Abstract
A role for serotonin in male sexual preference was recently uncovered by our finding that male mutant mice lacking serotonin have lost sexual preference. Here we show that female mouse mutants lacking either central serotonergic neurons or serotonin prefer female over male genital odors when given a choice, and displayed increased female–female mounting when presented either with a choice of a male and a female target or only with a female target. Pharmacological manipulations and genetic rescue experiments showed that serotonin is required in adults. Behavioral changes caused by deficient serotonergic signaling were not due to changes in plasma concentrations of sex hormones. We demonstrate that a genetic manipulation reverses sexual preference without involving sex hormones. Our results indicate that serotonin controls sexual preference.
Keywords: sexual behaviors, neurotransmitter, Tph2 knockout
Sexual behaviors are among the most important social behaviors. Although preference for the opposite sex is essential for reproduction, sexual behaviors toward members of the same sex have been observed in many animal species, indicating that there are potential evolutionary advantages (1, 2). The diversity of sexual preference has been of scientific interest to scholars from Aristotle to present-day scientists (2, 3).
Biologically, changes in sex hormones can change sexual behavior or sexual preference, resulting in either a loss of sexual preference or a reversal of sexual preference (4–14). Although a genetic component for homosexual orientation has been suggested (15), no specific genes have been identified in sexual preference in humans (16–20).
Same-sex preference was reported in female mice lacking the gene encoding estrogen-binding plasma protein alpha-fetoprotein (AFP) or those lacking the gene for aromatase through indirect effects on sex hormones (11, 12, 14, 21). Pheromone perception is important for sexual behaviors. Surgical removal of the vomeronasal organ or genetic inactivation of transient receptor potential channel 2 (TrpC2), which encodes a cation channel in the vomeronasal organ (22, 23), or cyclic nucleotide-gated channel α2 (Cgna2) in the main olfactory epithelium (24) resulted in loss of sexual preference in male mice. TrpC2−/− mutant females showed female–female mounting behavior (13, 25). There was an overall reduction of sexual behavior in Cgna2 mutant mice (19). However, none of these mice has been shown to prefer the same sex.
Our recent genetic studies have shown that 5-hydroxytryptamine (5-HT) in the male mouse brain is required for sexual preference because there was no sexual preference in mutant male mice lacking serotonergic neurons or 5-HT (26). We have now carried out experiments to determine whether 5-HT is involved in female sexual preference. Here we report a crucial role of serotonergic signaling in female sexual preference: strikingly, sexually differential olfactory preference indicated by several assays was reversed in female mice lacking serotonergic neurons or those unable to synthesize 5-HT in the brain. Our results suggest a role for 5-HT in sexual preference is separate from roles in sexual drive and discrimination. These studies have furthered our understanding of molecular mechanisms underlying neural control of sexual preference.
Results
Female Mice Lacking Serotonergic Neurons Preferred Female Over Male Mice.
It was known that conditional LIM homeobox transcription factor 1-beta (Lmx1b) knockout mice (Lmx1b−/−) could be generated by crossing Cre recombinase drived by Pet-1 enhancer (ePet-Cre) into Lmx1bfloxp/floxp mice, leading to the absence of serotonergic neurons in the brain without affecting 5-HT in the periphery (27). We have obtained female Lmx1b−/− mice and confirmed that the levels of 5-HT and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) were lower in the brains of homozygous (Lmx1b−/−) mutant females than those in the WT (Lmx1b+/+) and heterozygous (Lmx1b+/−) females (Fig. S1 A and B). The level of 5-HT in heterozygous females was also lower than that in the WT.
Sexual preference was first investigated by presenting a male and a female target mouse to a test female mouse. We have observed that head and genital areas in male rodents are highly attractive to WT female rodents, which is likely due to the presence of pheromones in exocrine glands in those areas (28, 29). We measured the latency, frequency, and duration of females attempting to sniff the genital and head areas of male and female targets. It was clear that WT females preferred male head and genital areas over female head and genital areas, whereas Lmx1b−/− females showed the opposite preference. The latency for sniffing target mice was not different among the Lmx1b+/+, Lmx1b+/−, and Lmx1b−/− females (Fig. S2A). When the duration of sniffing the whole body was compared, Lmx1b+/ + and Lmx1b+/− females spent longer time sniffing male target mice than female targets (Fig. 1A and Fig. S2C). By contrast, Lmx1b−/− females sniffed female targets longer than male targets (Fig. 1A and Fig. S2C). In sniff bouts, Lmx1b+/+ and Lmx1b+/− females showed preference for males over females, whereas Lmx1b−/− females showed preference for females over males (Fig. S2B).
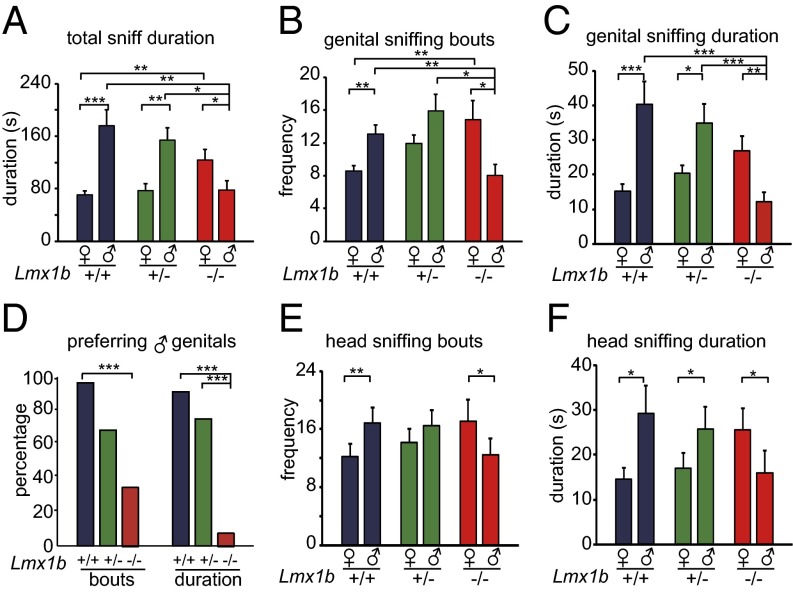
Fig. 1.
