mM: n = 5, 1 mM: n = 6, 10 mM: n = 6, 30 mM: n = 4). The voltage protocol is the same as in Fig. 2A. (F) Summary graph showing the time course of UCL2077 (3 μM) effect on KCNQ currents elicited at −30 mV (n = 5). (G) Voltage ramp (10 mV/s) reveals an outward current that is partially blocked by XE991. (H) Representative conductance–voltage relationship of XE991-sensitive current in control (dark gray) and tinnitus mice (light gray). Black and red lines represent Boltzmann fits. (I) Summary graph for Boltzmann fit parameters V1/2 and Gmax (control: n = 6, tinnitus: n = 6). All experiments were performed in the presence of excitatory and inhibitory receptor antagonists as in Fig. 1 H and I. *P < 0.05. Error bars indicate SEM. Detailed values in SI Materials and Methods (Values for main figures).
Plasticity of KCNQ2/3 Channels Is Crucial for the Induction of Tinnitus.
The KCNQ family of K+ channels comprises five members (KCNQ1–5). Given that mutations of KCNQ2 and KCNQ3 genes cause hyperexcitable epileptic states (29), and the expression of these subunits in the DCN (30, 31), we investigated whether KCNQ2/3 subunits mediate KCNQ currents in fusiform cells. We used tetraethylammonium chloride (TEA) and 3-(triphenylmethylaminomethyl)pyridine (UCL2077), two pharmacological agents that provide differential block to KCNQ currents mediated by different subunits. Compatible with the sensitivity of KCNQ2/3-mediated currents to these blockers (32, 33), application of TEA blocked KCNQ currents, with an IC50 of 1.8 mM (Fig. 3E, n = 4–6 for each TEA concentration), and application of UCL2077 (3 μΜ) blocked KCNQ currents by 23% (Fig. 3F, 77.3% ± 0.5% of baseline, n = 5). Given that KCNQ5-mediated currents are potentiated by UCL2077 (33, 34) and neither KCNQ1 nor KCNQ4 subunits are expressed in the DCN (35, 36), our findings suggest that KCNQ2/3 heteromers mediate the KCNQ currents in fusiform cells. Together, our results suggest that it is the plasticity of KCNQ2/3 channels that leads to the reduction of KCNQ channel activity in tinnitus mice.
Depolarizing Shift of V1/2 Causes Reduced KCNQ Channel Activity in Tinnitus Mice.
The reduction of KCNQ channel activity in tinnitus mice suggests a reduction of channel expression, a shift in the voltage dependence of channel activation, or both. To examine these possibilities, we compared maximal conductance (Gmax) and half-maximal activation voltage (V1/2) of KCNQ channels in control and tinnitus mice. Voltage ramps evoked an outward current that was partially suppressed by XE991 (Fig. 3G); by subtracting the trace after application of XE991 from the control trace, we determined the conductance–voltage relationship for KCNQ currents (Fig. 3H). Through fitting with a Boltzmann relationship, we revealed that in tinnitus mice Gmax is not reduced (Fig. 3I), but the V1/2 of KCNQ currents is shifted to more depolarized potentials (Fig. 3I, V1/2, control: −32.8 ± 1.7 mV, n = 6, tinnitus: −25.1 ± 1.7 mV, n = 6, P = 0.009; Gmax, control: 54.7 ± 14.2 nS, n = 6, tinnitus: 53.1 ± 12.9 nS, n = 6, P = 0.93). Although the Boltzmann fits—especially for determining maximal conductance—are limited by the range of voltages over which we were able to maintain voltage clamp, our results suggest that a depolarizing shift in V1/2 in tinnitus mice is important for the reduction in KCNQ currents that, in turn, leads to tinnitus-specific hyperactivity.
Retigabine, a KCNQ Channel Activator, Prevents the Development of Tinnitus.
