Abstract
Bisphenol A (BPA) is an estrogenic endocrine disruptor widely used in the production of plastics. Increasing evidence indicates that in utero BPA exposure affects sexual differentiation and behavior; however, the mechanisms underlying these effects are unknown. We hypothesized that BPA may disrupt epigenetic programming of gene expression in the brain. Here, we provide evidence that maternal exposure during pregnancy to environmentally relevant doses of BPA (2, 20, and 200 µg/kg/d) in mice induces sex-specific, dose-dependent (linear and curvilinear), and brain region-specific changes in expression of genes encoding estrogen receptors (ERs; ERα and ERβ) and estrogen-related receptor-γ in juvenile offspring. Concomitantly, BPA altered mRNA levels of epigenetic regulators DNA methyltransferase (DNMT) 1 and DNMT3A in the juvenile cortex and hypothalamus, paralleling changes in estrogen-related receptors. Importantly, changes in ERα and DNMT expression in the cortex (males) and hypothalamus (females) were associated with DNA methylation changes in the ERα gene. BPA exposure induced persistent, largely sex-specific effects on social and anxiety-like behavior, leading to disruption of sexually dimorphic behaviors. Although postnatal maternal care was altered in mothers treated with BPA during pregnancy, the effects of in utero BPA were not found to be mediated by maternal care. However, our data suggest that increased maternal care may partially attenuate the effects of in utero BPA on DNA methylation. Overall, we demonstrate that low-dose prenatal BPA exposure induces lasting epigenetic disruption in the brain that possibly underlie enduring effects of BPA on brain function and behavior, especially regarding sexually dimorphic phenotypes.
Keywords: environmental, fetal origin of adult disease
Bisphenol A (BPA) is an estrogenic endocrine disruptor widely used in the production of plastics and found in many consumer products (1). More than 90% of the examined US population has detectable levels of BPA in urine (2), with exposure occurring through diet, inhalation, and dermal absorption (1). Although potentially toxic throughout life, early-life exposure to BPA is of highest health concern (3, 4) as this compound may accumulate in fetal and infant tissues, disrupting normal developmental processes (1).
Animal studies have shown that in utero exposure to BPA produces prenatal and postnatal adverse effects on multiple tissues, including the brain (4). Prenatal BPA exposure affects brain development, sexual differentiation, social and anxiety-like behavior, and learning/memory (3). In humans, emerging evidence for BPA-associated disruption to neurodevelopment is consistent with the rodent data and has revealed sex-specific effects of gestational BPA levels on emotional regulation and aggression in children (5–7). Importantly, childhood BPA levels did not predict these measures, emphasizing the importance of gestational BPA exposure for neurobehavioral outcomes (5, 7).
Molecular mechanisms that underlie the neurodevelopmental toxicity and sex-specific effects of BPA are not well understood. BPA is a selective estrogen receptor (ER) modulator that binds both classic ERs, ERα and ERβ (8). BPA effects are typically attributed to its estrogenic or antiestrogenic action, although it is not clear how the low potency of BPA at ERs could account for the effects of low-dose exposures. Recent evidence suggests that long-lasting effects of prenatal BPA exposure likely involve disruption of epigenetic programming during development that may or may not be mediated by ERs (3).
Epigenetic gene regulation, involving posttranslational histone modifications and DNA methylation, is a process particularly sensitive to environmental cues during development (9). Animal studies have shown that maternal exposure to drugs (10), stress (11), and endocrine disruptors (12) during pregnancy can alter epigenetic gene programming in the brain and contribute to neurodevelopmental deficits in offspring. Several studies have linked gestational (13, 14) or neonatal (15, 16) BPA exposure to long-lasting changes in DNA methylation and altered gene expression in nonneuronal tissues. Investigation of the epigenetic effects of BPA in the brain has been very limited (17, 18) and has not explored whether environmentally relevant doses of BPA could induce enduring effects on the brain epigenome. In addition, the dose- and sex-dependent effects of BPA have not been thoroughly examined, although they have been suggested by the loss of sexual dimorphism and sex-biased neurodevelopmental effects observed in animals and humans.
In the present study, we examined the consequences of maternal exposure during pregnancy to multiple doses of BPA in BALB/c mice. We then determined the impact of in utero BPA for gene expression and DNA methylation in the brain of juvenile mice, and social/anxiety-like behavior among juvenile and adult offspring. Importantly, within these analyses we determined the sex-specificity of BPA-induced effects, the relationship between dose and outcome, and the potential modulation of BPA effects by postpartum maternal care.
Results
Study Design.
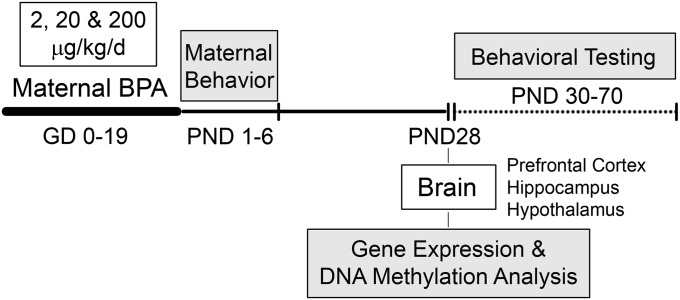
The study design is illustrated in Fig. 1. Before mating, female mice were assigned to each treatment group (2, 20, and 200 µg/kg BPA or vehicle; n = 24 per treatment), and mice that became pregnant (∼70%) were exposed daily (oral administration) to the assigned treatment throughout gestation [gestational days (GD) 0–19]. Multiple BPA treatments permitted assessment of dose-dependent effects at doses above and below the current reference dose for humans (50 μg/kg/d). From postpartum days 1 to 6, mother–infant interactions were observed to determine BPA-induced effects on maternal behavior and the effects of postnatal maternal behavior on BPA-induced outcomes in offspring. The breeding design generated a minimum of 12 litters per treatment [vehicle (n = 14), 2 µg/kg (n = 17), 20 µg/kg (n = 15), and 200 µg/kg (n = 12) BPA]. At weaning [postnatal day (PND) 28], six male and six female offspring per treatment [one or two pups per litter from a minimum of five litters per treatment for each sex; total litters, vehicle (n = 8), 2 µg/kg (n = 7), 20 µg/kg (n = 8), and 200 µg/kg (n = 7) BPA], were killed and whole brains were dissected (prefrontal cortex, hippocampus, hypothalamus) for gene expression and DNA methylation analyses. Remaining animals underwent behavioral testing from PND 30 to PND 70 [male and female offspring from vehicle (n = 8–10), 2 µg/kg (n = 10–12), 20 µg/kg (n = 10), and 200 µg/kg (n = 12) BPA litters]. Testing included behavioral domains (anxiety-like and social behavior) that are sexually dimorphic and have been previously examined in the context of BPA exposure studies (3).