Lmx1b−/− female mice preferred female over male mice. A test female was presented with a male and a female target. n = 18 for Lmx1b+/+ (+/+), n = 15 for Lmx1b+/ - (+/−), n = 15 for Lmx1b−/− (−/−). *P < 0.05, **P < 0.01, ***P < 0.001. (A) Lmx1b−/− females sniffed females longer than males, whereas their Lmx1b+/+ and Lmx1b+/− female littermates sniffed males longer. Lmx1b−/− females sniffed males for a shorter time than their female littermates. Lmx1b−/− female mice sniffed females longer than their Lmx1b+/+ littermates. (B) Lmx1b−/− females sniffed female genitals more often than male genitals, whereas their Lmx1b+/+ female littermates sniffed male genitals more often than female genitals. Lmx1b+/− female littermates did not show sexual preference in sniff bouts. Lmx1b−/− females sniffed male genitals less and female genitals more than Lmx1b+/+ females. (C) Lmx1b−/− females sniffed female genitals longer than male genitals, whereas their Lmx1b+/+ and Lmx1b+/− female littermates sniffed male genitals longer than female genitals. There is no significant difference among Lmx1b−/−, Lmx1b+/+, and Lmx1b+/− females in the duration of sniffing female genitals, but Lmx1b−/− females sniffed male genitals for a shorter time than their female littermates. (D) Percentage of mice of each genotype that sniffed male genitals more (bouts) or longer (duration) than female genitals. (E) Lmx1b−/− females sniffed female heads more than male heads, whereas their Lmx1b+/+ female littermates sniffed male heads more. Lmx1b+/− females had an intermediate phenotype: they had no preference for either males or females. (F) Lmx1b−/− females sniffed female heads for a longer duration than male heads, whereas their Lmx1b+/+ and Lmx1b+/− female littermates sniffed male heads longer than female heads.
The reversal of sexual preference was particularly obvious when sniffing of the genital and head areas was analyzed separately from the rest of the body. Lmx1b−/− mice showed a shorter latency to approach and sniff the genital area of female targets than that of male targets, whereas Lmx1b+/+ or Lmx1b+/ -females did not (Fig. S2D). Lmx1b+/+female littermates showed significant preference for male over female genital areas, both in the number of sniffing bouts (Fig. 1B and Fig. S2E) and in sniffing duration (Fig. 1C and Fig. S2F). This preference was reversed in Lmx1b−/− females (Fig. 1 B and C and Fig. S2 E and F): they sniffed the female genital area more frequently and with longer duration than the male genital area. Compared with their Lmx1b+/+ and Lmx1b+/− littermates, a significantly smaller percentage of Lmx1b−/− females preferred the male over female genital area (Fig. 1D). Lmx1b−/− female mice also showed reversed preference of head sniffing; Lmx1b−/− sniffed female heads more frequently (Fig. 1E and Fig. S2G) and for longer duration than male heads (Fig. 1F and Fig. S2H).
An intermediate phenotype was detected in Lmx1b+/− females: they were similar to Lmx1b+/+ females in sniff duration preference (Fig. 1 A, C, and F and Fig. S2F), but their preference was not statistically significant when the preference was analyzed with sniff bout frequency (Fig. 1 B and E). This is consistent with an intermediate level of 5-HT in Lmx1b+/− females (Fig. S1A), suggesting that this phenotype is sensitive to the dosage of 5-HT. The dosage sensitivity was also observed in some of the other assays (Fig. 2B).
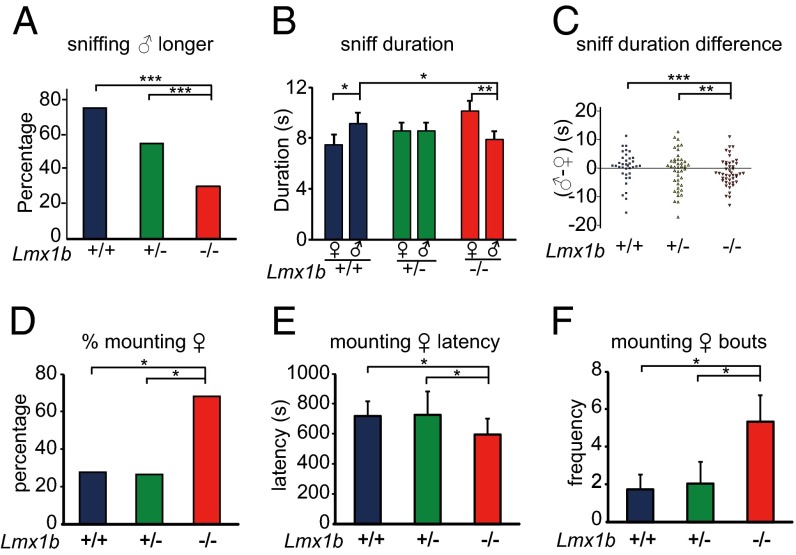
Fig. 2.
Lmx1b−/− females prefer estrous female genital odor over male genital odor and show more female–female mounting. A test female was presented with a slide smeared with male and estrous female genital excretions. (A–C) n = 31 for Lmx1b+/+ (+/+), n = 44 for Lmx1b+/− (+/−), n = 48 for Lmx1b−/− (−/−). *P < 0.05, **P < 0.01, ***P < 0.001. (A) Compared with their Lmx1b+/+ and Lmx1b+/− female littermates, a smaller percentage of Lmx1b−/− females sniffed male genital odor longer than estrous female genital odor. (B) Lmx1b−/− females preferred estrous female genital odor over male genital odor. Their Lmx1b+/+ female littermates sniffed male genital odor longer than estrous female genital odor. The sniff duration for male genital odor was shorter in Lmx1b−/− females than in their Lmx1b+/+ female littermates. Lmx1b+/− female littermates did not show a preference for either male or female genital odor. (C) Sniff duration of individual females was analyzed: sniff duration for male minus sniff duration for female. (D–F) A test estrous female was analyzed for its mounting of a female (+♀), n = 24 for Lmx1b+/+ (+/+), n = 15 for Lmx1b+/− (+/−), n = 19 for Lmx1b−/− (−/−). (D) A higher percentage of female–female mounting occurred in estrous Lmx1b−/− females than in their female Lmx1b+/+ and Lmx1b+/− littermates. (E) Female–female mounting latency of Lmx1b−/− females was modestly but significant less than their female Lmx1b+/+ and Lmx1b+/− littermates. (F) Female–female mounting bouts occurred more in Lmx1b−/− females than their female Lmx1b+/+ and Lmx1b+/− littermates.
These results indicate that mutant females lacking serotonergic neurons showed preference for females over males.
Preference of Female Mice Lacking Serotonergic Neurons for Female vs. Male Genital Odors.
When presented with live animals, the selection of sexual partners by rodents will be affected by behavioral feedback from target mice and by olfactory, visual, and acoustic cues of targets (1, 30). To avoid behavioral feedback from live animals and to examine pheromonal preference specifically, we used the genital odor assay to study the preference of female mice for male and female genital excretions.