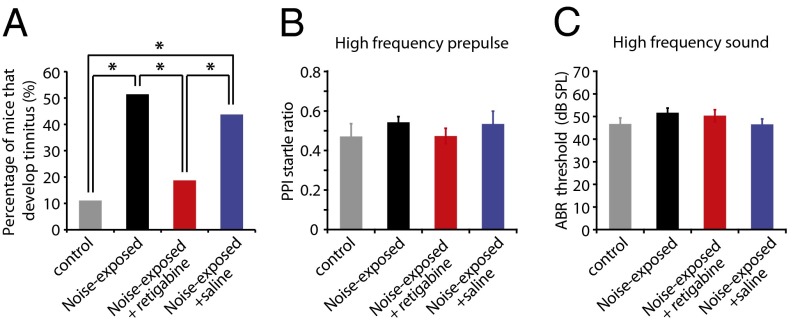
The critical role of the voltage dependence of KCNQ channels on generating tinnitus-specific hyperactivity suggests the provocative link between the biophysical properties of KCNQ channels and the development of perception of phantom sound. This hypothesis predicts that pharmacological shift in the voltage dependence of KCNQ channel activity to more hyperpolarized potentials will inhibit the development of tinnitus behavior. To test this hypothesis, we injected noise-exposed mice (starting 30 min after noise exposure and continuing injections twice per day for 5 d) with retigabine: retigabine specifically enhances KCNQ channel activation by causing a hyperpolarizing shift in the voltage dependence of the channel activation (37, 38). Intraperitoneal injection of retigabine (as its dihydrochloride salt) reduced the percentage of noise-exposed mice that develop tinnitus to the same level as in control mice [Fig. 4A, control: 11.1% (2 of 18; SI Materials and Methods), noise-exposed: 51.4% (18 of 35), noise-exposed + retigabine: 18.8% (3 of 16), noise-exposed + saline: 43.8% (7 of 16)]. Retigabine does not affect temporal processing or hearing, because it did not affect PPI or hearing thresholds (Fig. 4B, PPI, control: n = 16, noise-exposed: n = 33, noise-exposed + retigabine: n = 16, noise-exposed + saline: n = 16, P = 0.55; Fig. 4C, auditory brainstem response (ABR) threshold, control: n = 7, noise-exposed: n = 18, noise-exposed + retigabine: n = 7, noise-exposed + saline: n = 7, P = 0.35; Figs. S7 and S8). Although i.p. injection of retigabine affects KCNQ channels throughout the brain, these results, in combination with our physiological studies (Figs. 1–3) and the key role of DCN in tinnitus induction (14), support the notion that the pathogenic plasticity of subcortical KCNQ channel activity is crucial for the induction of tinnitus. Importantly, these results link the voltage dependence of KCNQ channel opening with the development of the perception of phantom sound.
Fig. 4.
Pharmacological enhancement of KCNQ channel activity prevents the development of tinnitus. All data were recorded 1 wk after sham- or noise exposure. (A) Percentage of mice that develop tinnitus (control: n = 18, noise-exposed: n = 35, noise-exposed + retigabine: n = 16, noise-exposed + saline: n = 16). (B and C) PPI and ABR thresholds for high-frequency testing sounds (20–32 kHz) (PPI startle ratio, control: n = 16, noise-exposed: n = 33, noise-exposed + retigabine: n = 16, noise-exposed + saline: n = 16; ABR thresholds, control: n = 7, noise-exposed: n = 18, noise-exposed + retigabine: n = 7, noise-exposed + saline: n = 7). *P < 0.05. Error bars indicate SEM. Detailed values in SI Materials and Methods (Values for main figures).
Discussion
KCNQ channels are slowly activating, noninactivating potassium channels that open at subthreshold membrane potentials. The time- and voltage dependent properties of KCNQ channels enable them to function as effective “brakes” that control excitability in neuronal, sensory, and muscular cells (23, 39). The importance of KCNQ channels is emphatically illustrated by the finding that mutations in KCNQ genes underlie multiple neurological diseases that are characterized by membrane hyperexcitability, such as epilepsy (29, 40). Here we report that tinnitus is a KCNQ channelopathy: a reduction in KCNQ2/3 channel activity leads to DCN tinnitus-specific hyperactivity and initiates the development of tinnitus.