Fig. 1.
Summary of study design.
BPA Effects on ER-Related Gene Expression.
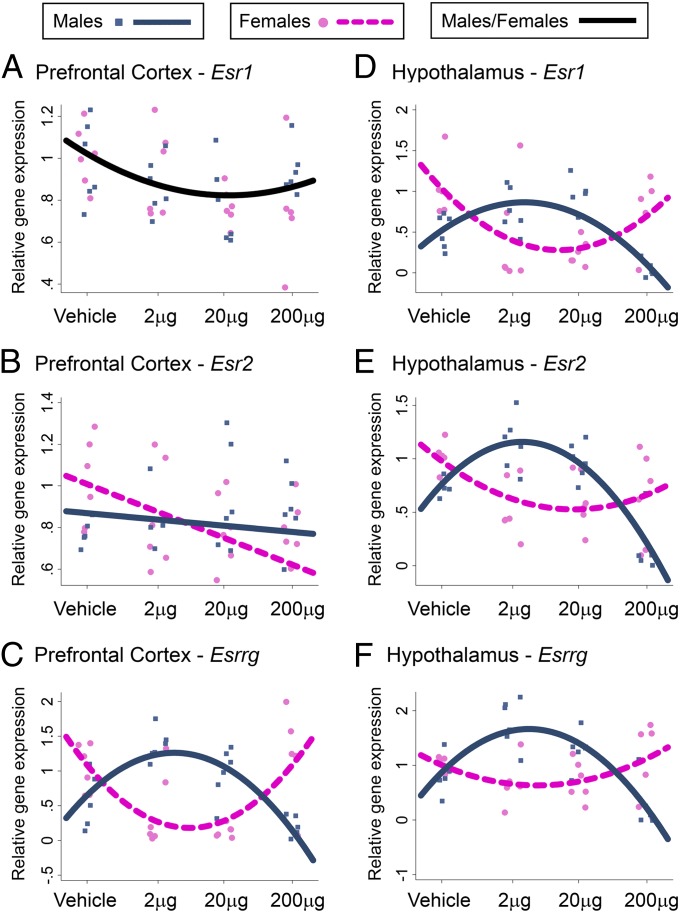
We examined the effects of prenatal BPA treatment on the expression of genes essential for sexual differentiation of the brain and behavior, including genes encoding ERα [estrogen receptor 1 (Esr1)] and ERβ [estrogen receptor 2 (Esr2)] as well as estrogen-related receptor γ (ERRγ; Esrrg) (Fig. 2 and Fig. S1 A–C). The initial statistical approach involved two-way ANOVA, which revealed significant dose-dependent and sex-specific effects of BPA on gene expression that varied across brain regions (SI Text, Complementary Data Analysis and Table S1). However, as ANOVA analysis treats the four BPA treatment groups (0, 2, 20, and 200 µg/kg) as categorically different from one another and cannot account for multiple observations within each litter, we used multilevel regression analysis to model the effect of BPA across the whole dose range examined (0–200 µg/kg) and to correct for pseudoreplication. This analysis treats BPA dosage as a continuous variable (logarithmic scale; Fig. 2, x axis) and derives the best-fit model for the dose-dependent (linear or curvilinear/quadratic) and sex-specific effects of BPA over the tested dose range (Fig. 2; SI Materials and Methods provides details of statistical approach). In the prefrontal cortex, BPA treatment led to a marginal quadratic change in Esr1 expression in both sexes (quadratic dose, P = 0.06; Fig. 2A), a sex-specific linear change in Esr2 (sex*linear dose, P < 0.05; Fig. 2B), and a sex-specific quadratic change in Esrrg (sex*quadratic dose: P < 0.0001; Fig. 2C). In the hippocampus, BPA treatment led to no change in Esr1 expression (Fig. S1A), a sex-specific linear change in Esr2 (sex*linear dose: P < 0.001; Fig. S1B), and a linear change in Esrrg in both sexes (linear dose, P < 0.05; Fig. S1C). In the hypothalamus, BPA treatment led to sex-specific quadratic changes in Esr1, Esr2, and Esrrg expression (sex*quadratic dose, all P < 0.0001; Fig. 2 D–F). Importantly, low doses of BPA (2 µg and 20 µg) generally led to a loss or reversal of sex differences in ER-related gene expression evident in vehicle-treated offspring (greater in females than in males) such that BPA-treated males had similar or elevated mRNA levels of Esr1 (hypothalamus), Esr2 (all three brain regions), and Esrrg (cortex, hypothalamus) compared with females (Fig. 2 and Fig. S1B).
Fig. 2.
Prenatal BPA treatment effects on ER-related gene expression. Esr1, Esr2, and Esrrg gene expression was analyzed in the prefrontal cortex (A–C) and hypothalamus (D–F). Graphs include individual data points and the best-fit (linear or curvilinear) model for these data [single black line, significant non–sex-specific effect of BPA; blue (male) and pink (female) lines, significant sex-specific effect of BPA] generated by multilevel regression analyses (Stata version 12.1).