As known previously (31), WT females were more attracted by pheromones present in the genital areas of the opposite sex than by those from the same sex: when given a choice between adult male genital excretion and estrous female genital excretion smeared on two sides of a slide, more Lmx1b+/+ and Lmx1b+/− females sniffed genital odor from males longer than from that of estrous females (Fig. 2A), whereas a significantly lower percentage of Lmx1b−/− females sniffed male genital odor longer than female genital odor (Fig. 2A). Lmx1b+/+ females sniffed male genital odor longer than female genital odor (Fig. 2B). Lmx1b+/− females sniffed male and female genital odor equivalently (Fig. 2B). Lmx1b−/− females sniffed female genital odor longer than male genital odor (Fig. 2B). Lmx1b−/− females sniffed male genital odor for a shorter duration than did the WT female littermates (Fig. 2B). By examining the difference in sniff duration of individual females, we also observed that Lmx1b−/− females were significantly different from Lmx1b+/+ and Lmx1b+/− females (Fig. 2C). The sniff latency of Lmx1b−/− mice was not significantly different from those of Lmx1b+/+ and Lmx1b+/− female littermates (Fig. S3A). When choosing between genital odors from diestrous females and males, Lmx1b−/− females also showed same-sex preference, whereas Lmx1b+/+ and Lmx1b+/− females showed no preference (Fig. S4 A–C).
When choosing between genital odor from intact males and that from castrated males, Lmx1b+/+ females preferred intact males over castrated males (Fig. S3B), whereas Lmx1b+/− and Lmx1b−/− females did not show a preference. The percentage of females preferring intact male genital odor was significantly lower in Lmx1b−/− females than either Lmx1b+/+ or Lmx1b+/− female littermates (Fig. S3C). Thus, Lmx1b−/− females were different from the WT females in their preference of odors present in the genital area of intact males.
An intermediate phenotype was also detected in Lmx1b+/− mice: they had lost preference between males and females (Fig. 2B) or between intact and castrated males (Fig. S3B). Thus, genital odor preference is also sensitive to the dosage of 5-HT.
When a test mouse was provided with a choice of male odor over saline or female odor over saline, no difference was detected among Lmx1b−/−, Lmx1b+/+, and Lmx1b+/− females (Fig. S5), indicating that Lmx1b−/− females were not generally defective in olfaction.
Female–Female Mounting by Mice Lacking Serotonergic Neurons.
When a test female mouse was presented with a target female, 68.4% of estrous Lmx1b−/− females mounted female intruders, whereas only ∼30% of Lmx1b+/+ and Lmx1b+/− female littermates exhibited this behavior (Fig. 2D). Lmx1b−/− females initiated mounting earlier and mounted more frequently than their female littermates (Fig. 2 E and F). The mounting behaviors of Lmx1b−/− females were similar to the male typical sexual behaviors: they sniffed female mice and tried to grasp the intruder females by the waist before mounting on their back. During mounting, Lmx1b−/− females showed pelvic thrusts toward the genital areas of the intruder females. If female intruders escaped from their grasp, Lmx1b−/− females often chased them and tried to mount again. This same-sex mounting behavior was not significantly changed by the estrous cycle. Diestrous Lmx1b−/− females also showed more mounting behavior than their female littermates (Fig. S6 A–C).
When presented with a male mouse, only a small percentage of estrous female mice mounted male intruders. Female–male mounting behavior was not significantly different among Lmx1b+/+, Lmx1b+/−, and Lmx1b−/− females (Fig. S6 D–F). Thus, elimination of central serotonergic neurons significantly increased female–female mounting.
Female Sexual Behaviors of Mice Lacking Serotonergic Neurons.
To investigate female-typical sexual behaviors, we presented a WT male to a test female. When mounted by males, Lmx1b−/− females were similar to Lmx1b+/+ and Lmx1b+/− female littermates: initially showing typical rejection behaviors, such as running and fighting, followed by proceptive and receptive behaviors.
During the first 10 mounts by male mice, Lmx1b−/− females showed proceptive behaviors about one time and lordosis behaviors about seven times (Fig. S6 G and H). These were not significantly different from their female littermates (Fig. S6 G and H). The receptive scores were not significantly different between Lmx1b−/− females and their littermates (Fig. S6I). Thus, lack of central serotonergic neurons did not change the female typical sexual behaviors when females encountered males.
Sexual Preference of Tph2 Knockout Female Mice.
Although studies of Lmx1b mutants have revealed a role for serotonergic neurons in female sexual preference, it did not show a role for 5-HT. We used mice mutant for tryptophan hydroxylases 2 (Tph2), which encodes the enzyme tryptophan hydroxylase required for the first step in the brain’s biosynthesis of 5-HT (26). The levels of 5-HT and 5-HIAA were significantly lower in the brains of Tph2−/− females than those in Tph2+/+ or Tph2+/− females (Fig. S1 C and D). Behavioral analysis of Tph2−/− females allowed us to examine the function of 5-HT.
When presented with a WT male and a WT female, Tph2−/− females displayed a change in sexual preference. Tph2+/+ females sniffed males with a shorter latency than females (Fig. S7A), but Tph2−/− females did not. Tph2+/+ females sniffed males longer than females, but Tph2−/− females sniffed females longer than males (Fig. 3A). The latency to sniff males was significantly increased in Tph2−/− females compared with that in Tph2+/+ females (Fig. S7A).