KCNQ channels, often KCNQ2 and KCNQ3, mediate the native neuronal M-type current (22, 32). M currents are strongly inhibited by activation of muscarinic acetylcholine receptors (mAChRs) and other G protein-coupled receptors that reduce membrane phosphatidylinositol-(4,5)-bisphosphate (PIP2) levels (41–43). Given the important role of cholinergic activity in DCN synaptic plasticity (44) and that noise exposure increases cholinergic activity in the DCN (45, 46), our results suggest that noise-induced up-regulation of mAChR signaling may underlie the reduced KCNQ channel activity in tinnitus mice.
Given that ablation of the DCN does not eliminate tinnitus once developed (47), we propose the existence of a “critical period” during which KCNQ channel enhancement is capable of preventing the development of tinnitus. Therefore, we suggest that plasticity of subcortical KCNQ2/3 channels is essential for the induction but not the expression of tinnitus. Recent studies show that cortical reorganization (6, 7) and aberrant thalamocortical rhythms (48) (thalamocortical dysrythmia) may underlie the expression of tinnitus; thalamocortical dysrythmia, by promoting stimulus-independent γ oscillations could maintain the conscious perception. Moreover, although it is evident that auditory system dysfunction is necessary for the generation of tinnitus, the maintenance of chronic tinnitus may involve pathological interactions between auditory and nonauditory structures, such as the limbic system (49, 50). Thus, our findings suggest that KCNQ-mediated, subcortical hyperactivity may be triggering cortical reorganization and thalamocortical dysrythmia, which, in combination with changes in the limbic system, lead to the maintenance of the perception of phantom sound.
Chronic neuropathic pain is another phantom perception that is initiated by a peripheral trauma that results in subcortical hyperexcitability and cortical reorganization (51, 52). Although changes in KCNQ channels have been associated with chronic neuropathic pain (53, 54), a detailed mechanistic scheme for the role of KCNQ channels in pain has yet to emerge. Given the similarities between tinnitus and central neuropathic pain (55–57), our studies suggest that plasticity of KCNQ biophysical properties is a promising site for investigating the induction mechanisms of chronic neuropathic pain.
Although our studies do not exclude other synaptic and intrinsic plasticity mechanisms that may contribute to the tinnitus-related changes in DCN excitability (18, 58, 59), our results highlight KCNQ2/3 channels as key players in the induction of tinnitus. KCNQ channels have been attractive targets for treating diseases associated with hyperexcitability (60); retigabine, a KCNQ channel activator, has been recently approved as an anticonvulsant. Our findings, by illustrating the role of reduced KCNQ channel activity in the induction of tinnitus, suggest that KCNQ2/3 channel activators are promising therapeutic drugs for preventing the development of tinnitus.
Materials and Methods
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Methods for inducing and behaviorally testing for tinnitus, criteria for the behavioral evidence of tinnitius, behavioral testing with retigabine, preparing brain slices, recording electrical signals from DCN slices, data analysis and statistics are provided in SI Materials and Methods. Moreover, values for main figures and quantification of intrinsic properties of fusiform cells are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Karl Kandler and Gordon Shepherd for manuscript comments; Drs. Laurence Trussell and Anastassios Tzingounis for helpful discussions; and all the members of the Tzounopoulos, Kandler, and Rubio laboratories for many helpful suggestions. This study was supported by US Department of Defense Peer Reviewed Medical Research Program Grant PR093405 and by National Institutes of Health/National Institute on Deafness and Other Communication Disorders Grant DC007905 (both to T.T.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1216671110/-/DCSupplemental.