BPA Effects on DNA Methyltransferase Gene Expression.
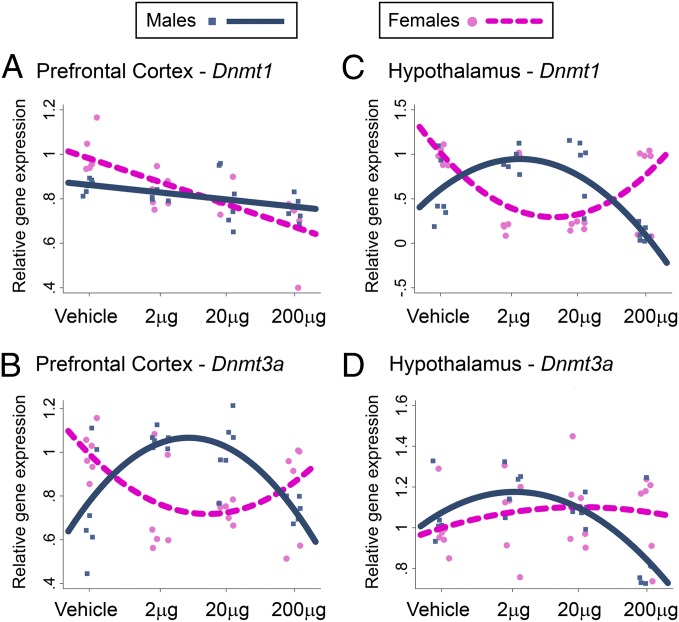
To examine whether epigenetic mechanisms may underlie BPA effects on ER-related gene expression, we examined the mRNA expression of enzymes that catalyze DNA methylation, DNA methyltransferase 1 (DNMT1) and DNMT3A (Fig. 3 and Fig. S1 D and E). In the prefrontal cortex, BPA treatment led to a sex-specific linear change in Dnmt1 expression (sex*linear dose, P < 0.001; Fig. 3A) and a sex-specific quadratic change in Dnmt3a (sex*quadratic dose, P < 0.0001; Fig. 3B). In the hippocampus, BPA treatment led to a marginal sex-specific quadratic change in Dnmt1 expression (sex*quadratic dose, P = 0.06; Fig. S1D) and no change in Dnmt3a (Fig. S1E). In the hypothalamus, BPA treatment led to a sex-specific quadratic change in Dnmt1 expression (sex*quadratic dose, P < 0.0001; Fig. 3C) and a marginal sex-specific quadratic change in Dnmt3a (sex*quadratic dose: P = 0.09; Fig. 3D). Overall, prenatal BPA treatment resulted in a sex-specific and dose-dependent disruption of epigenetic pathways in the cortex and the hypothalamus, paralleling changes in the mRNA expression of estrogen-related receptors.
Fig. 3.
BPA treatment effects on DNMT expression. Dnmt1 and Dnmt3a gene expression was analyzed in prefrontal cortex (A and B) and hypothalamus (C and D). Individual data points and best-fit (linear or curvilinear) model for data are represented [blue (male) and pink (female) lines, significant sex-specific BPA effect].
BPA Effects on DNA methylation of ERα (Esr1) Gene.
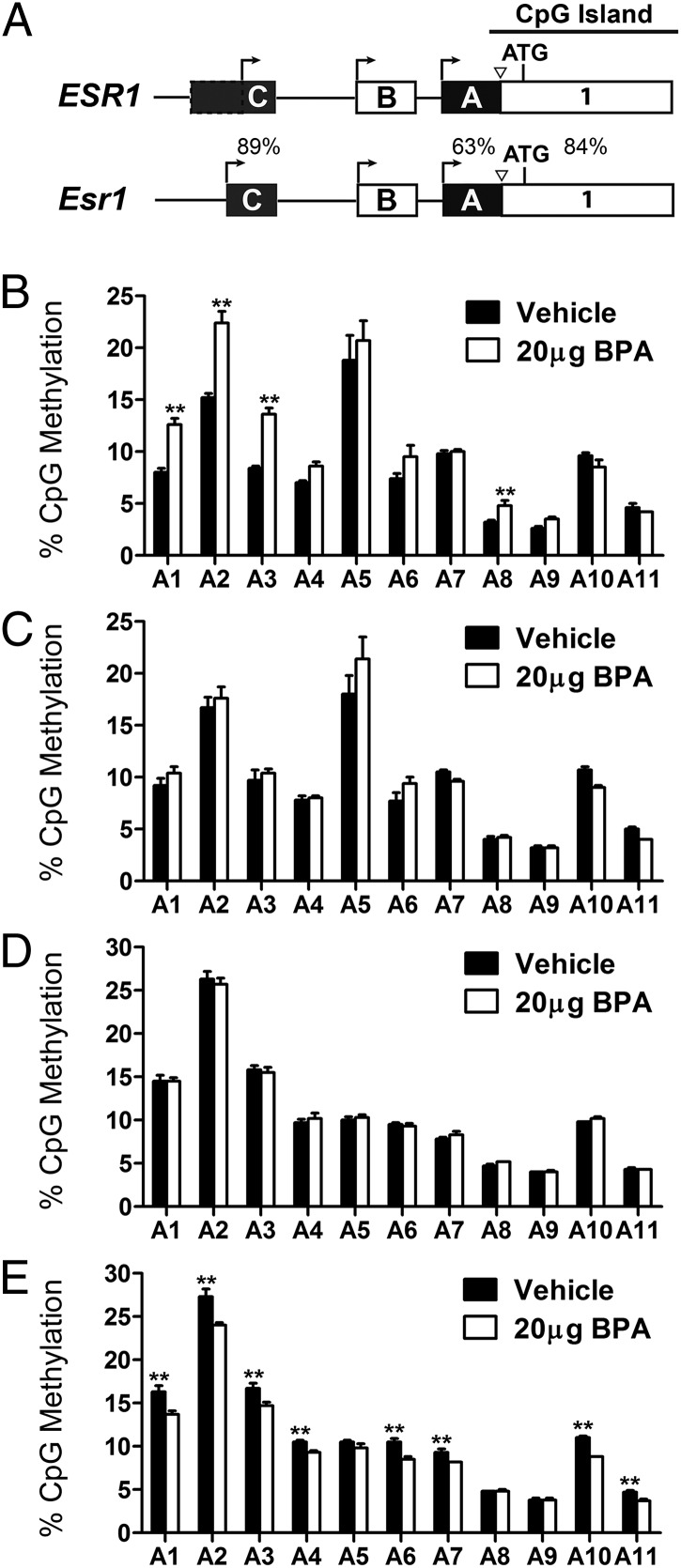
We examined DNA methylation of the Esr1 gene in two brain regions in which we observed significant changes in Esr1 mRNA levels (prefrontal cortex, hypothalamus) following 20 µg/kg prenatal BPA. We assessed methylation status of 17 CpG sites in untranslated exons A and C of Esr1 (Fig. 4A and Figs. S2 and S3), which are highly homologous to human (ESR1) counterparts (19). Methylation of these regions was previously shown to regulate Esr1 gene expression during development in the mouse brain (20). The majority of the examined sites lie within an Esr1 CpG island shore (Fig. 4A); CpG island shores are genomic areas up to 2 kb from CpG islands that have been shown to be involved in tissue-specific gene expression programming (21). We observed sex-specific and brain-region specific changes in the DNA methylation levels of exon A (Fig. 