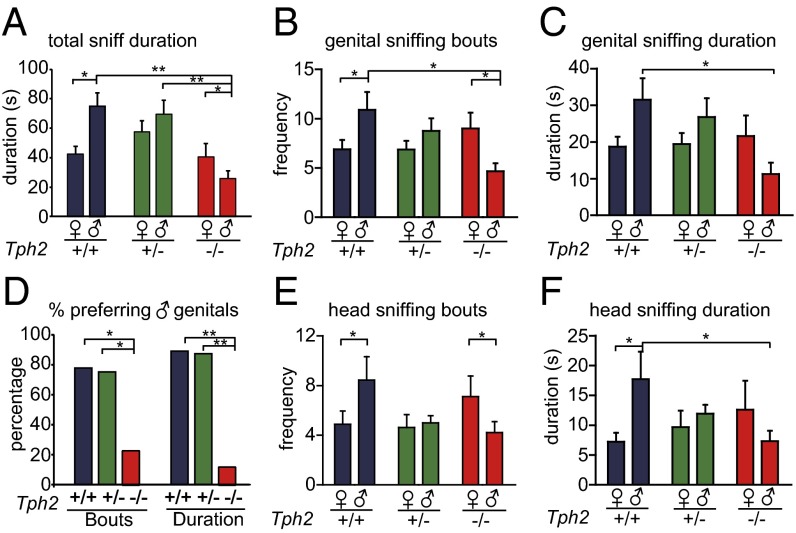
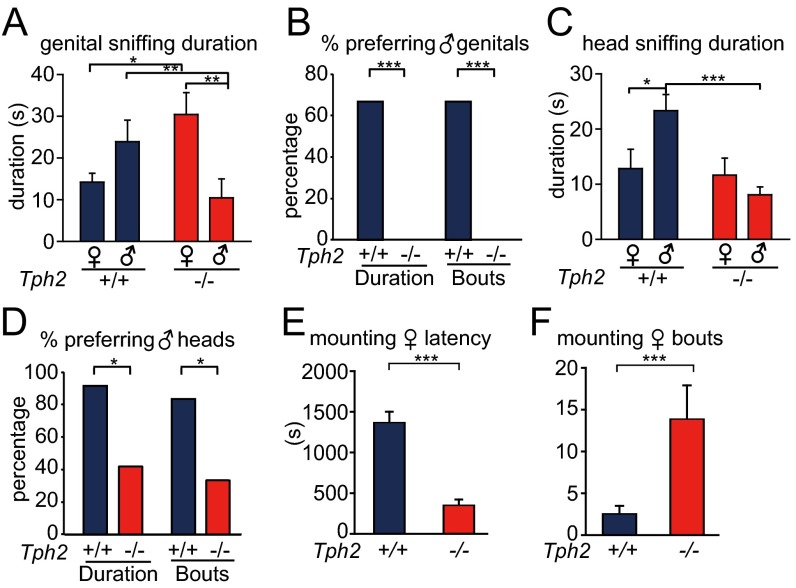
Fig. 3.
Sexual preference of Tph2 knockout female mice. n = 9 for Tph2+/+, n = 6 for Tph2+/−, n = 8 for Tph2−/−. *P < 0.05, **P < 0.01, ***P < 0.001. (A) Tph2−/− females sniffed female targets longer than male targets, whereas their Tph2+/+ littermates sniffed male targets longer than female targets. (B) Tph2−/− females sniffed female genitals more frequently than male genitals, whereas their Tph2+/+ littermates sniffed male genitals more frequently than female genitals. (C) Tph2−/− females sniffed male genitals for a shorter time than their Tph2+/+ littermates. (D) Percentage of mice of each genotype that sniffed male genitals more (bouts) or longer (duration) than female genitals. (E) Tph2−/− females sniffed female heads more frequently than male heads, whereas Tph2+/+ littermates sniffed male heads more. (F) Tph2−/− females sniffed male heads shorter than Tph2+/+ littermates. Tph2+/+ mice sniffed male heads longer than female heads.
The reversal of sexual preference by Tph2−/− females was more obvious when comparing the sniffing of the genital and head areas. Tph2+/+ females sniffed the genital and head areas of males more often than those of females, but Tph2−/− females sniffed the genital and head areas of females more than those of males (Fig. 3 B and E). Fewer Tph2−/− females than their Tph2+/+ and Tph2+/− littermate preferred male over female genital or head areas (Fig. 3E and Fig. S7 C and E). The duration of sniffing the genital and head areas of males was reduced in Tph2−/− females compared with Tph2+/+ females (Fig. 3 C and F). Tph2+/− females often displayed a phenotype between those of Tph2+/+ and Tph2−/− females.
When presented with female and male bedding, more Tph2−/− females than Tph2+/+ and Tph2−/− females had a longer duration of staying on the female bedding than male bedding (Fig. S8A). Although Tph2+/+ and Tph2+/− females stayed on male and female bedding for similar durations, Tph2−/− females stayed on female bedding for a longer duration than on male bedding (Fig. S8 B and C). Thus, Tph2−/− also showed preference for females in the bedding choice assay.
Mounting Preference of Tph2 Knockout Females.
When presented with a WT female and a male at the same time, Tph2−/− females displayed a strong mounting preference toward the females. Tph2−/− females mounted female targets with a shorter latency, higher frequency, and longer duration than the male targets, whereas their littermates showed no mounting preference (Fig. 4 A–C).
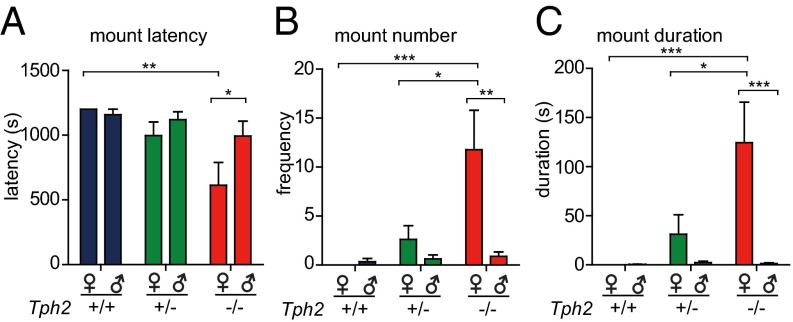
Fig. 4.
Mounting preference of Tph2 mice. n = 9 for Tph2+/+, n = 6 for Tph2+/−, n = 8 for Tph2−/−. *P < 0.05, **P < 0.01, ***P < 0.001. (A) Female–female mounting latency was shorter in Tph2−/− females than their Tph2+/+ littermates. Tph2−/− females mounted female targets earlier than male targets. (B) Tph2−/− females mounted female targets more than their littermates. Tph2−/− females mounted female targets more frequently than male targets. (C) Tph2−/− females mounted female targets longer than their littermates. Tph2−/− females mounted female targets longer than male targets.
Approximately 75% of Tph2−/− females mounted females, whereas none of their Tph2+/+ and 37.5% of Tph2+/− littermates displayed this behavior (Fig. S7F). Tph2−/− females mounted target females with a shorter latency, higher frequency, and longer duration than their littermates (Fig. 4 A–C). By contrast, there was no difference between Tph2−/− females and their Tph2+/+ littermates in mounting male targets: the mounting percentage, latency, bouts, and duration were not significantly different (Fig. 4 and Fig. S7F).
Sexual Preference in Serotonergic Defective Mice with Controlled Estrogen Level.
It is known that sex hormones influence sexual behaviors and sexual preference (4, 6, 14, 21, 32). We examined whether 5-HT functioned by changing the levels of sex hormones and found that neither estradiol nor testosterone was significantly different among estrous or diestrous Tph2−/−, Tph2+/−, and Tph2+/+ females (Fig. S9), suggesting that it is unlikely that 5-HT regulates sexual preference by controlling sex hormones.