References
- 1.Axelsson A, Ringdahl A. Tinnitus—a study of its prevalence and characteristics. Br J Audiol. 1989;23(1):53–62. doi: 10.3109/03005368909077819. [DOI] [PubMed] [Google Scholar]
- 2.Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123(8):711–718. doi: 10.1016/j.amjmed.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Roberts LE, et al. Ringing ears: The neuroscience of tinnitus. J Neurosci. 2010;30(45):14972–14979. doi: 10.1523/JNEUROSCI.4028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yankaskas K. Prelude: Noise-induced tinnitus and hearing loss in the military. Hear Res. 2013;295(295):3–8. doi: 10.1016/j.heares.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 5.Schaette R, McAlpine D. Tinnitus with a normal audiogram: Physiological evidence for hidden hearing loss and computational model. J Neurosci. 2011;31(38):13452–13457. doi: 10.1523/JNEUROSCI.2156-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engineer ND, et al. Reversing pathological neural activity using targeted plasticity. Nature. 2011;470(7332):101–104. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004;27(11):676–682. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear Res. 2000;147(1-2):261–274. doi: 10.1016/s0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- 9.Melcher JR, Sigalovsky IS, Guinan JJ, Jr, Levine RA. Lateralized tinnitus studied with functional magnetic resonance imaging: Abnormal inferior colliculus activation. J Neurophysiol. 2000;83(2):1058–1072. doi: 10.1152/jn.2000.83.2.1058. [DOI] [PubMed] [Google Scholar]
- 10.Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci. 2002;22(6):2383–2390. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finlayson PG, Kaltenbach JA. Alterations in the spontaneous discharge patterns of single units in the dorsal cochlear nucleus following intense sound exposure. Hear Res. 2009;256(1-2):104–117. doi: 10.1016/j.heares.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Middleton JW, et al. Mice with behavioral evidence of tinnitus exhibit dorsal cochlear nucleus hyperactivity because of decreased GABAergic inhibition. Proc Natl Acad Sci USA. 2011;108(18):7601–7606. doi: 10.1073/pnas.1100223108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang S, Weiner BD, Zhang LS, Cho SJ, Bao S. Homeostatic plasticity drives tinnitus perception in an animal model. Proc Natl Acad Sci USA. 2011;108(36):14974–14979. doi: 10.1073/pnas.1107998108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brozoski TJ, Wisner KW, Sybert LT, Bauer CA. Bilateral dorsal cochlear nucleus lesions prevent acoustic-trauma induced tinnitus in an animal model. J Assoc Res Otolaryngol. 2012;13(1):55–66. doi: 10.1007/s10162-011-0290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaltenbach JA, Zhang J, Finlayson P. Tinnitus as a plastic phenomenon and its possible neural underpinnings in the dorsal cochlear nucleus. Hear Res. 2005;206(1-2):200–226. doi: 10.1016/j.heares.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Kaltenbach JA, Zacharek MA, Zhang J, Frederick S. Activity in the dorsal cochlear nucleus of hamsters previously tested for tinnitus following intense tone exposure. Neurosci Lett. 2004;355(1-2):121–125. doi: 10.1016/j.neulet.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, et al. Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience. 2009;164(2):747–759. doi: 10.1016/j.neuroscience.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng C, Yang Z, Shreve L, Bledsoe S, Shore S. Somatosensory projections to cochlear nucleus are upregulated after unilateral deafness. J Neurosci. 2012;32(45):15791–15801. doi: 10.1523/JNEUROSCI.2598-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner JG, et al. Gap detection deficits in rats with tinnitus: A potential novel screening tool. Behav Neurosci. 2006;120(1):188–195. doi: 10.1037/0735-7044.120.1.188. [DOI] [PubMed] [Google Scholar]
- 20.Leao RM, Li S, Doiron B, Tzounopoulos T. Diverse levels of an inwardly rectifying potassium conductance generate heterogeneous neuronal behavior in a population of dorsal cochlear nucleus pyramidal neurons. J Neurophysiol. 2012;107(11):3008–3019. doi: 10.1152/jn.00660.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parham K, et al. Purkinje cell degeneration and control mice: Responses of single units in the dorsal cochlear nucleus and the acoustic startle response. Hear Res. 2000;148(1–2):137–152. doi: 10.1016/s0378-5955(00)00147-7. [DOI] [PubMed] [Google Scholar]