4 B–E). Prenatal BPA resulted in a significant increase in DNA methylation of exon A in the male prefrontal cortex (P < 0.05; Fig. 4B), with no effect observed in the cortex of females (Fig. 4C). In males, differential methylation associated with BPA was observed at CpG sites 1, 2, 3, and 8 (P < 0.01; Fig. 4B). In the hypothalamus, 20 µg/kg BPA treatment was associated with decreased DNA methylation of exon A in females (P < 0.01; Fig. 4E), with no effect observed in males (Fig. 4D). In females, differential hypothalamic DNA methylation associated with BPA treatment was observed at CpG sites 1, 2, 3, 4, 6, 7, 10, and 11 in exon A (P < 0.01; Fig. 4E). We found no evidence for an effect of BPA on exon C DNA methylation (all P > 0.17; Fig. S4).
Fig. 4.
Effects of gestational BPA exposure on DNA methylation of Esr1 exon A. (A) Schematic of the regulatory region of the human (ESR1) and mouse (Esr1) gene. Percentages represent the degree of homology between these two species. Methylation of 11 CpG sites in exon A (Fig. S2) was examined in the male (B) and female (C) prefrontal cortex and male (D) and female (E) hypothalamus at PND 28 (n = 6 per sex per brain region per treatment) following in utero exposure to vehicle or 20 µg/kg BPA.
BPA Effect on Home Cage Social Behavior.
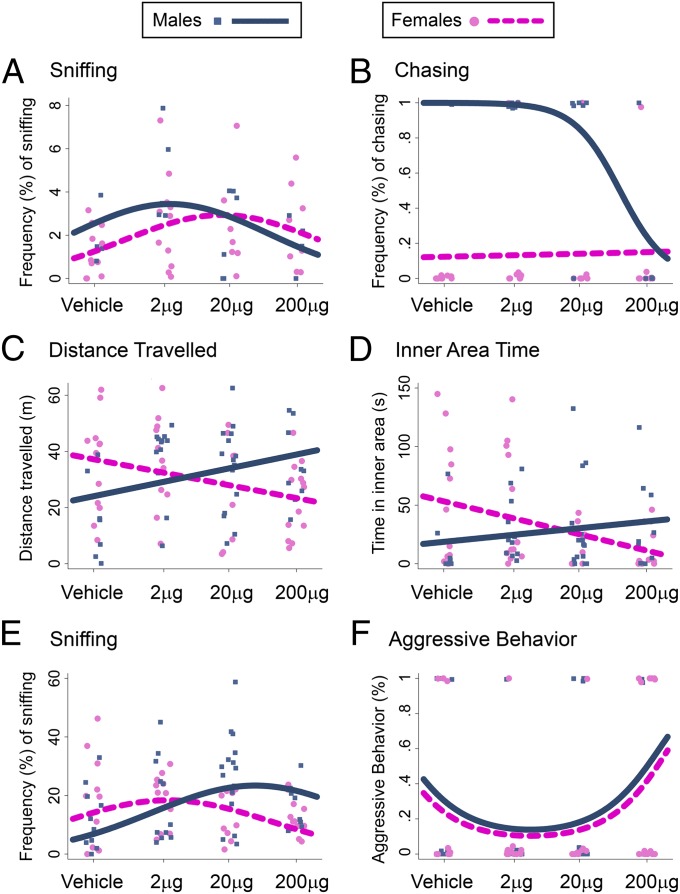
We examined the effects of prenatal BPA treatment on home cage behaviors between PND 30 and PND 40. Behaviors observed included huddling (side-by-side contact), sniffing or grooming another cage mate (allogrooming), and play behaviors (hopping, chasing, fighting). BPA treatment led to a marginal sex-specific quadratic change in huddling (P = 0.08; Fig. S5A) and sex-specific linear changes in sniffing, which followed a non–sex-specific quadratic change (quadratic term, P < 0.01; linear interaction term, P = 0.05; Fig. 5A). In both sexes, BPA led to quadratic changes in allogrooming (P < 0.01; Fig. S5B) and hopping (P < 0.01; Fig. S5C). Prenatal BPA exposure disrupted sexual dimorphism in play behaviors. It led to a sex-specific linear change in chasing (P < 0.05; Fig. 5B), such that sexual dimorphism in this behavior (greater in males than in females) was not observed at the 200-µg dose. Although fighting is typically a male-specific behavior, the only cage in which fighting occurred was a female cage that received the highest BPA dose, suggesting again the reversal of sexually dimorphic play behaviors.