We ovariotomized either Tph2+/+ or Tph2−/− females and restored their estradiol to the same level by injection (OVX+E). After such treatments, Tph2−/− females still displayed strong preference for females over males. They sniffed female genitals earlier, more frequently, and longer than male genitals (Fig. 5A and Fig. S10 A and B). More Tph2−/− females sniffed female heads or genitals longer and more frequently than their Tph2+/+ littermates (Fig. 5 B and D and Fig. S10 C and D). Tph2−/− females had a longer latency to sniffed male genitals than their Tph2+/+ littermates (Fig. S10A), and they sniffed male genitals less frequently and with shorter duration than their Tph2+/+ littermates (Fig. 5A and Fig. S10B). Tph2−/− also sniffed male heads less frequently and for a shorter duration (Fig. 5C and Fig. S10F). Tph2−/− sniffed female genitals more frequently and with a longer duration (Fig. 5A and Fig. S10B).
Fig. 5.
Sexual preference of estradiol treated ovariotomized (OVX+E) females. n = 12 for OVX+E Tph2+/+, n = 12 for OVX+E Tph2−/−.*P < 0.05, **P < 0.01, ***P < 0.001. (A) OVX+E Tph2−/− females sniffed female genitals longer than male genitals. OVX+E Tph2−/− sniffed female genitals longer than their OVX+E Tph2+/+ littermates. OVX+E Tph2−/− sniffed male genitals shorter than their OVX+E Tph2+/+ littermates. (B) Percentage of mice of each genotype that sniffed male genitals more (bouts) or longer (duration) than female genitals. (C) OVX+E Tph2−/− females sniffed male heads shorter than their littermates. OVX+E Tph2+/+ females sniffed male heads longer than female heads, whereas OVX+E Tph2−/− females did not display preference. (D) Percentage of mice of each genotype that sniffed male heads more (bouts) or longer (duration) than female heads. (E) OVX+E Tph2−/− mounted female targets earlier than their OVX+E Tph2+/+ littermates. (F) Tph2−/− females mounted female targets more frequently than their Tph2+/+ littermates.
When presented with a female alone, a higher percentage of Tph2−/− females than Tph2+/+ females mounted females (Fig. S10G). Tph2−/− females mounted females faster, more frequently, and longer than their Tph2+/+ littermates (Fig. 5 E and F and Fig. S10H).
Requirement of 5-HT in Adult Females for Sexual Preference.
Because Lmx1b−/− and Tph2−/− mice lacked central serotonergic neurons or 5-HT from embryogenesis to adulthood, it was unclear whether the phenotype in sexual preference was caused indirectly by developmental defects or directly by involvement of 5-HT in adult behaviors. We first used p-chlorophenylalanine (pCPA), a Tph inhibitor, to pharmacologically deplete 5-HT from WT adult animals (33).
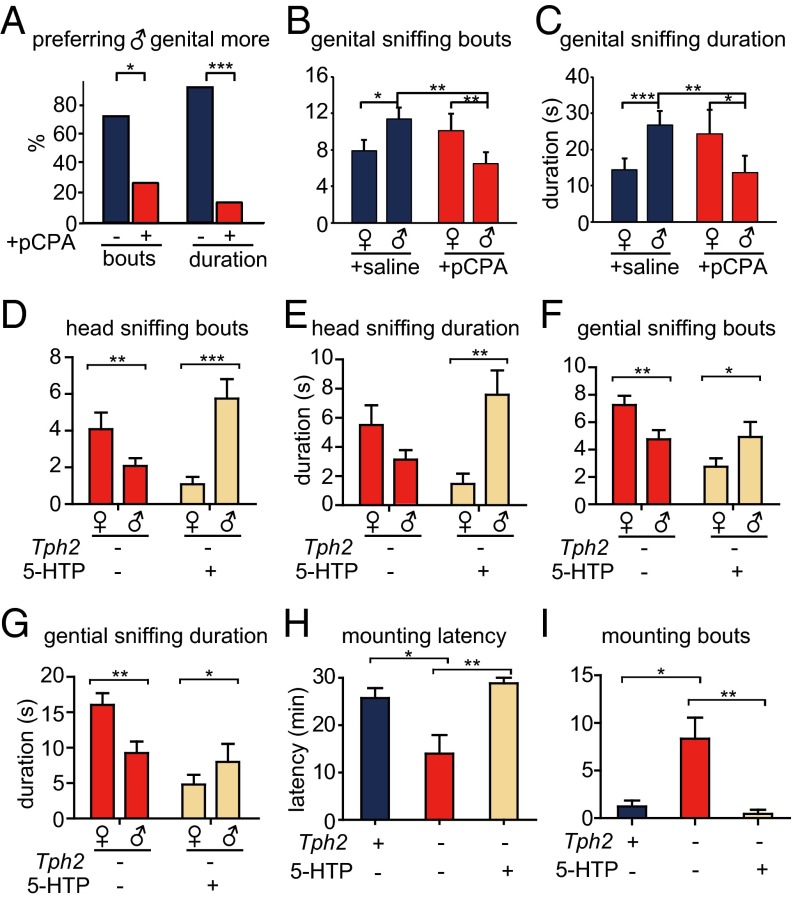
pCPA significantly lowered the levels of 5-HT and 5-HIAA in the adult brain (Fig. S1 E and F). In the mating choice assay, we found that 5-HT depletion significantly decreased the percentage of female mice preferring male over female genitals (Fig. 6A and Fig. S11 F and G). 5-HT depletion increased the latency of females to sniff male genitals, but did not change their latency to sniff female genitals (Fig. S11E). Control females injected with saline sniffed male genitals more frequently (Fig. 6B) and for a longer duration than female genitals (Fig. 6C). By contrast, female mice injected with pCPA sniffed female genitals more frequently and for a longer duration than male genitals (Fig. 6 B and C), resulting in a reversal of preference of sexually dimorphic odors (Fig. S11 F and G). 5-HT depletion also changed female preference in sniffing heads and the whole body (Fig. S11 A–D).
Fig. 6.