- 22.Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- 23.Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci. 2005;6(11):850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- 24.Hu H, Vervaeke K, Storm JF. M-channels (Kv7/KCNQ channels) that regulate synaptic integration, excitability, and spike pattern of CA1 pyramidal cells are located in the perisomatic region. J Neurosci. 2007;27(8):1853–1867. doi: 10.1523/JNEUROSCI.4463-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peters HC, Hu H, Pongs O, Storm JF, Isbrandt D. Conditional transgenic suppression of M channels in mouse brain reveals functions in neuronal excitability, resonance and behavior. Nat Neurosci. 2005;8(1):51–60. doi: 10.1038/nn1375. [DOI] [PubMed] [Google Scholar]
- 26.Shah MM, Migliore M, Valencia I, Cooper EC, Brown DA. Functional significance of axonal Kv7 channels in hippocampal pyramidal neurons. Proc Natl Acad Sci USA. 2008;105(22):7869–7874. doi: 10.1073/pnas.0802805105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yue C, Yaari Y. KCNQ/M channels control spike afterdepolarization and burst generation in hippocampal neurons. J Neurosci. 2004;24(19):4614–4624. doi: 10.1523/JNEUROSCI.0765-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tzingounis AV, Nicoll RA. Contribution of KCNQ2 and KCNQ3 to the medium and slow afterhyperpolarization currents. Proc Natl Acad Sci USA. 2008;105(50):19974–19979. doi: 10.1073/pnas.0810535105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jentsch TJ. Neuronal KCNQ potassium channels: Physiology and role in disease. Nat Rev Neurosci. 2000;1(1):21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- 30.Saganich MJ, Machado E, Rudy B. Differential expression of genes encoding subthreshold-operating voltage-gated K+ channels in brain. J Neurosci. 2001;21(13):4609–4624. doi: 10.1523/JNEUROSCI.21-13-04609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooper EC, Harrington E, Jan YN, Jan LY. M channel KCNQ2 subunits are localized to key sites for control of neuronal network oscillations and synchronization in mouse brain. J Neurosci. 2001;21(24):9529–9540. doi: 10.1523/JNEUROSCI.21-24-09529.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang HS, et al. KCNQ2 and KCNQ3 potassium channel subunits: Molecular correlates of the M-channel. Science. 1998;282(5395):1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- 33.Soh H, Tzingounis AV. The specific slow afterhyperpolarization inhibitor UCL2077 is a subtype-selective blocker of the epilepsy associated KCNQ channels. Mol Pharmacol. 2010;78(6):1088–1095. doi: 10.1124/mol.110.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang H, Trussell LO. KCNQ5 channels control resting properties and release probability of a synapse. Nat Neurosci. 2011;14(7):840–847. doi: 10.1038/nn.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kharkovets T, et al. KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proc Natl Acad Sci USA. 2000;97(8):4333–4338. doi: 10.1073/pnas.97.8.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldman AM, et al. Arrhythmia in heart and brain: KCNQ1 mutations link epilepsy and sudden unexplained death. Sci Transl Med. 2009;1(2):2ra6. doi: 10.1126/scitranslmed.3000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tatulian L, Delmas P, Abogadie FC, Brown DA. Activation of expressed KCNQ potassium currents and native neuronal M-type potassium currents by the anti-convulsant drug retigabine. J Neurosci. 2001;21(15):5535–5545. doi: 10.1523/JNEUROSCI.21-15-05535.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiong Q, Sun H, Zhang Y, Nan F, Li M. Combinatorial augmentation of voltage-gated KCNQ potassium channels by chemical openers. Proc Natl Acad Sci USA. 2008;105(8):3128–3133. doi: 10.1073/pnas.0712256105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soldovieri MV, Miceli F, Taglialatela M. Driving with no brakes: Molecular pathophysiology of Kv7 potassium channels. Physiology (Bethesda) 2011;26(5):365–376. doi: 10.1152/physiol.00009.2011. [DOI] [PubMed] [Google Scholar]