Fig. 5.
Effects of prenatal BPA treatment on behavior. BPA induced effects on home-cage social behaviors [frequency of sniffing (A) and chasing (B)], open-field behavior [distance traveled (C) and inner area time (D)], and social approach/aggression [frequency of sniffing (E) and aggression (F)]. Individual data points and the best-fit (linear or curvilinear) model are represented [blue (male) and pink (female) lines, significant sex-specific effect of BPA].
BPA Effects on Exploratory and Anxiety-Like Behavior.
At PND 60, offspring were assessed in the open-field test. BPA led to sex-specific linear dose-dependent changes in activity (distance traveled, linear interaction term, P < 0.001; Fig. 5C) and anxiety-like behavior (inner area time, linear interaction term, P < 0.01; Fig. 5D). Prenatal BPA was associated with a hyperactive phenotype in males and hypoactive phenotype in females (Fig. 5C). BPA exposure increased anxiety-like behavior in females (decreased inner area time) and decreased anxiety-like behavior in males (increased inner area time; Fig. 5D). Exploratory and anxiety-like behaviors were found to be sexually dimorphic, and BPA treatment reversed sex differences in these behaviors.
BPA Effects on Social Approach and Aggression.
Dyadic social interactions with a same-sex stimulus mouse (129Sv) were assessed at PND 70. Behaviors coded were frequency and duration of sniffing and frequency of aggressive behaviors toward stimulus mouse (tail rattling, biting, mounting). Each subject mouse was classified as dominant (aggressor), subordinate, or neutral in social status. For both sexes, BPA had a marginal quadratic effect on sniffing (P = 0.07), but had a differential linear effect by sex such that, at high doses, BPA treatment reversed sex differences in sniffing (linear interaction term, P < 0.001; Fig. 5E). For both sexes, BPA treatment had a quadratic effect on aggression (P < 0.05; Fig. 5F) and social dominance (P = 0.05; Fig. S6), increasing the probability of being aggressive/dominant at the highest dose.
BPA Effects on Maternal Behavior.
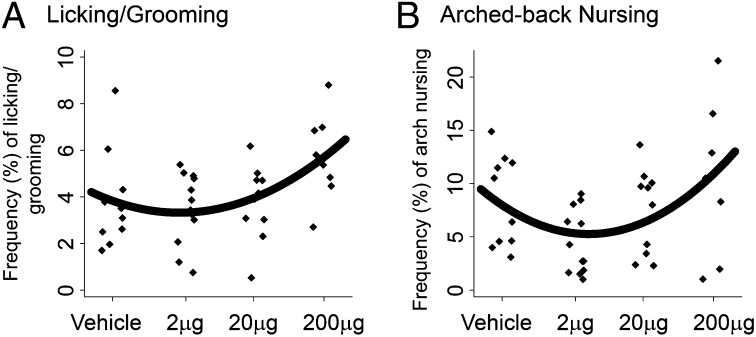
Low-dose BPA exposure during adulthood has been shown to reduce the frequency of maternal behavior in mice (22). Analyses of dose-dependent effects on maternal behavior indicated that BPA treatment led to quadratic changes in pup licking and grooming (P < 0.05; Fig. 6A) and arched-back nursing (P < 0.05; Fig. 6B), increasing these behaviors at the highest dose. BPA did not alter other postpartum behaviors (Table S2).
Fig. 6.
Effects of BPA treatment during pregnancy on postpartum maternal behavior. Regression curves showing quadratic change of (A) licking/grooming and (B) arched-back nursing behavior following BPA treatment.
Maternal Care Effects on BPA-Treated Offspring.
Variation in postpartum maternal behavior induces significant effects on molecular, neurobiological, and behavioral outcomes (23, 24). By using a normalized composite measure of maternal behavior combining licking/grooming and arched-back nursing, we found no evidence that maternal care mediated the effects of BPA on gene expression. Multiple regression multilevel models indicated that, along with BPA exposure, maternal care independently affected gene expression in the brain (inducing sex- and region-specific effects; Table S3).
We found no effect of maternal behavior on exon A methylation and controlling for this variable did not alter model parameters. Maternal care increased exon C methylation in the cortex (males and females), with no effect in the hypothalamus (cortex, P < 0.001; hypothalamus, P > 0.6). Interestingly, after controlling for maternal care, we found that BPA marginally decreased DNA methylation of exon C in the cortex of both sexes (P < 0.1) and significantly increased DNA methylation of exon C in the hypothalamus of both sexes (P < 0.001). The latter analysis indicated a suppression effect; accounting for maternal care nearly doubled the effect of BPA from 0.35 (P = 0.12) to 0.66 (P < 0.001).
Maternal care had no effect on open-field behavior, except in the 2-µg/kg BPA dose group, in which high maternal care increased distance traveled (P < 0.001). Maternal behavior had no effect on frequency of sniffing or aggression. Elevated maternal behavior increased the probability of both sexes becoming dominant (P < 0.01), and controlling for maternal behavior eliminated the BPA effect on this outcome, indicating that the BPA-dominance effect may be partially mediated by maternal behavior.
Discussion
This study provides evidence that low-dose maternal BPA exposure induces long-lasting disruption to epigenetic pathways in the brain of offspring. This disruption is associated with long-term changes in ER-related gene expression and DNA methylation in the brain and alteration in social and anxiety-like behavior. Importantly, our findings indicate that these BPA-induced changes occur in a sex-specific, brain region-specific, and dose-dependent manner.