Sexual preference of adult females after pCPA or 5-HTP injection. (A–C) Each adult C57BL/6J female was treated with saline (+saline, n = 15) or pCPA (+pCPA, n = 15) and presented with a male and female target. (D–G) Twelve Tph2−/− females (labeled as – for Tph2) were treated with 5-HTP (indicated as + for 5-HTP treatment), 12 Tph2−/− females were treated with saline. (H and I) Tph2+/+ were treated with saline (n = 9). Tph2−/− were treated with saline (n = 9) or 5-HTP (n = 9). *P < 0.05, **P < 0.01, ***P < 0.001. (A) pCPA decreased the percentage of females sniffing male genitals more frequent or longer than female genitals. (B) pCPA reversed the preference of females in genital sniffing bouts. Male genital sniffing bouts was significantly decreased in pCPA treated females than in control females. (C) pCPA reversed the preference of females in genital sniffing duration. Male genital sniffing duration was significantly decreased in pCPA treated females than in control females. (D and E) Injection of 5-HTP could rescue the same-sex preference in head sniffing bouts and duration of Tph2−/− females. (F and G) Injection of 5-HTP could rescue the same sex preference in genital sniffing bouts and duration of Tph2−/− females. The phenotype of head sniffing latency and genital sniffing latency was more variable and not as informative as those of sniffing frequency and duration. (H and I) Injection of 5HTP rescued the female–female mounting phenotype of Tph2−/− females.
When a female was tested with another female, 5-HT depletion also significantly increased the percentage of females with female–female mounting behavior (Fig. S11H). Latency for female–female mounting was decreased (Fig. S11I) and frequency was increased by 5-HT depletion (Fig. S11J).
Results with pCPA depletion in adults indicate that 5-HT functions in adulthood to regulate sexual preference of females.
Because pCPA can have nonspecific effect (34), we carried out genetic rescue experiments in adult females to demonstrate a role for 5-HT in adulthood. Injection of 5-HTP, an intermediate of 5-HT synthesis downstream of Tph2, into adult mice rescued the phenotype of Tph2−/− females in olfactory preference (Fig. 6 D–G and Fig. S12 A and B), female–female mounting (Fig. 6 H and I), and bedding preference (Fig. S12 C and D), further supporting a role for 5-HT in the preference behavior of adults.
Discussion
Our findings with Lmx1b−/− mice, Tph2−/− mice, and mice treated with pCPA have led to the conclusion that serotonergic signaling is involved in controlling sexual preference in adult females.
This article has extended significantly beyond the male sexual preference article in three aspects (26). (i) A genetic alternation made in the laboratory has reversed sexually preference without changing sex hormone levels. Previous studies in Drosophila and mammals have found genetic mutations causing an increased male–male (22) or female–female (13, 25) sexual activity, but none of these mutations has been shown to cause a reversal of preference in a behavioral assay. Lmx1b−/− mutant females studied here are different from AFP−/− females reported previously (13) in that the Lmx1b−/− females are as receptive as WT females to males when presented only with male partners. Our findings with Lmx1b−/− mutant females indicate that it is possible to observe same-sex preference in the laboratory as well as in the wild (13, 25). (ii) In the male sexual preference article, there is an important issue as to whether 5-HT only plays a role in inhibiting sexual drive or whether it has an additional role in sexual preference. It is possible that an overall increase in sexuality in serotonergic mutants can appear as more increase for male–male sexual activity in mutants because, with the baseline lower for male–male activity than that for male–female activity, an overall increase in both male–male and male–female activities can lead to more increase in male–male than male–female activity, thus appearing as a loss of sexual preference in the male mutants. In the present article, our finding of a reversal in sexual preference in female mutants clearly establishes a role for 5-HT in sexual preference because hypersexuality is very difficult to explain the reversal of sexual preference. (iii) Although our previous studies of male mutants did not detect a role for 5-HT in pheromone sensing in the periphery (26), it could not be completely ruled out that loss of sexual preference in males could be attributed to defective olfactory processing of innate sexual signals (35). However, the reversal of sexual preference observed in female mutants cannot be easily explained by a defective peripheral olfactory sensing and is more consistent with a central mechanism of 5HT in controlling sexual preference.
Sexual behaviors between members of the same sex have been observed after 5-HT depletion (36–42), but none of the previous studies has demonstrated a reversal of sexual preference. In fact, those experiments were carried out to investigate the control of sexual activities, not the regulation of sexual preference. Male crickets showed high-level courtship behavior to male crickets 7 d after antenna removal, when 5-HT was very low (43). Central 5-HT level also modulates sexual behaviors in ovariectomized Cnemidophorus uniparens (44, 45). 5-HT depletion causes male–male mounting in cats, rats, and rabbits (37, 41). Female rats also mounted female intruders after 5-HT depletion (42). Previous reports have interpreted the phenotype as hypersexuality and concluded that 5-HT inhibits male and female sexual activity. In Lmx1b−/− mutant females, no general increase in sexual activities has been observed: their receptivity toward males was similar to WT littermates when presented only with males. Rather, they showed increased activities toward females and decreased activities toward males when given a choice of a male and a female. Our results demonstrate that lack of central serotonergic neurons or 5-HT causes a reversal of sexual preference, revealing a role for 5-HT in regulating sexual preference.
There are 14 5-HT receptors distributed in different regions of the mouse brain. It remains to be determined which are involved in sexual preference. It will also be important to study signaling downstream of 5-HT.
Materials and Methods
Animals.
Lmx1b−/−, Lmx1bfloxp/floxp, ePet-Cre, and Tph2−/− mice were generated and genotyped as previously described (26, 27). All mice were maintained on a 12D:12L cycle with food and water ad libitum. All test mice were individually housed, 12- to 16-wk-old females. Lmx1b+/+ mice include Lmx1b+/+/ePet-Cre+, Lmx1bfloxp/floxp/ePet-Cre−, and Lmx1bfloxp/+/ePet–Cre− mice, which behaved similarly. Castrated males and ovariectomized females were used at least 2 wk after surgery. All behavioral assays were carried out 2–4 h after light was turned off and analyzed in a double-blind manner. Animal experiments have been approved by the Animal Review Board of Peking University.
Mating Choice Assay.
This assay was carried out essentially as previously described (22). Briefly, a male and an estrous female C57BL/6J mouse were placed into the home cage of the test mouse. Behaviors of the test female were recorded for 20 min and analyzed. To reduce the influence of the male intruders, we used castrated males swabbed with urine from an intact male on the genital area (80 μL) and back (20 μL). Contacts initiated by test mice using the snout were recorded as sniff.
Genital Odor Preference Assay.
A glass slide was smeared with the genitals of two donors, each on one side of the slide. The slide was clamped at the middle, which was clean, and hung in the middle of the home cage of the test mouse. Behaviors of the test mice were recorded for 3 min and analyzed. Sniff was recorded when test mice contacted the slide with the snout.
Bedding Preference Assay.