- 40.Maljevic S, Wuttke TV, Seebohm G, Lerche H. KV7 channelopathies. Pflugers Arch. 2010;460(2):277–288. doi: 10.1007/s00424-010-0831-3. [DOI] [PubMed] [Google Scholar]
- 41.Marrion NV. Control of M-current. Annu Rev Physiol. 1997;59:483–504. doi: 10.1146/annurev.physiol.59.1.483. [DOI] [PubMed] [Google Scholar]
- 42.Suh BC, Hille B. Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron. 2002;35(3):507–520. doi: 10.1016/s0896-6273(02)00790-0. [DOI] [PubMed] [Google Scholar]
- 43.Zhang H, et al. PIP(2) activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron. 2003;37(6):963–975. doi: 10.1016/s0896-6273(03)00125-9. [DOI] [PubMed] [Google Scholar]
- 44.Zhao Y, Tzounopoulos T. Physiological activation of cholinergic inputs controls associative synaptic plasticity via modulation of endocannabinoid signaling. J Neurosci. 2011;31(9):3158–3168. doi: 10.1523/JNEUROSCI.5303-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin YM, Godfrey DA, Wang J, Kaltenbach JA. Effects of intense tone exposure on choline acetyltransferase activity in the hamster cochlear nucleus. Hear Res. 2006;216-217:168–175. doi: 10.1016/j.heares.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Kaltenbach JA, Zhang J. Intense sound-induced plasticity in the dorsal cochlear nucleus of rats: evidence for cholinergic receptor upregulation. Hear Res. 2007;226(1-2):232–243. doi: 10.1016/j.heares.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Brozoski TJ, Bauer CA. The effect of dorsal cochlear nucleus ablation on tinnitus in rats. Hear Res. 2005;206(1-2):227–236. doi: 10.1016/j.heares.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 48.Llinás R, Urbano FJ, Leznik E, Ramírez RR, van Marle HJ. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci. 2005;28(6):325–333. doi: 10.1016/j.tins.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 49.Rauschecker JP, Leaver AM, Mühlau M. Tuning out the noise: Limbic-auditory interactions in tinnitus. Neuron. 2010;66(6):819–826. doi: 10.1016/j.neuron.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leaver AM, et al. Dysregulation of limbic and auditory networks in tinnitus. Neuron. 2011;69(1):33–43. doi: 10.1016/j.neuron.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306(5944):686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- 52.Vartiainen N, Kirveskari E, Kallio-Laine K, Kalso E, Forss N. Cortical reorganization in primary somatosensory cortex in patients with unilateral chronic pain. J Pain. 2009;10(8):854–859. doi: 10.1016/j.jpain.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 53.Passmore GM, et al. KCNQ/M currents in sensory neurons: Significance for pain therapy. J Neurosci. 2003;23(18):7227–7236. doi: 10.1523/JNEUROSCI.23-18-07227.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. Br J Pharmacol. 2009;156(8):1185–1195. doi: 10.1111/j.1476-5381.2009.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tonndorf J. The analogy between tinnitus and pain: A suggestion for a physiological basis of chronic tinnitus. Hear Res. 1987;28(2-3):271–275. doi: 10.1016/0378-5955(87)90054-2. [DOI] [PubMed] [Google Scholar]
- 56.Møller AR. Tinnitus and pain. Prog Brain Res. 2007;166:47–53. doi: 10.1016/S0079-6123(07)66004-X. [DOI] [PubMed] [Google Scholar]
- 57.De Ridder D, Elgoyhen AB, Romo R, Langguth B. Phantom percepts: Tinnitus and pain as persisting aversive memory networks. Proc Natl Acad Sci USA. 2011;108(20):8075–8080. doi: 10.1073/pnas.1018466108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang H, Brozoski TJ, Caspary DM. Inhibitory neurotransmission in animal models of tinnitus: Maladaptive plasticity. Hear Res. 2011;279(1-2):111–117. doi: 10.1016/j.heares.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pilati N, et al. Mechanisms contributing to central excitability changes during hearing loss. Proc Natl Acad Sci USA. 2012;109(21):8292–8297. doi: 10.1073/pnas.1116981109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wulff H, Castle NA, Pardo LA. Voltage-gated potassium channels as therapeutic targets. Nat Rev Drug Discov. 2009;8(12):982–1001. doi: 10.1038/nrd2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.