The nonmonotonic dose response to hormones and endocrine disruptors has been well documented by using in vitro and in vivo animal studies and supported by evidence from epidemiological studies (25). The analysis of multiple doses, with a logarithmic increase in dose, allowed for the assessment of these nonmonotonic effects in the present study. The doses analyzed include exposures lower than the current reference dose of 50 μg/kg/d, which is considered “safe” for humans (26). Although BPA metabolism in rodents differs from that in humans (27), recent pharmacokinetic scaling experiments estimate that exposures to ∼400 μg/kg/d produce blood concentrations of the unconjugated, bioactive form of BPA in the range of human blood concentrations (1, 25). Therefore, our data indicate that environmentally relevant doses of BPA may induce very different effects dependent on the level of exposure and support the view that appropriate risk assessment of BPA neurotoxicity should involve multiple low-dose exposures (25).
There is increasing concern over the effect of BPA on the developing human brain. One of the most striking neurobiological effects of BPA is the loss of sexual dimorphism in brain structure and behavior illustrated by animal studies (4, 28–31), findings concordant with human epidemiological studies (5–7). The molecular mechanisms underlying BPA-induced disruption of sexually dimorphic phenotypes are not understood. BPA interferes with the estrogen signaling via activation or inhibition of ERs (8). However, several studies have implicated altered expression of ERs in these BPA-induced effects (30, 32–34), which may or may not be mediated via estrogen signaling pathways. Classic ERs, ERα and ERβ, show sex-specific gene expression patterns throughout life and are essential for sexual differentiation, neuroendocrine regulation, and synaptic plasticity (35–37). In addition, ERRγ is an orphan estrogen-related receptor highly expressed in the adult brain (38), in a sex-specific pattern that parallels the expression of classic ER genes (Fig. 2). Here we provide evidence that (i) sexually dimorphic gene expression patterns of ERα, ERβ, and ERRγ are disrupted in the juvenile brain following in utero exposure to low doses (2 and 20 µg/kg) but not after the highest BPA dose (200 µg/kg; with the exception of the cortical and hippocampal ERβ) and (ii) epigenetic mechanisms may be among the molecular mechanisms that mediate these effects.
Epigenetic regulation via DNA methylation is involved in ERα (Esr1) gene expression programming during mouse brain development (20) and is sensitive to environmental cues such as postnatal maternal care in rats (39). Furthermore, studies in rats suggest that DNA methylation status of the Esr1 gene may be sexually dimorphic in the brain throughout life: it differs between sexes in the neonatal and adult hypothalamus (40) and can be altered in a sex-specific way by simulating certain aspects of maternal care (41). The potential of endocrine disruptors to affect epigenetic programming of Esr1 has been the subject of speculation (3, 32), and here we provide experimental evidence that in utero exposure to BPA disrupts DNA methylation patterns of Esr1 and demonstrate this effect to be sex-specific (effects occurring in males or females but not both) and brain region-specific (BPA-induced DNA methylation increases in the cortex and decreases in the hypothalamus). Although we observed that hypermethylation of Esr1 correlated with decreased Esr1 expression in the male prefrontal cortex, unexpectedly, hypomethylation was associated with reduced Esr1 mRNA levels in the female hypothalamus. These data suggest that additional levels of Esr1 gene regulation, such as changes in local histone modifications or levels of transcription factors, can fine-tune Esr1 transcription. It is also possible that DNA methylation within Esr1 gene regions not analyzed in the present study may be altered by BPA and contribute to induced changes in Esr1 gene expression. Within future studies of these effects, isolation of specific subpopulations of cells may enhance our capacity to detect BPA-induced epigenetic changes. Importantly, although postnatal maternal behavior was affected by BPA treatment during pregnancy, the effects of BPA on Esr1 gene expression and exon A methylation were not found to be mediated by postnatal maternal care. However, within exon C of the Esr1 gene, there was an indication that BPA altered methylation levels in the cortex and hypothalamus of both sexes, but these effects were masked by the effect of maternal care. Thus, our data clearly show that in utero BPA exposure has the ability to directly disrupt Esr1 epigenetic programming, and suggest that postnatal environmental factors such as the quality of maternal care may potentially be involved in the moderation of these effects, a finding with significant implications that merits further investigation using cross-fostering manipulations.
BPA-induced changes in DNA methyltransferases may serve as a mechanism for sex-specific epigenetic disruption of Esr1, with the potential for more global, genome-wide effects. Similar to changes in ER-related genes, we found that the sexual dimorphism in the expression of cortical Dnmt3a and hypothalamic Dnmt1 was reversed as a consequence of low-dose (2 µg and 20 µg) prenatal BPA exposure. In the male prefrontal cortex, up-regulation of Dnmt3a mRNA correlated with hypermethylation of Esr1 gene, whereas, in the female hypothalamus, down-regulation of Dnmt1 was associated with hypomethylation of the Esr1 gene. These results provide evidence that in utero BPA exposure may induce sex-specific disruption of DNA methylation pathways (although DNMTs may be only one of many mechanisms operating within these pathways) that may underlie enduring epigenetic changes within the brain.
Our findings of long-lasting disruption of DNA methylation pathways in the prefrontal cortex and hypothalamus may have additional impact when we consider the role of DNMT proteins in the brain beyond gene programming during development. DNMTs are highly expressed in the adult brain (42, 43), and deletion of Dnmt1 and Dnmt3a in mature forebrain neurons results in decreased DNA methylation, altered gene expression, and abnormal long-term hippocampal plasticity and memory deficits (44). Manipulation of Dnmt3a levels in the adult nucleus accumbens has been found to affect global DNA methylation levels and emotional behavior (45). The role of DNMTs in the mature hypothalamus is currently unknown, but Dnmt1 and Dnmt3a are highly expressed in this region and likely play an important role in hypothalamic gene regulation. Therefore, changes in Esr1 methylation that we observed may be the result of developmental disruption of Esr1 epigenetic programming, but they may also be maintained by persistent changes in the levels of DNMTs in mature neurons. However, even more importantly, our data suggest that in utero BPA treatment induces long-lasting epigenetic vulnerability in male and female brains and that numerous genes may be affected via epigenetic mechanisms contributing to BPA-induced brain dysfunction, including effects on behavior.