Bedding from group-housed adult C57BJ/6J males or females were not changed for 4 d. Ten grams of male or female bedding were put in one side on the bottom of a cage in an area of 11.5 × 17 cm2. The male and female beddings were prevented from mixing by a plastic bar of 6 cm. The size of cage was 29 × 17 × 15 cm (L × W × H). A grid of plastic bars separated the test mice from the bedding on the bottom of the cage. The bars were 5 mm wide with 5-mm intervals. The test mouse was put into the cage to be familiarized with the cage without bedding for 5 min before the mice were taken out and the bedding and a clean grid was put into the cage. After each assay, the cage was washed with water and then alcohol to remove odor.
Resident-Intruder Assay.
A C57BL/6J mouse was placed into the home cage of a test female. Behaviors of the test female were recorded for 30 min and analyzed. Two types of mice were used separately as the intruder: an intact female or a castrated male swabbed with urine from an intact male.
Lordosis.
This assay was carried out and analyzed essentially as previously described (46, 47). Ten-week-old sex-experienced C57BL/6J males were individually housed for 1 wk before being used. A vaginal smear was obtained from the test female 2–3 h before light-off every day. Test females were in estrous. A test female was placed into the home cage of a male. Behaviors of the test female were recorded and scored during 10 mounts exhibited by the male resident and analyzed as previously described (46). Briefly, unreceptive female behavior was defined as rearing, kicking, or fleeing response to mounting (score 0). Proceptive behavior was defined as the still posture of the female mice without dorsiflexion of vertebral column during mounting. Dorsiflexion of female vertebral column during mounting was recorded as receptive behavior (score 1–3 with 0.5 intervals as previously described in ref. 39). If the male exhibited less than 10 mounts during 15 min, the result was excluded from analysis.
Pharmacological Treatment.
Ten-week-old naive C57BL/6J females were used. pCPA (400 mg/kg body weight in saline, SIGMA-Aldrich) was injected intraperitoneally once per day for 4 d. Control mice were injected with saline. Behavioral assays were carried out on the fifth day.
For OVX+E females, estradiol (200 μg/kg body weight) (42) was injected s.c. daily after surgery. Resident-intruder assays were carried out 2 wk after surgery. Mating choice assays were done 1 wk later.
Statistics.
Data are presented as means ± SEM in all bar graphs. Behavioral data were analyzed using nonparametric Kruskal-Wallis or Mann Whitney U-tests. Dunn’s comparison was used if Kruskal-Wallis test was significant. Percentages were analyzed using Fisher’s exact test. ELISA and immunohistochemistry results were analyzed using the Wilcoxon signed-rank test. Significance was set as P < 0.05.
Supplementary Material
Acknowledgments
We thank Mr. Hongmin Yan for his involvement in the project, Dr. Zhou-Feng Chen for Lmx1bfloxp/floxp and Tph2−/− mice, Dr. Evan S. Deneris for ePet-Cre mice, Mr. Longhua Guo for helpful discussions and data analysis, and Yunxia Si and Jing Lang for help with mice. This work was supported by the Ministry of Science and Technology (973 Program 2010CB833900), the National Natural Science Foundation of China (31200833), the Beijing Municipal Commission on Science and Technology (Z111107067311058), and a postdoctoral fellowship from the Peking-Tsinghua Center for Life Sciences (to. Y.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.G.D. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1220712110/-/DCSupplemental.
References
- 1.Baum MJ. Mammalian animal models of psychosexual differentiation: when is ‘translation’ to the human situation possible? Horm Behav. 2006;50(4):579–588. doi: 10.1016/j.yhbeh.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Vasey VSPL, editor. Homosexual Behavior in Animals: An Evolutionary Perspective. New York: Cambridge Univ Press; 2006. [Google Scholar]
- 3.Bagemihl B. Biological Exuberance: Animal Homosexuality and Natural Diversity. New York: St. Martin’s Press; 1999. [Google Scholar]
- 4.Young WC, Rundlett B. The hormonal induction of homosexual behavior in the spayed female guinea pig. Psychosom Med. 1939;1:449–460. [Google Scholar]
- 5.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 6.Baum MJ, Erskine MS, Kornberg E, Weaver CE. Prenatal and neonatal testosterone exposure interact to affect differentiation of sexual behavior and partner preference in female ferrets. Behav Neurosci. 1990;104(1):183–198. doi: 10.1037//0735-7044.104.1.183. [DOI] [PubMed] [Google Scholar]
- 7.Brand T, Kroonen J, Mos J, Slob AK. Adult partner preference and sexual behavior of male rats affected by perinatal endocrine manipulations. Horm Behav. 1991;25(3):323–341. doi: 10.1016/0018-506x(91)90005-3. [DOI] [PubMed] [Google Scholar]
- 8.Bakker J, Brand T, van Ophemert J, Slob AK. Hormonal regulation of adult partner preference behavior in neonatally ATD-treated male rats. Behav Neurosci. 1993;107(3):480–487. doi: 10.1037//0735-7044.107.3.480. [DOI] [PubMed] [Google Scholar]
- 9.Bakker J, Van Ophemert J, Slob AK. Sexual differentiation of odor and partner preference in the rat. Physiol Behav. 1996;60(2):489–494. doi: 10.1016/s0031-9384(96)80023-0. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa S, et al. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139(12):5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- 11.Bakker J, Honda S, Harada N, Balthazart J. The aromatase knock-out mouse provides new evidence that estradiol is required during development in the female for the expression of sociosexual behaviors in adulthood. J Neurosci. 2002;22(20):9104–9112. doi: 10.1523/JNEUROSCI.22-20-09104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakker J, Honda S, Harada N, Balthazart J. Sexual partner preference requires a functional aromatase (cyp19) gene in male mice. Horm Behav. 2002;42(2):158–171. doi: 10.1006/hbeh.2002.1805. [DOI] [PubMed] [Google Scholar]
- 13.Bakker J, et al. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci. 2006;9(2):220–226. doi: 10.1038/nn1624. [DOI] [PubMed] [Google Scholar]
- 14.Brock O, Bakker J. Potential contribution of prenatal estrogens to the sexual differentiation of mate preferences in mice. Horm Behav. 2011;59(1):83–89. doi: 10.1016/j.yhbeh.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pillard RC, Weinrich JD. Evidence of familial nature of male homosexuality. Arch Gen Psychiatry. 1986;43(8):808–812. doi: 10.1001/archpsyc.1986.01800080094012. [DOI] [PubMed] [Google Scholar]