In the present study, BPA was found to induce persistent, sex-specific, and dose-dependent changes in social, exploratory, and anxiety-like behavior. Our results are consistent with previous studies showing that perinatal or prenatal BPA exposure abolished sex differences in open-field (29, 31) and play behavior (28). Similar to our findings, previous studies have shown that some aspects of social behavior are affected by BPA in a sex-specific way (46) whereas other domains are affected similarly in both sexes (47). Changes in ER expression may, at least in part, underlie behavioral changes that we observed, as ERs have been implicated in social and anxiety-like behaviors (37). ERβ has been associated with an anxiolytic effect in mice (37); anxiety-like behavior can be attenuated by the treatment with selective agonist of ERβ (48) whereas anxiety is increased in ERβ KO female mice (49). Accordingly, in the present study, BPA decreased female ERβ gene expression and increased anxiety-like behavior. The disruption of sexually dimorphic ERβ gene expression patterns we observed correlated well with the reversal of the sex differences in anxiety-like behavior. However, it is evident that changes in ER expression only partially correlate with behavioral changes, possibly because of the complex functional relationship between ERα and ERβ (37): these proteins may act in synergistic, compensatory, or antagonistic fashion, and this may vary with sex, brain region, or behavioral phenotype examined (37, 50). In addition, our study raises an important question about the role of ERRγ in neurobehavioral effects of BPA. Nevertheless, the sex-specific changes and the reversal of sexually dimorphic behaviors are reminiscent of the gene expression and DNA methylation changes we have observed, and suggest that in utero BPA exposure induces the long-lasting disruption of sexually dimorphic phenotypes via epigenetic mechanisms.
The emerging field of sex-specific epigenetic variation highlights the notion that epigenetic gene programming during development and responses to environmental cues are sex-specific and give rise to sex-specific epigenomes (51). This field offers significant promise to improve our understanding of sex-biased mental and cognitive disorders that are environmentally contributed, such as autism, attention deficit hyperactivity disorder, mood disorders, and schizophrenia. Thus, our finding that an in utero environmental exposure can induce sex-specific epigenetic disruption with long-lasting consequences for brain gene expression and behavior is a significant contribution to this area. To fully understand the mechanisms that underlie neurotoxicity of in utero BPA exposure, future studies will be needed to examine the timing and mechanisms of epigenetic disruption and identify the multiple genes and signaling pathways that may be involved. In addition, these studies should include a range of environmentally relevant doses and both sexes to properly assess the BPA potential to affect developing male and female human brain.
Materials and Methods
Pregnant BALB/c mice were orally exposed to BPA dissolved in tocopherol-stripped corn oil or only corn oil (vehicle control) during the entire gestational period. RNA and DNA were extracted simultaneously from dissected PND 28 offspring brain tissue. Quantitative real-time PCR was used to assess gene expression, and Esr1 CpG methylation was assessed by using the bisulfite-pyrosequencing method. Primers used for gene expression and DNA methylation analyses are provided in Tables S4 and S5. Behavioral assessment included (i) observations of home-cage social behavior, (ii) open-field testing, and (iii) social approach and aggression toward a stimulus mouse. Further details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
This research was supported by National Institutes of Health Grant DP2OD001674-01, National Institute of Environmental Health Sciences Grant 5P01ES09600, US Environmental Protection Agency Grant RD834509, the Trustees of the Blanchette Hooker Rockefeller Fund, and the Gladys and Roland Harriman Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1214056110/-/DCSupplemental.
References
- 1.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24(2):139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect. 2008;116(1):39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kundakovic M, Champagne FA. Epigenetic perspective on the developmental effects of bisphenol A. Brain Behav Immun. 2011;25(6):1084–1093. doi: 10.1016/j.bbi.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richter CA, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24(2):199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun JM, et al. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. 2011;128(5):873–882. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun JM, et al. Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect. 2009;117(12):1945–1952. doi: 10.1289/ehp.0900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perera F, et al. Prenatal bisphenol a exposure and child behavior in an inner-city cohort. Environ Health Perspect. 2012;120(8):1190–1194. doi: 10.1289/ehp.1104492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wetherill YB, et al. In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol. 2007;24(2):178–198. doi: 10.1016/j.reprotox.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447(7143):425–432. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- 10.Novikova SI, et al. Maternal cocaine administration in mice alters DNA methylation and gene expression in hippocampal neurons of neonatal and prepubertal offspring. PLoS One. 2008;3(4):e1919. doi: 10.1371/journal.pone.0001919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28(36):9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skinner MK, Anway MD, Savenkova MI, Gore AC, Crews D. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS ONE. 2008;3(11):e3745. doi: 10.1371/journal.pone.0003745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bromer JG, Zhou Y, Taylor MB, Doherty L, Taylor HS. Bisphenol-A exposure in utero leads to epigenetic alterations in the developmental programming of uterine estrogen response. FASEB J. 2010;24(7):2273–2280. doi: 10.1096/fj.09-140533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci USA. 2007;104(32):13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doshi T, Mehta SS, Dighe V, Balasinor N, Vanage G. Hypermethylation of estrogen receptor promoter region in adult testis of rats exposed neonatally to bisphenol A. Toxicology. 2011;289(2-3):74–82. doi: 10.1016/j.tox.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66(11):5624–5632. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang YJ, et al. High dose bisphenol A impairs hippocampal neurogenesis in female mice across generations. Toxicology. 2012;296(1-3):73–82. doi: 10.1016/j.tox.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Yaoi T, et al. Genome-wide analysis of epigenomic alterations in fetal mouse forebrain after exposure to low doses of bisphenol A. Biochem Biophys Res Commun. 2008;376(3):563–567. doi: 10.1016/j.bbrc.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 19.Koš M, Reid G, Denger S, Gannon F. Minireview: Genomic organization of the human ERalpha gene promoter region. Mol Endocrinol. 2001;15(12):2057–2063. doi: 10.1210/mend.15.12.0731. [DOI] [PubMed] [Google Scholar]
- 20.Westberry JM, Trout AL, Wilson ME. Epigenetic regulation of estrogen receptor alpha gene expression in the mouse cortex during early postnatal development. Endocrinology. 2010;151(2):731–740. doi: 10.1210/en.2009-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irizarry RA, et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat Genet. 2009;41(2):178–186. doi: 10.1038/ng.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palanza PL, Howdeshell KL, Parmigiani S, vom Saal FS. Exposure to a low dose of bisphenol A during fetal life or in adulthood alters maternal behavior in mice. Environ Health Perspect. 2002;110(suppl 3):415–422. doi: 10.1289/ehp.02110s3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Champagne FA. Epigenetic influence of social experiences across the lifespan. Dev Psychobiol. 2010;52(4):299–311. doi: 10.1002/dev.20436. [DOI] [PubMed] [Google Scholar]
- 24.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 25.Vandenberg LN, et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33(3):378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.US Environmental Protection Agency 1988. Bisphenol A (CASRN 80-05-7). Integrated Risk Information System. Available at www.epa.gov/iris/subst/0356.htm. Accessed July 4, 2012. [PubMed]
- 27.Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30(1):75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dessì-Fulgheri F, Porrini S, Farabollini F. Effects of perinatal exposure to bisphenol A on play behavior of female and male juvenile rats. Environ Health Perspect. 2002;110(suppl 3):403–407. doi: 10.1289/ehp.110-1241190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kubo K, et al. Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats. Neurosci Res. 2003;45(3):345–356. doi: 10.1016/s0168-0102(02)00251-1. [DOI] [PubMed] [Google Scholar]
- 30.Patisaul HB, Fortino AE, Polston EK. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol Teratol. 2006;28(1):111–118. doi: 10.1016/j.ntt.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Rubin BS, et al. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology. 2006;147(8):3681–3691. doi: 10.1210/en.2006-0189. [DOI] [PubMed] [Google Scholar]
- 32.Cao J, Mickens JA, McCaffrey KA, Leyrer SM, Patisaul HB. Neonatal bisphenol A exposure alters sexually dimorphic gene expression in the postnatal rat hypothalamus. Neurotoxicology. 2012;33(1):23–36. doi: 10.1016/j.neuro.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khurana S, Ranmal S, Ben-Jonathan N. Exposure of newborn male and female rats to environmental estrogens: Delayed and sustained hyperprolactinemia and alterations in estrogen receptor expression. Endocrinology. 2000;141(12):4512–4517. doi: 10.1210/endo.141.12.7823. [DOI] [PubMed] [Google Scholar]
- 34.Monje L, Varayoud J, Muñoz-de-Toro M, Luque EH, Ramos JG. Exposure of neonatal female rats to bisphenol A disrupts hypothalamic LHRH pre-mRNA processing and estrogen receptor alpha expression in nuclei controlling estrous cyclicity. Reprod Toxicol. 2010;30(4):625–634. doi: 10.1016/j.reprotox.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Handa RJ, Ogawa S, Wang JM, Herbison AE. Roles for oestrogen receptor β in adult brain function. J Neuroendocrinol. 2012;24(1):160–173. doi: 10.1111/j.1365-2826.2011.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci. 2011;14(6):677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tetel MJ, Pfaff DW. Contributions of estrogen receptor-α and estrogen receptor-ß to the regulation of behavior. Biochim Biophys Acta. 2010;1800(10):1084–1089. doi: 10.1016/j.bbagen.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lorke DE, Süsens U, Borgmeyer U, Hermans-Borgmeyer I. Differential expression of the estrogen receptor-related receptor gamma in the mouse brain. Brain Res Mol Brain Res. 2000;77(2):277–280. doi: 10.1016/s0169-328x(00)00063-2. [DOI] [PubMed] [Google Scholar]
- 39.Champagne FA, et al. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology. 2006;147(6):2909–2915. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- 40.Schwarz JM, Nugent BM, McCarthy MM. Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span. Endocrinology. 2010;151(10):4871–4881. doi: 10.1210/en.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurian JR, Olesen KM, Auger AP. Sex differences in epigenetic regulation of the estrogen receptor-α promoter within the developing preoptic area. Endocrinology. 2010;151(5):2297–2305. doi: 10.1210/en.2009-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng J, Chang H, Li E, Fan G. Dynamic expression of de novo DNA methyltransferases Dnmt3a and Dnmt3b in the central nervous system. J Neurosci Res. 2005;79(6):734–746. doi: 10.1002/jnr.20404. [DOI] [PubMed] [Google Scholar]
- 43.Goto K, et al. Expression of DNA methyltransferase gene in mature and immature neurons as well as proliferating cells in mice. Differentiation. 1994;56(1-2):39–44. doi: 10.1046/j.1432-0436.1994.56120039.x. [DOI] [PubMed] [Google Scholar]
- 44.Feng J, et al. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat Neurosci. 2010;13(4):423–430. doi: 10.1038/nn.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LaPlant Q, et al. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci. 2010;13(9):1137–1143. doi: 10.1038/nn.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolstenholme JT, et al. Gestational exposure to low dose bisphenol A alters social behavior in juvenile mice. PLoS ONE. 2011;6(9):e25448. doi: 10.1371/journal.pone.0025448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolstenholme JT, et al. Gestational exposure to bisphenol a produces transgenerational changes in behaviors and gene expression. Endocrinology. 2012;153(8):3828–3838. doi: 10.1210/en.2012-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146(2):797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- 49.Imwalle DB, Gustafsson JA, Rissman EF. Lack of functional estrogen receptor beta influences anxiety behavior and serotonin content in female mice. Physiol Behav. 2005;84(1):157–163. doi: 10.1016/j.physbeh.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Ogawa S, et al. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139(12):5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- 51.McCarthy MM, et al. The epigenetics of sex differences in the brain. J Neurosci. 2009;29(41):12815–12823. doi: 10.1523/JNEUROSCI.3331-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.