- 16.Hamer DH, Hu S, Magnuson VL, Hu N, Pattatucci AM. A linkage between DNA markers on the X chromosome and male sexual orientation. Science. 1993;261(5119):321–327. doi: 10.1126/science.8332896. [DOI] [PubMed] [Google Scholar]
- 17.Hu S, et al. Linkage between sexual orientation and chromosome Xq28 in males but not in females. Nat Genet. 1995;11(3):248–256. doi: 10.1038/ng1195-248. [DOI] [PubMed] [Google Scholar]
- 18.Rice G, Anderson C, Risch N, Ebers G. Male homosexuality: Absence of linkage to microsatellite markers at Xq28. Science. 1999;284(5414):665–667. doi: 10.1126/science.284.5414.665. [DOI] [PubMed] [Google Scholar]
- 19.DuPree MG, Mustanski BS, Bocklandt S, Nievergelt C, Hamer DH. A candidate gene study of CYP19 (aromatase) and male sexual orientation. Behav Genet. 2004;34(3):243–250. doi: 10.1023/B:BEGE.0000017870.77610.52. [DOI] [PubMed] [Google Scholar]
- 20.Mustanski BS, et al. A genomewide scan of male sexual orientation. Hum Genet. 2005;116(4):272–278. doi: 10.1007/s00439-004-1241-4. [DOI] [PubMed] [Google Scholar]
- 21.Brock O, Baum MJ, Bakker J. The development of female sexual behavior requires prepubertal estradiol. J Neurosci. 2011;31(15):5574–5578. doi: 10.1523/JNEUROSCI.0209-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leypold BG, et al. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci USA. 2002;99(9):6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295(5559):1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- 24.Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat Neurosci. 2005;8(12):1660–1662. doi: 10.1038/nn1589. [DOI] [PubMed] [Google Scholar]
- 25.Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448(7157):1009–1014. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, et al. Molecular regulation of sexual preference revealed by genetic studies of 5-HT in the brains of male mice. Nature. 2011;472(7341):95–99. doi: 10.1038/nature09822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao ZQ, et al. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci. 2006;26(49):12781–12788. doi: 10.1523/JNEUROSCI.4143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimoto H, Haga S, Sato K, Touhara K. Sex-specific peptides from exocrine glands stimulate mouse vomeronasal sensory neurons. Nature. 2005;437(7060):898–901. doi: 10.1038/nature04033. [DOI] [PubMed] [Google Scholar]
- 29.Ninomiya K, Kimura T. Male odors that influence the preference of female mice: Roles of urinary and preputial factors. Physiol Behav. 1988;44(6):791–795. doi: 10.1016/0031-9384(88)90064-9. [DOI] [PubMed] [Google Scholar]
- 30.Kelliher K, Baum M. Effect of sex steroids and coital experience on ferrets’ preference for the smell, sight and sound of conspecifics. Physiol Behav. 2002;76(1):1–7. doi: 10.1016/s0031-9384(02)00691-1. [DOI] [PubMed] [Google Scholar]
- 31.Ferkin MH, Li HZ. A battery of olfactory-based screens for phenotyping the social and sexual behaviors of mice. Physiol Behav. 2005;85(4):489–499. doi: 10.1016/j.physbeh.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 32.McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci. 2011;14(6):677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koe BK, Weissman A. p-Chlorophenylalanine: A specific depletor of brain serotonin. J Pharmacol Exp Ther. 1966;154(3):499–516. [PubMed] [Google Scholar]
- 34.Dailly E, Chenu F, Petit-Demoulière B, Bourin M. Specificity and efficacy of noradrenaline, serotonin depletion in discrete brain areas of Swiss mice by neurotoxins. J Neurosci Methods. 2006;150(1):111–115. doi: 10.1016/j.jneumeth.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Kobayakawa K, et al. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450(7169):503–508. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]
- 36.Tagliamonte A, Tagliamonte P, Gessa GL, Brodie BB. Compulsive sexual activity induced by p-chlorophenylalanine in normal and pinealectomized male rats. Science. 1969;166(3911):1433–1435. doi: 10.1126/science.166.3911.1433. [DOI] [PubMed] [Google Scholar]
- 37.Ferguson J, et al. “Hypersexuality” and behavioral changes in cats caused by administration of p-chlorophenylalanine. Science. 1970;168(3930):499–501. doi: 10.1126/science.168.3930.499. [DOI] [PubMed] [Google Scholar]
- 38.Whalen RE, Luttge WG. P-chlorophenylalanine methyl ester: An aphrodisiac? Science. 1970;169(3949):1000–1001. doi: 10.1126/science.169.3949.1000. [DOI] [PubMed] [Google Scholar]
- 39.Malmnas CO, Meyerson BJ. p-chlorophenylalanine and copulatory behaviour in the male rat. Nature. 1971;232(5310):398–400. doi: 10.1038/232398a0. [DOI] [PubMed] [Google Scholar]
- 40.Salis PJ, Dewsbury DA. p-chlorophenylalanine facilitates copulatory behaviour in male rats. Nature. 1971;232(5310):400–401. doi: 10.1038/232400a0. [DOI] [PubMed] [Google Scholar]
- 41.Fratta W, Biggio G, Gessa GL. Homosexual mounting behavior induced in male rats and rabbits by a tryptophan-free diet. Life Sci. 1977;21(3):379–383. doi: 10.1016/0024-3205(77)90518-5. [DOI] [PubMed] [Google Scholar]
- 42.van de Poll NE, van Dis H, Bermond B. The induction of mounting behavior in female rats by p-chlorophenylalanine. Eur J Pharmacol. 1977;41(2):225–229. doi: 10.1016/0014-2999(77)90214-x. [DOI] [PubMed] [Google Scholar]
- 43.Murakami S, Itoh MT. Removal of both antennae influences the courtship and aggressive behaviors in male crickets. J Neurobiol. 2003;57(1):110–118. doi: 10.1002/neu.10255. [DOI] [PubMed] [Google Scholar]
- 44.Dias BG, Crews D. Regulation of pseudosexual behavior in the parthenogenetic whiptail lizard, Cnemidophorus uniparens. Endocrinology. 2008;149(9):4622–4631. doi: 10.1210/en.2008-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dias BG, Crews D. Serotonergic modulation of male-like pseudocopulatory behavior in the parthenogenetic whiptail lizard, Cnemidophorus uniparens. Horm Behav. 2006;50(3):401–409. doi: 10.1016/j.yhbeh.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogawa S, et al. Survival of reproductive behaviors in estrogen receptor beta gene-deficient (betaERKO) male and female mice. Proc Natl Acad Sci USA. 1999;96(22):12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sisk CL, Meek LR. Sexual and reproductive behaviors. Curr Protoc Neurosci. 2001 doi: 10.1002/0471142301.ns0802s00. Chapter 8:Unit 8.2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.